Abstract
Background
Previous research has established connections between gut microbiota, immune modulation, and several virus-related diseases. However, no study has explored the relationships between gut microbiota and herpes zoster and postherpetic neuralgia (PHN).
Methods
A total of 205 taxa of gut microbiota were regarded as exposures. The occurrences of herpes zoster and PHN were selected as outcomes. The causal effects of gut microbiota on herpes zoster and PHN were estimated with multiple methods for two-sample Mendelian randomization, such as inverse variance weighted (IVW), MR–Egger, and weighted median. All results were subjected to FDR correction to prevent from possibility of multiple comparison.
Results
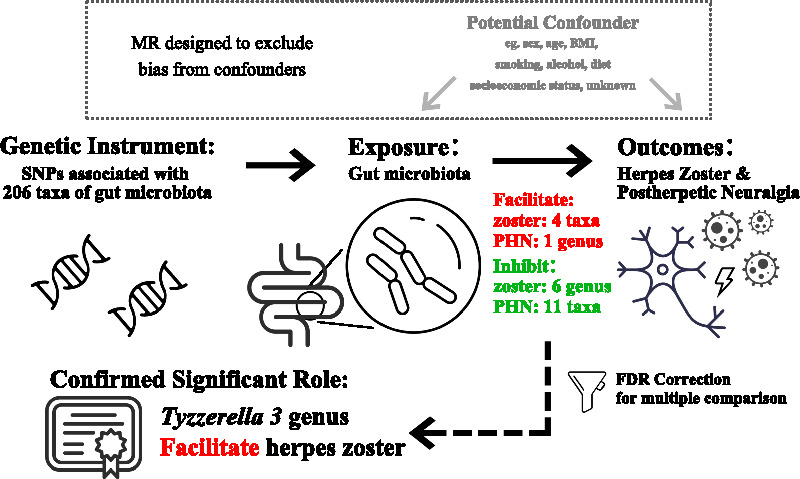
Among the significant findings, four taxa and one genus were identified as facilitators of herpes zoster and PHN, respectively. Conversely, six genera and eleven taxa were found to inhibit herpes zoster and PHN, respectively. The causal effect of the Tyzzerella 3 was confirmed through FDR correction, making it a key focus in this study. Specifically, it was found to causally facilitate herpes zoster primarily with IVW (OR 1.420, 95% CI 1.174–1.718, p < 0.001, q = 0.039), as there is no heterogeneity or horizontal pleiotropy found.
Conclusions
With investigation of the causal association between gut microbiota, and herpes zoster/PHN, significant findings were identified in 22 different taxa. Among them, Tyzzerella 3 keeps significant after multiple comparison correction, and displays potential to facilitate the occurrence of herpes zoster.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-024-02106-w.
Keywords: Mendelian randomization, Gut microbiota, Herpes zoster, Postherpetic neuralgia
Background
The gut microbiota has been found to actively participate in numerous physiological processes. It is involved in the digestion of food, the integrity maintenance o warranted f intestinal mucosal barrier, and the synthesis of essential nutrients [1, 2]. Furthermore, the gut microbiota is also critical in immune regulation. Its intricate interaction with the immune system highlights the significance of the gut microbiota in immune modulation, and resistance to virus [3], like influenza infection [4].
Varicella-zoster virus (VZV) is the agent leading to both chickenpox and herpes zoster, of which shingles is another well-known name. After an episode of herpes zoster, individuals may experience postherpetic neuralgia (PHN), a debilitating condition that severely affects the life quality and is notoriously difficult to treat [5]. The involvement of the immune system in the pathogenesis of VZV is significant. Not only does the gut microbiota interacts with the immune cells, but many immune diseases like inflammatory bowel disease (IBD) and immunosuppression status have also been found to be associated with herpes zoster [6]. This suggests the need to explore whether the gut microbiota can impact the occurrence of herpes zoster and PHN.
Gut microbiota has been found associated with multiple viruses. To our knowledge, there have been studies reporting associations with COVID-19, respiratory syncytial virus, rotavirus, and influenza [4, 7–9]. For example, Yeoh et al. reported that gut microbiota composition was found altered in COVID-19 patients, and the perturbed microbiota composition shows stratification phenomenon with disease severity [7]. As for mechanisms, it has been proven that gut microbiota can prime systemic antiviral immunity by Erttmann et al. who also explored the related signaling pathways. They found cGAS–STING–IFN-I axis plays a key role, and reported that membrane vesicles which have DNA insides and are derived from gut microbiota can enter circulation and facilitate clearance of both vesicular stomatitis virus and herpes simplex virus-1 in a cGAS-dependent manner [10].
Therefore, it is logical and rational that gut microbiota can influence the occurrence of herpes zoster and PHN. However, the relationship between them remains unknown. This study is the first to conduct Mendelian randomization (MR) analyses to find out whether there is causality between gut microbiota, herpes zoster, and neuropathic pain. Although there have been vaccines for herpes zoster, we believe that the investigation in this study can also help to improve the zoster vaccines, since gut microbiota composition has been found associated with COVID-19 vaccine immunogenicity [11]. In addition, the findings may hold the potential to provide new insights for VZV researchers, as well as for new preventive and therapeutic strategy development.
Methods
Study design
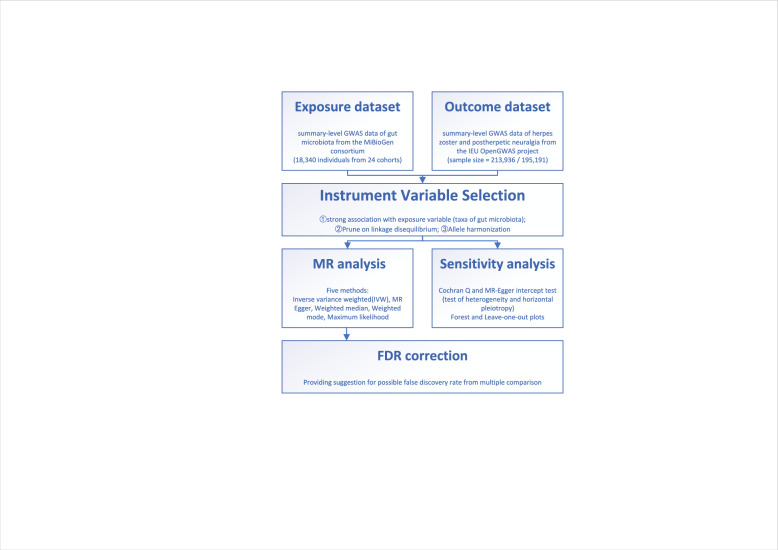
The present study is a two-sample MR study to find out whether there is causality between gut microbiota and herpes zoster while mitigating the impact of confounding factors and avoiding horizontal pleiotropy. MR analysis uses genetic variants to ascertain if the observational correlation between a risk factor and an outcome aligns with a causal relationship. This approach hinges on the random assortment of genetic variants during meiosis, resulting in a random dispersion of genetic variants within a population, which is well reviewed and introduced by Emdin et al. on JAMA. [12] The flowchart of design of this study is displayed in Fig. 1.
Fig. 1.
Flowchart of Mendelian randomization (MR) analysis in the present study
Data source
We utilized summary-level data from two different genome-wide association studies (GWAS) sources, which also summarized in Table 1.
Table 1.
Summary of data source
| Trait | Sample size | Population | Data source | |
|---|---|---|---|---|
| Gut microbiota | Phylum × 9 | 18,340 |
European (16 cohorts, N = 13,266), Middle-Eastern (1 cohort, N = 481), East Asian (1 cohort, N = 811), American Hispanic/Latin (1 cohort N = 1097), African American (1 cohort, N = 114) multi-ancestry (4 cohorts, N = 2571) |
MiBioGen consortium; (PMID:33462485) |
| Class × 16 | ||||
| Order × 20 | ||||
| Family × 35 | ||||
| Genus × 125 | ||||
| Herpes Zoster | 213,936 | European |
IEU OpenGWAS project; gwas.mrcieu.ac.uk · finn-b-AB1_ZOSTER · finn-b-G6_POSTZOST |
|
| Post-herpetic Neuralgia | 195,191 | European | ||
Genetic variations of the gut microbiota referred to a comprehensive GWAS conducted by Kurilshikov et al. [13] This extensive GWAS encompassed a vast participant pool of 18,340 individuals from 24 diverse cohorts. The study meticulously explored 205 taxa (125 genera, 35 families, 20 orders, 16 classes, and 9 phyla) providing thorough analyses across multiple taxonomic levels of the gut microbiota.
The IEU Open GWAS project in 2021 has contributed the largest publicly available GWAS dataset for herpes zoster and postherpetic neuropathic pain, which is provided by FinnGen, a database built by the University of Helsinki. This extensive GWAS comprises a total sample size of 213,936 for herpes zoster (Dataset ID: finn-b-AB1_ZOSTER) and 195,191 for post-zoster neuralgia (Dataset ID: finn-b-G6_POSTZOST) collected from European ancestry.
Selection of instrument variables
To obtain IVs for our analysis, we performed a screening of SNPs related to the gut microbiota. Because it is necessary to have an adequate number of genetic variations included as instrument variables (IVs) for sensitivity analysis and horizontal pleiotropy test, and IVs has to be strongly associated with gut microbiota. Therefore, the significance threshold for IVs used in this study was finally set at 1 × 10–5, which also refers to published high-quality MR studies with the same exposure data source [14, 15]. To address potential SNP correlations, we conducted LD-clumping using a threshold of r2 < 0.001 and a maximum distance of 10,000 kb. This process resulted in a final set of 2702 independent SNPs associated with 205 bacterial traits, identified by their significant p values. These 2702 SNPs were then utilized as IVs to model the effects of specific taxa within the gut microbiota. Prior to conducting the MR analysis, we implemented data harmonization measures to make sure that the effects of the SNPs on the taxa of gut microbiota and outcome variables corresponded to the same allele.
MR analysis
For our study, we conducted an extensive two-sample MR analysis investigating the causality between gut microbiota and both of herpes zoster and post-zoster neuralgia. We employed five distinct analytical methods, namely random-effects inverse variance weighted (IVW), MR Egger, and weighted median, as well as weighted mode and maximum likelihood. IVW analysis using a random-effect model was conducted regrading as the primary analysis. The IVW method can ensure accurate causal estimates but only if the SNPs fully adhered to the three fundamental principles of MR studies. Other analysis methods were employed to complement the IVW estimates.
Since there are 205 taxa of gut microbiota, the false discovery rate (FDR) correction for multiple comparison was performed. The q values in results are adjusted values of p values.
MR–Egger methods are used to find the existence of horizontal pleiotropy, and indicated heterogeneity by Cochran’s Q-statistic. A sensitivity analysis with leave-one-out method was performed to assess the reliability, stability, and robustness of the causal estimates, specifically investigating whether the results were disproportionately influenced by any individual SNP. Calculation of all analyses was done with the “TwoSampleMR” package (version 0.5.6) on R (version 4.2.2).
Results
Results of all taxa
We selected SNPs as IVs for each included taxon, and the specific details of these SNPs are listed in Table S1. The results of the causal analysis for all taxa can be found in Table S2, while Table S3 presents the assessment of heterogeneity and horizontal pleiotropy. In addition, the MR Steiger directionality test revealed that all taxa were positioned upstream of herpes zoster and PHN, as indicated in Table S4.
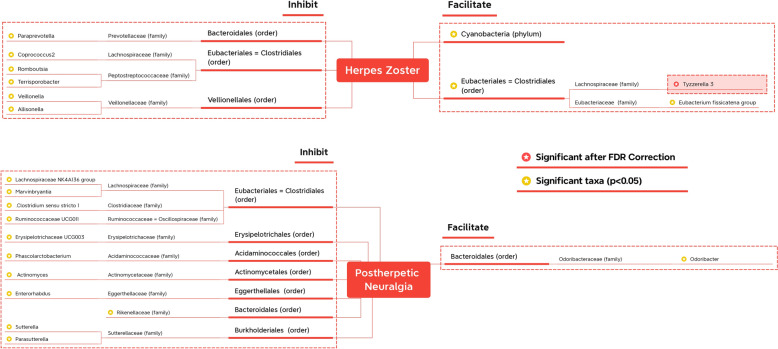
Given the numerous taxa analyzed, interpreting the results from the tables can be challenging. Therefore, to provide a concise summary of the findings, we have presented them in Fig. 2. Only taxonomic levels with star marks have significance. But to make it easier to know the significant taxa belonging to which order or family, the upper orders/families without significance are also written (but without star marks). For example, Marvinbryantia genus might significantly inhibit PHN. But Lachnospiraceae family or Eubacteriales order do not have significance, which is displayed just to show what taxa Marvinbryantia genus belongs to.
Fig. 2.
Significant finding summary. The taxa with yellow-star marks have statistical significance, while the red-star mark beside the Tyzzerella 3 means still significant after FDR correction
Upon examining all taxa, ranging from phyla to genera, we identified 1 phylum, 1 order, and 8 genera exhibiting nominal significance with herpes zoster in at least one MR method. For post-zoster neuralgia, significant results were observed for 1 family and 11 genera (excluding unknown genera). Among these nominal significant findings, one phylum, one order, and two genera were found to facilitate herpes zoster, while the remaining six genera demonstrated inhibitory effects on zoster. Only the Odoribacter genus exhibited nominal significant causality with enhancing post-zoster neuropathic pain, while other nominal significant results for families or genera indicated a preventive role against neuralgia. Furthermore, the assessment shows that there is heterogeneity in Veillonella genus. Thus, the significant results of Veillonella genus on herpes zoster should be considered more cautiously.
In addition, after applying the FDR correction, only one result remained significant, supporting the notion that the Tyzzerella3 genus contributes to the occurrence of herpes zoster (IVW: OR 1.420, 95% CI 1.174–1.718, p < 0.001, q = 0.039). This finding underscores the emphasis placed on the Tyzzerella3 genus in subsequent reports.
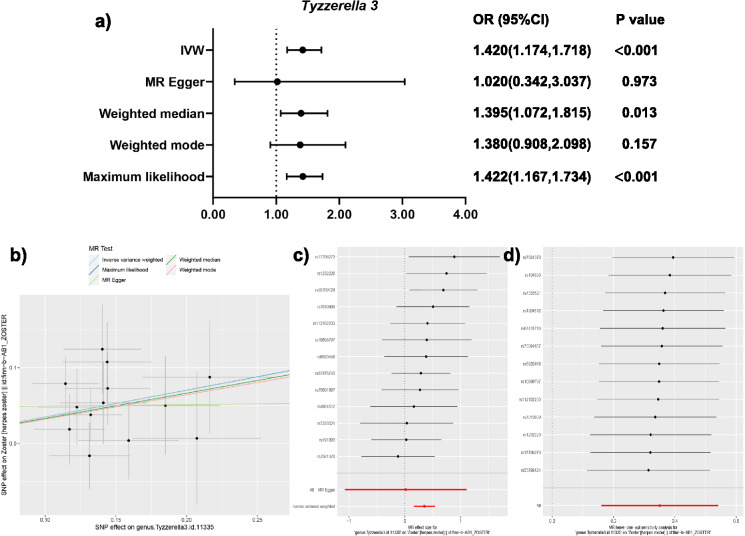
Results of Tyzzerella 3 on herpes zoster
The analysis screened out a total of 13 selected SNPs for the Tyzzerella 3. The results, presented in Table 2, demonstrate significant associations between the Tyzzerella 3 and herpes zoster. These findings are visually depicted in Fig. 3a, b. The positive slopes observed in Fig. 3b support the hypothesis that the presence of Tyzzerella 3 may potentially facilitate the occurrence of herpes zoster.
Table 2.
Causal estimate results of Tyzzerella 3 evaluated in MR analyses
| Outcome | Exposure | Method | NSNP | Beta | SE | p value | FDR | OR | Lower limit | Higher limit |
|---|---|---|---|---|---|---|---|---|---|---|
| Herpes Zoster | genus.Tyzzerella3.id.11335 | Maximum likelihood | 13 | 0.352 | 0.101 | < 0.001 | 0.061 | 1.422 | 1.167 | 1.734 |
| Herpes Zoster | genus.Tyzzerella3.id.11335 | Weighted median | 13 | 0.333 | 0.134 | 0.013 | 0.966 | 1.395 | 1.072 | 1.815 |
| Herpes Zoster | genus.Tyzzerella3.id.11335 | Inverse variance weighted | 13 | 0.351 | 0.097 | < 0.001 | 0.039 | 1.420 | 1.174 | 1.718 |
| Herpes Zoster | genus.Tyzzerella3.id.11335 | Weighted mode | 13 | 0.322 | 0.214 | 0.157 | 0.982 | 1.380 | 0.908 | 2.098 |
| Herpes Zoster | genus.Tyzzerella3.id.11335 | MR–Egger | 13 | 0.019 | 0.557 | 0.973 | 0.981 | 1.020 | 0.342 | 3.037 |
| Postzoster Neuralgia | genus.Tyzzerella3.id.11335 | Maximum likelihood | 13 | − 0.457 | 0.375 | 0.223 | 0.854 | 0.633 | 0.304 | 1.320 |
| Postzoster Neuralgia | genus.Tyzzerella3.id.11335 | Weighted median | 13 | − 0.906 | 0.511 | 0.076 | 0.931 | 0.404 | 0.149 | 1.100 |
| Postzoster Neuralgia | genus.Tyzzerella3.id.11335 | Inverse variance weighted | 13 | − 0.449 | 0.379 | 0.237 | 0.911 | 0.638 | 0.303 | 1.343 |
| Postzoster Neuralgia | genus.Tyzzerella3.id.11335 | Weighted mode | 13 | − 0.986 | 0.748 | 0.212 | 0.961 | 0.373 | 0.086 | 1.618 |
| Postzoster Neuralgia | genus.Tyzzerella3.id.11335 | MR–Egger | 13 | 0.951 | 2.230 | 0.678 | 0.981 | 2.588 | 0.033 | 204.798 |
P-values less than 0.05 are displayed in bold to indicate statistical significance
Fig. 3.
MR results of Tyzzerella 3. (a) ORs of different MR methods; (b) scatter plot of causal estimation; (c) forest plot of causal estimation, demonstrating the absence of outlier SNPs; (d) leave-one-out plot which display the reliability of the findings
Specifically, three methods show significance, which are IVW (OR 1.420, 95% CI 1.174–1.718, p < 0.001, q = 0.039), weighted median (OR 1.395, 95% CI 1.072–1.815, p = 0.013), and maximum likelihood (OR 1.422, 95% CI 1.167–1.734, p < 0.001). Table 3 displays the absence of evidence of horizontal pleiotropy between Tyzzerella 3 and herpes zoster. Therefore, the primary method, IVW, is considered the main result of this analysis. In addition, p values were adjusted to q values using FDR correction to prevent from bias of multiple comparison. The q value for the IVW method is calculated to be 0.039, which is less than 0.05, further confirming the causal relationship between Tyzzerella 3 and herpes zoster.
Table 3.
Heterogeneity and directional horizontal pleiotropy of single-nucleotide polymorphism effect of Tyzzerella 3
| Exposure | Outcomes | Heterogeneity tests | Test for directional horizontal pleiotropy | ||||
|---|---|---|---|---|---|---|---|
| Q statistics | p value of Q statistics | Intercept of MR–Egger | Standard error | p value | |||
| Tyzzerella 3 | Herpes Zoster | MR–Egger | 7.799 | 0.731 | 0.04757 | 0.07871 | 0.558 |
| IVW | 8.164 | 0.772 | |||||
| Postherpetic Neuralgia | MR–Egger | 12.427 | 0.332 | − 0.20088 | 0.31513 | 0.537 | |
| IVW | 12.886 | 0.377 | |||||
Figure 3c presents the causal estimates of individual SNPs, demonstrating the absence of outlier SNPs. Figure 3d displays the results of sensitivity analyses. It is seen that when any SNP is omitted, the results remain significant, indicating the reliability of the findings.
Discussion
The incidence of herpes zoster is considerable, with an overall annual incidence of 1.2–3.4 cases per 1000 person-years in the US and 1.85–3.9 cases per 1000 person-years in the UK [6]. Its impact on daily life is significant and is of great importance. Furthermore, herpes zoster can give rise to a condition known as PHN, characterized by enduring dermatomal pain lasting for more than about 3 months after the acute herpes zoster rash. Approximately 20% patients of herpes zoster will experience pain, and of those, 6% will suffer from clinically significant PHN that may last for years or even a lifetime [16]. Despite extensive research, the mechanisms responsible for PHN have not been fully elucidated. The prevailing hypotheses include persistent chronic ganglionitis, neuronal damage resulting from the replication of VZV in ganglia, and altered sodium channels. [17] Although vaccines for herpes zoster are available recently, their coverage remains limited. Surveys indicate a herpes zoster vaccination willingness rate of only 55.74% among 14,066 individuals from 4 WHO regions [18]. Besides, these vaccines do not offer complete prevention against the occurrence of herpes zoster and PHN. Hence, it is crucial to conduct further research on herpes zoster to develop more effective solutions.
The research findings related to gut microbiota hold promise for practical clinical applications, primarily due to the availability of interventions that can influence the composition of gut microbiota. Researchers have already applied fecal microbiota transplantation (FMT) in attempts to treat diseases like Clostridioides difficile infection [19] and IBDs [20, 21]. Interestingly, there was a specific study conducted on patients with Crohn’s disease, where one case of adverse event, namely herpes zoster, was reported in the FMT cohort, while none occurred in the non-FMT cohort. Although it is not certain if this case of herpes zoster was directly related to FMT, it has sparked interest and calls for further investigation [21].
Upon our investigation, the most significant results obtained were related to various genera, with only one family, one order, no class, and one phylum showing significant results attributed to the inherent heterogeneity within these taxa. However, Cyanobacteria, as a phylum, emerged as a factor significantly facilitating occurrence of herpes zoster. Cyanobacteria is well-known in ecological and environmental fields as the only prokaryotes to have developed oxygenic photosynthesis, thereby profoundly influencing the earth’s biological and chemical cycles [22]. However, the non-photosynthetic Cyanobacteria in human gut was just found in recent years. Over the period from 2016 to 2021, ten studies explored the potential association between Cyanobacteria and health issues, and these studies were comprehensively reviewed by Hu et al. [23]. Remarkably, approximately 80% of these studies indicated a higher abundance of Cyanobacteria in individuals with worsened health status, suggesting a possible link between Cyanobacteria and health complications.
Among the significant genera identified, Tyzzerella remains significant even after FDR correction, indicating that this significance is unlikely to be a result of multiple comparisons. Tyzzerella is a relatively new genus, having been reclassified from the Clostridiaceae family to Lachnospiraceae as a distinct genus by Yutin et al. in 2013 [24]. The genus was named in honor of Ernest Tyzzer, a pathologist who first isolated Tyzzerella piliformis, formerly known as Clostridium piliforme, the pathogenic bacteria of Tyzzer’s disease. This disease is an acute epizootic disease affecting mammals, characterized by necrotic liver lesions, and is usually fatal. Although it is found worldwide, infections in humans are rare [25, 26]. As a result, Tyzzerella and its implications may not be well-known among physicians. Although Tyzzerella is not sufficient to cause diseases in humans, there still might be potential effects. In previous studies of human gut microbiota, there are only several mentioned Tyzzerella 3. In detail, it has been found that patients with acute myocardial infraction [27] or those living around mining/smelting areas [28] have a higher abundance of Tyzzerella 3. Moreover, Tyzzerella 3 is associated with a lower frequency but higher severity of postnatal depression [29]. In addition, the only study demonstrated a causal relationship, showing Tyzzerella 3 can inhibit preeclampsia–eclampsia. However, there is currently no information available on the relationship between Tyzzerella 3 and virus-related diseases. Pang et al. published the only article mentioned the relationship between Tyzzerella 3 and immune cells [30]. In spinal cord injury patients, it was found that Tyzzerella 3 is linked to fewer NK cells, but no significant correlations were observed with T cells, which are crucial in the pathogenesis of herpes zoster and PHN. As such, the findings of Tyzzerella 3 in the present study may require further confirmation through additional research, and the underlying mechanisms also remain unclear.
By contrast, there are fewer significant findings for PHN in this study, and none remains significant after FDR correction. Several genera were identified as potentially inhibiting PHN, while only one genus, Odoribacter, was found to facilitate PHN. In other studies, Odoribacter has been reported to improve glucose tolerance and limit inflammation in mice, leading to its consideration as a probiotic [31, 32]. However, the research on Odoribacter is still limited. We cannot confirm whether Odoribacter is beneficial for human health. Nonetheless, based on the findings of the present study, it appears that Odoribacter is not favorable for individuals with PHN.
This study used MR analysis to investigate causality, effectively mitigating the interference of potential confounders and addressing issues of reverse causation. Association studies often struggle to distinguish between two possibilities: whether “herpes zoster causes alterations in gut microbiota” or “changes in gut microbiota contribute to herpes zoster and PHN.” However, the MR analyses conducted in this study provided insight into the direction of causality. As a result, researchers can refer to this study to consider potential new strategies or further exploration with greater confidence.
However, it is important to acknowledge several limitations in this study that warrant consideration. First, the present study analyzed summary-level data, instead of individual-level data, and therefore, cannot make stratified analyses based on covariates such as sex. In addition, two-sample MR studies were designed to evaluating linear associations, thereby not adequately capturing the nonlinear effects of SNPs originating from gut microbiota on herpes zoster and PHN. Finally, the majority of cases included in the analysis are of European descent. As such, the generalization capacity of the findings to other ethnicities may be limited. It should be cautious when extrapolating the results to diverse ethnic groups, and further studies on different populations are warranted.
Conclusion
With investigation of the causal association between gut microbiota, and herpes zoster/PHN, significant findings were identified in 22 different taxa. Among them, Tyzzerella 3 keeps significant after multiple comparison correction, and displays potential to facilitate the occurrence of herpes zoster.
Supplementary Information
Additional file 1: Table S1. Details of SNPs selected as IVs, including IVs of all taxa. Table S2. Results of causality evaluated in MR analyses, including results of all taxa, both outcomes, and all MR methods. Table S3. Results of heterogeneity and directional horizontal pleiotropy of single-nucleotide polymorphism effect of all taxa on both outcomes. Table S4. MR Steiger directionality test indicated that all taxa were upstream of both outcomes.
Acknowledgements
Not applicable.
Abbreviations
- CI
Confidence interval
- FMT
Fecal microbiota transplantation
- GWAS
Genome-wide association study
- IBD
Inflammatory bowel disease
- IV
Instrumental variable
- IVW
Inverse-variance weighted
- MR
Mendelian randomization (analysis)
- OR
Odd ratio
- PHN
Postherpetic neuralgia
- SNP
Single-nucleotide polymorphism
- VZV
Varicella-zoster virus
Author contributions
XZ, ZL, and GL designed the study, analyzed and interpreted the data, and drafted the manuscript. ZQ, ZW, RL, YL, ZJ, and YM analyzed and interpreted the data. XP concepted and designed the study and revised the manuscript. The authors read and approved the final manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (Grants 82172842, 81803104 and 81672386); the Sichuan Province Science and Technology Support Program (Grants 2021YFSY008 and 2020YFS0276); Natural Science Foundation of Sichuan Province (2024NSFSC1934); West China Nursing Discipline Development Special Fund Project (Grant HXHL21008); the Technology Innovation Project of Chengdu Science and Technology Bureau (Grant 2019-YF05-00459-SN); and Postdoctoral Research and Development Fund and Translational medicine fund of West China Hospital (Grants 2020HXBH119 and CGZH19002).
Availability of data and materials
The data sets analyzed during the current study are available in the MiBioGen, https://mibiogen.gcc.rug.nl/, and the IEU OpenGWAS project, https://gwas.mrcieu.ac.uk/.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xin Zhang, Zheran Liu and Guihong Liu have contributed equally to this work.
References
- 1.Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med. 2018;50(8):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowland I, Gibson G, Heinken A, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57(1):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30(6):492–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosshart SP, Vassallo BG, Angeletti D, et al. Wild mouse gut microbiota promotes host fitness and improves disease resistance. Cell. 2017;171(5):1015-28.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le P, Rothberg M. Herpes zoster infection. BMJ. 2019;364:k5095. [DOI] [PubMed] [Google Scholar]
- 6.Guillo L, Rabaud C, Choy EH, et al. Herpes zoster and vaccination strategies in inflammatory bowel diseases: a practical guide. Clin Gastroenterol Hepatol. 2022;20(3):481–90. [DOI] [PubMed] [Google Scholar]
- 7.Yeoh YK, Zuo T, Lui GC, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70(4):698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim AH, Hogarty MP, Harris VC, et al. The complex interactions between rotavirus and the gut microbiota. Front Cell Infect Microbiol. 2020;10:586751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yagi K, Asai N, Huffnagle GB, et al. Early-life lung and gut microbiota development and respiratory syncytial virus infection. Front Immunol. 2022;13:877771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erttmann SF, Swacha P, Aung KM, et al. The gut microbiota prime systemic antiviral immunity via the cGAS-STING-IFN-I axis. Immunity. 2022;55(5):847-61.e10. [DOI] [PubMed] [Google Scholar]
- 11.Ng SC, Peng Y, Zhang L, et al. Gut microbiota composition is associated with SARS-CoV-2 vaccine immunogenicity and adverse events. Gut. 2022;71(6):1106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. 2017;318(19):1925–6. [DOI] [PubMed] [Google Scholar]
- 13.Kurilshikov A, Medina-Gomez C, Bacigalupe R, et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet. 2021;53(2):156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li P, Wang H, Guo L, et al. Association between gut microbiota and preeclampsia-eclampsia: a two-sample Mendelian randomization study. BMC Med. 2022;20(1):443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song J, Wu Y, Yin X, et al. The causal links between gut microbiota and COVID-19: a Mendelian randomization study. J Med Virol. 2023;95(5): e28784. [DOI] [PubMed] [Google Scholar]
- 16.Johnson RW, Rice AS. Clinical practice. Postherpetic neuralgia. N Engl J Med. 2014;371(16):1526–33. [DOI] [PubMed] [Google Scholar]
- 17.Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiol Rev. 2021;101(1):259–301. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, Yang L, Li L, et al. Willingness to vaccinate against herpes zoster and its associated factors across who regions: global systematic review and meta-analysis. JMIR Public Health Surveill. 2023;9:e43893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yadegar A, Pakpoor S, Ibrahim FF, et al. Beneficial effects of fecal microbiota transplantation in recurrent Clostridioides difficile infection. Cell Host Microbe. 2023;31(5):695–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benech N, Kapel N, Sokol H. Fecal microbiota transplantation for ulcerative colitis. JAMA. 2019;321(22):2240. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Cui B, Li Q, et al. The safety of fecal microbiota transplantation for Crohn’s disease: findings from a long-term study. Adv Ther. 2018;35(11):1935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sánchez-Baracaldo P, Bianchini G, Wilson JD, et al. Cyanobacteria and biogeochemical cycles through Earth history. Trends Microbiol. 2022;30(2):143–57. [DOI] [PubMed] [Google Scholar]
- 23.Hu C, Rzymski P. Non-photosynthetic melainabacteria (Cyanobacteria) in human gut: characteristics and association with health. Life (Basel). 2022;12(4):476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yutin N, Galperin MY. A genomic update on clostridial phylogeny: gram-negative spore formers and other misplaced clostridia. Environ Microbiol. 2013;15(10):2631–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith KJ, Skelton HG, Hilyard EJ, et al. Bacillus piliformis infection (Tyzzer’s disease) in a patient infected with HIV-1: confirmation with 16S ribosomal RNA sequence analysis. J Am Acad Dermatol. 1996;34(2 Pt 2):343–8. [DOI] [PubMed] [Google Scholar]
- 26.Pritt S, Henderson KS, Shek WR. Evaluation of available diagnostic methods for Clostridium piliforme in laboratory rabbits (Oryctolagus cuniculus). Lab Anim. 2010;44(1):14–9. [DOI] [PubMed] [Google Scholar]
- 27.Han Y, Gong Z, Sun G, et al. Dysbiosis of gut microbiota in patients with acute myocardial infarction. Front Microbiol. 2021;12:680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao M, Zhu Y. Long-term metal exposure changes gut microbiota of residents surrounding a mining and smelting area. Sci Rep. 2020;10(1):4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y, Chen C, Yu H, et al. Fecal microbiota changes in patients with postpartum depressive disorder. Front Cell Infect Microbiol. 2020;10:567268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pang R, Wang J, Xiong Y, et al. Relationship between gut microbiota and lymphocyte subsets in Chinese Han patients with spinal cord injury. Front Microbiol. 2022;13:986480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huber-Ruano I, Calvo E, Mayneris-Perxachs J, et al. Orally administered Odoribacter laneus improves glucose control and inflammatory profile in obese mice by depleting circulating succinate. Microbiome. 2022;10(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lima SF, Gogokhia L, Viladomiu M, et al. Transferable immunoglobulin A-coated odoribacter splanchnicus in responders to fecal microbiota transplantation for ulcerative colitis limits colonic inflammation. Gastroenterology. 2022;162(1):166–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Details of SNPs selected as IVs, including IVs of all taxa. Table S2. Results of causality evaluated in MR analyses, including results of all taxa, both outcomes, and all MR methods. Table S3. Results of heterogeneity and directional horizontal pleiotropy of single-nucleotide polymorphism effect of all taxa on both outcomes. Table S4. MR Steiger directionality test indicated that all taxa were upstream of both outcomes.
Data Availability Statement
The data sets analyzed during the current study are available in the MiBioGen, https://mibiogen.gcc.rug.nl/, and the IEU OpenGWAS project, https://gwas.mrcieu.ac.uk/.