Abstract
Background
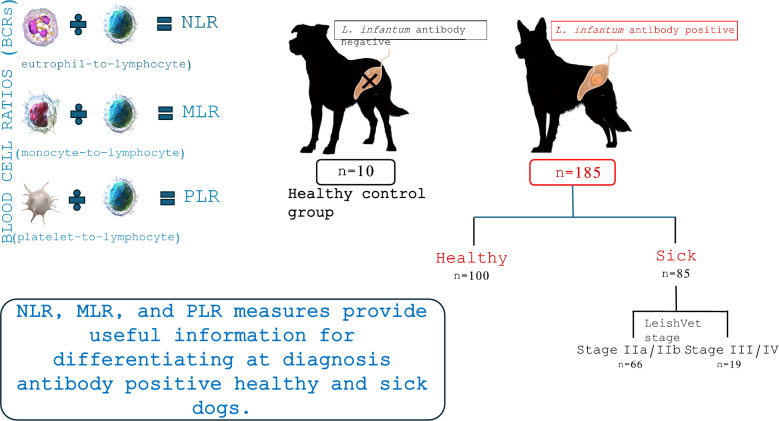
The accuracy of blood cell ratios (BCRs) as cost-effective and easily accessible diagnostic and prognostic markers of inflammatory conditions has been investigated in veterinary medicine in recent years.
Methods
Neutrophil-to-lymphocyte (NLR), monocyte-to-lymphocyte (MLR), and platelet-to-lymphocyte (PLR) ratios were studied in 195 dogs clinically evaluated and tested for anti-Leishmania infantum (Li) antibodies (Li-seronegative (Li−), n = 10; Li-seropositive clinically healthy (Li+healthy), n = 100; Li-seropositive with clinical and/or clinicopathological abnormalities (Li+sick), n = 85). The Li+sick dogs were classified in LeishVet stages IIa/IIb (Li+IIa/IIb) (n = 66) and III/IV (Li+III/IV) (n = 19). BCR relationships with LeishVet clinical stage, antibody levels, and serum protein electrophoretic fraction concentrations were investigated.
Results
Higher NLR values were found in Li+, Li+healthy, and Li+IIa/IIb sick dogs compared to Li− dogs (P < 0.001). Higher NLR and MLR were found in Li+sick (NLR, P < 0.001; MLR, P = 0.034) and Li+III/IV dogs (NLR, P < 0.001; MLR, P = 0.005) compared to Li− dogs, and in Li+III/IV dogs (NLR, P = 0.002; MLR, P < 0.001) compared to Li+healthy. All three BCRs were higher in Li+sick (NLR, MLR, P < 0.001; PLR, P = 0.023) and Li+IIa/IIb dogs (NLR P < 0.001; MLR P = 0.001; PLR, P = 0.012) compared to Li+healthy dogs. The BCRs failed to distinguish dogs with moderate (Li+IIa/IIb) and severe or very severe disease (Li+III/IV). BCRs demonstrated weak positive correlations with serum globulin fractions and antibody levels, and weak negative correlations with serum albumin level were found. Li+sick dogs presenting hypoalbuminemia showed higher MLR ratios (P = 0.001) than those with normal albumin values.
Conclusions
This study shows that BCR measures provide useful information for differentiating antibody-positive healthy and sick dogs at diagnosis. Dogs with hypoalbuminemia showed higher MLR values despite monocytosis being very rare.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-024-06522-z.
Keywords: Canine leishmaniosis, Serum protein electrophoresis, Neutrophil-to-lymphocyte ratio, Monocyte-to-lymphocyte ratio, Platelet-to-lymphocyte ratio, Clinical staging and LeishVet
Background
Canine leishmaniosis (CanL) is a zoonotic disease caused by Leishmania infantum with a severe fatal course in some dogs [1]. Leishmaniosis is endemic in more than 70 countries, including those in Southern Europe, Northern Africa, the Middle East, Central Asia, China, and South America, and dogs represent the main domestic reservoir for L. infantum infection [2, 3]. The main route of transmission in endemic areas is vectorial, through the bite of female phlebotomine sand flies [1, 2]. However, other modes of transmission have been documented such as transplacental and venereal infections, or by transfusion of infected canine blood products. The non-vectorial transmission has a primary role in the epidemiology of foci of CanL in non-endemic areas where competent vectors are not present [1].
The course of CanL is influenced by the type of the dog immune response [4, 5]. In fact, the development of a progressive infection underlying the disease is associated with a marked humoral immune response and downregulation in host cell-mediated immunity [4, 5]. Individual dogs show different levels of both antibody and cellular adaptive immune responses. In endemic areas, most infected dogs are apparently healthy or have slight clinicopathological abnormalities, while others have a variably severe course of disease. Therefore, clinical staging systems are useful for treatment choice and to formulate prognosis [4]. The LeishVet clinical staging system takes into consideration the antibody level and the type of clinical signs and clinicopathological abnormalities detected [1].
Dogs with leishmaniosis may present various clinical signs and clinicopathological abnormalities that reflect an intense systemic inflammatory response. Markers of inflammation have been investigated primarily in dogs with clinical leishmaniosis, and increased levels of positive acute-phase proteins (APPs) such as serum ferritin [6–14], C-reactive protein (CRP) [6, 8–17], haptoglobin (Hp) [8, 11, 13–16], serum amyloid A (SAA) [8, 15, 16], and ceruloplasmin [8, 15] have been observed. Similarly, hypoalbuminemia and a decrease in other negative APPs such as transferrin (or total iron-binding capacity: TIBC) [6, 8] and paraoxonase 1 (PON-1) [8, 10, 11, 14] were reported. Increased levels of α2-globulins and γ-globulins were found in serum protein electrophoresis (SPE) analysis [8, 11, 13, 15, 17], and increases were found in the total level of immunoglobulins G (IgG) and M (IgM) [16].
The complete blood count (CBC) of dogs with clinical leishmaniosis may show a mild-normocytic normochromic non-regenerative anemia as a consequence of the chronic inflammation and sequestration of iron in macrophages; however, anemia can be moderate or severe in dogs with advanced chronic renal disease as additional patho-mechanisms occur [8]. White blood cell abnormalities are variable and may include neutrophilia, lymphopenia, lymphocytosis, and eosinophilia [1, 3, 8]. Moderate thrombocytopenia [8] or thrombocytosis [18] can also be detected.
There is great interest in cost-effective and easily accessible markers of inflammation because of their clinical relevance in prognosis and monitoring of diseases. Blood cell ratios (BCRs) have been extensively investigated with this aim in human medicine [19–36]. In dogs, BCRs have been examined in various infectious [37–40] and non-infectious [41–48] inflammatory conditions. Two studies [49, 50] evaluated BCRs in dogs with L. infantum infection comparing healthy and sick dogs [49] and assessing the prognostic potential of BCRs in dogs with chronic renal disease associated with L. infantum infection that were followed up [50].
This study considered some BCRs in L. infantum antibody-positive dogs, based on the hypothesis that differences may exist with L. infantum antibody-negative dogs and also between L. infantum antibody-positive healthy and sick dogs and among dogs with different severity of disease. With this aim, we studied neutrophil-to-lymphocyte (NLR), monocyte-to-lymphocyte (MLR), and platelet-to-lymphocyte (PLR) ratios in healthy seronegative dogs and L. infantum antibody-positive healthy and sick dogs. Specifically, we analyzed (a) differences between L. infantum-negative and seropositive dogs, (b) differences between L. infantum antibody-positive healthy and sick dogs, and (c) differences between dogs with different severity of leishmaniosis. Additionally, we analyzed (d) differences between L. infantum antibody-positive dogs presenting abnormalities in concentrations of the electrophoretic fractions and those with values within the reference intervals, and (e) relationships of BCRs with antibody levels and serum protein electrophoretic fraction concentrations.
Methods
Study description
A review of medical records of 185 apparently healthy dogs partly included in a previous published study was performed [51]. Specifically, data from 172 seropositive dogs (91 healthy dogs and 81 sick dogs) were from a multicentric study on the clinical status of L. infantum antibody-positive apparently healthy dogs in endemic areas [51]. Only dogs with CBC and SPE data available were selected. Among stage II sick dogs, only dogs substaged according to the measurement of proteinuria were included [1]. Data from an additional 13 L. infantum antibody-positive, apparently healthy dogs clinically evaluated by practitioners participating in the multicentric study were also included (nine healthy and four sick dogs). Ten beagles purchased from a breeder for research use (Isoquimen, Sant Feliu de Codines, Spain) were included as controls. They were housed indoors at UAB (Autonomous University of Barcelona) Veterinary School and were enrolled as control dogs because they were clinically healthy based on physical examination, CBC, biochemical profile with urinalysis and urine protein-to-creatinine ratio (UPC), SPE, and antibody negative to L. infantum antigen. Dogs were sampled between February 2020 and June 2021 in different areas of Spain (n = 161) and Italy (n = 34). Data regarding signalment, history, and physical examination findings were recorded. From the database of studied dogs, data related to their LeishVet clinical stage [1], CBC, SPE, and anti-L. infantum antibody levels were selected for the present study [51]. A total of 195 dogs (anti-L. infantum antibody-negative healthy dogs, Li− n = 10; anti-L. infantum antibody-positive dogs, Li+ n = 185 including both healthy dogs, Li+healthy n = 100 and dogs with clinical and/or clinicopathological abnormalities, Li+sick n = 85) were studied. The Li− group (median age: 24, range: 12–24 months; 25th–75th percentile = 15–24) included five male and five female Beagle dogs, classified as healthy seronegative according to physical examination and laboratory testing (CBC, SPE, biochemistry, and urinalysis with UPC). The Li+ dogs included more males (Li+healthy n = 63; Li+sick, n = 53) than females (Li+healthy, n = 37; Li+sick, n = 32), with 82 crossbreed and 103 purebred dogs of 34 different breeds (Supplementary Table 1). The age range was 12–168 months in Li+healthy dogs (median age = 48 months; 25th–75th percentile = 36–75) and 5–144 months in Li+sick dogs (median age = 60 months; 25th–75th percentile = 36–96). The Li+sick dogs were classified according to the LeishVet clinical staging system [1], and two groups of staged dogs were considered for statistical analysis: stage IIa/IIb group (Li+IIa/IIb, n = 66) including dogs with moderate disease and the stage III/IV group (Li+III/IV, n = 19) including dogs with severe/very severe disease.
Clinicopathological and serological evaluation
The CBC was performed using the XN-1000 analyzer (Sysmex España SL, Sant Just Desvern, Spain) or Advia 2120 (Siemens Healthcare SRL, Milan, Italy), and blood smears were also examined for cell morphological abnormalities, detection of hemoparasites, and to exclude samples from the statistical analysis when platelet clumps were observed. The absolute concentrations of lymphocytes, neutrophils, monocytes, and platelets were evaluated. Neutrophil, monocyte, and platelet values were divided by absolute concentrations of lymphocytes, and neutrophil-to-lymphocytes (NLR), monocytes-to-lymphocytes (MLR), and platelet-to-lymphocyte (PLR) ratios were calculated. The PLR was calculated in overall 100 dogs, as in 95 dogs platelet aggregates were detected in blood smears and platelet concentration could not be used. The SPE was evaluated using the Capillarys 3 (Sebia Dubai SA, Dubai, UAE), and reference intervals are reported in Supplementary Table S2. An in-house enzyme-linked immunosorbent assay (ELISA) was performed on the sera of all dogs studied for the detection of anti-Leishmania antibodies as previously described [51]. The result was quantified as ELISA units (EU) and sera were classified as high positive when having a positivity percentage equal to or higher than 300 EU, medium positive when having a positive percentage equal to or higher than 150 EU and less than 300 EU, and low positive when having a positivity percentage lower than 150 EU and equal to or higher than 35 EU [51]. ELISA endpoint values were measured in all samples classified as medium or high positive, performing twofold serial dilutions [51].
Statistical analysis
Statistical analysis was performed using Jamovi 2.3.28.0 statistical software. The distribution of continuous variables was evaluated by the Shapiro–Wilk test and descriptive statistics were obtained for all the investigated variables.
The Mann–Whitney U-test was used to evaluate differences in endpoint ELISA levels, SPE fractions, and BCRs between groups of dogs as follows: Li− vs. Li+, Li− vs. Li+healthy, Li− vs. Li+sick, Li+healthy vs. Li+sick, Li− vs. Li+IIa/IIb, Li− vs. Li+III/IV, Li+healthy vs. Li+IIa/IIb, Li+healthy vs. Li+III/IV, Li+IIa/IIb vs. Li+III/IV. Similarly, the Mann–Whitney U-test was used to evaluate differences in lymphocyte, neutrophil, monocyte, and platelet concentrations among Li− and Li+, Li+healthy and Li+sick, Li+healthy and Li+IIa/IIb, Li+healthy and Li+III/IV, Li+IIa/IIb and Li+III/IV. The number of Li+sick dogs with out-of-range lymphocyte, monocyte, neutrophil, and platelet concentrations was evaluated, and the prevalence in Li+IIa/IIb and Li+III/IV dogs was compared by Fisher’s exact test.
Spearman’s rho test was used to measure the strength of the correlations between NLR, MLR, and PLR values and SPE fractions in the total cohort and endpoint ELISA levels in the Li+ dogs. The strength of this relationship, according to the correlation coefficient absolute value (rs), was qualified as follows: rs = 1: perfect correlation; 1 > rs ≥ 0.8: strong correlation; 0.8 > rs ≥ 0.4: moderate correlation; 0.4 > rs > 0.141 (NLR, MLR) or > 0.199 (PLR): weak correlation; rs < 0.141 (NLR, MLR) or < 0.199 (PLR): no correlation [52]. The critical value of rs was established on the basis of the number of degrees of freedom for each parameter evaluated [53]. Differences were considered significant if P-values were < 0.05.
Results
Descriptive statistics and significant Mann–Whitney U-test of ELISA levels, NLR, MLR, PLR, and SPE fractions results are presented in Table 1. All three BCRs were higher in Li+sick and Li+IIa/IIb dogs compared to Li+healthy dogs. Higher NLR and MLR were found in Li+sick and Li+III/IV dogs compared to Li− dogs and in Li+III/IV dogs compared to Li+healthy dogs. Li− dogs had significantly lower NLR values than any category of antibody-positive dogs considered, except for Li+III/IV dogs.
Table 1.
Descriptive statistics of blood cell ratios, ELISA levels (ELISA units), and serum electrophoretic fractions (g/l) for the enrolled dogs
|
Li− Median (Min–Max) [25th–75th] (n = 10) |
Li+ Median (Min–Max) [25th–75th] (n = 185) |
Li+healthy Median (Min–Max) [25th–75th] (n = 100) |
Li+sick Median (Min–Max) [25th–75th] (n = 85) |
Li+IIa/IIb Median (Min–Max) [25th–75th] (n = 66) |
Li+III/IV Median (Min–Max) [25th–75th] (n = 19) |
Mann–Whitney U-test P |
|
|---|---|---|---|---|---|---|---|
| NLR |
1.49 (1.05–3.91) [1.28–1.73]A,B,C,E,F |
3.1 (1–48) [2.3–4.5]A |
2.8 (1.3–30) [2.1–3.8]B,D,G,H |
3.7 (1–48) [2.6–5.7]C,D |
3.7 (1–48) [2.52–5.78]E,G |
4 (1.6–14.3) [3.3–5.55]F,H |
< 0.001A,B,C,D,E,F,G 0.002H |
| MLR |
0.18 (0.12–0.35) [0.15–0.22]C,F |
0.2 (0.0–1.3) [0.1–0.4] |
0.2 (0.1–1.3) [0.1–0.3]D,G,H |
0.3 (0–1.2) [0.2–0.4]C,D |
0.3 (0–1.2) [0.2–0.4]G |
0.3 (0.1–1.1) [0.3–0.5]F,H |
0.001G < 0.001D,H 0.005 F 0.034C |
| PLR |
123 (60.4–217) [94.1–160] |
123 (17.2–1290) [77.3–182] |
107 (40.5–267) [73.5–158]D, G |
150 (17.2–1290) [87.9–197]D |
163 (31.8–1290) [101–197]G |
136 (17.2–484) [82.4–193] |
0.012G 0.023D |
| ELISA |
5.79 (4.42–7.65) [4.97–6.24] |
241 (4–11,114) [119–813] |
142 (4–1210) [101–250]D,G,H |
752 (4.4–11,114) [197–2933]D |
616 (87.2–8594) [187–2136]G |
1794 (4.4–11,114) [281–3405]H |
< 0.001D,G,H |
| Albumin |
32.5 (29.7–35.7) [31.8–33.3]B,F |
34.6 (15.3–49.1) [31.1–37.8] |
36.5 (25.5–45.4) [34.3–39]B,D,G,H |
31.6 (15.3–49.1) [27.1–34.6]D |
32.9 (21.2–49.1) [28.8–35.3]G,I |
27.3 (15.3–34.1) [22.6–30.7]F,H,I |
< 0.001B,D,F,G,H,I |
| α1-Globulin |
3.45 (3–4) [3.18–3.65] |
3.5 (1.7–10.2) |
3.4 (1.7–4.8) [2.9–3.73]D,G,H |
3.7 (2–10.2) [3.2–4.3]D |
3.7 (2–5.9) [3.2–4.27]G |
3.7 (2.8–10.2) [3.25–4.6]H |
0.008G 0.002D 0.013H |
| α2-globulin |
6.9 (5.3–8.6) [5.95–7.38]C,E,F |
7.2 (2.8–18.1) [6.2–8.7] |
6.5 (2.8–12.7) [5.7–7.5]D,G,H |
8.4 (4.5–18.1) [7.1–9.5]C,D |
8.45 (4.9–18.1) [7.23–9.17]E,G |
8.1 (4.5–13.6) [6.85–11]F,H |
< 0.001D,G,H 0.004C,E 0.020 F |
| β-globulin |
7.1 (6.5–8.3) [6.95–7.73]A,B,C,E,F |
12.9 (1.4–37.9) [11.4–15.4]A |
12.1 (1.4–24.3) [10.4–14.2]B,D,G,H |
14.1 (9.2–37.9) [12.1–17.7]C,D |
13.9 (9.2–37.9) [12.1–16.9]E,G |
15.3 (10.3–24.8) [13.1–19.9]F,H |
< 0.001A,B,C,D,E,F,G,H |
| γ-globulin |
3.45 (2.6–4.7) [3.2–4.17]A,B,C,E,F |
9.8 (4.4–56) [7.7–14.1]A |
8.2 (4.4–13.7) [7–9.4]B,D,G,H |
14.5 (5.3–56) [11–21.6]C,D |
14.1 (5.3–50.8) [11–19]E,G,I |
22.1 (8.6–56) [12.3–30]F,H,I |
< 0.001A,B,C,D,E,F,G,H 0.024I |
Min minimum, Max maximum, 25th 25th percentile, 75th 75th percentile, P P-values, NLR neutrophil-to-lymphocyte ratio, MLR monocyte-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio, Li− anti-L. infantum antibody-negative healthy dogs, Li+ anti-L. infantum antibody-positive dogs, Li+healthy Li-seropositive healthy dogs, Li+sick dogs with clinical and/or clinicopathological abnormalities, Li+IIa/IIb dogs in LeishVet stage IIa/IIb, Li+III/IV dogs in LeishVet stage III/IV. § = PLR was calculated in 100 dogs, 7 Li−, 54 Li+healthy, 39 Li+sick, 27 Li+IIa/IIb dogs, and 12 Li+III/IV dogs respectively. Endpoint ELISA results were evaluated for each group of Li+ dogs. Significant comparisons: A = Li+ > Li−; B = Li+healthy > Li−; C = Li+sick > Li−; D = Li+sick > Li+healthy; E = Li+IIa/IIb > Li−; F = Li+III/IV > Li−; G = Li+IIa/IIb > Li+healthy; H = Li+III/IV > Li+healthy; I = Li+III/IV > Li+IIa/IIb
Descriptive statistics and significant Mann–Whitney U-test results for lymphocyte, neutrophil, monocyte, and platelet concentrations are shown in Supplementary Table 3 (Table S3), and the number of Li+sick dogs with out-of-range values of these blood cell concentrations are reported in Supplementary Table 4 (Table S4).
Correlations of BCRs with ELISA and SPE results evaluated in the total cohort are described in Table 2. BCRs demonstrated weak positive correlations with serum globulin fractions and antibody levels, and weak negative correlations with serum albumin level were found. Li+sick dogs with hypoalbuminemia had higher MLR (median = 0.450; range = 0.3–0.7; 25th–75th percentile = 0.325–0.575) (P = 0.001) than those with albumin values within the reference interval (median = 0.3; range = 0.0–1.2; 25th–75th percentile = 0.2–0.4). No other differences were found concerning the other electrophoretic fractions, and NLR and PLR.
Table 2.
Spearman’s rho test between neutrophil-to-lymphocyte (NLR), monocyte-to-lymphocyte (MLR), platelet-to-lymphocyte (PLR) ratios, and endpoint ELISA or serum protein electrophoresis in the total cohort
| NLR | MLR | PLR | |||||
|---|---|---|---|---|---|---|---|
| Albumin | rs | −0.150* | –0.369* | −0.159 | |||
| P | 0.036 | < 0.001 | 0.114 | ||||
| α1-Globulins | rs | 0.158* | 0.184* | 0.244* | |||
| P | 0.027 | 0.010 | 0.014 | ||||
| α2-Globulins | rs | 0.361* | 0.309* | 0.299* | |||
| P | < 0.001 | < 0.001 | 0.003 | ||||
| β-Globulins | rs | 0.143* | 0.076 | −0.081 | |||
| P | 0.047 | 0.294 | 0.425 | ||||
| γ-Globulins | rs | 0.306* | 0.276* | 0.116 | |||
| P | < 0.001 | < 0.001 | 0.251 | ||||
| ELISAa | rs | 0.185* | 0.294* | 0.248* | |||
| P | 0.012 | < 0.001 | 0.017 | ||||
rs = Spearman’s rho. * Significant difference. a Spearman’s rho test between NLR, MLR, PLR, and ELISA levels was evaluated only in Li+ dogs
Discussion
The purpose of this study was to evaluate selected BCRs (NLR, MLR, and PLR) as markers for differentiating L. infantum antibody-positive healthy and sick dogs and for staging the severity of disease in sick dogs. We found higher NLR values in Li-seropositive compared to Li-seronegative dogs. Interestingly, in the Li+ sick dogs ,the NLR, MLR, and PLR values were higher than in Li+ healthy dogs, and (excluding the PLR) in Li-seronegative animals as well. Furthermore, Li+ sick dogs presenting hypoalbuminemia showed higher MLR ratios than animals with normal albumin values.
Clinically staged sick dogs with both severe/very severe (stages III/IV) and moderate (stages IIa/IIb) disease had higher NLR values than Li+ healthy and Li− dogs. However, NLR failed to distinguish dogs between moderate (stages IIa/IIb) and severe or very severe disease (stages III/IV). Stage II is the more frequently observed LeishVet stage in dogs receiving a diagnosis of clinical leishmaniosis in endemic areas and the number of sick dogs with severe or very severe disease was low because all the studied dogs were from a population of apparently healthy dogs from L. infantum-endemic areas [4, 51]. Moreover, LeishVet stage I dogs were not studied as they have mild clinical signs and can be antibody negative, while we considered data from antibody-positive apparently healthy dogs, and this is a limitation for the aim to consider BCRs as markers of disease severity [1, 4, 51, 54, 55]. However, significant correlations of BCRs with two markers of disease severity (high antibody levels and abnormalities in SPE fractions) were found (Table 2) [1, 4]. As could be expected, hypoalbuminemia and increases in γ-globulins were confirmed to be markers of disease severity, as their values were significantly different among dogs with moderate and severe disease (Table 1).
Only two previous investigations have evaluated BCR values in CanL. Ferreira et al. (2021) compared NLR values among symptomatic and asymptomatic L. infantum-positive dogs and with L. infantum-negative control dogs [49]. They reported higher values in symptomatic than in asymptomatic dogs and in both groups compared to control dogs, in agreement with the present results [49]. Duran-Galea et al. (2024) focused on the prognostic value of NLR and PLR in leishmaniotic dogs with chronic kidney disease (CKD) staged according to the Immune Reconstitution Inflammatory Syndrome (IRIS) stage system [50]. Interestingly, they reported that the progression of CKD was positively correlated with NLR values and a short-term fatal course of disease [50]. In the present study, the NLR was useful for differentiating seropositive healthy and sick dogs and correlated with markers of disease severity.
BCRs as diagnostic and prognostic markers of various inflammatory conditions in dogs have been more extensively investigated [37–45]. In CanL, both acute and chronic inflammation may occur [56, 57], and increases in neutrophil and monocyte concentrations can be observed [58]. Neutrophilia and monocytosis were both rarely found in the present study; however, the group of dogs with more severe disease had significantly higher values of monocytes than Li+healthy dogs. Conversely, lymphopenia contributed to the increases in BCRs observed in the Li+sick dogs and it occurred in dogs with both moderate and severe disease, likely due to stress leukogram. Importantly, we found no cases of lymphocytosis that are reported in mild forms of CanL [59]. The relationship between NLR and hypoalbuminemia has been investigated in other inflammatory canine diseases [41, 44]. Benvenuti et al. (2020) evaluated NLR values in dogs affected by inflammatory bowel disease (IBD) and found a negative correlation between NLR and albumin values [44]. Becher et al. (2021) reported the same result in dogs with chronic enteropathy [41]. To the best of our knowledge, no data are available in the literature on the relationship between NLR, MLR, and PLR values with the other serum electrophoretic fractions.
In the present study, PLR values of the total cohort were positively correlated with ELISA and α-globulin measures (Table 2), and they differentiated healthy and sick dogs (Table 1). Other differences were not found, and we have to consider that this ratio was obtained in a lower number of dogs (n = 100) compared to NLR and MLR (n = 195), as PLR values were not calculated when platelets clumps were observed in blood smears. This is a limitation for the feasibility of platelet concentration that may occur in practice. Additionally, thrombocytopenia was more frequent than thrombocytosis, and significantly higher numbers of platelets were observed only in dogs with moderate disease versus Li+healthy dogs. From the overall evaluation of the significant correlations, it appears that NLR and MLR could be more useful in the clinicopathological evaluation of disease severity.
Reference intervals for NLR, MLR, and PLR in healthy dogs are not currently available, so their use in routine practice is not generally performed. However, some studies have proposed reference intervals for NLR in healthy control dogs, using different methodologies, and reporting vastly different upper limits: 10.91 [38] and 4.1 [41]. Defining a reference interval for healthy dogs was not one of the aims of the present study; however, our data add more information to the literature. The upper limit for NLR recorded in negative healthy dogs of the present study (3.91) was similar to that reported by Becher et al. (2021) [41]. Conversely, no published data are available on MLR and PLR upper limits in healthy dogs; therefore, comparisons with other results are not possible, and additional large studies are needed to establish reference intervals.
We considered these data preliminary, as we only evaluated the relationship between some BCRs and changes in serum electrophoretic fractions. Other BCRs and the relationship among BCRs and additional clinicopathological parameters were not determined. Apart from the relationship of BCRs with other CBC, biochemical, and urinary data, those with APP changes currently available from commercial laboratories and studied in dogs with leishmaniosis could be considered in further studies [6–17]. Breed-related differences in absolute concentrations of white blood cells and platelets were not recognized among the pure breeds of enrolled dogs. The demographic data for studied dogs could be a limitation, as control dogs were all beagles and significantly younger than the antibody-positive dogs. Moreover, no beagle breed dog was among the L. infantum antibody-positive dogs. Bourgès-Abella et al. [60] found moderate differences between the reference intervals obtained in a large group of laboratory beagles and those previously reported for various breeds [60]. However, Kimura and Kotani [61] did not find age-related variations in white blood cell and platelet concentrations in beagles from 6 to 60 months of age [61].
Validation of the BCRs as diagnostic and prognostic markers of CanL is of great practical interest, particularly when clinical decisions have to be made on the basis of cost-effective and easily accessible diagnostic investigations. The BCRs are easily calculated from the CBC report and are available with no additional blood tests. This means that no additional blood volume is required, and no extra costs are charged to owners. In fact, apart from some patients with critical conditions, the CBC is always included in the minimal clinicopathological evaluation database of dogs, in toy breed dogs, and in cases of severe anemia when the volume of blood taken is restricted. The overall results of this study support the hypothesis that the studied BCRs could be an additional marker for CanL.
Conclusions
The BCRs measured provided useful information for differentiating antibody L. infantum-positive healthy and sick dogs, but with the limitations of the present study, a clear differentiation among dogs with different severity of disease was not possible. However, dogs with hypoalbuminemia showed higher MLR values despite the fact that monocytosis was very rare.
Supplementary Information
Acknowledgements
Angela Burrascano (University of Messina) for technical collaboration. The paper has been sponsored by Elanco Animal Health in the framework of the CVBD World Forum Symposium.
Author contributions
Conceptualization: GD, MGP, LSG; methodology: MGP, GD, MB, LSG; formal analysis: GD, MGP; investigation: GD, MB, IMF; resources: MGP, LSG, CM; data curation: GD, MGP, MB; writing —original draft preparation: GD, MGP; writing—review and editing: GD, MGP, LSG, MB, CM; supervision: MGP, LSG. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partly funded by Ecuphar Veterinaria SLU.
Availability of data and materials
No datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
Study authorization was obtained from the Spanish authority Agencia Española de Medicamentos y Productos Sanitarios (AEMPS), with authorization number 008/EPA-2383ESP, the Ethics Committee of Comissió d’Ètica en l’Experimentació Animal i Humana de la Universitat Autònomade Barcelona (CEAAH 4526, November 2018), and the Generalitat de Catalunya (FUE-2018- 0944112 i ID KSHYD6LVR, April 2019).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Solano-Gallego L, Koutinas A, Miró G, Cardoso L, Pennisi MG, Ferrer L, et al. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet Parasitol. 2009;165:1–18. [DOI] [PubMed] [Google Scholar]
- 2.Baneth G, Koutinas AF, Solano-Gallego L, Bourdeau P, Ferrer L. Canine leishmaniosis - new concepts and insights on an expanding zoonosis: part one. Trends Parasitol. 2008;24:324–30. [DOI] [PubMed] [Google Scholar]
- 3.Baneth G, Solano-Gallego L. Leishmaniasis. Vet Clin North Am Small Anim Pract. 2022;52:1359–75. [DOI] [PubMed] [Google Scholar]
- 4.Solano-Gallego L, Montserrrat-Sangrà S, Ordeix L, Martínez-Orellana P. Leishmania infantum-specific production of IFN-γ and IL-10 in stimulated blood from dogs with clinical leishmaniosis. Parasit Vectors. 2016;9:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez-Orellana P, Marí-Martorell D, Montserrat-Sangrà S, Ordeix L, Baneth G, Solano-Gallego L. Leishmania infantum-specific IFN-γ production in stimulated blood from dogs with clinical leishmaniosis at diagnosis and during treatment. Vet Parasitol. 2017;248:39–47. [DOI] [PubMed] [Google Scholar]
- 6.Silvestrini P, Zoia A, Planellas M, Roura X, Pastor J, Cerón JJ, et al. Iron status and C-reactive protein in canine leishmaniasis. J Small Anim Pract. 2014;55:95–101. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Subiela S, Cerón JJ, Strauss-Ayali D, Garcia-Martinez JD, Tecles F, Tvarijonaviciute A, et al. Serum ferritin and paraoxonase-1 in canine leishmaniosis. Comp Immunol Microbiol Infect Dis. 2014;37:23–9. [DOI] [PubMed] [Google Scholar]
- 8.Paltrinieri S, Gradoni L, Roura X, Zatelli A, Zini E. Laboratory tests for diagnosing and monitoring canine leishmaniasis. Vet Clin Pathol. 2016;45:552–78. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Subiela S, Pardo-Marín L, Tecles F, Baneth G, Cerón JJ. Serum C-reactive protein and ferritin concentrations in dogs undergoing leishmaniosis treatment. Res Vet Sci. 2016;109:17–20. [DOI] [PubMed] [Google Scholar]
- 10.Rubio CP, Martinez-Subiela S, Tvarijonaviciute A, Hernández-Ruiz J, Pardo-Marin L, Segarra S, et al. Changes in serum biomarkers of oxidative stress after treatment for canine leishmaniosis in sick dogs. Comp Immunol Microbiol Infect Dis. 2016;49:51–7. [DOI] [PubMed] [Google Scholar]
- 11.Cantos-Barreda A, Escribano D, Cerón JJ, Bernal LJ, Furlanello T, Tecles F, et al. Relationship between serum anti-Leishmania antibody levels and acute phase proteins in dogs with canine leishmaniosis. Vet Parasitol. 2018;260:63–8. [DOI] [PubMed] [Google Scholar]
- 12.Ceron JJ, Pardo-Marin L, Caldin M, Furlanello T, Solano-Gallego L, Tecles F, et al. Use of acute phase proteins for the clinical assessment and management of canine leishmaniosis: general recommendations. BMC Vet Res. 2018;14:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daza González MA, Fragío Arnold C, Fermín Rodríguez M, Checa R, Montoya A, Portero Fuentes M, et al. Effect of two treatments on changes in serum acute phase protein concentrations in dogs with clinical leishmaniosis. Vet J. 2019;245:22–8. [DOI] [PubMed] [Google Scholar]
- 14.Pardo-Marin L, Ceron JJ, Tecles F, Baneth G, Martínez-Subiela S. Comparison of acute phase proteins in different clinical classification systems for canine leishmaniosis. Vet Immunol Immunopathol. 2020;219:109958. [DOI] [PubMed] [Google Scholar]
- 15.Martínez-Subiela S, Bernal LJ, Cerón JJ. Serum concentrations of acute-phase proteins in dogs with leishmaniosis during short-term treatment. Am J Vet Res. 2003;64:1021–6. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Subiela S, Strauss-Ayali D, Cerón JJ, Baneth G. Acute phase protein response in experimental canine leishmaniasis. Vet Parasitol. 2011;180:197–202. [DOI] [PubMed] [Google Scholar]
- 17.Cavalera MA, Gernone F, Uva A, D’Ippolito P, Roura X, Paltrinieri S, et al. Effect of domperidone (leisguard®) on antibody titers, inflammatory markers and creatinine in dogs with leishmaniosis and chronic kidney disease. Parasit Vectors. 2021;14:525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirschmann LC, Simon CF, Brod CS, Radin J, Rosa CS, Recuero ALC. Clinical and hematological evaluation of leishmaniasis serum-positive dogs in Rio Grande do Sul. Sci Anim Heath. 2016;4:179–97. [Google Scholar]
- 19.Chuang SH, Chang CH. Platelet-to-lymphocyte ratio and lymphocyte-to-monocyte ratio in glaucoma: a meta-analysis. Biomark Med. 2024;18:39–49. [DOI] [PubMed] [Google Scholar]
- 20.Ye JH, Zhang Y, Naidoo K, Ye S. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in psoriasis: a systematic review and meta-analysis. Arch Dermatol Res. 2024;316:85. [DOI] [PubMed] [Google Scholar]
- 21.Choi ME, Jung JM, Kim DH, Won CH, Chang SE, Lee MW, et al. Baseline Serum neutrophil-to-lymphocyte ratio in acral melanoma compared with nonacral melanoma and its prognostic significance. J Am Acad Dermatol. 2024;90:977–85. [DOI] [PubMed] [Google Scholar]
- 22.Firment J, Hulin I. Zahorec index or neutrophil-to-lymphocyte ratio, valid biomarker of inflammation and immune response to infection, cancer and surgery. Bratisl Lek Listy. 2024;125:75–83. [DOI] [PubMed] [Google Scholar]
- 23.Nairn L, Sivaratnam S, Bali K, Wood TJ. Neutrophil to lymphocyte ratio as an indicator of periprosthetic joint infection: a retrospective cohort study. J Am Acad Orthop Surg. 2024;32:271–8. [DOI] [PubMed] [Google Scholar]
- 24.Wang D, Wang Y, Dong X, Yu M, Cai H. The significance of preoperative neutrophil-to-lymphocyte ratio in predicting short-term complications and survival benefits of pancreaticoduodenectomy: A systematic review and meta-analysis. Am J Surg. 2024;229:76–82. [DOI] [PubMed] [Google Scholar]
- 25.Xie J, Guo Z, Zhu Y, Ma M, Jia G. Peripheral blood inflammatory indexes in breast cancer: a review. Medicine (Baltimore). 2023;102:e36315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diana R, Pierluigi M, Dardo M, Claudia A, Rosario R, Luigi L. The prognostic role of pre-treatment platelet-to-lymphocyte ratio in head and neck squamous cell carcinoma: meta-analysis and trial sequential analysis. J Evid Based Dent Pract. 2023;23:101898. [DOI] [PubMed] [Google Scholar]
- 27.Yang N, Yang K, Pan S, He Q, Jin J. Progress in the application of the neutrophil-to-lymphocyte ratio in dialysis-related complications. Ren Fail. 2023;45:2259996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tudurachi BS, Anghel L, Tudurachi A, Sascău RA, Stătescu C. Assessment of inflammatory hematological ratios (NLR, PLR, MLR, LMR and monocyte/HDL-cholesterol ratio) in acute myocardial infarction and particularities in young patients. Int J Mol Sci. 2023;24:14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wladis EJ, Bohnak CE, Law JJ, Adam AP, Rothschild MI, Pauze DR. Neutrophil-to-lymphocyte ratios distinguish idiopathic orbital inflammation from orbital infectious disease. Ophthalmic Plast Reconstr Surg. 2024;40:178–80. [DOI] [PubMed] [Google Scholar]
- 30.Shavakhi M, Nourigheimasi S, Dioso E, Goutnik M, Lucke-Wold B, Khanzadeh S, et al. Prognostic role of neutrophil to lymphocyte ratio in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Can J Gastroenterol Hepatol. 2022;2022:1554079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.da Silva RMFL, Borges LE. Neutrophil-lymphocyte ratio and red blood cell distribution width in patients with atrial fibrillation and rheumatic valve disease. Curr Vasc Pharmacol. 2023;21:367–77. [DOI] [PubMed] [Google Scholar]
- 32.van Holstein Y, van den Berkmortel PJE, Trompet S, van Heemst D, van den Bos F, Roemeling-van Rhijn M, et al. The association of blood biomarkers with treatment response and adverse health outcomes in older patients with solid tumors: a systematic review. J Geriatr Oncol. 2023;14:101567. [DOI] [PubMed] [Google Scholar]
- 33.Adane T, Melku M, Worku YB, Fasil A, Aynalem M, Kelem A, et al. The association between neutrophil-to-lymphocyte ratio and glycemic control in type 2 diabetes mellitus: a systematic review and meta-analysis. J Diabetes Res. 2023;2023:3117396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishna Reddy CH, Achari PK, Nisha B, Radha AR. Significance of laboratory markers in predicting the severity of COVID-19 in the central reserve police force front-line workers with a review of literature. Indian J Public Health. 2022;66:512–5. [DOI] [PubMed] [Google Scholar]
- 35.Farias JS, Villarreal EG, Savorgnan F, Acosta S, Flores S, Loomba RS. The use of neutrophil-lymphocyte ratio for the prediction of refractory disease and coronary artery lesions in patients with Kawasaki disease. Cardiol Young. 2023;33:1409–17. [DOI] [PubMed] [Google Scholar]
- 36.Hirahara N, Matsubara T, Kaji S, Hayashi H, Sasaki Y, Kawakami K, et al. Novel inflammation-combined prognostic index to predict survival outcomes in patients with gastric cancer. Oncotarget. 2023;14:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rejec A, Butinar J, Gawor J, Petelin M. Evaluation of complete blood count indices (NLR, PLR, MPV/PLT, and PLCRi) in healthy dogs, dogs with periodontitis, and dogs with oropharyngeal tumors as potential biomarkers of systemic inflammatory response. J Vet Dent. 2017;34:231–40. [DOI] [PubMed] [Google Scholar]
- 38.Hodgson N, Llewellyn EA, Schaeffer DJ. Utility and prognostic significance of neutrophil-to-lymphocyte ratio in dogs with septic peritonitis. J Am Anim Hosp Assoc. 2018;54:351–9. [DOI] [PubMed] [Google Scholar]
- 39.Pierini A, Gori E, Lippi I, Ceccherini G, Lubas G, Marchetti V. Neutrophil-to-lymphocyte ratio, nucleated red blood cells and erythrocyte abnormalities in canine systemic inflammatory response syndrome. Res Vet Sci. 2019;126:150–4. [DOI] [PubMed] [Google Scholar]
- 40.Pierini A, Gori E, Lippi I, Lubas G, Marchetti V. Are leukocyte and platelet abnormalities and complete blood count ratios potential prognostic markers in canine sepsis? Front Vet Sci. 2020;7:578846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becher A, Suchodolski JS, Steiner JM, Heilmann RM. Blood neutrophil-to-lymphocyte ratio (NLR) as a diagnostic marker in dogs with chronic enteropathy. J Vet Diagn Investig. 2021;33:516–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cagnasso F, Borrelli A, Bottero E, Benvenuti E, Ferriani R, Marchetti V, et al. Comparative evaluation of peripheral blood neutrophil to lymphocyte ratio, serum albumin to globulin ratio and serum c-reactive protein to albumin ratio in dogs with inflammatory protein-losing enteropathy and healthy dogs. Animals. 2023;13:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skor O, Fuchs-Baumgartinger A, Tichy A, Kleiter M, Schwendenwein I. Pretreatment leukocyte ratios and concentrations as predictors of outcome in dogs with cutaneous mast cell tumours. Vet Comp Oncol. 2017;15:1333–45. [DOI] [PubMed] [Google Scholar]
- 44.Benvenuti E, Pierini A, Gori E, Lucarelli C, Lubas G, Marchetti V. Neutrophil-to-lymphocyte ratio (NLR) in canine inflammatory bowel disease (IBD). Vet Sci. 2020;7:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiti LE, Ferrari R, Boracchi P, Morello E, Marconato L, Roccabianca P, et al. Prognostic impact of clinical, haematological, and histopathological variables in 102 canine cutaneous perivascular wall tumours. Vet Comp Oncol. 2021;19:275–83. [DOI] [PubMed] [Google Scholar]
- 46.Gavazza A, Cremonini V, Miglio A, Starita C, Rossi G, Antognoni MT. Hematological ratios and indices in canine large B-cell lymphoma. Open Vet J. 2024;14:980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kocartuk M, Saril A, Oz AD, Rubio CP, Ceron JJ, Ylmaz Z. Neutrophil-to-lymphocyte ratio and red blood cell distribution width to platelet ratio and their relationships with inflammatory and antioxidant status in dogs with different stages of heart failure due to myxomatous mitral valve disease. Vet Res Commun. 2024;48:2477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yun S, Yun T, Cha S, Oh J, Lee D, Koo Y, et al. Can neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios be used as markers for hypercortisolism in dogs? Top Companion Anim Med. 2024;61:100890. [DOI] [PubMed] [Google Scholar]
- 49.Ferreira TMV, Oliveira ATC, de Carvalho VM, Pinheiro ADN, de Carvalho Sombra TCF, Ferreira TC, et al. Leukocytes and albumin in canine leishmaniasis. Acta Sci Vet. 2021;49:1–7. [Google Scholar]
- 50.Durán-Galea A, Cristóbal-Verdejo JI, Barrera-Chacón R, Macías-García B, González-Solís MA, Nicolás-Barceló P, et al. Clinical importance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and systemic immune-inflammation index in dogs with leishmaniasis. Comp Immunol Microbiol Infect Dis. 2024;107:102148. [DOI] [PubMed] [Google Scholar]
- 51.Baxarias M, Jornet-Rius O, Donato G, Mateu C, Alcover MM, Pennisi MG, et al. Signalment, immunological and parasitological status and clinicopathological findings of Leishmania-seropositive apparently healthy dogs. Animals (Basel). 2023;13:1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Numeracy, maths and statistics academic skills kit. Strength of correlation. https://www.ncl.ac.uk/webtemplate/ask-assets/external/maths-resources/statistics/regression-and-correlation/strength-of-correlation.html. Accessed 15 Apr 2024.
- 53.Ramsey PH. Critical values for Spearman’s rank order correlation. J Educ Stat. 1989;14:245. [Google Scholar]
- 54.Lombardo G, Pennisi MG, Lupo T, Chicharro C, Solano-Gallego L. Papular dermatitis due to Leishmania infantum in seventeen dogs: diagnostic features, extent of the infection and treatment outcome. Parasit Vectors. 2014;7:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martínez-Flórez I, Guerrero MJ, Dalmau A, Cabré M, Alcover MM, Berenguer D, et al. Effect of local administration of meglumine antimoniate and polyhexamethylene biguanide alone or in combination with a Toll-like receptor 4 agonist for the treatment of papular dermatitis due to Leishmania infantum in dogs. Pathogens. 2023;12:821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cavalera MA, Gusatoaia O, Uva A, Gernone F, Tarallo VD, Donghia R, et al. Erythrocyte sedimentation rate in heartworm naturally infected dogs “with or without” Leishmania infantum seropositivity: an observational prospective study. Front Vet Sci. 2024;11:1371690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verçosa BLA, Muniz-Junqueira MI, Menezes-Souza D, Fujiwara RT, Borges LF, Melo MN, et al. MCP-1/IL-12 ratio expressions correlated with adventitial collagen depositions in renal vessels and IL-4/IFN-γ expression correlated with interstitial collagen depositions in the kidneys of dogs with canine leishmaniasis. Mol Immunol. 2023;156:61–76. [DOI] [PubMed] [Google Scholar]
- 58.Almeida V, Lima I, Fraga D, Carrillo E, Moreno J, Dos-Santos WLC. Hematological changes in dogs with visceral leishmaniasis are associated with increased IFN-γ and TNF gene expression levels in the bone marrow. Microorganisms. 2021;9:1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nicolato Rde C, de Abreu RT, Roatt BM, Aguiar-Soares RD, Reis LE, Carvalho Md, et al. Clinical forms of canine visceral leishmaniasis in naturally Leishmania infantum-infected dogs and related myelogram and hemogram changes. PLoS One. 2013;8:e82947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bourgès-Abella NH, Gury TD, Geffré A, Concordet D, Thibault-Dupray KC, Dauchy A, et al. Reference intervals, intraindividual and interindividual variability, and reference change values for hematological variables in laboratory beagles. J Am Assoc Lab Anim Sci. 2015;54:17–24. [PMC free article] [PubMed] [Google Scholar]
- 61.Kimura T, Kotani K. Perinatal veterinary medicine-related evaluation in hematological and serum biochemical profiles of experimental beagles throughout pregnancy and parturition. Anim Model Exp Med. 2018;1:282–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.