Abstract
Filter-grown Madin-Darby canine kidney (MDCK) cells labelled for 24 h with [35S]sulphate were found to secrete macromolecules [35S]sulphated on their carbohydrate moieties predominantly into the basolateral medium, whereas the tyrosine-[35S]sulphated proteins synthesized were predominantly secreted into the apical medium. In contrast with the predominant apical secretin of tyrosine-[35S]sulphated proteins, the free tyrosine O-[35S]sulphate (Tyr[35S]) was released mostly into the basolateral medium. A time-lapse study using prelabelled MDCK cells incubated in fresh medium revealed that, during the 48 h time course monitored, the release of tyrosine-[35S]sulphated proteins into the apical medium was faster and quantitatively greater than that into the basolateral medium. During the same time there was a concomitant release, predominantly into the basolateral medium, of the free Tyr[35S] derived from the degradation of tyrosine-[35S]sulphated proteins. An endocytotic degradation experiment was performed to demonstrate the endocytosis of tyrosine-sulphated proteins and their degradation to generate free TyrS. It was found that free Tyr[35S] was generated and released when an apically secreted (or basolaterally secreted) tyrosine-[35S]sulphated protein preparation was added to the apical medium (or the basolateral medium) of unlabelled filter-grown MDCK cells. In both cases, the free Tyr[35S] generated was predominantly released into the basolateral medium similar to the results obtained in the time-lapse study.
Full text
PDF


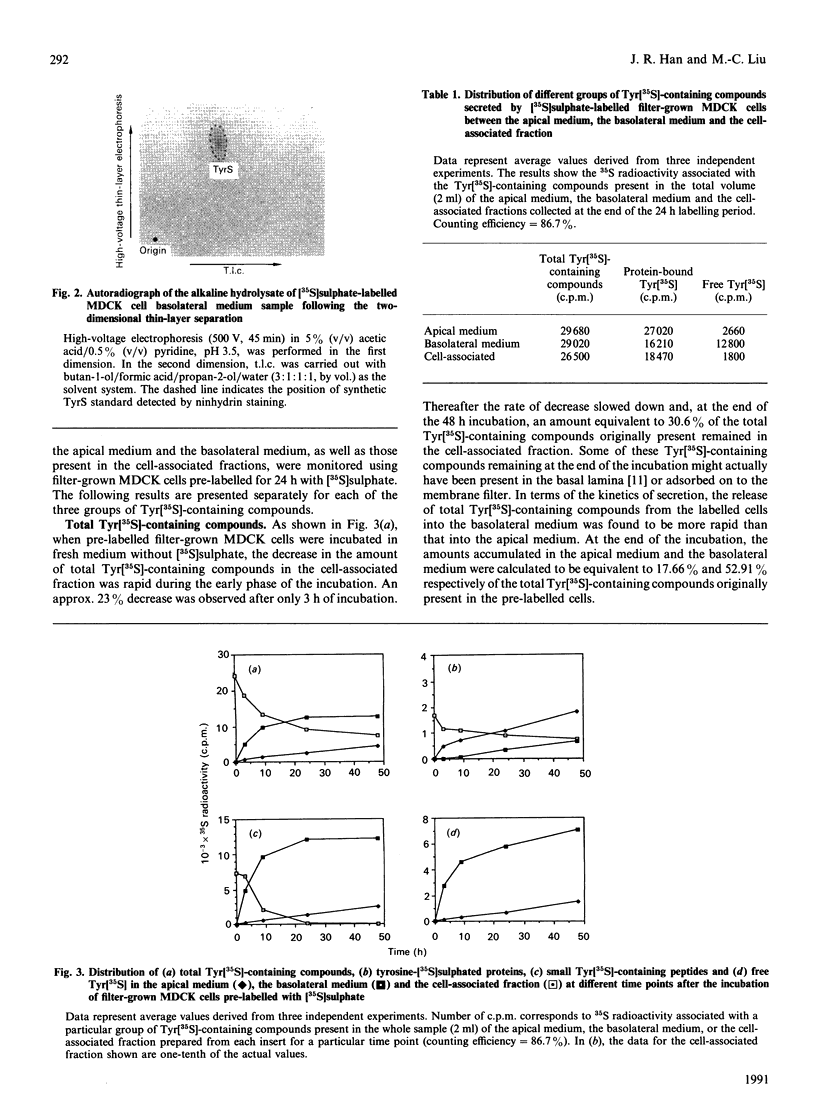
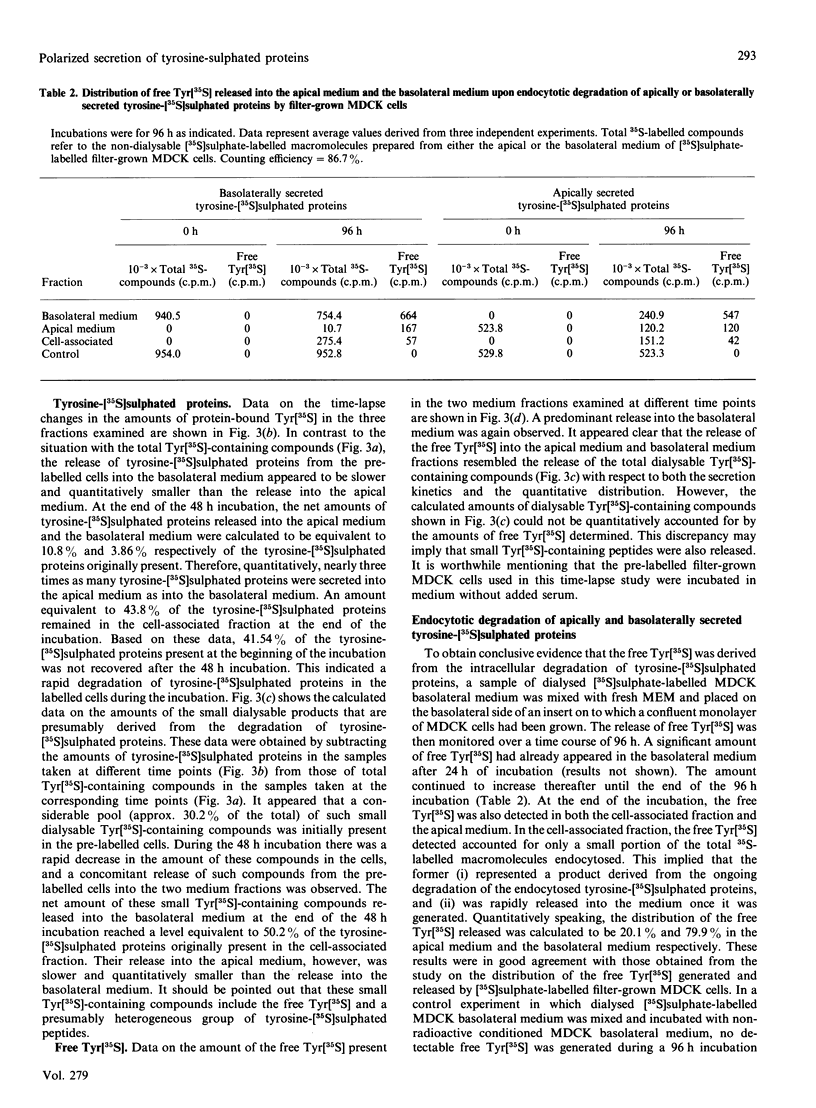


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Huttner W. B. Inhibition of N-glycosylation induces tyrosine sulphation of hybridoma immunoglobulin G. EMBO J. 1984 Oct;3(10):2209–2215. doi: 10.1002/j.1460-2075.1984.tb02118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle P. A., Huttner W. B. Tyrosine sulfation is a trans-Golgi-specific protein modification. J Cell Biol. 1987 Dec;105(6 Pt 1):2655–2664. doi: 10.1083/jcb.105.6.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartles J. R., Hubbard A. L. Plasma membrane protein sorting in epithelial cells: do secretory pathways hold the key? Trends Biochem Sci. 1988 May;13(5):181–184. doi: 10.1016/0968-0004(88)90147-8. [DOI] [PubMed] [Google Scholar]
- Caplan M. J., Stow J. L., Newman A. P., Madri J., Anderson H. C., Farquhar M. G., Palade G. E., Jamieson J. D. Dependence on pH of polarized sorting of secreted proteins. Nature. 1987 Oct 15;329(6140):632–635. doi: 10.1038/329632a0. [DOI] [PubMed] [Google Scholar]
- Fuller S., von Bonsdorff C. H., Simons K. Vesicular stomatitis virus infects and matures only through the basolateral surface of the polarized epithelial cell line, MDCK. Cell. 1984 Aug;38(1):65–77. doi: 10.1016/0092-8674(84)90527-0. [DOI] [PubMed] [Google Scholar]
- Gottlieb T. A., Beaudry G., Rizzolo L., Colman A., Rindler M., Adesnik M., Sabatini D. D. Secretion of endogenous and exogenous proteins from polarized MDCK cell monolayers. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2100–2104. doi: 10.1073/pnas.83.7.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeve L., Drickamer K., Rodriguez-Boulan E. Polarized endocytosis by Madin-Darby canine kidney cells transfected with functional chicken liver glycoprotein receptor. J Cell Biol. 1989 Dec;109(6 Pt 1):2809–2816. doi: 10.1083/jcb.109.6.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille A., Braulke T., von Figura K., Huttner W. B. Occurrence of tyrosine sulfate in proteins--a balance sheet. 1. Secretory and lysosomal proteins. Eur J Biochem. 1990 Mar 30;188(3):577–586. doi: 10.1111/j.1432-1033.1990.tb15438.x. [DOI] [PubMed] [Google Scholar]
- Hille A., Rosa P., Huttner W. B. Tyrosine sulfation: a post-translational modification of proteins destined for secretion? FEBS Lett. 1984 Nov 5;177(1):129–134. doi: 10.1016/0014-5793(84)80996-5. [DOI] [PubMed] [Google Scholar]
- JEVONS F. R. TYROSINE O-SULPHATE IN FIBRINOGEN AND FIBRIN. Biochem J. 1963 Dec;89:621–624. doi: 10.1042/bj0890621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John R. A., Rose F. A., Wusteman F. S., Dodgson K. S. The detection and determination of L-tyrosine O-sulphate in rabbit and other mammalian urine. Biochem J. 1966 Jul;100(1):278–281. doi: 10.1042/bj1000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondor-Koch C., Bravo R., Fuller S. D., Cutler D., Garoff H. Exocytotic pathways exist to both the apical and the basolateral cell surface of the polarized epithelial cell MDCK. Cell. 1985 Nov;43(1):297–306. doi: 10.1016/0092-8674(85)90035-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee R. W., Huttner W. B. Tyrosine-O-sulfated proteins of PC12 pheochromocytoma cells and their sulfation by a tyrosylprotein sulfotransferase. J Biol Chem. 1983 Sep 25;258(18):11326–11334. [PubMed] [Google Scholar]
- Liu M. C., Lipmann F. Decrease of tyrosine-O-sulfate-containing proteins found in rat fibroblasts infected with Rous sarcoma virus or Fujinami sarcoma virus. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3695–3698. doi: 10.1073/pnas.81.12.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. C., Lu R. L., Han J. R., Tang X. B., Suiko M., Liu C. C. Identification of complexes between the tyrosine-O-sulphate-binding protein and tyrosine-sulphated proteins in bovine liver membrane lysates. Biochem J. 1991 Apr 1;275(Pt 1):259–262. doi: 10.1042/bj2750259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. C., Suiko M., Lipmann F. Rapid catabolism of tyrosine-O-sulphated proteins and the formation of free tyrosine O-sulphate as an end product in rat embryo fibroblasts. Biochem J. 1987 Apr 15;243(2):555–559. doi: 10.1042/bj2430555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. C., Suiko M., Tang X. B. Isolation and characterization of a bovine liver tyrosine-O-sulfate-binding protein--a putative receptor molecular for tyrosine-sulfated proteins? Biochem Biophys Res Commun. 1988 Oct 31;156(2):964–969. doi: 10.1016/s0006-291x(88)80938-0. [DOI] [PubMed] [Google Scholar]
- Simons K., Fuller S. D. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Simons K., Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990 Jul 27;62(2):207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- TALLAN H. H., BELLA S. T., STEIN W. H., MOORE S. Tyrosine-O-sulfate as a constituent of normal human urine. J Biol Chem. 1955 Dec;217(2):703–708. [PubMed] [Google Scholar]