Abstract
Background
Coffee and tea consumption account for most caffeine intake and 2–3 billion cups are taken daily around the world. Caffeine dependence is a widespread but under recognized problem.
Objectives
To conduct a systematic review on the genetic susceptibility factors affecting caffeine metabolism and caffeine reward and their association with caffeine intake.
Methodology
We conducted PubMed and Embase searches using the terms “caffeine”, “reward”, “gene”, “polymorphism”, “addiction”, “dependence” and "habit" from inception till 2024. The demographics, genetic and clinical data from included studies were extracted and analyzed. Only case-control studies on habitual caffeine drinkers with at least 100 in each arm were included.
Results
A total of 2552 studies were screened and 26 studies involving 1,851,428 individuals were included. Several genes that were involved with caffeine metabolism such as CYP1A2, ADORA2A, AHR, POR, ABCG2, CYP2A6, PDSS2 and HECTD4 rs2074356 (A allele specific to East Asians and monomorphic in Europeans, Africans and Americans) were associated with habitual caffeine consumption with effect size difference of 3% to 32% in number of cups of caffeinated drink per day per effect allele. In addition, ALDH2 was linked to the Japanese population. Genes associated with caffeine reward included BDNF, SLC6A4, GCKR, MLXIPL and dopaminergic genes such as DRD2 and DAT1 which had around 2–5% effect size difference in number of cups of caffeinated drink for each allele per day.
Conclusion
Several genes that were involved in caffeine metabolism and reward were associated with up to 30% effect size difference in number of cups of caffeinated drink per day, and some associations were specific to certain ethnicities. Identification of at-risk caffeine dependence individuals can lead to early diagnosis and stratification of at-risk vulnerable individuals such as pregnant women and children, and can potentially lead to development of drug targets for dependence to caffeine.
Introduction
Caffeine (1,3,7-trimethylxanthine, 137X), a purine alkaloid, is a widely consumed psychostimulant worldwide, with around 2–3 billion cups drunk daily [1, 2]. Almost 90% of US adults consume caffeine through sources such as coffee and tea products [3]. Besides such beverages, caffeine can also be found in soft drinks, chocolates and energy drinks [3, 4]. The average caffeine consumption is around 70–76 mg/person per day worldwide with caffeine consumption projected to increase due to population growth [5, 6]. Caffeine’s popularity worldwide is often due to its stimulatory nature, increasing alertness and improving cognitive function, including learning and memory [7, 8]. It also enhances physical performance and has been known to have a positive effect on endurance and high-intensity sports [9]. Increasing research into the health outcomes of caffeine has also shown caffeine’s positive health outcomes on type 2 diabetes mellitus, kidney stones, gout, non-alcoholic fatty liver disease, liver cirrhosis and liver cancer [10].
Caffeine’s psychostimulant effects are brought about by its nonselective and competitive antagonism of adenosine A1 and A2A receptors [11]. Adenosine is widely distributed in the body and brain and is thought to play a role in homeostatic sleep–wake regulation [4, 12]. In the Central Nervous System (CNS), by removing adenosine’s inhibition of dopamine neurons, caffeine increases dopaminergic input on mesocorticolimbic structures [13–15]. Through modulating dopaminergic pathways and reducing dopaminergic neuronal loss, caffeine induces neuroprotective effects on conditions such as Alzheimer’s disease and Parkinson’s disease [16]. However, there is individual variation in the amount of neuroprotection that caffeine can offer, likely due to the involvement of multiple genes [17]. Thus, studies are needed to understand and identify genes that could possibly mediate caffeine neuroprotection.
In addition, the dopaminergic neurons that caffeine activates are essential for brain reward processing and can be found in the ventral tegmental area (VTA), nucleus accumbens (NAc), hippocampus and medial prefrontal cortex of the brain [18]. Despite the positive effects of caffeine, it is also a double-edged sword. By activating reward pathways, caffeine has a strong potential for dependence and addiction. Excessive caffeine intake can lead to withdrawal symptoms such as headaches, depressed mood or irritability and difficulty concentrating [19]. Such symptoms often vanish after caffeine ingestion, which produces psychological satisfaction [19]. Previous research has shown that a dose of 25–50 mg of caffeine per cup of coffee can reinforce the behaviour of consuming caffeine [19]. Heavy caffeine users are thought to be individuals who consume six or more cups of coffee per day (around 600–1000 mg of caffeine) [20]. Habitually consuming caffeine can lead to caffeine dependence syndrome, a behavioural disorder recognized by the World Health Organization (WHO) [21]. Physical dependence can also occur and has been defined as the body’s normal physiological adaptation to continued drug presence within the body, consisting of processes like receptor up-regulation or down-regulation [18]. Caffeine “abuse” is thought to occur when individuals have an “uncontrolled need to consume caffeine, even if it is harmful to their health” [9]. The Fifth Edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) suggested that “Caffeine Use Disorder” be considered a condition for further study [22]. As such, more investigations are needed to further understand the nature of caffeine dependence and its pathophysiology, including the genes that can make an individual susceptible to greater caffeine intake.
Moreover, some individuals are highly sensitive to caffeine which can lead to neurotoxicity with various impacts on physical and mental health [23]. As a stimulant, it can interfere with sleep–wake cycles, leading to prolonged sleep latency, poor sleep quality and insomnia at night [24, 25]. Over time, this insomnia can lead to fatigue and impaired cognitive function. Studies have found that those who have poor sleep with caffeine ingestion are more likely to metabolize caffeine slowly [19, 26]. Besides insomnia, caffeine’s stimulant properties also serve to increase the body’s flight-or-fight response, increasing stress and anxiety, precipitating panic attacks in those with anxiety and panic disorder [27]. Acute caffeine exposure at high doses was also found to induce seizures [28]. While many factors could explain why some individuals are more sensitive than others to caffeine including age and sleep habits, genetics has been receiving increased attention.
Studies have revealed the impact of gene polymorphisms on caffeine intake, particularly CYP1A2 which is responsible for the hepatic metabolism of caffeine [29]. Faster metabolism of caffeine is thought to increase caffeine consumption [30]. With the ubiquitous consumption of caffeine worldwide, potential for dependence and its numerous effects on the body, understanding the genetics behind caffeine metabolism and reward are of particular interest. Furthermore, understanding the genes that affect caffeine’s neurological mechanisms in the CNS could provide novel pharmacological therapies against neurological diseases. However, to our knowledge, there are no studies systematically examining the specific genes and Single Nucleotide Polymorphisms (SNPs) involved in caffeine metabolism and caffeine reward processing and how they relate to caffeine dependency. To address the current gaps in knowledge, we conducted a systematic review of the current literature to identify and discuss current evidence of genetic polymorphisms affecting caffeine metabolism and caffeine reward and thus how they affect caffeine intake.
Methods
This systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement guidelines (http://www.prisma-statement.org) [31]. Research papers were identified through a systematic computerized literature search using PubMed and Embase. Articles published up to August 2024 were reviewed. The following search terms were used: “caffeine”, “reward”, “gene”, “polymorphism”, “dependence”, “addiction”, “habit”. Inclusion criteria were that articles have been (1) published in English (2) examined genetic polymorphisms of individuals who habitually consumed caffeine (3) case-control study with at least 100 in each arm. Exclusion criteria were (1) studies examining effects of caffeine on anxiety or sleep or performance (2) No full text found (3) comments or editorials on defining caffeine as a substance use disorder. Articles that did not meet inclusion criteria and/or met exclusion criteria were removed. An outline of the records identified, included and excluded is shown in the PRISMA flow diagram in Fig. 1. In total, 2582 articles were screened and a total of 26 studies were included in the final review.
Fig. 1.
PRISMA flowchart
Results
In total, 26 studies reporting data on 1,851,428 individuals were included. In general, the cardinal genes involved with caffeine metabolism were CYP1A2, ADORA2A, AHR, POR, ABCG2 and CYP2A6. Other caffeine metabolism genes that were found to be associated with caffeine metabolism were PDSS2 and in Asian populations, HECTD4 and ALDH2. The pertinent genes associated with caffeine reward were BDNF, SLC6A4, GCKR, MLXIPL and dopaminergic genes such as DRD2 and DAT1. Other genes linked to caffeine as a bitter and addictive beverage, like SEC16B, and rare genetic variations such as OR2G2 and SNCAIP will also be briefly covered. The genes involved in caffeine intake and metabolism are summarized in Tables 1 and 2.
Table 1.
Genetic studies of caffeine intake and metabolism
| No | Study design | No. of participants | Gene/Nearest gene | SNP-effect allele/non-effect allele | Genetic ancestry | Mechanism of gene | Result | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Case-control | 39,196 individual participants aged more than 40 years, as part of the Korean Genome and Epidemiology Study | GCKR | rs126036-C/T | Korean | Caffeine metabolism | Individuals with the GCKR C allele were more likely to consume more coffee than those with the T allele, in line with other studies where C allele was associated with higher caffeine consumption | Kim et al. [141] |
| 2 | GWAS | 130,153 23andMe participants of European Ancestry | CYP1A1/2 | rs2472297-C/T | European | Caffeine metabolism | The study found 7 GWAS-significant loci that had strong associations with coffee intake | Thorpe et al. [42] |
| AHR, AGR3 | rs4410790-C/T | European | Caffeine metabolism | |||||
| ADORA2A, UPB1 | rs199612805-D/I | European | Caffeine metabolism | |||||
| STYXL1 | rs28634426-G/T | European | Exact role unknown | |||||
| MMS22L, POU3F2 | rs34645063-D/I | European | Exact role unknown | |||||
| PCMTD2 | rs11474881-D/I | European | Exact role unknown | |||||
| CTC-490E21.12 | rs117824460-A/G | European | Exact role unknown | |||||
| 3 | GWAS | 2830 participants through 2 cohorts recruited through Coriell Personalized Medicine Collaborative and United States Air Force | CYP1A1/2 | rs2472297-A | American | Caffeine metabolism | Rs2472297’s association with caffeine consumption was replicated in this study | Kusic et al. [142] |
| 4 | GWAS | 336,517 individuals from the UK Biobank study | CYP1A1/2 | rs2472297-T | European | Caffeine metabolism | rs4410790, and rs2472297, were found in associations with both cereal and coffee intake. Additionally, rs4410790 (the C-allele) and rs2472297 (the T-allele) were also strongly associated with higher intake of tea | Kang et al. [143] |
| rs4410790-C | European | Caffeine metabolism | ||||||
| 5 | GWAS | 370,193 individuals from the UK Biobank study | 2p23.3 GCKR | rs1260326-C/T | European | Caffeine reward | All SNPs were associated with habitual coffee intake in both men (P < 0.002) and women (P < 0.0006). Similar patterns were observed for regular, instant, ground/filtered and decaffeinated coffee as well as tea | Cornelis et al. [38] |
| 4q22.1 ABCG2 | rs1481012-A/G | European | Caffeine metabolism | |||||
| 7p21.1 AHR | rs6968554-G/A | European | Caffeine metabolism | |||||
| 7q11.23 POR | rs17685-A/G | European | Caffeine metabolism | |||||
| 15q24.1 CYP1A1-CYP1A2 |
rs2472297-T/C rs762551-A |
European | Caffeine metabolism | |||||
| 22q11.23 SPECC1L-ADORA2A | rs2330783-G/T | European | Caffeine metabolism | |||||
| 22q11.23 ADORA2A | rs5751876-T/C | European | Caffeine metabolism | |||||
| 7q11.23 MLXIPL | rs7800944-C/T | European | Caffeine reward | |||||
| 6 | GWAS | 7868 Korean Individuals | HECTD4 | rs2074356 | Korean | Exact role unknown | The significant SNPs discovered to be related to habitual coffee consumption in the Korean population in this GWAS were all introns. The strongest significant variant found in this study was rs2074356 | Jin et al. [53] |
| ACAD10 | rs11066015 | Korean | Exact role unknown | |||||
| MYL2 | rs12229654 | Korean | Exact role unknown | |||||
| CUX2 | rs12229654 | Korean | Caffeine metabolism | |||||
| CUX2 | rs12229654 | Korean | Caffeine metabolism | |||||
| 7 | GWAS | 165,084 Japanese individuals | CYP1A2, CSK | rs58806801-G/A | Japanese | Caffeine metabolism | These loci were found to be associated with coffee consumption. Other loci nominally associated with caffeine consumption include ABCG2, MIR2113, MLXIPL, POR, APOE5 | Matoba et al. [71] |
| ADORA2A-AS1 | rs5760444-T/C | Japanese | Caffeine metabolism | |||||
| AGR3-AHR | rs4410790-T/C | Japanese | Caffeine metabolism | |||||
| ABCG2 | rs75544042-A/G | Japanese | Caffeine metabolism | |||||
| POR | rs3815455-C/T | Japanese | Caffeine metabolism | |||||
| MCL1, ENSA | rs6681426-G/A | Japanese | Exact role unknown | |||||
| GCKR | rs1260326-T/C | Japanese | Caffeine reward | |||||
| ALDH2 | rs671-G/A | Japanese | Exact role unknown | |||||
| MLXIPL | rs13234378-A/T | Japanese | Caffeine reward | |||||
| APOE5 | rs662799-G/A | Japanese | Exact role unknown | |||||
| MIR2113 | rs12189679-G/A | Japanese | Exact role unknown | |||||
| 8 | GWAS | 6264 individuals for GWAS discovery and 5975 for replication analysis | 7p21 AHR | rs10252701-A/C | Japanese | Caffeine metabolism | 2 loci (7p21 and 12q24) were associated with habitual coffee consumption and achieved genome-wide significance (P < 5 × 10 −8) | Jia et al. [55] |
| 12q24 (ALDH2 and CUX) | rs7910528-A/C | Japanese | Caffeine metabolism | |||||
| 9 | GWAS | 337 542 individuals from the UK Biobank Study | 15q24.1 CYP1A1/2 | rs2472297-T/C | European | Caffeine metabolism | In this study, 5 loci that were associated with plasma caffeine metabolites in previous literature were replicated (CYP1A1/2, GCKR, ABCG2, AHR, POR). Other novel loci, SEC16B, TMEM18, OR8U8, AKAP6, MC4R, SPECC1L-ADORA2A were found to be associated with caffeine consumption | Zhong et al. [47] |
| 2p23.3 GCKR | rs1260326-C/T | European | Caffeine reward | |||||
| 7p21.1 AHR | rs117692895-C/G | European | Caffeine metabolism | |||||
| rs4410790-C/T | European | |||||||
| rs4719497-T/C | European | |||||||
| 4q22.1 ABCG2 | rs1481012-A/G | European | Caffeine metabolism | |||||
| 7q11.23 POR | rs1057868-T/C | European | Caffeine metabolism | |||||
|
22q11.23 SPECC1L-ADORA2A |
rs2330783-G/T | European | Caffeine metabolism | |||||
| 1q25.2 SEC16B | rs574367-T/G | European | Exact role unknown | |||||
| 2p25.3 TMEM18 | rs10866548-G/A | European | Exact role unknown | |||||
| 11q12.1 OR8U8 | rs597045-A/T | European | Exact role unknown | |||||
| 14q12 AKAP6 | rs1956218-G/A | European | Exact role unknown | |||||
| 18q21.32 MC4R | rs66723169-A/C | European | Exact role unknown | |||||
| 10 | GWAS | Japanese population of 11,261 participants as part of the Japan Multi-Institutional Collaborative cohort | ALDH2 |
rs4646776-C/G rs671-A/G |
Japanese | Exact role unknown | 24 SNPS on the 12q24 locus were found to have genome-wide significance with habitual caffeine consumption. The lead variant for the 12q24.12-13 locus, rs2074356-A, had the strongest significance and its effect size was estimated at 0.20 | Senda et al. [54] |
| ACAD10 |
rs60125993-C/CT rs11066008-G/A rs11066015-A/G |
Japanese | Exact role unknown | |||||
| BRAP |
rs11065992-C/T rs3782886-C/T rs11066001-C/T |
Japanese | Exact role unknown | |||||
| NAA25 |
rs11066132-T/C rs116873087-C/G rs11066150-A/G rs147992802-T/C |
Japanese | Exact role unknown | |||||
| TRAFD1 | rs12231737-T/C | Japanese | Exact role unknown | |||||
| MYL2-CUX2 (intergenic) |
Rs12227162-T/C rs149607519-G/C rs148177611-T/TAGAA |
Japanese | Exact role unknown | |||||
| CUX2 (intron) | rs3809297-T/G | Japanese | Caffeine metabolism | |||||
| OAS2-DTX1 (intergenic) | rs139144808-TA/T | Japanese | Exact role unknown | |||||
| MAPKAPK5 | rs78069066-A/G | Japanese | Exact role unknown | |||||
| HECTD4 |
rs2074356-A Rs144504271-A/G rs77768175-G/A rs11066280-A/T |
Japanese | Exact role unknown | |||||
| HECTD4-RPL6 (intergenic) | rs11537471-G/A | Japanese | Exact role unknown | |||||
| 11 | Meta-Analysis | 415,530 participants and 300,760 coffee drinkers from 10 meta-analysed European ancestry cohorts | CYP1A1/2 | rs2472297-T/C | European | Caffeine metabolism | CYP1A1/2, AHR showed the expected patterns with the T alleles for both genes being associated with higher habitual coffee consumption | Zhou et al. [144] |
| AHR | rs6968865-T | European | ||||||
| 12 | GWAS | 9876 individuals of European ancestry from 6 population-based studies | 7p21 AHR |
rs4410790-C/T rs6968554-G/A rs10275488-C/T rs2892838-A/C rs11400459-A/AT rs10683220 –G/GTTAACA |
European | Caffeine metabolism | AHR and CYP1A2 variants were associated with higher plasma caffeine, slow caffeine metabolism and low caffeine consumption. CYP2A6 variants was associated with lower caffeine consumption | Cornelis et al. [30] |
| 15q24 CYP1A2 |
rs12909047-A/G rs35107470-G/A rs62005807-C/G rs2470893-T/C rs2472297-T/C |
European | Caffeine metabolism | |||||
| 19q13.2 CYP2A6 |
rs66500423-T/C rs4803373-C/G rs78011401-C/T rs11668399-C/G rs56113850-T/C rs56267346-G/A rs28399442-A/C rs67210567-T/G rs7260629-T/G rs5828081-A/AT rs200292835-TTTTG/T rs28602288-C/T rs79600176-C/T rs184589612-C/T rs72480748-G/A rs10425738-G/A rs56881024-T/A |
European | Caffeine metabolism | |||||
| 6p23 CD83 | rs62391270-C/T | European | Exact role unknown | |||||
| 13 | Meta analysis | 7 studies (N = 6778) included subjects of Caucasian ethnicity and 3 studies (N = 1750) included subjects of Asian ethnicity | CYP1A2 | rs762551-A | Association was significant in Caucasian subjects but not Asian subjects | Caffeine metabolism | CYP1A2 rs762551 AA genotype may lead to higher coffee intake, especially in males, younger age groups, and individuals of Caucasian ethnicity | Denden et al. [145] |
| 14 | GWAS | 2938 Italian individuals | PDSS2 |
rs2216084-T/C rs6942255-A/G rs7745311-C/T rs7754744-G/A rs9386630-G/T |
Italian | Caffeine metabolism | Conditional knockout of PDSS2 in the liver has been shown to increase the expression of the genes of the caffeine metabolism pathway. It was thus hypothesized that higher PDSS2 expression would inhibit the expression of the genes in the caffeine metabolism pathway thus inhibiting caffeine degradation | Pirastu et al. [52] |
| 15 | Genome-wide (GW) meta-analysis | 30 062 and 7964 coffee consumers of European and African American ancestry respectively | CYP1A1/2 |
rs2472297-T/C rs2470893-T/C |
European | Caffeine metabolism | Eight loci met GW significance with per-allele effect sizes of 0.03–0.14 cups per day. 4 genes, ABCG2, AHR, POR and CYP1A2, are involved in pharmacokinetics and 2 genes are involved in pharmacodynamics (BDNF and SLC6A4) of caffeine. GCKR and MLXIPL genes are related to metabolic traits but lack roles in coffee consumption | Cornelis et al. [1] |
| AHR |
rs4410790-C/T rs6968554-G/A |
European | Caffeine metabolism | |||||
| POR | rs17685-A/G | European | Caffeine metabolism | |||||
| ABCG2 | rs1481012-A/G | European | Caffeine metabolism | |||||
| BDNF | rs6265-C/T | European | Caffeine reward | |||||
| SLC6A4, EFCAB5 | rs9902453-G/A | European | Caffeine reward | |||||
| GCKR | rs1260326-C/T | European | Caffeine reward | |||||
| MLXIPL | rs7800944-C/T | European | Caffeine reward | |||||
| 16 | GWAS | 10,015 Individuals from the Avon Longitudinal Study of Parents and Children | 15q24 CYP1A1/2 | rs2472297 | European | Caffeine metabolism | Both genotypes were individually associated with total caffeine consumption, and with coffee and tea consumption | McMahon et al. [43] |
| 7p21 AHR | rs6968865 | European | Caffeine metabolism | |||||
| 17 | Cross sectional | 4066 individuals from different parts of Europe and Central Asia | TAS2R43 |
rs71443637-T (H212R) rs35720106 (synonymous variant) rs68157013-C (W35S) |
Europeans and Central Asians | Caffeine reward | rs71443637 -T and rs68157013-C, both wild type alleles, were found to be associated with higher caffeine liking | Pirastu et al. [78] |
| 18 | Cross sectional | 158 women and 59 men (n = 217) between the ages of 24 and 47 years of Caucasian and African descent living in North America | ANKK1 | Taq1 (rs1800497) | 80% Caucasian, 15% African | Caffeine reward | A multilocus genetic profile score reflecting the additive effects of alleles known to confer relatively increased dopamine signaling in the ventral striatum was related to more frequent engagement in addictive behaviours, including caffeine | Davis et al. [61] |
| DRD2 |
rs12364283 rs6277 (C957T) rs1799732 (141C Ins/Del) |
80% Caucasian, 15% African | Caffeine reward | |||||
| DAT1 | NA | 80% Caucasian, 15% African | Caffeine reward | |||||
| COMT | Rs4680 (Val158Met) | 80% Caucasian, 15% African | Caffeine reward | |||||
| 19 | GWAS | > 18 000 individuals of Northern European ancestry | CYP1A1/2 |
rs2470893 rs2472296 |
Caucasian | Caffeine metabolism | Genome-wide significant association was observed for two SNPs in the 15q24 region, rs2470893 and rs2472297, which were also in strong linkage disequilibrium. They have a commonly shared 5' flanking region between CYP1A1 and CYP1A2 genes. Significant evidence of association was also detected at rs382140 near NRCAM-a gene implicated in vulnerability to addiction | Amin et al. [33] |
| NRCAM | rs382140 | Caucasian | Addiction | |||||
| NA | rs6495122 | Caucasian | Independent Hit | |||||
| 20 | Case-control | Hispanic Americans living in the Central Valley of Costa Rica | CYP1A1/1A2 | rs2472297 (n = 2570) | Costa Rican | Caffeine metabolism | Subjects who drank more caffeine were more likely to be carriers of the T, C, or T allele for rs6968865, rs4410790, and rs2472297, respectively | Josse et al. [40] |
| AHR |
rs6968865 rs4410790 |
Costa Rican | Caffeine metabolism | |||||
| 21 | Cohort | 6288 participants from the Rotterdam Study (RS), a cohort study of inhabitants of Ommoord, Rotterdam, Netherlands with age more than 55 years old | CYP1A1/2 |
rs2472297-A rs2470893-A |
Dutch | Caffeine metabolism | rs2472297G > A, the female sex, and non-smoking habits were significantly inversely related to coffee intake | Rodenburg et al. [39] |
| 22 | Genome-wide association study (GWAS) | 47,341 individuals of European descent | CYP1A1/2 (15q24) | rs2470893-T/C | European | Caffeine metabolism | 2 Loci received genome-wide significance: 7p21 near AHR and 15q24, between CYP1A1 and CYP1A2. CYP1A2 metabolizes caffeine and AHR regulates CYP1A2 | Cornelis et al. [41] |
| AHR (7p21) | rs4410790-C/T | European | Caffeine metabolism | |||||
| 23 | Meta analysis of 4 genome wide association studies | 5110 individuals from Iceland, 2791 individuals from the Netherlands, 1620 Danish women, 771 individuals from the Sorbs Slavonic populate in Germany, 369 individuals from the USA | CYP1A1/2 | rs2472297-T | European | Caffeine metabolism | Two sequence variants significantly associated with increased coffee consumption: rs2472297-T located between CYP1A1 and CYP1A2 at 15q24 and rs6968865-T near aryl hydrocarbon receptor (AHR) at 7p21. An effect of ∼0.2 cups a day per allele was observed for both SNPs | Sulem et al. [36] |
| AHR | rs6968865-T | European | Caffeine metabolism | |||||
| 24 | Randomized, double-blind, parallel groups design | 379 predominantly white Europeans | ADORA2A |
rs5751876-T rs3761422-T |
European | Caffeine metabolism | In the participants who habitually consumed at least moderate amounts of caffeine, caffeine intake from coffee was higher in the TT genotype group compared with the combined CC and CT group. Results were similar for rs3761422, including higher habitual coffee consumption in the TT genotype group | Rogers et al. [46] |
| 25 | Cross-sectional study | 2873 Hispanic Americans living in Costa Rica | ADORA2A | rs5751876 | Costa Rican | Caffeine metabolism | Persons with the ADORA2A TT genotype were significantly more likely to consume less caffeine (ie. < 100 mg/day) than were carriers of the C allele | Cornelis et al. [48] |
Table 2.
Rare genetic variations associated with caffeine dependency
| No. | Study design | No. of participants | Genes | SNP-effect allele/non-effect allele | Genetic ancestry | Mechanism of gene | Result | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Exome-wide association study/GWAS |
Exome data after quality control (n = 20,566) GWAS (n = 438,860 individuals of European ancestry) |
OR2G2 |
rs12737801-C rs1151687-G |
European | Olfactory receptor activity | Six candidate SNPs corresponding to OR2G2, VEZT, IRGC, and SNCAIP were verified with a GWAS dataset (p < 0.05) and were observed to be associated with habitual coffee consumption | Cheng et al. [81] |
| VEZT | rs201317857-C | European | Olfactory receptor activity | |||||
| IRGC |
rs34439296-C rs346049-C |
European | Exact Role Unknown | |||||
| SNCAIP | rs55712196-G | European | Exact Role Unknown |
Genes involved in Caffeine Metabolism
The reproducibility of the most pertinent genes involved in caffeine metabolism are as follows: CYP1A2 was found across 15 studies, AHR across 11 studies, ADORA2A across 5 studies, ABCG2 across 5 studies and POR across 5 studies.
CYP1A2
CYP1A2 is a major caffeine metabolism enzyme, responsible for an estimated 95% of caffeine metabolism in humans [1]. The CYP1A gene is found on chromosome 15q22 and CYP1A1 and CYP1A2 are closely connected, sharing a common 5′-flanking region [32]. CYP1A1 encodes P1-450 of the cytochrome P450 superfamily of enzymes, which is closely associated with polycyclic-hydrocarbon-induced aryl hydrocarbon hydroxylase (AHH) activity [33]. With AHH activity, CYP1A1 can metabolize benzo(a)pyrene, a chemical found in coffee involved in cancer [33]. CYP1A2 has been reported to have up to 60-fold variation in caffeine demethylation between individuals, possibly due to genetic and environmental factors [34]. In a twin study, performed in 378 Danish mono- and di-zygotic twins, there was a higher correlation in CYP1A2 theophylline metabolism between monozygotic twins (r = 0.798) compared with dizygotic twins (r = 0.394), suggesting some genetic and inheritable component to CYP1A2 activity [35].
The genotypes found to be associated with increased CYP1A2 inducibility include rs762551-A and rs2472297-T [36–39]. rs2470893 also plays a role in the transcriptional activation of CYP1A1 and CYP1A2 [1]. CYP1A2 enzyme activity is often significantly associated with paraxanthine (1,7 dimethylxanthine [17X]) and other caffeine metabolites in the plasma and urine [30, 39]. It has been suggested that in individuals with genotypes that slowly metabolize caffeine, such as those with rs762551 AC/GC genotype, caffeine persists in the blood for a longer time, enhancing dopamine signalling and neuroprotection via adenosine 2A antagonistic effects [37].
AHR
The Aryl Hydrocarbon Receptor (AHR) is closely linked to CYP1A1/2 through the same biochemical pathway [36]. Rs6968865 at 7p21, near AHR, was found to be associated with increased caffeine consumption in 2 studies involving European, African American and Costa Rican individuals [1, 36, 40]. Another two studies revealed that rs4410790-C was associated with higher caffeine intake [41, 42]. Both CYP1A1/2 rs2472297 and AHR rs6968865 were thought to increase caffeine consumption by around 0.2 cups per day per risk (T) allele, with an Icelandic population having an effect size of up to 0.32 cups of caffeine per day [36, 43].
ABCG2
Variants at 4q22 (rs1481012) map to ABCG2, encoding a xenobiotic efflux transporter [1]. As compared to the minor G allele, rs1481012-A is associated with higher caffeine habitual consumption and higher levels of caffeine and its metabolites, 17X and theophylline (1,3 dimethylxanthine [13X]) [1, 30]. The ABCG2 protein was first identified from its elevated expression in breast cancer and acute myeloid leukemia and has been known to confer multidrug resistance to tumour cells [44, 45]. Inhibition of ABCG2 has been explored to improve cancer therapeutic efficacy and studies have identified xanthines, including caffeine, that can induce the lysosomal degradation of ABCG2, suggesting the role of caffeine in further understanding cancer and the degradation of ABCG2 [44].
ABCG2 also facilitates biliary excretion of substrates but it is unclear if biliary excretion of caffeine is common in healthy individuals [30]. ABCG2 also functions at the blood–brain barrier (BBB) but little is known about the distribution properties of caffeine across the BBB [30].
POR
Variants at 7q11.23 (rs17685) are responsible for the 3′UTR of POR, encoding P450 oxidoreductase which displaces electrons to CYP450 enzymes [1]. The rs17685 A variant is associated with larger caffeine intake and higher POR expression [1]. POR rs17685 was also associated with total cholesterol, low-density lipoprotein (LDL) and triglycerides [38].
ADORA2A
The ADORA2A rs5751876-TT genotype is known for high sensitivity to caffeine and was associated with higher caffeine intake [46]. Besides SNP rs5751876, other SNPs rs2330783, rs3761422 and rs199612805 have also been found to be associated with caffeine consumption [38, 42, 46, 47]. However, some other studies have also reported that the rs5751876-TT genotype was less likely to have higher caffeine intake [48]. This difference has been attributed to differences in frequency of rs5751876-TT in different populations [46]. The ADORA2A-TT genotype was also linked to the anxiogenic effect of caffeine, with subjects reporting a higher level of anxiety compared to the C/C genotype [49]. ADORA2A genetic polymorphisms also play a role in glucose metabolism in muscles, affecting performance tests [49].
CYP2A6
CYP2A6 is expressed almost only in the liver and is responsible for the metabolism of caffeine, along with steroids, nicotine and other clinically important drugs such as antiretrovirals and antimalarial drugs [50]. CYP2A6 is responsible for hydroxylating 17X to 1,7,-dimethyluric acid (17U) and the ratio of 17U/17X is often used as a marker of CYP2A6 activity [30]. In a GWAS study by Cornelis et al., rs56113850 was found to be the most significant SNP [30]. Rs56113850-T variant was thought to reduce CYP2A6-mediated hydroxylation of 17X, leading to an increased plasma concentration of caffeine metabolite paraxantine [30]. The rs56113860-T genotype was also associated with increased caffeine consumption, in line with how genetic variants with increased caffeine consumption have higher paraxanthine-to-caffeine ratios [30, 51].
PDSS2
Pirastu et al. found that the gene PDSS2 has an association with caffeine consumption [52]. PDSS2 encodes for the prenyl side chain of coenzyme Q10 and it was hypothesized that higher expression would inhibit expression of caffeine metabolism genes and thus decrease caffeine metabolism in the body [52].
Caffeine Metabolism Genes in Asian Populations
HECTD4
Most of the studies discussed so far were conducted in European populations. Two GWAS studies from Asia have reported that HECTD4 rs2074356-A, an intronic variant located in 12q24.12-13, was strongly associated with habitual caffeine consumption with effect sizes of up to 0.20–0.32 [53, 54]. The rs2074356 A allele was found to be specific to East Asians and monomorphic in Europeans, Africans and Americans [54]. HECTD4 encodes the E3 ubiquitin protein ligase, responsible for the final step of the ubiquitination cascade [54]. Besides the association with habitual caffeine consumption, rs2074356 in HECTD4 was also associated with drinking behaviour in a Chinese population [54]. HECTD4 was also suggested to be associated with Type 2 Diabetes and blood sugar, generating great interest on how caffeine can influence blood sugar control [53].
ALDH2
Rs671 is a missense mutation in the ALDH2 gene and encodes for a functional Glu504Lys polymorphism [54]. Coffee consumption among Japanese men was found to be higher with the ALDH2 504Lys variant [54]. It was also found to be associated with smoking, which has also been associated with caffeine consumption [54]. Another study found that the rs79105258-C allele at the 12q24 locus near both ALDH2 and Cut-Like Homeobox 2 (CUX2) genes also influences caffeine consumption and this association is independent of other potential confounding factors such as BMI, smoking and alcohol consumption [55]. In addition, the 12q24 locus was found to have a different genetic effect in males versus females [55]. Despite its association with addictive behaviour like smoking and caffeine consumption, the ALDH2 504Lys variant protects individuals from excessive alcohol intake through increased blood concentrations of toxic acetaldehyde, producing the alcohol flush reaction [54].
Genes involved in Caffeine’s Rewarding Effects
BDNF
Brain-Derived Neurotrophic Factor (BDNF) influences serotonin, dopamine and glutamate circuits in the brain [1]. As these neurotransmitters are involved in reward and motivation, they potentially impact consumption behaviour by modulating the acute behavioural and reinforcing properties of caffeine. The SNP at rs6265 is a Val66Met missense mutation [1]. The Met66 allele is thought to decrease BDNF secretion and may weaken the rewarding effects of coffee and thus, motivation to consume caffeine [1].
SLC6A4
The SLC6A4 gene spans 37,809 base pairs and is located on 17q11.1-q12 [56]. The protein that SLC6A4 encodes for transports serotonin from synaptic spaces into presynaptic neurons [1, 56].The G allele variant rs9902453 is thought to be associated with higher caffeine intake [1]. Both acute and chronic caffeine intake are known to increase activity in the serotonergic raphe nuclei [57]. Serotonergic neurotransmission regulates a wide range of physiological and behavioural responses including sensory processing, food intake, mood and impulse control [1, 58].
Dopaminergic System Genes
Variants in genes encoding the DA D2 receptor (DRD2) and DA transporter (DAT1) are thought to be responsible for dopaminergic signalling in reward mechanisms involved in addiction, including compulsive eating [59, 60]. D2 receptors are present in the pre-synaptic and post-synaptic terminals to bind dopamine [59]. Rs1799732 is a SNP in the DRD2 promoter region and the DelC minor allele has been associated with lower expression of DRD2 and thus increased ventral striatal reactivity which was related to more frequent addictive behaviours [61–64]. Rs12364283 can also be found in the DRD2 gene where the minor T allele is associated with increased D2 receptor density [61]. Rs6277 is also found in the DRD2 gene and is believed to affect DRD2 binding potential with the homozygous T genotype having the highest binding potential [61, 65].
On the other hand, dopamine transporters (DATs) are important for eliminating dopamine from the synaptic cleft, directly modulating post-synaptic dopaminergic signalling [59]. The DAT gene contains a variable number tandem repeat (VNTR) polymorphism, which can have 3–13 repeats [59, 66]. Although the VNTR function is not well characterized, different alleles are postulated to be involved in variation of DAT mesolimbic levels with the 9-repeat allele showing reduced transporter protein expression and thus greater synaptic dopamine levels and addictive behaviour [59, 61, 66].
In addition, Taq1A is a C/T SNP (rs1800497) of the Ankyrin Repeat and Kinase-Domain Containing 1 (ANKK1) gene downstream of the DRD2 region on chromosome 11 [61]. It encodes for D2 receptors and is often researched as the T allele has been associated with reduced D2 receptor binding affinity, reduced dopamine and lower reward processing [59, 67]. The C allele has been associated with elevated dopamine and higher likelihood of addictive behaviours like caffeine consumption, as compared to the T allele [61].
The catechol-O-methyltransferase (COMT) gene has the SNP rs4680 which involves a valine to methionine substitution at position 158 [61]. A higher number of Met alleles has been associated with reduced dopamine catabolism and thus higher dopamine levels and increased activation reward processing regions like the basal ganglia [61, 68, 69].
GCKR
GCKR is expressed in the liver and encodes the protein glucokinase regulatory protein (GKRP) that phosphorylates glucose in the liver [30, 70]. The GCKR rs1260326-C variant has been associated with higher caffeine consumption [1, 38]. Besides glucose, the variant rs1260326 (p.Leu446Pro) has also been associated with both fat and glucose fat metabolism [1, 71]. The gene GCKR was replicated in 5 studies.
MLXIPL
Max-Like Protein X Interacting Protein-Like (MLXIPL) is a transcription factor involved in the regulation of plasma triglycerides and lipogenesis [38]. The C allele has been linked to higher coffee and alcohol drinking behaviour compared to the T allele [38]. The rs7800944 CC genotype was also associated with larger glucose levels after ingesting caffeine compared to the TT or TC genotype, especially in women [38].
Genes linked to Caffeine as a Bitter and Addictive Beverage
Zhong et al. found six novel loci, 1q25.2 (SEC16B), 2p25.3 (TMEM18), 11q12.1 (OR8U8), 14q12 (AKAP6), 18q21.32 (MC4R) and 22q11.23 (SPECC1L-ADORA2A), to be associated with both bitter non-alcoholic beverage and caffeine consumption [47]. SEC16B, TMEM18, AKAP6 and MC4R are loci involved in Body Mass Index (BMI) [47, 72, 73]. TMEM18 and MC4R variants associated with increased caffeine consumption are also linked with increased BMI and are thought to be related to the rewarding aspect of drinking caffeine as they are highly expressed in the hypothalamus [47].
In addition, rs382140 near NRCAM (neuronal cell adhesion molecule) was also associated with caffeine drinking [33]. NRCAM is expressed in the brain, playing a role in axonal growth and the development of thalamocortical projections, and thus is thought to be important in addiction [33, 74, 75]. GWAS Studies have already found links between NRCAM and drug or alcohol dependence [74, 76, 77]. Given that twin studies have shown relations between heritability of caffeine consumption and alcohol and nicotine addiction, genetic variants like NRCAM that influence addiction can also possibly increase caffeine consumption and the likelihood of caffeine dependency [33].
Furthermore, Pirastu et al. found 3 SNPs that were significantly associated with caffeine liking on the TAS2R43 gene, a gene encoding for bitter receptors [78]. Rs68157013-C (W35S) and rs71443637-T (H212R) were both associated with a higher liking of caffeine and higher perception of caffeine bitterness [78]. The SNP rs35720106 was a synonymous variant, with strong linkage disequilibrium with rs71443637 [78]. Previous studies have also found associations between caffeine and the TAS2R bitter receptor gene cluster on chromosome 12. TAS2R43 was activated by caffeine and contributed to bitter aftertastes [79, 80]. Thus, bitter receptor genes could also contribute to a higher liking of caffeine and increased habitual caffeine consumption.
Rare Genetic Variations associated with Caffeine Dependency
Cheng et al. recently identified rare genetic variations to be associated with caffeine dependency (Table 2). The gene-based exome-wide association study found that six SNPs corresponding to OR2G2, VEZT, IRGC, and SNCAIP genes were observed to be linked with habitual coffee consumption [81]. OR2G2 (Olfactory receptor family 2 subfamily G member 2) is related to olfactory receptor activity [81]. SNCAIP (Synphilin-1) has been associated with hyperphagia and is thought to produce a protein that could be neuroprotective in nature, preserving mitochondrial activity in dopaminergic cells [81–83]. In addition, Thorpe et al. found SNPs near the genes STYXL1, MMS22L, PCMTD2 and CTC-490E21.12 that were thought to be associated with coffee intake in participants of European ancestry [42]. However, the mechanisms of these genes are not well understood, and further research is required to explore the exact molecular mechanisms of these genes in habitual caffeine consumption.
Discussion
Metabolism of caffeine
Upon consumption, caffeine is absorbed throughout the stomach and small intestine within 45 minutes [29, 84]. Caffeine is mainly metabolised by the CYP1A2 enzyme on chromosome 15q22, a liver enzyme part of the inducible cytochrome P450s enzymatic complex [29]. With the CYP1A2 enzyme, caffeine is demethylated via the N3-demethylation reaction into its secondary metabolites, paraxanthine (~ 80%), theobromine (~ 10%) and theophylline (~ 5%) [29] (Fig. 2). Within a day, around 50–60% of administered caffeine is eliminated as its metabolites, mainly through renal excretion in urine [85]. Caffeine has several influences on molecular pathways and neurotransmitters in the brain (Fig. 3). In both humans and rats, caffeine metabolism can be mediated by other enzymes such as CYP3A2 and CYP2C6 [86]. Studies have suggested that variants of certain genes can lead to differing rates of caffeine metabolism [87]. Thus, recently, there has been great interest in examining specific SNPs and their effects on how caffeine is metabolized and processed in the body. In this review, we have found that the main genes associated with caffeine metabolism were CYP1A2, ADORA2A, AHR, POR, ABCG2 and CYP2A6. The salient genes associated with caffeine reward processing were BDNF, SLC6A4, GCKR, MLXIPL and dopaminergic genes such as DRD2 and DAT1. The effects of genetic variants on caffeine dependence are summarized in Fig. 4.
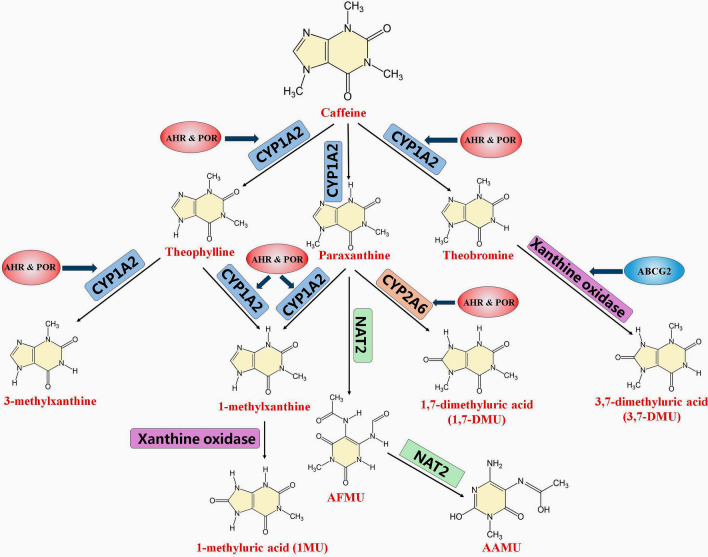
Fig. 2.
Caffeine metabolism pathway and metabolites. Caffeine is primarily metabolized in the liver, undergoing demethylation and oxidation. The main route of caffeine metabolism in humans is via CYP1A2 catalyzed N-3 demethylation to paraxanthine (around 84%), N-1 demethylation to theophylline (around 8%) and N-7 demethylation to theobromine (around 8%). Other than theobromine, paraxanthine, and theophylline, the major metabolites in urine are 3-methylxanthine, 1-methylxanthine, 1-methyl uric acid, 5-acetylamine-6-formylamine-3-methyluracil (AFMU), 5-acetylamino-6-amino-3-methyluracil, 1,7-dimethyl uric acid and 3,7-dimethyl uric acid, which are secondary metabolites of theobromine, paraxanthine, and theophylline catalyzed by CYP1A2, CYP2A6, N-acetyltransferase 2 and xanthine oxidase
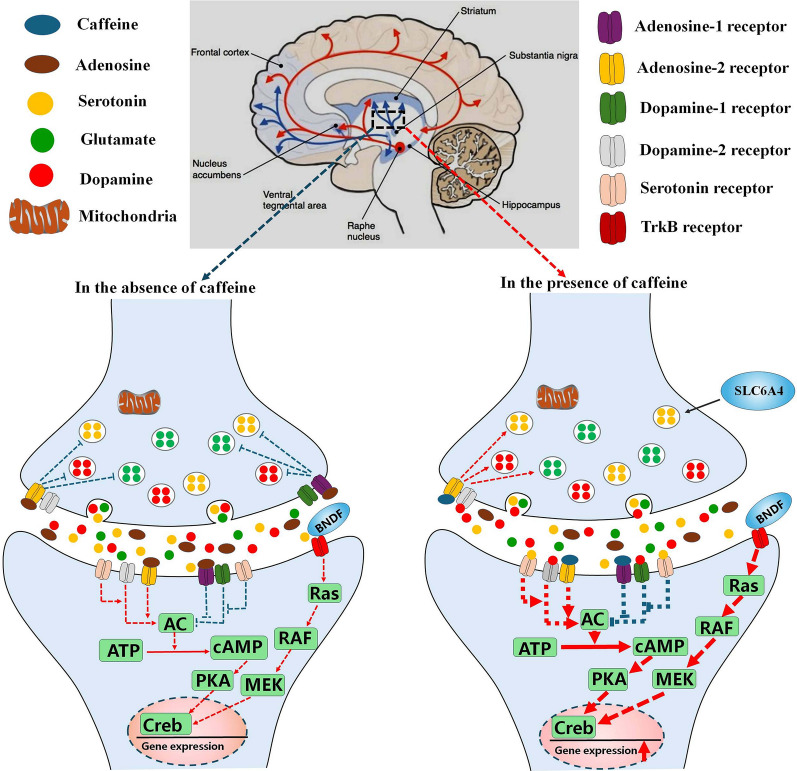
Fig. 3.
The effect of caffeine on molecular pathways and neurotransmitters. In the absence of caffeine, adenosine acts as an inhibitory modulator of neuronal activity. Adenosine binds to its presynaptic receptor to inhibit the release of neurotransmitters including dopamine, serotonin and glutamate. It also binds to its postsynaptic receptor to disrupt the interaction of dopamine and serotonin with their receptors. Dopamine, serotonin and glutamate induce neuronal signal transduction and inhibit neuronal function. Caffeine abrogates the interaction of adenosine with its presynaptic and postsynaptic receptors, promoting the release of dopamine, serotonin and glutamate and enhances the interaction of dopamine and serotonin with their postsynaptic receptors to increase neuron activity and functions. Caffeine abolishes the inhibitory effects of adenosine on dopaminergic pathway, increasing dopaminergic inputs on mesocorticolimbic structures and promoting human psychomotor activity
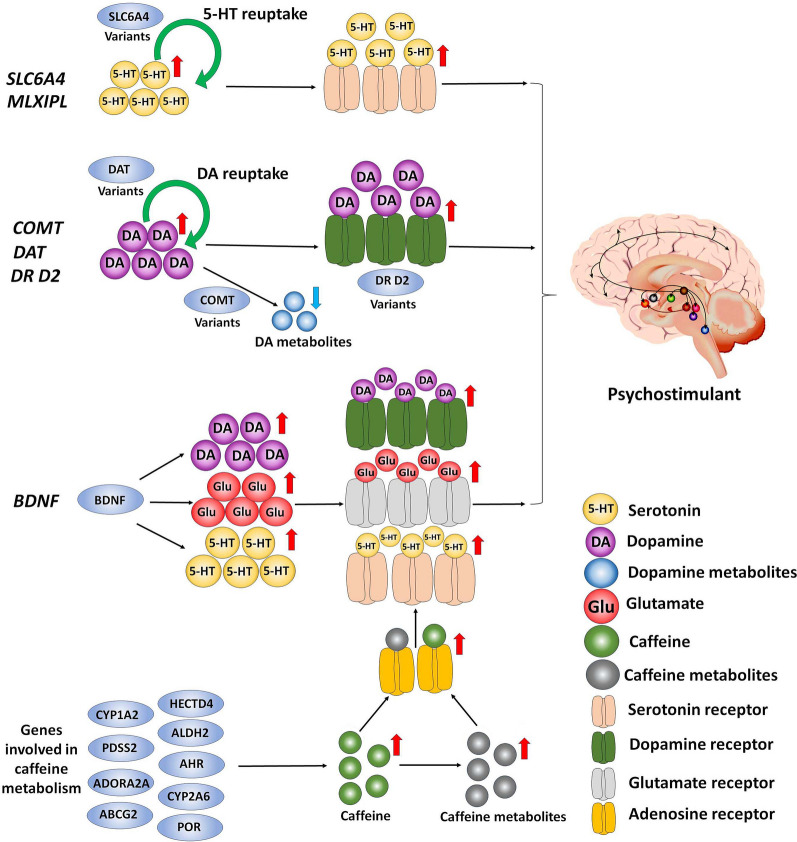
Fig. 4.
Genetic variants and their effects on caffeine dependence. The SLC6A4 gene encodes an integral membrane protein that transports serotonin (5-hydroxytryptpamine, 5-HT) from synaptic spaces into presynaptic neurons. SLC6A4 variant is associated with higher caffeine intake and increased activity in the serotonergic raphe nuclei. Genes encoding the DA D2 receptor (DRD2), DA transporter (DAT) and catechol-O-methyltransferase (COMT) are thought to be responsible for dopaminergic signaling in reward mechanisms implicated in caffeine addiction. DRD2 regulates the expression of dopamine D2 receptors on dopaminergic neurons. DRD2 variants regulate the expression of D2 receptors or their binding potential to DA, leading to higher DA levels and addictive behaviors. Meanwhile, the DAT eliminates DA at synapses and modulates post-synaptic dopaminergic signaling. DAT variants are associated with reduced expression of DAT, resulting in elevated synaptic DA levels and addictive behavior. COMT mediates the expression of COMT which is involved in DA degradation. COMT variants have been associated with higher DA levels and enhanced activation in the regions related to addictive action. Brain-derived neurotrophic factor (BDNF) is a member of growth factors in the neurotrophin family expressed throughout the nervous system. Caffeine consumption promotes the release of BDNF. The released BDNF can bind the TrkB receptor on the dopaminergic neurons, activate its downstream signaling pathway and promote the release of neurotransmitters, including DA, glutamate and serotonin, to enhance addictive behavior. The genes involved in caffeine metabolism, including CYP1A2, ADORA2A, AHR, POR, ABCG2, CYP2A6, PDSS2, HECTD4, and ALDH2 genes, promote caffeine metabolism. Caffeine and its metabolites act as adenosine receptor antagonists to block adenosine receptors, eventually promoting the release of neurotransmitters, including DA, serotonin, and glutamate, and enhancing addictive action
Role of Body Metabolism and BMI
As an activator of the sympathetic nervous system, caffeine is commonly known to decrease body weight by increasing resting metabolic rate, fat metabolism and energy consumption [88–90]. A previous study found that CYP1A2 rs762551 metabolizer status affects the association between caffeine ingestion and BMI. For those with rapid metabolizer status (rs762551-A), they were more likely to have higher caffeine intake and lower BMI [91]. Given that genes conferring rapid metabolizer status can lead to higher caffeine metabolism, individuals with a baseline high metabolic rate and without any caffeine rapid metabolizer genes may naturally metabolize caffeine faster, leading to greater consumption of caffeine to achieve the same stimulant effects, possibly resulting in more caffeine dependence.
In addition to body metabolism, the body weight of individuals could also affect caffeine pharmacokinetics and thus caffeine’s metabolism in the body. It was found that at rest, obese subjects had higher caffeine absorption rate constant and lower elimination rate constant compared to lean subjects [89]. This could possibly result in more caffeine excretion and lower caffeine remaining in lean subjects, resulting in greater consumption of caffeine in lean subjects and thus more caffeine dependence.
Animal Models involving Caffeine Intake
As caffeine pharmacokinetics are similar after consumption of caffeine in humans and animals, many studies involving caffeine intake have been conducted in animal models [19]. The gene CYP1A2 that contributes greatly to caffeine metabolism in humans, was also found to mediate caffeine metabolism in rats, with significantly lower caffeine clearance and metabolic velocity in CYP1A2 knockout rat models [92]. Caffeine withdrawal signs in rats, cats and monkeys include decreases in locomotor activity and operant behaviour [19]. In adult rodents, caffeine has been known to modulate reward circuitry, especially in the NAc and prefrontal cortex [22]. In the NAc, compared to controls, rats that ingested caffeine exhibited significantly elevated expression of genes such as Drd3, which is responsible for a dopamine receptor involved in the pathogenesis and maintenance of addiction [93]. Another two separate studies found that female mice and rat models that ingested caffeine had an increase in expression of the dopamine 2 receptor (D2R) gene [94, 95]. In Davis et al. the DRD2 gene was found to be significantly associated with the caffeine reward pathway, underscoring the importance of dopamine receptor genes in mediating caffeine addiction [61].
As part of the mesolimbic dopaminergic system, caffeine also increases dopamine, stimulating reward-related structures of the brain, in line with its reinforcing nature [19, 96]. A study found that caffeine decreased the transcription of the ADORA2A gene in the hippocampi of rats, which facilitates the activation of dopamine receptors and reward pathways [97]. The VTA is another part of the brain that contains a large population of DA neurons [98]. Studies have shown that the VTA is crucial for reward processing in the brain and is associated with substance dependency [98]. It was found that in male Wistar rats, a low dose of systemic caffeine injected into the rostral VTA produced increased reward processing, indicated by an increase in conditioned place preferences [98]. The chronic release of dopamine and the resulting rewarding effects are thought to encourage future consumption of other abusive substances, such as heroin, underlying the transition to dependency and addiction [99].
Apart from increasing dopamine, caffeine also increases extracellular glutamate concentrations in the NAc of male rats by blocking the adenosine A1 receptor [96]. Increased glutamate concentrations have been previously associated with chronic exposure to other addictive substances such as alcohol, nicotine and cocaine and was thought to be involved in the development of alcohol addiction [100]. A study found that chronic caffeine ingestion starting from adolescence in mice had increased reward-seeking behaviour and increased ethanol drinking habits in adulthood, postulated to be due to increased dopamine and glutamate levels [4]. However, even within animal models, there are individual differences in vulnerability to dependency, thought to be due to reasons such as stochastic gene expression [99]. Another possible reason is that the life experiences of mice may lead to the expression of genes that reinforce dependence neuronal pathways, affecting development of addictive behaviour [99]. Understanding the genes that distinguish between habitual caffeine users and non-users would be invaluable in predicting who would be more likely to habitually consume caffeine. Knowledge of such genes would also be helpful in the development of new treatments for neurodegenerative conditions and understanding dependency on other substances.
Pathophysiology of Important Genes associated with Caffeine Intake
CYP1A2
Individuals with the substitution of A to C allele at position 163 (rs762551) are known to be “slow metabolizers”, compared to homozygous A individuals [101]. Those with slow caffeine metabolism are more likely to have higher caffeine levels and lower paraxanthine levels and it was previously suggested that they may need less caffeine compared to “fast metabolizers” to achieve the same stimulant effects of caffeine, possibly resulting in lower habitual caffeine consumption [30]. They may also get more adverse stimulant-related effects at lower doses, deterring them from habitually drinking more caffeine [30]. Moreover, in “slow metabolizers”, caffeine intake was also previously shown to be associated with higher risk of myocardial infarction and when more than 3 cups of coffee per day was consumed, there was a higher risk of albuminuria, hyperfiltration and hypertension, compared to “fast metabolizers” [102–104]. Thus, it could also be more favourable for “slow metabolizers” to consume less caffeine in the long run.
One of the SNPs that has high inducibility of CYP1A2 is rs2472297 which was found to be closely related to a promoter region of both CYP1A1 and CYP1A2, possibly accounting for the higher metabolism of caffeine [30]. As genotypes associated with higher CYP1A2 inducibility, such as rs762551-AA and rs2472297-T, are more likely to rapidly metabolize caffeine, they could possibly lead to lower plasma caffeine levels and increased caffeine consumption compared to those with slow caffeine metabolism genotypes [36–38]. Over time, this increased caffeine consumption may lead “fast metabolizers” to develop a tolerance, lowering sensitivity to caffeine and resulting in habitual caffeine intake.
AHR
AHR at 7p21 encodes a ligand-activated transcription factor, AhR, that binds to a dioxin responsive element (DRE) on DNA, upregulating transcription of CYP1A1 and CYP1A2 in the nucleus [36, 41]. It was found in previous studies on human placenta samples that there can be as much as 20-fold differences in AhR affinity for ligand binding, affecting whether the CYP1 family of genes has “high” or “low” inducibility phenotypes [105]. Thus, given its role in inducing the CYP1 family of genes, AHR can determine the activity of CYP1A2 and its metabolism of caffeine.
ABCG2
ABCG2 is one of the main ATP-binding cassette (ABC) transporters in the CNS. ABCG2 is found in the membranes of various organs including the liver, kidney and brain [30]. As a broad-spectrum pump, it prevents excessive amounts of xenobiotic substances from building up in the brain, regulating the transport of a wide variety of substances across plasma membranes, including the BBB endothelium [30, 106]. For example, the transporters were found to prevent blood-to-brain transport of many opioids [107]. Other substances that can affect the CNS such as cannabinoids and stimulants have been found to interact with ABC transporters [106]. Sustained abuse of such drugs can lead to enhanced ABC transporter expression at the BBB, such that individuals would need more drugs to overcome the upregulation of ABC transporters, leading to drug dependence and tolerance [106, 107]. This mechanism can possibly also explain the role of ABCG2 in habitual caffeine intake.
POR
POR encodes for P450 oxidoreductase (POR) which transfers electrons to many cytochrome P450 (CYP) enzymes, including many drug-metabolizing enzymes, such as CYP1A2, CYP2C9, CYP2D6 and CYP3A4 [108]. With more than 140 variants, POR is highly polymorphic and different POR variants have been shown to significantly change activity levels of CYP1A2 with suggestions that genetic variation in POR may be at least as important as variations in CYP alleles [108]. Variants such as POR A287P can greatly decrease CYP1A2 activities, possibly impacting the CYP1A2 metabolism of xenobiotics like caffeine in the liver, leading to decreased caffeine consumption compared to variants that can increase CYP1A2 caffeine metaoblism [109].
ADORA2A
Adenosine is ubiquitously present in all cells and is responsible for many of caffeine’s effects. Caffeine’s blockage of adenosine A2 receptors plays a part in caffeine’s effects such as increased wakefulness, enhanced memory, psychomotor stimulation and anxiety [27, 110]. In particular, rs5751876 T/T and rs35320474 T/T polymorphisms have been associated with anxiety after acute caffeine intake [27]. In rodents, caffeine has been shown to increase exercise performance and ergogenic effects [111].
ADORA2A is also involved in caffeine’s rewarding effects via its modulation of dopaminergic transmission. Normally, when adenosine binds to adenosine receptors, adenylyl cyclase and Ca2 + channels are activated, consequently activating the cAMP-PKA signalling pathway, which induces the phosphorylation of dopamine, inhibiting its release, leading to many downstream biological changes in the brain and the CNS, [112]. The ADORA2A gene encodes the adenosine 2A receptor, which is antagonized by caffeine, potentiating downstream D2 receptors, leading to increased dopamine release in the brain, enhancing dopaminergic input on the mesocorticolimbic pathway [11, 19, 27, 47]. Studies have shown that chronic caffeine intake can lead to changes in tolerance and sensitization of dopamine-mediated pathways in rats, emphasizing adenosine’s role in habitual caffeine intake [27].
CYP2A6
As an enzyme that also belongs to the cytochrome P450 system, CYP2A6 is also in charge of metabolizing xenobiotics like caffeine, converting paraxanthine(17X) to 17U [113]. The SNP rs56113850-T was previously associated with lower caffeine consumption and higher plasma paraxanthine/caffeine levels reflecting slow paraxanthine metabolism [30]. In other studies, the C allele has also been more strongly linked with elevated caffeine consumption [114]. Moreover, with CYP2A6, around 75% of nicotine is converted to cotinine (COT) which is then converted to 3-hydroxycotinine (THOC) [30]. Rs56113850-C was also strongly associated with nicotine smoking, another addictive behaviour [115]. The same variants involved in higher caffeine consumption were also involved in heavy smoking behaviour, reflecting the role of CYP2A6 in both addictive behaviours.
BDNF
Mesolimbic fibers start from the VTA and extend to the NAc and prefrontal cortex. In response to drugs and other rewarding-related stimuli like food, dopamine is released in the NAc and prefrontal cortex. BDNF, a neutrophin, and its receptor, tropomyosin-related kinase B (TrkB) are expressed in dopaminergic neurons in structures of the mesolimbic reward neurocircuit, like the VTA and medial prefrontal cortex [116]. BDNF-TrkB binding results in autophosphorylation of tyrosine residues and the downstream signalling induces the Ras-mitogen-activated protein kinase (MAPK) pathway [117]. BDNF and the pathway it induces have been implicated in antidepressant treatments, underscoring its role in enhancing reward in the brain [117].
There is some evidence that addictive substances like cocaine and nicotine can increase BDNF mRNA levels in the NAc and striatum of rats and was associated with increased motivation to consume and self-administer these addictive substances, indicating BDNF’s role in the development of behaviour that could lead to habitual caffeine intake [118, 119]. Previous studies have shown that the BDNF met allele decreases trafficking of BDNF transcripts to dendrites, decreasing BDNF signaling in the NAc, thus decreasing reward-seeking behaviour and also possibly motivation to consume caffeine [116, 117, 120].
SLC6A4
SLC6A4 is thought to directly affect caffeine drinking behaviour by influencing the psychostimulant and rewarding effects of caffeine [1]. Caffeine is a methylxanthine and its structure is similar to tryptophan which is a precursor to serotonin [121]. Thus, consuming caffeine can directly to an increase in tryptophan and serotonin [121, 122]. Caffeine’s inhibition of adenosine A1 receptors also triggers the release of neurotransmitters including serotonin [57].
In addition, the raphe nuclei have strong linkage with structures involved in reward processing including the VTA, NAc and medial prefrontal cortex [123]. There are also strong connections with the dopaminergic system which is well-recognized to be involved in reward processing [123]. Studies of alcohol consumption in zebrafish, rats and humans have shown parallels between dopamine and serotonin signalling with increased levels of 5-HT and the serotonin metabolite 5-HIAA [124]. Serotonin has been linked to various substance use disorders, including alcohol, heroin and cocaine, and the long arm of chromosome 17 was thought to increase heroin addiction susceptibility [56, 124]. Thus, given the close relationship between dopamine and serotonin and the links to other substance use disorders, it is likely that SLC6A4 also has an important role in mediating habitual caffeine intake.
Dopaminergic Pathway Genes
Dopaminergic projections from the VTA to the NAc are involved in reward processing [99, 125]. This pathway has the largest effect on addiction and many addictive substances have been shown to increase dopamine in the NAc along this path [125]. Through the antagonism of adenosine receptors, caffeine is able to increase dopamine released in the CNS [27]. With chronic usage of addictive substances, neuroadaptations, such as less dopamine receptors and lower reward-related dopamine release, take place [125]. Thus, over time, larger amounts of addictive substances, including caffeine, are needed to achieve homeostasis, to prevent withdrawal symptoms such as anxiety and depression [125]. As such, variants such as Rs179732 DelC minor allele that are associated with lower expression of the DA D2 receptor (DRD2) are more likely to result in habitual caffeine intake [61]. Moreover, DRD2-KO mice were also found to be bradykinetic, like in Parkinson’s Disease, and were more likely to self-administer other addictive substances like cocaine compared to their wild-type counterparts [126].
The dopamine transporter (DAT) belongs to a family of monoamine transporters and is responsible for re-uptaking dopamine from the synaptic cleft, thus influencing the strength and time span of dopamine signalling [126]. Thus, lower amounts of transporter proteins result in greater synaptic dopamine levels over time and greater addictive behaviour [126]. With the creation of DAT-KO mice, research has shown that DAT is involved in dopamine synthesis, homeostasis and storage, among others [126]. Moreover, the hyperactive behaviour in DAT-KO mice was similar to normal mice who were given high amounts of CNS stimulants like amphetamine, highlighting how DAT blockade leads to addictive behaviour, including habitual caffeine intake [127].
GCKR
It has been suggested that GCKR which encodes glucokinase regulatory protein regulates the metabolism and sensing of glucose in the brain, potentially influencing cerebral reward pathways affected by various coffee compounds, although its exact role in coffee intake is not yet clear [1, 30].
MLXIPL
MLXIPL is a transcription factor crucial for glucose and lipid metabolism [128]. However, its exact role in caffeine intake is not clear yet and future studies are needed to fully elucidate its function.
Challenges and Limitations
There are several challenges when investigating genetic susceptibility to caffeine consumption. While the proposed DSM-5 diagnostic criteria for caffeine use disorder (such as persistent desire to control caffeine use, continued caffeine use despite knowing the problems that can be exacerbated by caffeine, withdrawal syndrome for caffeine, etc.) are useful for clinical classification, there are no objective biological markers with high diagnostic accuracy [129, 130]. Some criteria may be open to subjective interpretation and the diagnostic approach has been suggested to be more “conservative” than for other substance abuse diagnosis [129]. A recent study of 1006 caffeine-consuming adults in the USA found only 8% fulfilled DSM-proposed criteria for caffeine use disorder, and these subjects were younger and more likely to smoke cigarettes. The findings suggest that the diagnostic criteria could only identify a relatively small fraction of those with caffeine use disorder in the general population [130]. Coffee and tea consumption constitutes the main source of caffeine intake and this represents the most obvious measurable clinical outcome. However, there are many other caffeinated drinks (such as chocolate and energy drinks etc.) and caffeine is also found naturally in some foods. These are not fully captured in most studies. Moreover, even the quantification of intake or exposure (based on number of cups, number of times, duration of intake etc.) is frequently analyzed differently.
One major issue is recall bias, as most studies are retrospective in nature and ask respondents to recollect estimated information over the previous few weeks, or months. This method of collecting data is subject to reliability and consistency concerns. Such data can vary widely and there has not been a validated standardized evaluation scale of caffeine intake that has been universally adopted. The challenge is to develop and validate non-invasive methods to assess caffeine consumption accurately and in a consistent manner.
Current studies have also frequently based caffeine consumption data at one time point, but this does not reflect or capture the actual consumption data prospectively over a prolonged period. Caffeine use disorders do not arise over a short time interval and in the context of genetic predisposition and caffeine exposure interaction, there is an intricate interplay over time and studies have generally not considered potential extrinsic (e.g. the effect of seasonal weather) and intrinsic (e.g. social and medical factors) confounders.
Both coffee and tea and other beverages contain other active components which may influence addictive behaviour. It is not clear if the different preparation of these beverages (such as filtered/brewed/instant) or if the added sugar, milk and additives in the beverages can influence the outcome measures.
The association between caffeine use disorder and non-drug psychiatric disorders can also be a confounding variable. Moderate caffeine intake (< 6 cups/day) may help with depressive symptoms, and suicide risk whereas high caffeine intake has been associated with anxiety, psychotic and manic symptoms [131]. Like other substance use disorders, the relapsing and remitting trajectory of caffeine use disorder has not been sufficiently examined. Hence, dissecting out the background noise and overlap can facilitate better stratification of more homogenous groups of subjects and this can increase the chance of uncovering more accurate genetic susceptibility signals for caffeine dependence.
The association of HECTD4 rs2074356 with habitual caffeine consumption highlighted the importance of ethnicity and population stratification in gene-caffeine interaction studies. In this instance, the A allele was specific to East Asians and monomorphic in Europeans, Africans and Americans. When there are more than one genetic modifying variants in specific populations, the application of polygenic scores may increase the chance of successfully finding genes that interact with caffeine intake in those populations. In recent years, machine learning-based Bayesian and other mixed-model methods have been developed. Such methods can better evaluate gene–gene and gene-lifestyle interactions. Thus, future large-scale epidemiology studies across different populations will be better poised to identify novel gene-caffeine interactions when more in-depth analyses are available [132, 133]. Measurements of metabolites (such as paraxanthine, theophylline, theobromine and paraxanthine/caffeine ratio etc.) in the caffeine pathway will provide more robust real time evidence regarding the amount of caffeine in the body and its correlation in those with addictive behaviour. In addition, it will also allow correlational analysis with other lifestyle and genetic factors. For example, a recent study using > 400,000 subject data from the UK Biobank data found that those taking caffeine within about 1 h of blood sampling had higher glucose levels than non-caffeine drinkers. Interestingly, age, adiposity, fasting time and genetic factors (such as CYP1A2 and MLXIPL) involved in caffeine metabolism and drinking behaviour influenced the findings [38]. These observations suggest that gene caffeine interaction studies are likely to be complex and accurate details on caffeine exposure time, type and composition of the beverage sources and blood sampling timing can also be some of the key considerations for future studies. Furthermore, the possibility of selection bias of apparent healthy individuals who are more likely to participate in such surveys also needs to be considered.
Given that most studies on caffeine intake have been based on Europeans, more future studies are needed to characterize the genes involved in caffeine intake in non-European populations. Newer studies from Japan and Korea on genes affecting caffeine intake have been done in recent years, however, these studies do not account for the heterogeneity of genes in Asian populations, much less other underrepresented populations. An issue regarding gene characterization from non-European populations includes a smaller than optimal sample size, resulting in studies that may lack the statistical power necessary to detect meaningful genes that affect caffeine intake. Nevertheless, with the increasing demand for caffeine around the world, more studies on caffeine-related genes in non-European populations may arise. Such newer studies can also independently replicate existing studies, adding to their credibility.
Another significant challenge is the definition of caffeine dependence. As the mechanism underlying caffeine dependence/caffeine use disorder has not been fully elucidated and harm from caffeine is extremely varied across individuals, it is tough to accurately quantify and define caffeine dependence. There is limited research on caffeine dependence prevalence, with only a few studies from a limited number of countries such as the USA, Italy, Hungary and New Zealand [134]. More studies are needed to estimate the prevalence and understand the negative biopsychosocial effects of caffeine dependence, which is in line with how the DSM-5 recognises caffeine use disorder as a condition for further study [135].
In addition, many studies currently have data for caffeine intake but lack data for excessive consumption of caffeine. Other studies have also recognized how the number of individuals with caffeine intake higher than 400 mg per day is rarely documented, leading to difficulties in assessing exactly when individuals start experiencing various side effects of caffeine and thus difficulty in assessing for caffeine use disorder and caffeine withdrawal [134]. Moreover, given the ubiquity of caffeine in popular drinks and foods from chocolate to energy drinks, it can also be difficult to accurately assess caffeine intake, and the amount of caffeine needed to produce harm.
Impact on Health
Caffeine intoxication can present with anxiety, insomnia, psychomotor agitation and irritability and consuming elevated amounts for a prolonged period can lead to withdrawal symptoms including headaches and lethargy [136]. From 2000 to 2019, per coffee capita consumption increased about 37% with total global coffee consumption reaching around 13 teragrams in 2019 [6]. Along with that, the prevalence of Caffeine Use Disorder has been estimated to be around 6% to 14% [134, 137]. Given caffeine’s psychoactive properties, widespread popularity and omnipresence in common drinks and food, this systematic review examining caffeine dependence genes can have significant implications for global health and personalised medicine, especially for Caffeine Use Disorder. Knowledge of whether specific populations are at higher risk of caffeine dependence can allow for the early implementation of targeted screening or preventative programmes and educational initiatives, reducing future burden of care. In addition, knowing the genes that predispose individuals to caffeine addiction can contribute to greater understanding of the complex interplay between genes, environment and behaviour, paving the way for better addiction prevention and treatment, both at the individual and population levels.
Unfortunately, current treatment availability for Caffeine Use Disorder is limited, partly due to the lack of research on caffeine use disorder and treatment options that work best to reduce caffeine consumption [129]. However, we hope that through this study, the genetic factors contributing to caffeine dependence are better understood. Insights from this systematic review can help identify individuals with genetic predispositions to caffeine dependence. This identification could enable researchers and clinicians to better predict clinical phenotypes, helping them to diagnose and risk stratify patients, especially vulnerable individuals such as pregnant women and children, for whom the risks of dependence and addiction are greater [138]. With such information, healthcare providers can tailor addiction prevention and interventions to patients’ specific needs, adjusting caffeine consumption based on genetic predisposition, leading to more effective and personalised therapies, including lifestyle modifications and pharmacological interventions. Moreover, since habitual caffeine consumption is positively associated with consumption of other addictive substances such as alcohol and smoking, personalised addiction therapies for caffeine dependence could also be applied to other substances, guiding broader healthcare interventions to address addiction and its associated health consequences [139, 140].
Conclusions
In our systematic review, we identified 26 studies (comprising > 1.8 million individuals) with sample sizes of at least 200 subjects each that examined genetic susceptibility to caffeine dependence. Genes involved with caffeine metabolism such as CYP1A2, ADORA2A, AHR, POR, ABCG2, CYP2A6, PDSS2 and HECTD4 rs2074356 (A allele specific to East Asians and monomorphic in Europeans, Africans and Americans) were associated with habitual caffeine consumption. Genes associated with caffeine reward were BDNF, SLC6A4, GCKR, MLXIPL and dopaminergic genes (potentially affect dopamine neurotransmission) such as DRD2 and DAT1.
Since caffeine dependence can lead to various forms of functional impairment and social issues, resulting in many seeking therapies, identification of genes associated with caffeine intake and metabolism will provide novel insights on the biological pathways that can potentially lead to development of drug targets for dependency to caffeine or other addictive substances. Drug and non-pharmacologic intervention can also be potentially tailored for specific healthy presymptomatic gene carriers to reduce the caffeine dependency risk, using a precision medicine approach.
Acknowledgements
We thank the National Medical Research Council for their support.
Author contributions
All authors contributed equally to the manuscript. All authors read and approved the final manuscript.
Funding
None to declare.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coffee and Caffeine Genetics Consortium, Cornelis MC, Byrne EM, et al. Genome-wide meta-analysis identifies six novel loci associated with habitual coffee consumption. Mol Psychiatry. 2015;20(5):647–56. 10.1038/mp.2014.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts A. Caffeine: an evaluation of the safety database. In: Gupta RC, Lall R, Srivastava A, editors. Nutraceuticals. 2nd ed. Cambridge: Academic Press; 2021. p. 501–18. 10.1016/B978-0-12-821038-3.00032-X. [Google Scholar]
- 3.Frary CD, Johnson RK, Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc. 2005;105(1):110–3. 10.1016/j.jada.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 4.Martins RS, Rombo DM, Gonçalves-Ribeiro J, et al. Caffeine has a dual influence on NMDA receptor–mediated glutamatergic transmission at the hippocampus. Purinergic Signal. 2020;16(4):503–18. 10.1007/s11302-020-09724-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fredholm BB. Astra Award Lecture: adenosine, adenosine receptors and the actions of caffeine. Pharmacol Toxicol. 1995;76(2):93–101. 10.1111/j.1600-0773.1995.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 6.Quadra GR, Paranaíba JR, Vilas-Boas J, et al. A global trend of caffeine consumption over time and related-environmental impacts. Environ Pollut. 2020;256:113343. 10.1016/j.envpol.2019.113343. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman HR, Tharion WJ, Shukitt-Hale B, Speckman KL, Tulley R. Effects of caffeine, sleep loss, and stress on cognitive performance and mood during U.S. Navy SEAL training. Sea-Air-Land Psychopharmacol. 2002;164(3):250–61. 10.1007/s00213-002-1217-9. [DOI] [PubMed] [Google Scholar]
- 8.Haskell CF, Kennedy DO, Wesnes KA, Scholey AB. Cognitive and mood improvements of caffeine in habitual consumers and habitual non-consumers of caffeine. Psychopharmacology. 2005;179(4):813–25. 10.1007/s00213-004-2104-3. [DOI] [PubMed] [Google Scholar]
- 9.Gutiérrez-Hellín J, Varillas-Delgado D. Energy drinks and sports performance, cardiovascular risk, and genetic associations; future prospects. Nutrients. 2021;13(3):715. 10.3390/nu13030715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolb H, Kempf K, Martin S. Health effects of coffee: mechanism unraveled? Nutrients. 2020;12(6):1842. 10.3390/nu12061842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kowiański P, Lietzau G, Steliga A, et al. Nicotine-induced CREB and DeltaFosB activity is modified by caffeine in the brain reward system of the rat. J Chem Neuroanat. 2018;88:1–12. 10.1016/j.jchemneu.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Huang ZL, Zhang Z, Qu WM. Roles of adenosine and its receptors in sleep-wake regulation. Int Rev Neurobiol. 2014;119:349–71. 10.1016/B978-0-12-801022-8.00014-3. [DOI] [PubMed] [Google Scholar]
- 13.Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51(1):83–133. [PubMed] [Google Scholar]
- 14.Acquas E, Tanda G, Di Chiara G. Differential effects of caffeine on dopamine and acetylcholine transmission in brain areas of drug-naive and caffeine-pretreated rats. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2002;27(2):182–93. 10.1016/S0893-133X(02)00290-7. [DOI] [PubMed] [Google Scholar]
- 15.Lazarus M, Shen HY, Cherasse Y, et al. Arousal effect of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J Neurosci Off J Soc Neurosci. 2011;31(27):10067–75. 10.1523/JNEUROSCI.6730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manalo RVM, Medina PMB. Caffeine protects dopaminergic neurons from dopamine-induced neurodegeneration via synergistic adenosine-dopamine D2-like receptor interactions in transgenic Caenorhabditis elegans. Front Neurosci. 2018;12:137. 10.3389/fnins.2018.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh K, Singh S, Singhal NK, Sharma A, Parmar D, Singh MP. Nicotine- and caffeine-mediated changes in gene expression patterns of MPTP-lesioned mouse striatum: implications in neuroprotection mechanism. Chem Biol Interact. 2010;185(2):81–93. 10.1016/j.cbi.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther. 2005;108(1):18–58. 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Nehlig A. Are we dependent upon coffee and caffeine? A review on human and animal data. Neurosci Biobehav Rev. 1999;23(4):563–76. 10.1016/S0149-7634(98)00050-5. [DOI] [PubMed] [Google Scholar]
- 20.Favrod-Coune T, Broers B. Addiction to caffeine and other xanthines. In: El-Guebaly N, Carrà G, Galanter M, Baldacchino AM, editors. Textbook of addiction treatment: international perspectives. Berlin: Springer International Publishing; 2021. p. 215–28. 10.1007/978-3-030-36391-8_16. [Google Scholar]
- 21.Zduńska A, Cegielska J, Zduński S, Domitrz I. Caffeine for headaches: helpful or harmful? A brief review of the literature. Nutrients. 2023;15(14):3170. 10.3390/nu15143170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferré S. Mechanisms of the psychostimulant effects of caffeine: implications for substance use disorders. Psychopharmacology. 2016;233(10):1963–79. 10.1007/s00213-016-4212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alasmari F. Caffeine induces neurobehavioral effects through modulating neurotransmitters. Saudi Pharm J. 2020;28(4):445–51. 10.1016/j.jsps.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Temple JL, Hostler D, Martin-Gill C, et al. Systematic review and meta-analysis of the effects of caffeine in fatigued shift workers: implications for emergency medical services personnel. Prehosp Emerg Care. 2018;22(sup1):37–46. 10.1080/10903127.2017.1382624. [DOI] [PubMed] [Google Scholar]
- 25.Jessel CD, Narang A, Zuberi R, Bousman CA. Sleep quality and duration in children that consume caffeine: impact of dose and genetic variation in ADORA2A and CYP1A. Genes. 2023. 10.3390/genes14020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiffin P, Ashton H, Marsh R, Kamali F. Pharmacokinetic and pharmacodynamic responses to caffeine in poor and normal sleepers. Psychopharmacology. 1995;121(4):494–502. 10.1007/BF02246500. [DOI] [PubMed] [Google Scholar]
- 27.Yang A, Palmer AA, de Wit H. Genetics of caffeine consumption and responses to caffeine. Psychopharmacology. 2010;211(3):245–57. 10.1007/s00213-010-1900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chwedorowicz R, Łukawski K, Raszewski G, Czuczwar SJ. Caffeine impairs anticonvulsant effects of levetiracetam in the maximal electroshock seizure threshold test in mice. J Basic Clin Physiol Pharmacol. 2023;34(3):357–64. 10.1515/jbcpp-2022-0224. [DOI] [PubMed] [Google Scholar]
- 29.Saunders B, da Costa LR, de Souza RAS, Barreto G, Marticorena FM. Caffeine and sport. In: Toldrá F, editor. Advances in food and nutrition research, vol. 106. Cambridge: Academic Press; 2023. p. 95–127. 10.1016/bs.afnr.2023.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Cornelis MC, Kacprowski T, Menni C, et al. Genome-wide association study of caffeine metabolites provides new insights to caffeine metabolism and dietary caffeine-consumption behavior. Hum Mol Genet. 2016;25(24):5472–82. 10.1093/hmg/ddw334. [DOI] [PubMed] [Google Scholar]
- 31.Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: an R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Campbell Syst Rev. 2022. 10.1002/cl2.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corchero J, Pimprale S, Kimura S, Gonzalez FJ. Organization of the CYP1A cluster on human chromosome 15: implications for gene regulation. Pharmacogenetics. 2001;11(1):1–6. 10.1097/00008571-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Amin N, Byrne E, Johnson J, et al. Genome-wide association analysis of coffee drinking suggests association with CYP1A1/CYP1A2 and NRCAM. Mol Psychiatry. 2012;17(11):1116–29. 10.1038/mp.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunes A, Dahl ML. Variation in CYP1A2 activity and its clinical implications: influence of environmental factors and genetic polymorphisms. Pharmacogenomics. 2008;9(5):625–37. [DOI] [PubMed] [Google Scholar]
- 35.Rasmussen BB, Brix TH, Kyvik KO, Brøsen K. The interindividual differences in the 3-demthylation of caffeine alias CYP1A2 is determined by both genetic and environmental factors. Pharmacogenetics. 2002;12(6):473–8. 10.1097/00008571-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Sulem P, Gudbjartsson DF, Geller F, Prokopenko I, Feenstra B, Aben KK, Franke B, den Heijer M, Kovacs P, Stumvoll M, Mägi R. Sequence variants at CYP1A1–CYP1A2 and AHR associate with coffee consumption. Hum Mol Genet. 2011;20(10):2071–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kukal S, Thakran S, Kanojia N, Yadav S, Mishra MK, Guin D, Singh P, Kukreti R. Genic-intergenic polymorphisms of CYP1A genes and their clinical impact. Gene. 2023;857:147171. [DOI] [PubMed] [Google Scholar]
- 38.Cornelis MC. Recent consumption of a caffeine-containing beverage and serum biomarkers of cardiometabolic function in the UK Biobank. Br J Nutr. 2021;126(4):582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodenburg EM, Eijgelsheim M, Geleijnse JM, et al. CYP1A2 and coffee intake and the modifying effect of sex, age, and smoking123. Am J Clin Nutr. 2012;96(1):182–7. 10.3945/ajcn.111.027102. [DOI] [PubMed] [Google Scholar]
- 40.Josse AR, Da Costa LA, Campos H, El-Sohemy A. Associations between polymorphisms in the AHR and CYP1A1-CYP1A2 gene regions and habitual caffeine consumption. Am J Clin Nutr. 2012;96(3):665–71. 10.3945/ajcn.112.038794. [DOI] [PubMed] [Google Scholar]
- 41.Cornelis MC, Monda KL, Yu K, et al. Genome-wide meta-analysis identifies regions on 7p21 (AHR) and 15q24 (CYP1A2) as determinants of habitual caffeine consumption. PLoS Genet. 2011. 10.1371/journal.pgen.1002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Genome-wide association studies of coffee intake in UK/US participants of European ancestry uncover cohort-specific genetic associations. Neuropsychopharmacology. https://www.nature.com/articles/s41386-024-01870-x#MOESM2. Accessed 4 Sept 2024. [DOI] [PMC free article] [PubMed]
- 43.McMahon G, Taylor AE, Davey Smith G, Munafò MR. Phenotype refinement strengthens the association of AHR and CYP1A1 genotype with caffeine consumption. PLoS ONE. 2014;9(7): e103448. 10.1371/journal.pone.0103448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding R, Shi J, Pabon K, Scotto KW. Xanthines down-regulate the drug transporter ABCG2 and reverse multidrug resistance. Mol Pharmacol. 2012;81(3):328–37. 10.1124/mol.111.075556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ross DD, Karp JE, Chen TT, Doyle LA. Expression of breast cancer resistance protein in blast cells from patients with acute leukemia. Blood. 2000;96(1):365–8. [PubMed] [Google Scholar]
- 46.Rogers PJ, Hohoff C, Heatherley SV, et al. Association of the anxiogenic and alerting effects of caffeine with ADORA2A and ADORA1 polymorphisms and habitual level of caffeine consumption. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2010;35(9):1973–83. 10.1038/npp.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong VW, Kuang A, Danning RD, et al. A genome-wide association study of bitter and sweet beverage consumption. Hum Mol Genet. 2019;28(14):2449–57. 10.1093/hmg/ddz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cornelis MC, El-Sohemy A, Campos H. Genetic polymorphism of the adenosine A2A receptor is associated with habitual caffeine consumption. Am J Clin Nutr. 2007;86(1):240–4. 10.1093/ajcn/86.1.240. [DOI] [PubMed] [Google Scholar]
- 49.Martins GL, Guilherme JPLF, Ferreira LHB, de Souza-Junior TP, Lancha AH. Caffeine and exercise performance: possible directions for definitive findings. Front Sports Act Living. 2020. 10.3389/fspor.2020.574854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aklillu E, Djordjevic N, Carrillo JA, Makonnen E, Bertilsson L, Ingelman-Sundberg M. High CYP2A6 enzyme activity as measured by a caffeine test and unique distribution of CYP2A6 variant alleles in Ethiopian population. OMICS J Integr Biol. 2014;18(7):446. 10.1089/omi.2013.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cornelis MC, Munafo MR. Mendelian randomization studies of coffee and caffeine consumption. Nutrients. 2018;10(10):1343. 10.3390/nu10101343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pirastu N, Kooyman M, Robino A, et al. Non-additive genome-wide association scan reveals a new gene associated with habitual coffee consumption. Sci Rep. 2016;6:31590. 10.1038/srep31590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin T, Youn J, Kim AN, et al. Interactions of habitual coffee consumption by genetic polymorphisms with the risk of prediabetes and type 2 diabetes combined. Nutrients. 2020;12(8):2228. 10.3390/nu12082228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakagawa-Senda H, Hachiya T, Shimizu A, et al. A genome-wide association study in the Japanese population identifies the 12q24 locus for habitual coffee consumption: The J-MICC Study. Sci Rep. 2018;8(1):1493. 10.1038/s41598-018-19914-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jia H, Nogawa S, Kawafune K, et al. GWAS of habitual coffee consumption reveals a sex difference in the genetic effect of the 12q24 locus in the Japanese population. BMC Genet. 2019;20(1):61. 10.1186/s12863-019-0763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao J, Hudziak JJ, Li D. Multi-cultural association of the serotonin transporter gene (SLC6A4) with substance use disorder. Neuropsychopharmacology. 2013;38(9):1737–47. 10.1038/npp.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arnold MR, Williams PH, McArthur JA, et al. Effects of chronic caffeine exposure during adolescence and subsequent acute caffeine challenge during adulthood on rat brain serotonergic systems. Neuropharmacology. 2019;148:257–71. 10.1016/j.neuropharm.2018.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ducci F, Goldman D. The genetic basis of addictive disorders. Psychiatr Clin North Am. 2012;35(2):495–519. 10.1016/j.psc.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carlier N, Marshe VS, Cmorejova J, Davis C, Müller DJ. Genetic similarities between compulsive overeating and addiction phenotypes: a case for “food addiction”? Curr Psychiatry Rep. 2015;17:1–1. [DOI] [PubMed] [Google Scholar]
- 60.Heber D, Carpenter CL. Addictive genes and the relationship to obesity and inflammation. Mol Neurobiol. 2011. 10.1007/s12035-011-8180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis C, Loxton NJ. Addictive behaviors and addiction-prone personality traits: associations with a dopamine multilocus genetic profile. Addict Behav. 2013;38(7):2306–12. 10.1016/j.addbeh.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 62.Forbes E, Brown S, Kimak M, Ferrell R, Manuck S, Hariri A. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry. 2009;14(1):60–70. 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parsian A, Cloninger CR, Zhang ZH. Functional variant in the DRD2 receptor promoter region and subtypes of alcoholism. Am J Med Genet. 2000. 10.1002/1096-8628(20000612)96:3%3c407::aid-ajmg32%3e3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 64.Arinami T, Gao M, Hamaguchi H, Toru M. A functional polymorphism in the promoter region of the dopamine D2 receptor gene is associated with schizophrenia. Hum Mol Genet. 1997;6(4):577–82. 10.1093/hmg/6.4.577. [DOI] [PubMed] [Google Scholar]
- 65.Hirvonen M, Laakso A, Någren K, Rinne JO, Pohjalainen T, Hietala J. C957T polymorphism of the dopamine D2 receptor (DRD2) gene affects striatal DRD2 availability in vivo. Mol Psychiatry. 2004;9(12):1060–1. [DOI] [PubMed] [Google Scholar]
- 66.van Dyck CH, Malison RT, Jacobsen LK, et al. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. J Nucl Med Off Publ Soc Nucl Med. 2005;46(5):745–51. [PubMed] [Google Scholar]
- 67.Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry. 1991;48(7):648–54. [DOI] [PubMed] [Google Scholar]
- 68.Williams LM, Gatt JM, Grieve SM, et al. COMT Val(108/158)Met polymorphism effects on emotional brain function and negativity bias. Neuroimage. 2010;53(3):918–25. 10.1016/j.neuroimage.2010.01.084. [DOI] [PubMed] [Google Scholar]
- 69.Chen J, Lipska BK, Halim N, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75(5):807–21. 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clarke TK, Adams MJ, Davies G, et al. Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank (N=112,117). Mol Psychiatry. 2017;22(10):1376–84. 10.1038/mp.2017.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matoba N, Akiyama M, Ishigaki K, et al. GWAS of 165,084 Japanese individuals identified nine loci associated with dietary habits. Nat Hum Behav. 2020;4(3):308–16. 10.1038/s41562-019-0805-1. [DOI] [PubMed] [Google Scholar]
- 72.Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akiyama M, Okada Y, Kanai M, et al. Genome-wide association study identifies 112 new loci for body mass index in the Japanese population. Nat Genet. 2017;49(10):1458–67. 10.1038/ng.3951. [DOI] [PubMed] [Google Scholar]
- 74.Muskiewicz DE, Uhl GR, Hall FS. The role of cell adhesion molecule genes regulating neuroplasticity in addiction. Neural Plast. 2018;2018: e9803764. 10.1155/2018/9803764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ishiguro H, Liu QR, Gong JP, et al. NrCAM in addiction vulnerability: positional cloning, drug-regulation, haplotype-specific expression, and altered drug reward in knockout mice. Neuropsychopharmacology. 2006;31(3):572–84. 10.1038/sj.npp.1300855. [DOI] [PubMed] [Google Scholar]
- 76.Uhl GR, Liu QR, Naiman D. Substance abuse vulnerability loci: converging genome scanning data. Trends Genet. 2002;18(8):420–5. 10.1016/S0168-9525(02)02719-1. [DOI] [PubMed] [Google Scholar]
- 77.Long JC, Knowler WC, Hanson RL, et al. Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet. 1998;81(3):216–21. 10.1002/(SICI)1096-8628(19980508)81:3%3c216::AID-AJMG2%3e3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 78.Pirastu N, Kooyman M, Traglia M, et al. Association analysis of bitter receptor genes in five isolated populations identifies a significant correlation between TAS2R43 variants and coffee liking. PLoS ONE. 2014;9(3): e92065. 10.1371/journal.pone.0092065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuhn C, Bufe B, Winnig M, et al. Bitter taste receptors for saccharin and acesulfame K. J Neurosci Off J Soc Neurosci. 2004;24(45):10260–5. 10.1523/JNEUROSCI.1225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ledda M, Kutalik Z, Souza Destito MC, et al. GWAS of human bitter taste perception identifies new loci and reveals additional complexity of bitter taste genetics. Hum Mol Genet. 2014;23(1):259–67. 10.1093/hmg/ddt404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheng B, Pan C, Cheng S, et al. Whole exome sequencing study identifies novel rare risk variants for habitual coffee consumption involved in olfactory receptor and hyperphagia. Nutrients. 2022;14(20):4330. 10.3390/nu14204330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu J, Wang X, Ma R, et al. AMPK signaling mediates synphilin-1-induced hyperphagia and obesity in Drosophila. J Cell Sci. 2021;134(3): jcs247742. 10.1242/jcs.247742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shishido T, Nagano Y, Araki M, et al. Synphilin-1 has neuroprotective effects on MPP+-induced Parkinson’s disease model cells by inhibiting ROS production and apoptosis. Neurosci Lett. 2019;690:145–50. 10.1016/j.neulet.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 84.Saimaiti A, Zhou DD, Li J, et al. Dietary sources, health benefits, and risks of caffeine. Crit Rev Food Sci Nutr. 2023;63(29):9648–66. 10.1080/10408398.2022.2074362. [DOI] [PubMed] [Google Scholar]
- 85.Carrillo JA, Benítez J. Caffeine metabolism in a healthy Spanish population: N-acetylator phenotype and oxidation pathways. Clin Pharmacol Ther. 1994;55(3):293–304. 10.1038/clpt.1994.30. [DOI] [PubMed] [Google Scholar]
- 86.Kot M, Daniel WA. Relative contribution of rat cytochrome P450 isoforms to the metabolism of caffeine: the pathway and concentration dependence. Biochem Pharmacol. 2008;75(7):1538–49. 10.1016/j.bcp.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 87.Fulton JL, Dinas PC, Carrillo AE, Edsall JR, Ryan EJ, Ryan EJ. Impact of genetic variability on physiological responses to caffeine in humans: a systematic review. Nutrients. 2018;10(10):1373. 10.3390/nu10101373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Conger SA, Tuthill LM, Millard-Stafford ML. Does caffeine increase fat metabolism? A systematic review and meta-analysis. Int J Sport Nutr Exercise Metabol. 2022. 10.1123/ijsnem.2022-0131. [DOI] [PubMed] [Google Scholar]
- 89.Harpaz E, Tamir S, Weinstein A, Weinstein Y. The effect of caffeine on energy balance. J Basic Clin Physiol Pharmacol. 2017;28(1):1–10. 10.1515/jbcpp-2016-0090. [DOI] [PubMed] [Google Scholar]
- 90.LeBlanc J, Jobin M, Cote J, Samson P, Labrie A. Enhanced metabolic response to caffeine in exercise-trained human subjects. J Appl Physiol. 1985;59(3):832–7. 10.1152/jappl.1985.59.3.832. [DOI] [PubMed] [Google Scholar]
- 91.Gkouskou KG, Georgiopoulos G, Vlastos I, et al. CYP1A2 polymorphisms modify the association of habitual coffee consumption with appetite, macronutrient intake, and body mass index: results from an observational cohort and a cross-over randomized study. Int J Obes. 2022;46(1):162–8. 10.1038/s41366-021-00972-6. [DOI] [PubMed] [Google Scholar]
- 92.Sun D, Lu J, Zhang Y, et al. Characterization of a novel CYP1A2 knockout rat model constructed by CRISPR/Cas9. Drug Metab Dispos. 2021;49(8):638–47. 10.1124/dmd.121.000403. [DOI] [PubMed] [Google Scholar]
- 93.Thompson SM, Rakoczy RJ, Duffy MA, Kiss AJ, McMurray MS. Differential consumption of alcohol, caffeine, and caffeinated alcohol by adolescent rats, and effects on post-adolescent gene expression signatures in the nucleus accumbens and orbitofrontal cortex. Drug Alcohol Depend. 2023;251:110921. 10.1016/j.drugalcdep.2023.110921. [DOI] [PubMed] [Google Scholar]
- 94.Stonehouse AH, Adachi M, Walcott EC, Jones FS. Caffeine regulates neuronal expression of the dopamine 2 receptor gene. Mol Pharmacol. 2003;64(6):1463–73. 10.1124/mol.64.6.1463. [DOI] [PubMed] [Google Scholar]
- 95.Horvath G, Adam G, Tuboly G, et al. Caffeine–treat or trigger? Disparate behavioral and long-term dopaminergic changes in control and schizophrenia-like Wisket rats. Physiol Behav. 2021;236:113410. 10.1016/j.physbeh.2021.113410. [DOI] [PubMed] [Google Scholar]
- 96.Solinas M, Ferre S, You ZB, Karcz-Kubicha M, Popoli P, Goldberg SR. Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens. J Neurosci. 2002;22(15):6321–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.dos Santos Sanna PL, Bernardes Carvalho L, dos Santos Afonso CC, et al. Adora2A downregulation promotes caffeine neuroprotective effect against LPS-induced neuroinflammation in the hippocampus. Brain Res. 2024;1833:148866. 10.1016/j.brainres.2024.148866. [DOI] [PubMed] [Google Scholar]
- 98.Yee M, Maal-Bared G, Ting-A-Kee R, Chwalek M, Mackay-Clackett I, Bergamini M, Grieder TE, van der Kooy D. Segregation of caffeine reward and aversion in the rat nucleus accumbens shell versus core. Eur J Neurosci. 2020. 10.1111/ejn.14718. [DOI] [PubMed] [Google Scholar]
- 99.Lüscher C, Janak PH. Consolidating the circuit model for addiction. Ann Rev Neurosci. 2021. 10.1146/annurev-neuro-092920-123905. [DOI] [PubMed] [Google Scholar]
- 100.Das SC, Yamamoto BK, Hristov AM, Sari Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 2015;97:67–74. 10.1016/j.neuropharm.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang J, Dewi L, Peng Y, Hou CW, Song Y, Condello G. Does ergogenic effect of caffeine supplementation depend on CYP1A2 genotypes? A systematic review with meta-analysis. J Sport Health Sci. 2023. 10.1016/j.jshs.2023.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cornelis MC, El-Sohemy A, Kabagambe EK, Campos H. Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA. 2006;295(10):1135–41. 10.1001/jama.295.10.1135. [DOI] [PubMed] [Google Scholar]
- 103.Mahdavi S, Palatini P, El-Sohemy A. CYP1A2 genetic variation, coffee intake, and kidney dysfunction. JAMA Netw Open. 2023;6(1): e2247868. 10.1001/jamanetworkopen.2022.47868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Palatini P, Ceolotto G, Ragazzo F, et al. CYP1A2 genotype modifies the association between coffee intake and the risk of hypertension. J Hypertens. 2009;27(8):1594. 10.1097/HJH.0b013e32832ba850. [DOI] [PubMed] [Google Scholar]
- 105.Hermann R, Rostami-Hodjegan A, Zhao P, Ragueneau-Majlessi I. Seeing what is behind the smokescreen: a systematic review of methodological aspects of smoking interaction studies over the last three decades and implications for future clinical trials. Clin Transl Sci. 2023;16(5):742–58. 10.1111/cts.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hussein NA, Muskiewicz DE, Terrero D, Malla S, Hall FS, Tiwari AK. The effects of drugs of abuse on ABC transporters. In: Patel VB, Preedy VR, editors. Handbook of substance misuse and addictions: from biology to public health. Berlin: Springer International Publishing; 2022. p. 609–34. 10.1007/978-3-030-92392-1_184. [Google Scholar]
- 107.Yang J, Reilly BG, Davis TP, Ronaldson PT. Modulation of opioid transport at the blood-brain barrier by altered ATP-binding cassette (ABC) transporter expression and activity. Pharmaceutics. 2018;10(4):192. 10.3390/pharmaceutics10040192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tomalik-Scharte D, Maiter D, Kirchheiner J, Ivison HE, Fuhr U, Arlt W. Impaired hepatic drug and steroid metabolism in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. Eur J Endocrinol. 2010;163(6):919–24. 10.1530/EJE-10-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Agrawal V, Huang N, Miller WL. Pharmacogenetics of P450 oxidoreductase: effect of sequence variants on activities of CYP1A2 and CYP2C19. Pharmacogenet Genom. 2008;18(7):569. 10.1097/FPC.0b013e32830054ac. [DOI] [PubMed] [Google Scholar]
- 110.Ribeiro JA, Sebastião AM. Caffeine and adenosine. J Alzheimers Dis. 2010;20(s1):S3–15. 10.3233/JAD-2010-1379. [DOI] [PubMed] [Google Scholar]
- 111.Aguiar AS, Speck AE, Canas PM, Cunha RA. Neuronal adenosine A2A receptors signal ergogenic effects of caffeine. Sci Rep. 2020;10(1):13414. 10.1038/s41598-020-69660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ferré S. An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem. 2008;105(4):1067–79. 10.1111/j.1471-4159.2007.05196.x. [DOI] [PubMed] [Google Scholar]
- 113.Tanner JA, Tyndale RF. Variation in CYP2A6 activity and personalized medicine. J Pers Med. 2017;7(4):18. 10.3390/jpm7040018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cornelis MC, van Dam RM. Genetic determinants of liking and intake of coffee and other bitter foods and beverages. Sci Rep. 2021;11(1):23845. 10.1038/s41598-021-03153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Buchwald J, Chenoweth MJ, Palviainen T, et al. Genome-wide association meta-analysis of nicotine metabolism and cigarette consumption measures in smokers of European descent. Mol Psychiatry. 2021;26(6):2212–23. 10.1038/s41380-020-0702-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Homan P, Grob S, Milos G, et al. The role of BDNF, leptin, and catecholamines in reward learning in bulimia nervosa. Int J Neuropsychopharmacol. 2015;18(5): pyu092. 10.1093/ijnp/pyu092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci. 2012;35(1):47–56. 10.1016/j.tins.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leão RM, Cruz FC, Carneiro-de-Oliveira PE, et al. Enhanced nicotine-seeking behavior following pre-exposure to repeated cocaine is accompanied by changes in BDNF in the nucleus accumbens of rats. Pharmacol Biochem Behav. 2013;104:169–76. 10.1016/j.pbb.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 119.Corominas-Roso M, Roncero C, Daigre C, et al. Changes in brain-derived neurotrophic factor (BDNF) during abstinence could be associated with relapse in cocaine-dependent patients. Psychiatry Res. 2015;225(3):309–14. 10.1016/j.psychres.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 120.Green CR, Corsi-Travali S, Neumeister A. The role of BDNF-TrkB signaling in the pathogenesis of PTSD. J Depress Anxiety. 2013. 10.4172/2167-1044.S4-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chandio ZA, Sidiqua A, Khaskheli MI, Waghani A, Metlo WA. Review effect of caffeine overdose. RADS J Biol Res Appl Sci. 2020;11(2):154–8. 10.37962/jbas.v11i2.266. [Google Scholar]
- 122.Fernstrom MH, Bazil CW, Fernstrom JD. Caffeine injection raises brain tryptophan level, but does not stimulate the rate of serotonin synthesis in rat brain. Life Sci. 1984. 10.1016/0024-3205(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 123.Courtiol E, Menezes EC, Teixeira CM. Serotonergic regulation of the dopaminergic system: implications for reward-related functions. Neurosci Biobehav Rev. 2021;128:282–93. 10.1016/j.neubiorev.2021.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Clayman CL, Connaughton VP. Neurochemical and behavioral consequences of ethanol and/or caffeine exposure: effects in zebrafish and rodents. Curr Neuropharmacol. 2022;20(3):560–78. 10.2174/1570159X19666211111142027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bogdan R, Hatoum AS, Johnson EC, Agrawal A. The genetically informed neurobiology of addiction (GINA) model. Nat Rev Neurosci. 2023;24(1):40. 10.1038/s41583-022-00656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Solinas M, Belujon P, Fernagut PO, Jaber M, Thiriet N. Dopamine and addiction: what have we learned from 40 years of research. J Neural Transm. 2019;126(4):481–516. 10.1007/s00702-018-1957-2. [DOI] [PubMed] [Google Scholar]
- 127.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379(6566):606–12. 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 128.Kooner JS, Chambers JC, Aguilar-Salinas CA, et al. Genome-wide scan identifies variation in MLXIPL associated with plasma triglycerides. Nat Genet. 2008;40(2):149–51. 10.1038/ng.2007.61. [DOI] [PubMed] [Google Scholar]
- 129.Meredith SE, Juliano LM, Hughes JR, Griffiths RR. Caffeine use disorder: a comprehensive review and research agenda. J Caffeine Res. 2013;3(3):114–30. 10.1089/jcr.2013.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sweeney MM, Weaver DC, Vincent KB, Arria AM, Griffiths RR. Prevalence and correlates of caffeine use disorder symptoms among a United States sample. J Caffeine Adenosine Res. 2020;10(1):4–11. 10.1089/caff.2019.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lara DR. Caffeine, mental health, and psychiatric disorders. J Alzheimers Dis JAD. 2010;20(Suppl 1):S239-248. 10.3233/JAD-2010-1378. [DOI] [PubMed] [Google Scholar]
- 132.Moore R, Casale FP, Jan Bonder M, et al. A linear mixed-model approach to study multivariate gene-environment interactions. Nat Genet. 2019;51(1):180–6. 10.1038/s41588-018-0271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sun N, Wang Y, Chu J, Han Q, Shen Y. Bayesian approaches in exploring gene-environment and gene-gene interactions: a comprehensive review. Cancer Genom Proteom. 2023;20(6 Suppl):669–78. 10.21873/cgp.20414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Booth N, Saxton J, Rodda SN. Estimates of caffeine use disorder, caffeine withdrawal, harm and help-seeking in New Zealand: a cross-sectional survey. Addict Behav. 2020;109:106470. 10.1016/j.addbeh.2020.106470. [DOI] [PubMed] [Google Scholar]
- 135.Diagnostic and statistical manual of mental disorders. DSM Library. 10.1176/appi.books.9780890425596.
- 136.Amer SA, AlAmri FA, AlRadini FA, et al. Caffeine addiction and determinants of caffeine consumption among health care providers: a descriptive national study. Eur Rev Med Pharmacol Sci. 2023;27(8):3230–42. 10.26355/eurrev_202304_32093. [DOI] [PubMed] [Google Scholar]
- 137.Verster JC, Koenig J. Caffeine intake and its sources: a review of national representative studies. Crit Rev Food Sci Nutr. 2018. 10.1080/10408398.2016.1247252. [DOI] [PubMed] [Google Scholar]
- 138.Ennis D. The effects of caffeine on health: the benefits outweigh the risks. 2014.
- 139.Treur JL, Taylor AE, Ware JJ, et al. Associations between smoking and caffeine consumption in two European cohorts. Addiction. 2016;111(6):1059–68. 10.1111/add.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lau-Barraco C, Milletich RJ, Linden AN. Caffeinated alcohol consumption profiles and associations with use severity and outcome expectancies. Addict Behav. 2014;39(1):308–15. 10.1016/j.addbeh.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim MJ, Jin HS, Eom YB. Coffee consumption affects kidney function based on GCKR polymorphism in a Korean population. Nutr Res. 2024;122:92–100. 10.1016/j.nutres.2023.12.008. [DOI] [PubMed] [Google Scholar]
- 142.Kusic DM, Zajic SC, Gharani N, et al. Genome-wide association study of caffeine consumption using coriell personalized medicine collaborative data. Genet Mol Med. 2023. 10.33425/2689-1077.1020. [Google Scholar]
- 143.Kang J, Jia T, Jiao Z, et al. Increased brain volume from higher cereal and lower coffee intake: shared genetic determinants and impacts on cognition and metabolism. Cereb Cortex. 2022;32(22):5163–74. 10.1093/cercor/bhac005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhou A, Taylor AE, Karhunen V, et al. Habitual coffee consumption and cognitive function: a Mendelian randomization meta-analysis in up to 415,530 participants. Sci Rep. 2018;8(1):7526. 10.1038/s41598-018-25919-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Denden S, Bouden B, Haj Khelil A, Ben Chibani J, Hamdaoui MH. Gender and ethnicity modify the association between the CYP1A2 rs762551 polymorphism and habitual coffee intake: evidence from a meta-analysis. Genet Mol Res. 2016. 10.4238/gmr.15027487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.