Abstract
Temperate forest soils are considered significant methane (CH4) sinks, but other methane sources and sinks within these forests, such as trees, litter, deadwood, and the production of volatile organic compounds are not well understood. Improved understanding of all CH4 fluxes in temperate forests could help mitigate CH4 emissions from other sources and improve the accuracy of global greenhouse gas budgets. This review highlights the characteristics of temperate forests that influence CH4 flux and assesses the current understanding of the CH4 cycle in temperate forests, with a focus on those managed for specific purposes. Methane fluxes from trees, litter, deadwood, and soil, as well as the interaction of canopy-released volatile organic compounds on atmospheric methane chemistry are quantified, the processes involved and factors (biological, climatic, management) affecting the magnitude and variance of these fluxes are discussed. Temperate forests are unique in that they are extremely variable due to strong seasonality and significant human intervention. These features control CH4 flux and need to be considered in CH4 budgets. The literature confirmed that temperate planted forest soils are a significant CH4 sink, but tree stems are a small CH4 source. CH4 fluxes from foliage and deadwood vary, and litter fluxes are negligible. The production of volatile organic compounds could increase CH4’s lifetime in the atmosphere, but current in-forest measurements are insufficient to determine the magnitude of any effect. For all sources and sinks more research is required into the mechanisms and microbial community driving CH4 fluxes. The variability in CH4 fluxes within each component of the forest, is also not well understood and has led to overestimation of CH4 fluxes when scaling up measurements to a forest or global scale. A roadmap for sampling and scaling is required to ensure that all CH4 sinks and sources within temperate forests are accurately accounted for and able to be included in CH4 budgets and models to ensure accurate estimates of the contribution of temperate planted forests to the global CH4 cycle.
Keywords: CH4 flux, CH4 sink, CH4 source, Greenhouse gas, Soil, Foliage, Stem, Litter, Deadwood, Volatile organic compounds
Background
Methane and its role in global climate change
Methane (CH4) is a powerful greenhouse gas (GHG). Each molecule has 28–35 times the warming potential equivalent of carbon dioxide (CO2) over 100 years [1–3]. As such, methane is the second largest contributor to global warming after CO2 and is responsible for ∼ 30% of the rise in global temperatures since industrialisation [4]. Furthermore, CH4 oxidation in the troposphere produces ozone (O3) [5]. Ozone has strong greenhouse gas potential and influence on climate regulation (211 × stronger than CO2 over a 100 year period [6]), and also has complex effects on atmospheric chemistry and air quality through interaction with nitrogen oxides (NOx), volatile organic compounds (VOCs), formation of smog and respiratory illness, and the absorption of ultraviolet radiation. As such, CH4 emissions are regulated under international climate agreements, such as the United Nations Framework Convention on Climate Change and also the convention on Long-Range Transboundary Air Pollution established to address air pollution that travels across national boundaries [4].
Annual CH4 emissions are estimated at 550–594 million tonnes (CH4), 60% of which is produced by human activity [4]. These anthropogenic sources have driven a 260% increase in atmospheric CH4 concentrations relative to pre-industrial times (sensu 1750), and concentrations continue to increase. The current emissions trajectory is noteworthy, as it tracks midway between the two warmest Intergovernmental Panel on Climate Change (IPCC) scenarios (RCP6 and 8.5) [7] with concomitant impacts expected on ocean acidification, sea level rise, occurrence of extreme weather, biodiversity loss, agriculture and food safety, and other areas. Rapid and deep reductions in all GHGs, including CH4, are needed to meet the targets to limit global warming to 2 °C above pre-industrial times.
Although CH4 absorbs much more infrared radiation than CO2, it is also short-lived in the atmosphere. Consequently, most of the impact (radiative forcing/GHG effect) associated with CH4 occurs in the first few decades after release. For example, CH4 global warming potential (GWP) in the first 20 years of release is 80.8–82.5 × equivalent of CO2 [8]. Accordingly, reducing methane emissions will result in significant short-term effects on climate change, constituting one of the most efficient measures to help meet short-term emissions targets [9]. Realistic pathways to keeping the planet within safe thermal boundaries include substantial, early reductions in atmospheric CH4 concentrations [4].
Methane sources and sinks
Methane is produced from a wide range of natural and anthropogenic sources, including both biological and non-biological processes [10]. As such, the range of sources and sinks (Sensu biological and/or other processes that remove CH4 from the atmosphere) are diverse. An important definition exists between natural and anthropogenic sources. Natural sources are defined as ‘pre-agricultural’ emissions, i.e. those before adoption of wide-spread agriculture (Neolithic/agricultural revolution ca. 10,000 years before present (BP)). These include wetlands (76% of natural CH4 emissions), termites (11%), oceans (8%) and CH4 hydrates (5%). Anthropogenic sources (currently 60% of total emissions; as before) include fossil fuel production (33% of manmade CH4 emissions), livestock production (27%), rice cultivation (7%), wastewater treatment (7%), and landfills (11%) [11]. Accounting for these emissions involves quantification and estimation of emissions from natural and anthropogenic sources, as well as considering land-use changes, both historic (pre-agriculture) and contemporary.
Biogenic CH4 emissions (natural or anthropogenic) are a result of microbial activity, primarily methanogenic archaea, during the decomposition of organic matter in anaerobic environments such as in animal gut/intestines or wetland, and peatland soils [12]. More recently, other CH4-producing microbial groups have been discovered, including fungi [13] and bacteria [14]. There is also a growing literature regarding non-methanogenesis oxic CH4 production at plant and litters surfaces generating emissions (reviewed by Liu, Xie [15] and Putkinen, Siljanen [16]).
Methane is highly reactive and contributes to the chemistry of the troposphere and stratosphere. Reactions with hydroxyl radicals (OH) comprises the primary sink of CH4, accounting for 90% of total losses [17]. The other major sink is through biological CH4 oxidation in soils. This biological sink occurs mainly in aerobic soils and is estimated to remove about 3–10% of annual CH4 released to the atmosphere [18, 19]. Biological CH4 oxidation is carried out by methanotrophic microorganisms. Until recently, these were considered to use CH4 as a primary source of both carbon and energy source and be active under aerobic/oxic conditions [20]. It is now recognised a diverse range of microorganisms can oxidise CH4 with or without oxygen [11, 21]. Methanotrophs are nearly ubiquitous across natural ecosystems including soils and sediments.
Different land uses affect the size of the soil CH4 sink. Significant research has focussed on land use systems such as rice production, wetlands, and peatlands which are significant CH4 sources at a global scale. Conversely, soils in other (typically oxic) systems are net sinks; for example, CH4 uptake in temperate zones accounts for nearly half of the global soil sink (10.4 Tg CH4 yr− 1) [19]. Globally, soils under temperate forested land have the highest methane uptake compared with other land-uses [18, 22]. For example, in New Zealand higher CH4 oxidation has been measured in soils from pine plantations compared with pasture [23, 24]. Saggar, Tate [22] also found that pine forest soils had higher methane uptake rates compared with pasture and cropping soils, with uptake rates of 4–6 kg CH4 ha− 1yr− 1 and < 1–1.5 kg CH4 ha− 1yr− 1, respectively. Considering the overall global CH4 budget, temperate forest soils represented the largest terrestrial CH4 sink, with an average uptake of 7.64 (4.55–10.73) Tg CH4 yr− 1, accounting for 51% of the total global forest soil CH4 uptake [4, 25, 26].
The importance of methane cycling in temperate forests
Despite temperate forest soils being the largest terrestrial CH4 sink, when compared with other components of the global CH4 cycle/budget, the relative contribution of temperate planted forests to the global CH4 budget is minor. Indeed, in a tabulation of sources and sinks the impact of temperate forests was considered insignificant relative to other land uses (e.g., wetlands) or activities (agriculture and waste) and was not recorded individually (Table 3, Saunois, Stavert [4]). However, this belies the importance of forest-related CH4 cycling at regional to jurisdictional scales; i.e., those at which land management and carbon reporting often occur. For example, in New Zealand nearly 24% of the land area is temperate natural forest, and a further 5% is planted temperate forest [27]. As such, although New Zealand holds only a small amount of Earth’s temperate forest (∼ 0.95%; Fig. 1, under Temperate forests and forest soils), the total carbon budget of the country is highly sensitivite to any changes in carbon exchange in temperate forests. Similarly, Tasmania (Australia), British Colombia (Canada), Washington and Oregon (USA), through to Hokkaido and Honshu (Japan), parts of South Korea, Chile and so forth all have high proportions of temperate forest as part of the total land area. Understanding the carbon balance in these systems is, by extrapolation, an important part of regional carbon budgets and policy informing land use change and management.
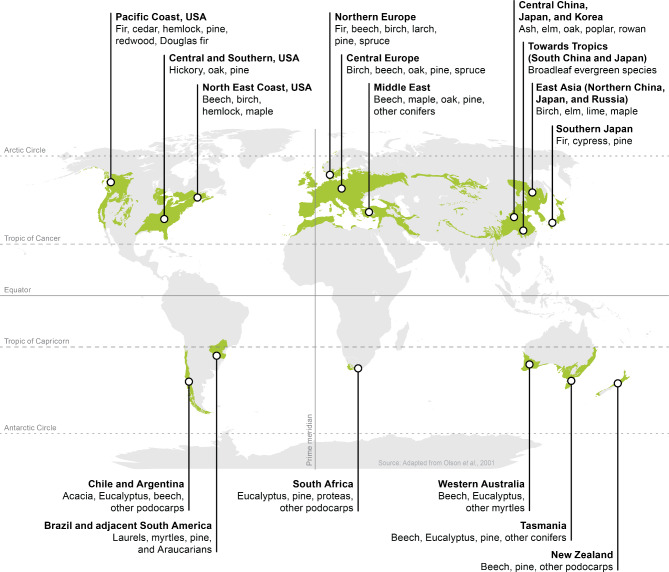
Fig. 1.
Global distribution of temperate forests and main tree species, either naturally occurring or planted, in each region
Providing a more accurate understanding of the CH4 flux in temperate forests is needed and will allow better total budgeting of GHG emissions, particularly for sensitive regions (i.e., as above). However, research on CH4 emissions in forested ecosystem reveals a far more complex story than previously thought, with an interplay of productive-consumptive, aerobic-anaerobic, biotic-abiotic processes occurring between upland-wetland soils, trees, and atmosphere [4]. That is, the CH4 cycle within forests is complex, with many individual pathways occurring; nett CH4 balance at any point in time must integrate these.
The aims of this review are to (1) highlight the features that define temperate forests and have the potential to influence CH4 flux and (2) assess the current state of knowledge around the CH4 cycle in temperate forests with particular focus on those being managed for specific purposes. This review considers processes and magnitude of CH4 fluxes from trees, litter and deadwood, and soil, as well as interaction of canopy-released volatile organic compounds on atmospheric CH4 chemistry. Methane fluxes will be quantified and factors (biological, climatic, management) affecting the magnitude and variance of these fluxes will be discussed. Based on the finding’s recommendations will be made on where research should be focused to accurately account for CH4 in greenhouse gas budgets.
Temperate forests and forest soils
Approximately 26% of the ice-free land mass is under forest, but historically this was much greater. About 8,000 years BP, for example, Earth held 6 billion ha of forest; this has declined to ∼ 4 billion ha today [28, 29]. These forests encompass the major biome types, spanning tropical, sub-tropical, temperate, to boreal systems. Within these, ‘primary forests’ are those that are largely free from human impact and have established and grown naturally; these ecosystems maintain high levels of biodiversity due to continuity and range of microhabitats resulting from the complex structure of the forests. They are more typical in tropical and boreal biomes, and most underrepresented in temperate biomes.
This underrepresentation is due to the impact of human activity in temperate forests, which is significant and persistent. These impacts range from entire alteration of landscapes and shift in land use types, through to the directed planting, management, and harvesting of forests themselves. This signature of human activity on temperate forest is evident globally and can be traced back to ancient times. From the Roman empire across Europe, agricultural expansion in China, to fire in North America and impacts elsewhere [30], the expansion of civilisation and alteration of temperate forest ecosystems is a defining characteristic of Earth’s recent history.
Temperate forests cover about ∼ 1.5 billion ha of Earth’s surface (∼ 16% of total forests). The extensive coverage of temperate forests underscores their importance in Earth system processes, encompassing biogeochemical cycles and the provision of ecosystem services. Approximately 17% of global net primary productivity is supported by temperate forests, and more than 15% of all Earth’s terrestrial carbon is held in these forest systems [31]. As such, the exchange of carbon held in temperate forests (spanning the living biomass, to deadwood, litter and the forest floor, as well as the extensive soil reserves) with the atmosphere comprises an important factor influencing the trajectory of climate change [32, 33].
Distribution
Temperate forests are mostly distributed between 30° − 60° latitude in both the northern and southern hemispheres [34] (see Fig. 1). This latitudinal band largely spans the range of climatic conditions that define temperate biomes; typically, a 4–6 month frost free growing season (a key difference to the shorter season in boreal forests) and mean annual temperature of ∼ 5–20 °C. Compared with tropical forests, temperate forests do experience periods of 0 °C or colder and precipitation typically exceeds potential evaporation. However, there are various approaches to defining ‘temperate’, and these are expanded on by de Gouvenain and Silander [31].
The distribution of temperate forest is weighted to the northern hemisphere (∼ 80%) due to the larger extent of land north of the equator. This includes eastern United States, the Pacific Northwest and into Canada. Northern Europe, Turkey, Iran, southern European Russia, southern parts of eastern China, Japan, and Korea (Fig. 1). In the southern hemisphere, temperate forests are distributed through South America including Chile and parts of Patagonia, Tasmania and south-eastern Australia, and the entire extent of New Zealand [30, 31, 34].
Forest structure
In temperate forests under natural conditions, a wide floristic range occurs and this is primarily driven by ecoregion-associated climatic variation [34]. Areas with warm to hot continental climate, and those with marine climates such as the Eastern US and parts of Europe, support mixed-deciduous forests. However, in rainy subtropical temperate zones and where ocean moisture is trapped by mountain ranges and caught by the canopy or forms meteoric rain (temperate rainforest conditions), mixed evergreen forests predominate.
Within ecoregions, landform influences overlay these broader (e.g., climatic) factors affecting tree suitability. Pinaceae and Eucalyptus spp., for example, are well suited to soils of low nutrient status, low organic matter, or dryer (xeric) sites, whereas in moderately wet sites (mesic), beech, oak, and other species are better adapted [28]. However, the drivers of forest structure and composition are complex; local macroclimatic and ecophysiological conditions are expressed against geologic and biogeographic backgrounds and paleoclimatic history [31]. Indicative temperate forest species, either naturally occurring or those planted, in different ecozones are given in Fig. 1. Pests and diseases, fire, windstorm, drought, elevated nitrogen deposition/eutrophication, and other disturbances also influence forest structure and function [35]. These latter factors now have a strong signal of human activity driving the frequency and severity of occurrence [36, 37].
The legacy of human influence on temperate forests is so extensive (likely more than any other forest type) it is difficult to determine what comprises a ‘natural’ or ‘pristine’ state [30]. Indeed, the evolution of these forest systems should be recognised as occurring alongside human activity. Humans imprint is seen in their former and current distributions, but also the tree species that are present. Ironically the rate of human induced climate change may actually necessitate that human intervention is needed to support temperate forests by way of assisted migration or climate conscious planting to increase forest resilience. Natural rate of recruitment/replacement cannot keep pace with change in the habitat conditions (sensu Hutchinsonian niche space [38]). Seedlings growing today may be unsuitable for the conditions of tomorrow. An example are the coniferous temperate forests in California’s Sierra Nevada biome [39], where climate change has led to a vegetation-climate mismatch, effectively stranding forests in locations unsuitable for their future regeneration (sometimes colloquially referred to as ‘zombie forests’). Trees are sessile and long-lived; they cannot migrate from climate change. Paradoxically, the change brought about by humans on Earth’s systems means that the fate of temperate forests will be more connected with human activity than ever before.
Soils
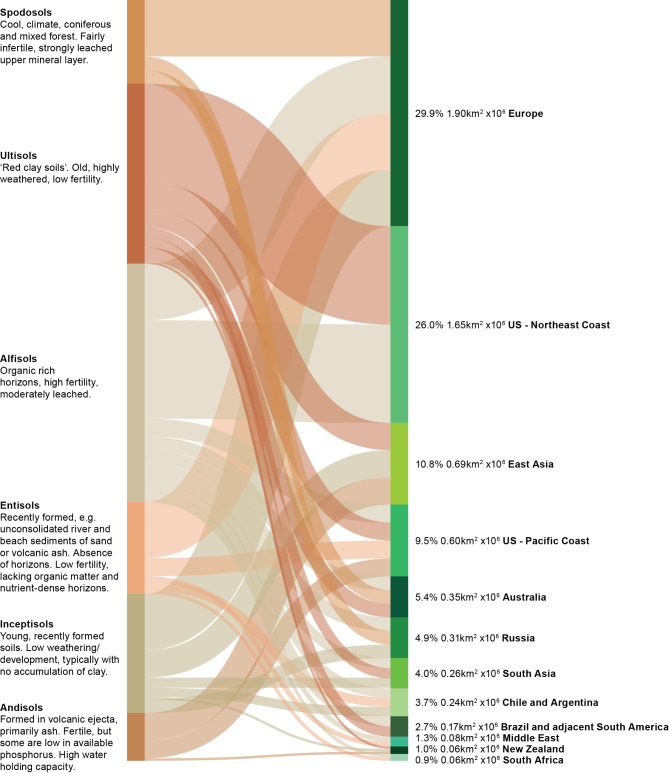
Soil types are typical of those distributed through the latitudinal range. These include five main orders: Alfisols, Entisols, Inceptisols, Spodosols, and Ultisols (Fig. 2), and range in fertility, age and extent of weathering, water retention and other primary properties. However, just as the floristic diversity of forests can exhibit strong local diversity due to spatiotemporal and other factors, soil types too are highly diverse, even at local scale. Similarly, whilst five soil orders support most temperate forests globally, other soils type can be important regionally. In New Zealand’s North Island, for example, ash and tephra deposited by volcanic activity has weathered to form extensive Andosols (Fig. 2). These soils support the growth of extensive natural and planted temperate forests and are therefore an important soil type in forested regions with a history of volcanic activity.
Fig. 2.
Sankey plot connecting the main soil types that support temperate forests and the regions they are found. The left-hand side has the main soil types and key features of each soil type. On the right-hand side, regions are listed along with the proportion of global temperate forests (%) and the area of temperate forests (km2 × 106) in each region. Classification based in USDA soil taxonomy.
Like forests themselves (see later), soils can be subject to significant modification from their natural state due to human management. In productive planted forests, this includes alterations to fundamental properties such as pH, inputs to redress nutrient deficiencies, site alteration to increase draining and so forth. More broadly, human intervention in the matching of tree species to sites has been one of the defining themes of temperate forests historically through to today. Many temperate forests are managed to some extent, including choice of tree for site or, at least, interventions such as thinning or selective removal which advantage different forests structures and compositions. Choices such as planting deciduous or conifer-based forests have clear impact on the soil. Podsols (order within Spodosols), for example, form under coniferous or mixed forest with unique properties related to organic matter accumulation, horizon formation, and mobilisation of iron and aluminium oxides or hydroxides. The types of trees planted can strongly influence soil pedogenesis.
Temperate forest soils typically hold much greater levels of organic matter than those in tropical regions. Much of this organic matter rests on the soil surface, comprising a ‘forest floor’ layer of varying thickness. The top of the forest floor comprises the most recently fallen litter such as leaves/needles and wood debris. As this extends downwards to the surface of mineral soil horizon, the state of this resource changes, becoming increasingly humified, with a wider ratio of carbon to nitrogen (C: N) and other elemental changes, and increased moisture content [40]. Microbial biomass in the lower regions of the forest floor are often rich in fungal mycelium, other microorganisms, invertebrates, and other life. Plant roots extend laterally into the lower portions of this layer (particularly the interface of mineral soil and organic matter layer), recapturing nutrients and energy fallen from the canopy and other parts of the forest. Inputs of fresh litter into the forest floor seasonally vary among different temperate forest systems. In the USA, needle fall of conifers and deciduous broad-leaved trees leaf-fall occurs in Autumn, while in New Zealand conifer needle fall occurs in Autumn, and for the native beech forests (Nothofagus spp.) leaf fall is primarily in Spring [41]. Litter-fall is a key process of the nutrient and energy recycling within such forest systems.
Summary of prominent features of temperate forests that influence methane flux
A defining characteristic of temperate forests is the significant influence of human activity on these ecosystems globally. In comparison to other forest systems, extensive areas of temperate forests have been and continue to be profoundly shaped by human intervention, resulting in alterations to their structure and function, including changes in location, species composition, and environmental conditions. Human influence of these forests spans a continuum, from historic influences of land use alteration, agricultural expansion, and global movement in trees species (and pests, diseases, weeds, and herbivores), through to harvest of trees from naturally regenerated stands, selection systems and plantation forests intensively managed for timber production [30]. With increasing global demand for wood and fibre, energy, carbon storage, and other materials/resources that forests can provide, temperate forests are increasingly managed for production of fast-growing tree species.
A further prominent feature of temperate forests is the occurrence of distinct seasonality. Temperate forests can be defined via their latitudinal zone which provides conditions for winter induced dormancy and a defined growth season during the warmer months (as before). This seasonal variability is important as it underpins strong phenological events that drive temperate forest ecology and functionality. As discussed above, litter fall is a key seasonally-triggered event. During this period large shifts in resource allocation occur from canopy to the forest floor and soil, shifting soil nutrient cycling and energy flow through the trophic food webs. Such events profoundly impact biogeochemical processes related to carbon flow and, therefore, are likely to impact exchange of trace gases such as CH4. Other phenology events include timing of leaf emergence, tree sap flow (sometimes harvested; e.g., maple), fungal sporocarp emergence and senescence, emergence of invertebrates (e.g., cicadas) and so forth. These and other biological events triggered by environmental cues and changes play crucial roles in temperate forest ecosystem ecophysiology and dynamics.
These prominent features mean that processes such as production and consumption of CH4 are particularly challenging to assess in temperate forests, as the ecophysiological conditions change dramatically over the course of each year. Strong seasonal cycles of temperature and moisture drive biogeochemical cycles and phenological events, such as episodic reallocation of nutrients and energy within the forest system (as described above); these alter carbon cycling dynamics among primary producers, decomposers, and the wider food web. These unique elements of temperate forests must be explicitly considered in CH4 cycling and budgets. Furthermore, given the extent of human interaction with temperate forests, including extensive management of these for production of wood, fibre, and environmental products [42], there exists significant potential for intervention practices targeted towards influencing ecosystem CH4 budgets.
Forest methane fluxes
The biological cycling of CH4 within forests ecosystems comprises a range of processes from soils, canopy, stems and other components. These act as various sources or sinks of CH4, with each process varying in relative magnitude and direction over time and as conditions change [43]. There are also indirect processes such as the production of volatile organic compounds by trees that can interfere with the natural atmospheric processes that degrade CH4 [44, 45]. While the critical zones in CH4 cycling interactions are known - i.e., soil, tree stem, canopy, deadwood, and VOCs – the relative size contribution of each of these to the overall cycle is not well characterised. Soil is the exception and has been reasonably well studied with soils in temperate/upland forests generally considered CH4 sinks [4, 18, 46–49].
The most common method for measuring CH4 flux of different forest compartments in situ is with chambers [22, 43, 50]. Chamber measurements are well suited to process-level studies of individual components within the ecosystem [51] and therefore the majority of studies mentioned in the below sections use this method. Another technique for measuring forest CH4 flux is the eddy covariance method. This method integrates fluxes over a larger area, which results in measurements that are more representative of the ecosystem as a whole [52]. Wang, Murphy [51] used this method to measure CH4 fluxes in a temperate forest in Ontario, Canada and found that over the measurement period (June to October 2011), the site was a net CH4 sink. Although, this method and others like it (flux gradient, Eddy accumulation etc [53]). can measure net CH4 flux at a forest level, this method is generally expensive and not commonly used to measure CH4 flux in temperate forests.
Forest soils
Size of soil flux
Methane consumption by forest soils is considered the greatest among all land use types [46, 47]. Global annual mean soil CH4 consumption in temperate forest soils is reported to be 3.6 kg CH4 ha− 1 y− 1 [47]. However, this average belies considerable regional variation. For example, estimates range from 4 to 6 kg CH4 ha− 1 y− 1 in single-species planted forests [22]. This large uncertainty existing within systems largely constrained for tree species and management practices, shows the importance of understanding local and even site-specific variation influencing forest CH4 emissions and regional CH4 budgets.
Factors that affect soil flux
Soil CH4 flux is the net product of the activity of CH4 producing archaea (methanogens) and CH4 consuming bacteria (methanotrophs). Factors that influence CH4 flux are those that affect either gas diffusion and/or microbial activity. For a forest soil to be a CH4 sink, conditions need to be favourable for methanotrophs and CH4 oxidation. The ability of methanotrophs to access CH4 via diffusion is the main factor influencing CH4 oxidation activity [54–56]. Methane flux in temperate planted forest soils is affected by both environmental conditions and management practices. These factors differ between and even within temperate forests resulting in variation in CH4 flux. The environmental factors covered in this review are soil water content, soil bulk density, oxygen availability, Eh (redox potential) and pH, temperature, and soil physicochemical properties (e.g., fertility, organic carbon, C:N ratio and other soil nutrients). Management practices include stand age, stand density, use of nitrogen (N) + phosphorus (P) fertiliser, tree species and compaction of soil due to the use of heavy machinery at harvesting.
Environmental factors
Soil water content
Soil water content has been found to affect CH4 uptake in temperate forests [57–62]. As soil moisture increases, soil aeration decreases and macropores become blocked limiting methanotrophic activity [18, 63]. In extremely wet conditions (e.g. saturated soil), the reduced supply of oxygen can also create anaerobic conditions suitable for methanogens resulting in CH4 emissions [18, 64]. Drier soils allow for increased diffusion of gas through the soil [57, 59, 65]. However, if soil moisture reduces too much (e.g. below wilting point) CH4 uptake is limited due to the microbes being physiologically stressed [57]. In forests, tree roots help increase CH4 uptake by reducing soil moisture [66].
Ni and Groffman [67] found that CH4 uptake by soils was declining in forests in the northern hemisphere (0 to 60 °N latitude) as precipitation increased. This reduction in the forest CH4 uptake could mean the total forest sink has been overestimated in several regions across the globe. These results show the sensitivity of soils to changes in precipitation and, therein, CH4 cycling. There is an urgent need for up to date regional CH4 budgets, including for New Zealand nationally, to accurately quantify the forest soil CH4 sink. There is also a need to understand the magnitude and direction change of forest CH4 cycling in response to predicted future climate conditions to help improve the accuracy of future CH4 budgeting.
Soil bulk density/soil structure
Methane uptake in forests soils is dependent on physical factors controlling gas diffusion through the soil such as soil moisture (discussed above) and soil bulk density/structure, and compaction (discussed in forest management and silviculture section). Soil bulk density and soil structure directly influence gas diffusion and, therefore, exchange of trace gases such as CH4. In general, there is a strong association between increased soil porosity and uptake of atmospheric CH4; soils with medium and fine texture have reduced gas transport [68]. In forest soils, Smith, Dobbie [69] found that CH4 oxidation rates were greater in coarse-textured soils with well-developed soil structure and permeable surface organic layer. Tate, Saggar [62] also found that afforestation of pastures increased CH4 oxidation and concluded that this increase was related to the higher proportion of macropores in soils growing pine trees. Tree roots help increase CH4 uptake in soils by changing soil texture, increasing porosity and therefore increasing gas diffusion [70].
Oxygen availability, eh and pH
Gradients of dissolved oxygen concentration directly affect methanotrophs. Methane consumption occurs under oxic conditions. However, when oxygen is depleted (anoxic conditions, Eh + 750 - -220), microorganisms can utilise alternative electron acceptors (NO3−, NO2, Fe(III), Mn(IV) and SO42−) for CH4 oxidation [71]. Anoxic conditions establish when oxygen supply cannot meet oxygen demand. Oxygen supply in soils is predominantly controlled by soil water content and structure (as above). However, even in seemingly aerobic, well drained soils, the complex physical structure of soils results in an abundance of anoxic microsites/hotspots and associated metabolic gradients [72, 73]. For example, significant anoxic CH4 oxidation has been identified at depths ranging from 0 to 60 cm in temperate forest soil profiles [73]. Methane oxidation occurring under anoxic conditions (microsites and spatial and temporal scales) is now recognised as important but ill-defined control on CH4 cycling in soils [72, 73].
Although temperate forest soils are generally considered CH4 sinks, CH4 production could still be occurring within hotspots in the soil. Biological CH4 production occurs under anaerobic, Eh -240 conditions but methanogens have also been found to be abundant and active in oxic soils and broadly represented in soil metagenome datasets [74]. This highlights the need for high resolution studies across sites to capture these hotspots [75] when measuring site CH4 flux. Microbial studies are needed to better understand anaerobic CH4 uptake and aerobic CH4 production.
In forests, most soils exhibit soil pH levels 6 but range from pH > 3.5 to pH < 8.0 [46]. Although pH generally has a first-order influence on many soil biogeochemical cycles, its influence on CH4 cycling is relatively minor. Methanotrophic microorganisms typically adapt to local forest soil pH conditions [56] and, as such, rates are relatively pH insensitive.
Temperature and season
The distinct seasonality of temperate forests is a prominent feature that influences CH4 (discussed above). Seasonal variation in CH4 uptake is driven by changing temperature and soil moisture throughout the year [22]. Despite this the effect of temperature alone is generally small within the ranges associated with temperate forests [69]. Methane oxidation has been found to vary little over a wide range of temperatures (1–30 °C) [18]. The optimum temperature for CH4 oxidation is between 25 and 30 °C [76, 77]. However, at temperatures outside this range (e.g. -5 °C [78] and 40 °C [77]) CH4 oxidation was reduced/or completely inhibited.
Soil fertility and soil organic carbon
Temperate forests are found on soils that range in fertility. High fertility soil can exhibit greater CH4 uptake than low fertility soil [78, 79]. The processes responsible for this effect are unknown, but it may be that the greater availability of nutrients reduces restrictions on methanotroph maintenance and growth relative to low fertility soils.
Soil organic carbon in forest soils has been found to have a contrasting effect on CH4 flux. Global studies incorporating all forest biomes by Feng, Guo [25] and Gatica, Fernández [80] found that increasing soil organic carbon reduced CH4 uptake, or even caused greater CH4 emissions. This was thought to be due to carbon stimulating methanogenesis [81]. In contrast, Borken, Xu [79] found no correlation between soil organic carbon and CH4 uptake and Lee, Oh [82] found that forest soils with greater levels of soil organic carbon (0 to 20 g kg− 1 vs. 60 to 100 g kg− 1) had greater CH4 uptake (study included all forest biomes). Along with soil carbon content, soil C:N ratio in forest soils have also been found to affect CH4 flux, with greater C:N ratios associated with reduced CH4 uptake [83, 84]. More research is required exploring the influence of soil organic carbon and C:N ratios on CH4 flux as the mechanisms behind these variable responses are largely unknown.
Other soil nutrients
The availability of ferric ion (Fe3+), sulphate ion (SO42−) and Manganese (Mn4+) determine whether CH4 can be oxidised under oxygen limiting conditions (see section on oxygen availability). This relationship was thought to be more relevant to tropical soils/rice paddies [84] but is now considered to be relevant in all soils due to their heterologous nature [73, 85]. It is important to note that the availability of these alternative electron acceptors depends on the presence of other microorganisms that can reoxidise/replenish/recycle these nutrients. Production and release of reactive intermediates by methanotrophs are strongly influenced by metals (copper, iron manganese, lanthanides) which in turn influence the dynamic fluxes of nitrous oxide and CH4 to the atmosphere [86, 87].
Forest management and silviculture
Use of nitrogen and phosphorus fertilisers
The addition of N fertiliser has been found to inhibit CH4 oxidation in temperate forest soils [88–92]. Annual applications of N fertiliser of 150 kg N ha− 1 yr− 1 as ammonium nitrate (NH4NO3) have been reported to decrease CH4 uptake by soils relative to the control by 64% [78] to 86% [93]. Lower rates of 50 kg NH4NO3-N ha− 1yr− 1 decreased annual CH4 uptake by 16% compared with unfertilised soils [78]. A meta-analysis by Xia, Du [94] found that N addition significantly decreased soil CH4 uptake by 39% in temperate forests. However, this was dependent on N level with CH4 uptake increasing at N additions below 27 kg N ha− 1 yr− 1.
There are also studies that show N input from fertilisation can either stimulate forest soil CH4 uptake or have no effect on uptake at all [89]. A single application of N fertiliser to temperate forest soils has been shown to cause an initial decrease in CH4 uptake compared with unfertilised soils but after 2 months [95] – 1 year [96] there was no difference in CH4 uptake between the soils that received fertiliser and the control soils that did not. After an initial suppression in CH4 uptake following N fertiliser addition some studies have shown an increase in CH4 uptake [96–98].
Mechanisms for inhibition/stimulation of CH4 uptake in response to N fertiliser addition are poorly understood. There are multiple proposed mechanisms for the inhibition of CH4 uptake as a result on N fertiliser addition. The inhibiting effect of NH4NO3fertiliser could be due to ammonia oxidisers competing with methanotrophs [99] or due to competition between NH4+ and CH4 at the binding site of the catalysing enzyme CH4 monooxygenase in the first step of CH4 oxidation pathway [76]. The intermediates and end products of methanotrophic ammonia oxidation, i.e. hydroxylamine and nitrate, can be toxic to methanotrophic bacteria and lead to inhibition of CH4 consumption [89].
The response may also be dependent on soil N status. Soil CH4 flux is less sensitive to N addition in N-limited ecosystems [92] with less reduction or even an increase in CH4 uptake [95, 97, 98]. One possible explanation is that the addition of N corrects a N limitation that was reducing methanotroph activity [98] or the addition of N corrects other soil properties such as C:N ratio that makes conditions more favourable for methanotrophs [97]. In contrast, N addition in forest ecosystems could also lead to increased litter accumulation on soil surface reducing the diffusion of CH4 and oxygen into the soil reducing CH4 uptake [100].
It has also been suggested that the recovery of CH4 uptake after an initial period of decline following N fertilisation could be due to a shift in the methanotroph community to one that is tolerant to excess NH4+ [93]. For example, Mohanty, Bodelier [101] found that N addition had little effect on CH4 oxidation in soils where type I methanotrophs predominated but could markedly affect oxidation in type II-dominated soils. Others have reported that increased N availability does not impact methanotrophic community structure but did reduce the abundance and activity of CH4 consuming methanotrophs [102]. There are many different members of the microbial community contributing to cycling of N and CH4 in soils [103]. Complex interactions and multiple factors mean that mixed results have been obtained from use of N fertilisers.
Type of N fertiliser may also influence the response of CH4 uptake to N fertiliser addition. Urea has been found to reduce CH4 uptake by 5 to 20 times compared with unfertilised soils [91]. However, urea has been found to cause the least suppression of CH4 uptake in upland ecosystems (not specifically forests) compared with other forms of N [92].
Little is known about the effect of P addition on CH4 oxidation, particularly in temperate forest soils. The few studies that have been done show contrasting results. Winsborough, Thomas [104] found that the addition of P in a high N soil caused a decrease in CH4 uptake whereas Borken, Xu [79] found that total P content in the soil was not correlated with CH4 uptake and Burke, Smemo [105] showed no effect of P on the methanotroph community. The review by Veraart, Steenbergh [106] concluded that a better mechanistic understanding is needed about the effect of P on methanotroph community structure and on CH4 oxidation, not just in planted forests but in soils generally.
Globally, fertiliser use in plantation forests is low, with many receiving no fertiliser at all [107]. In New Zealand for example, the average application rate of fertiliser (N + P) across all planted forests in 2017, including those with no applications) was 8 kg ha− 1 [108]. Fertiliser application is infrequent. Applications in the first year are common but after this application frequency ranges from once per year to not at all. For N, the per application rate is ∼ 200 kg N ha− 1, applied in the form of urea, ammonium sulphate and di-ammonium sulphate [108]. For P, the per application rate is ∼ 75 kg P ha− 1 for phosphorus [108].
When N fertiliser is applied to plantation forests the rate is generally at rates ∼ 200 kg N ha− 1 yr− 1 therefore it is likely that N fertiliser use in planted forests will reduce CH4 uptake. However, if N fertilisation is infrequent the response may not be permanent and CH4 uptake rates could recover to pre-fertilisation levels within 1 year. In N limited environments, the addition of N could even increase CH4 uptake. However, more research is required to better understand the mechanisms behind these processes before site specific response predictions can be made.
Stand age
Soil CH4 uptake increases with stand age [109, 110]. Smith, Dobbie [69] found that after afforestation CH4 uptake increased over 100 years. This could be due to decreased soil moisture content resulting from increased tree transpiration and rainfall interception by canopy and litter cover. Using a 120-year afforestation chronosequence, Hiltbrunner, Zimmermann [111] found that soil CH4 oxidation increased with stand age because atmospheric CH4 diffusion into the soil was enhanced as soil water content decreased.
Stand density
Thinning is the management practice of reducing the stand density which leads to increased tree size and improved timber quality. The timing and intensity of thinning varies greatly across forest types and management objectives globally. In New Zealand, radiata pine is typically planted at about 1,000 to 1,250 stems per hectare and pruned or thinned to 200–500 stems per ha. However, stand density has not been found to affect soil CH4 uptake. De Bernardi, Priano [112] found there was no difference in CH4 uptake between a Pinus radiata stand in Argentina with a density of 727 trees ha− 1 and one with 977 trees ha− 1 despite differences in canopy cover and ground cover (the lower stocking rate had a grass understory). Forest thinning has also been found to have no significant effect on soil CH4 uptake in ponderosa pine forests [113]. Despite these results, thinning does affect soil moisture due to decreased canopy cover and less rainfall interception and therefore it would be expected that thinning would have an impact on CH4 uptake. There are limited studies on the effect of stand density and thinning in temperate planted forests on CH4 flux, and this is an area where more research is required.
Tree species
A range of trees are grown in temperate forests. These include deciduous, coniferous and broadleaved evergreen trees. Tree species affects soil CH4 uptake in planted temperate forests [25, 114]. Coniferous forests have been found to have lower CH4 uptake rates compared with deciduous beech trees [79, 115] and mixed stands with both broadleaved and coniferous trees [116]. In contrast, Christiansen and Gundersen [117] reported that CH4 uptake did not differ between deciduous (oak (Quercus robur)) and coniferous (Norway spruce (Picea L. Karst)) tree stands. Modelling of global CH4 flux by Feng, Guo [25] estimated soil CH4 uptake rate of coniferous forests to be much greater than broadleaf forests and similar to that of mixed broadleaf/conifer forest however, this comparison included tropical and boreal forests as well as temperate. How tree species effect CH4 uptake is not well understood. Methanotrophic communities in deciduous forest soils differ from those of mixed and coniferous forest soils suggesting that tree species might affect the methanotrophic community structure and activity [56]. Borken, Xu [79] speculated that organic compounds produced by the spruce and pine trees may have diminished the activity or population of methanotrophs in the soil. Maurer, Kolb [44] found Norway Spruce (Picea abies) released monoterpenes into the soil at rates high enough to explain reduced CH4 uptake. It could also be due to the different composition of the litter under the different trees or the greater atmospheric deposition of N in coniferous forests influencing soil factors such as pH, carbon content and C:N ratio and therefore influencing methanotroph growth and activity [115, 116]. Further studies investigating the mechanisms behind the effect of tree type on soil-atmosphere CH4 exchange are needed.
Compaction
The use of heavy machinery during the harvesting of temperate planted forests leads to severe compaction of the soil. This results in a change in soil structure, increasing soil bulk density, decreasing soil porosity and air permeability, and increasing water retention and potential for waterlogging [118]. This affects gas diffusivity and the microbial community in the soil, reducing CH4 uptake or even facilitating CH4 emissions in planted forests. In three beech stands in Germany, compaction reduced CH4 uptake by 90% in wheel tracks [119] and in a mixed beech-spruce forest in Switzerland compacted soil vehicle tracks was found to be a CH4 source, also supporting greater abundances of methanogenic archaea [120]. The severity of compaction depends on factors such as harvesting equipment, operation conditions and site characteristics [118]. This is likely to result in site specific impacts of compaction on CH4 flux. Reducing heavy machinery within forests is a key factor that could be used to minimize the impact at harvesting on CH4 uptake, and this should be reflected in research priorities. An example is the development of suitable information to allow the incorporation of site conditions and characteristics into harvest plans to minimise negative impacts on CH4 flux.
Stem methane fluxes
Tree stems are most commonly a source of CH4 [43] although there have also been reports of CH4 uptake from tree stems [16, 121, 122]. Tree stem CH4 flux has been extensively reviewed by Covey and Megonigal [43]. Table 1 provides a summary of stem CH4 emissions in dry, temperate, upland forests (adapted from Covey and Megonigal [43]), highlighting the relatively small contribution of this CH4 source to atmospheric pools. Tree emissions are commonly measured per stem. Studies that have upscaled per stem measurements from upland temperate forests have estimates of stem emissions ranging from 0.2628 g CH4 ha− 1 yr− 1 to 288 g CH4 ha− 1 yr− 1 (references in Covey and Megonigal [43]). Stem emissions have been estimated to offset soil CH4 uptake by roughly 3.5% by Warner, Villarreal [123], 1–6% by Pitz and Megonigal [121] and Wang, Gu [124] estimated a much higher 63% from a temperate poplar forest in Beijing, China. In contrast, a recent study by Gauci, Pangala [122] estimated woody surfaces in upland temperate forests to be a small CH4 sink with a total annual CH4 uptake of 1.96 Tg CH4 yr− 1. It is important to note that calculating stand level CH4 flux from tree stem measurements taken from single stems is difficult due to the variability of stem emissions/uptake within a forest (discussed in section below). Currently this variability is not routinely considered in scaling up calculations leading to overestimation of CH4 emissions from tree stems at a global, stand or even tree level.
Table 1.
Methane stem emissions from trees in upland temperate forests (adapted from Covey and Megonigal, 2019 [43]).
| Study | Site Description | Source | Rate | Units | Notes |
|---|---|---|---|---|---|
| [133] | Upland | Stem-based |
0.009 (± 0.006) |
nmol m− 2 s− 1 | Low stem surface emissions from young plantation oak trees |
| [129] | Upland and Wetland | Stem/soil-based | 1.19–9.84 | nmol m− 2 s− 1 | Fluxes vary along a gradient with highest in wetland areas |
| [123] | Upland | Stem-based | 0.11 (± 0.21) | nmol m− 2 s− 1 | Tree species were mainly tulip, maple, beach, and birch |
| [121] | Upland | Stem/soil-based | 0.44 (± 0.24) | nmol m − 2 s− 1 | Looked at 17 trees, mostly beach, tulip, hickory. Stem emissions were 1–6% of soil sink |
| [134] | Upland | Stem-based | 0.52 (± 0.92) | nmol m− 2 s− 1 | Fagus sylvatica forests |
| [128] | Upland | Stem-based | 0.003 (± 0.001) | nmol m− 2 s− 1 | Tree species included pine, hemlock, oak, birch and maple. They found widespread microbial production in living stems producing CH4 |
The processes that regulate CH4 flux from tree stems are not well understood. The emission of CH4 from tree stems is thought to be driven by two possible mechanisms. Firstly, methanogenic microbes can colonise the stems of trees, resulting in CH4 production and release from the tree [16, 125]. There is also some evidence that methanotrophs are also present in tree stems [16, 122, 126] suggesting that the direction of CH4 flux of tree stems is driven by the balance of co-occurring methanogenesis and methanotrophy.
In addition, CH4 produced in the soil can be absorbed by the roots and transported to the stem of the tree where it diffuses into the atmosphere [123, 127, 128]. This second pathway is common in wetland and tropical environments in which the anaerobic nature of the soil promotes CH4 production, creating a large pool of CH4 that can be absorbed by roots. Consequently, studies which report CH4 fluxes in these wetter environments often provide greater CH4 stem emission values than what would be produced in a temperate climate [129]. This trend is supported by Warner, Villarreal [123] who observed stem CH4 fluxes 1–2 orders of magnitude less in temperate forests than those reported for wetland systems.
Stem emissions and uptake in temperate forests are extremely variable. This variation in CH4 fluxes arises from multiple factors including tree species, age, tissue type, site characteristics and environmental conditions [43, 130]. Stem emissions have been found to vary between and within tree species. Epron, Mochidome [127] found that coniferous species emitted almost no CH4 whereas four broadleaved species had high intraspecific variability (0-3.7 nmol m− 2 s− 1). Warner, Villarreal [123] also found significant differences in CH4 fluxes between 6 different tree species. Gauci, Pangala [122] measured mean CH4 flux of sycamore (Acer pseudoplatanus) and ash (Fraxinus excelsior) trees and found that on average sycamore trees were emitting CH4 and ash trees were up taking CH4. There was also a wide flux range for each tree species (sycamore: -50.41–27.69 µg CH4 m− 2 h− 1, oak: -39.79–27.08 µg CH4 m− 2 h− 1) showing within species variability. Even on the same tree CH4 emissions vary by tissue type with CH4 emissions decreasing from the main stem > branches > leaves [127] and differing between sapwood and hardwood [124]. With increasing height up the stem CH4 emissions have been shown to decrease [121] and Gauci, Pangala [122] found that measurements taken above 1.3 m on ash and sycamore trees in a temperate forest, showed CH4 uptake whereas measurements below this showed CH4 emissions. Some studies have shown temporal (seasonal and/or diurnal) variation [121, 131], while others have not [123]. Size and/or age of the tree has also been shown to influence stem CH4 emissions [129, 132].
To improve estimates of stem CH4 flux at a tree, stand, regional or global level, research should focus on characterisation of the microorganisms and biochemical pathways associated with CH4 production and investigation into the biogeochemical processes involved in CH4 production and transport through stems [130]. There also needs to be long term studies with frequent measurements (i.e., monthly, daily, hourly) to capture temporal variability along with studies that take measurements across individuals, tree species and locations to understand other sources of variability.
Foliage methane fluxes
The potential for tree foliage to play a role in CH4 fluxes is uncertain. The few studies that have measured tree foliage in situ were in boreal forests and suggest that foliage may be involved in non-trivial fluxes but no consistent patterns have been reported with studies showing positive, negative and nondetectable fluxes [16, 135–137]. Recently, a study by Gorgolewski, Caspersen [138], in an upland temperate forest in Ontario, found that tree foliage was consistently a CH4 sink. This is the first in situ observation of consistent CH4 uptake across a range of tree species and sizes in a temperate forest. The foliage consumed CH4 at a rate of − 0.54 nmol m− 2 s− 1 in direct sunlight, which was approximately 62% of the soil consumption rate and represented 38% of nett daytime ecosystem CH4 consumption. The mechanism for potential foliar CH4 uptake is hypothesised to be consumption by endophytic methanotrophic bacteria. Photosynthetically active radiation was also found to influence foliar CH4 uptake [135, 138].
Tree foliage has also been found to be a CH4 source. Gorgolewski, Caspersen [138] also measured CH4 flux at a lowland temperate forest site where soil was a CH4 source. They found that foliage was a CH4 source (6.06 nmol m-2 s-1 ± 2.47 SE in direct sunlight) with an emission rate about 8% that of the soil. A possible mechanism for this is that CH4 produced by methanogens in the soil or in the tree stem was transported to foliage through the transpiration stream [137]. It is unlikely methanogens on the tree foliage played a significant role in the nett CH4 flux as they need anoxic conditions to survive [16]. Although it should be noted that there are microbes that have been shown to produce CH4 in oxic environments [15, 16]. Another possible mechanism is aerobic CH4 production by plants [139]. This process is induced by cutting injuries, increasing temperature, ultraviolet radiation and reactive oxygen species [140]. However, the possibility of aerobic CH4 emissions by plants has been a contentious topic in the literature [139–141] because studies have mostly been lab studies not representative of natural conditions [140]. Tree foliage fluxes appear to be important and could be a potential sink in temperate forests, but further research is required to confirm fluxes and mechanisms.
Litter and deadwood methane fluxes
Temperate forests can hold significant masses of litter and deadwood both as a consequence of natural events such as leaf fall, shedding of branches (self-pruning/autonomic abscission), windthrow, and management such as thinning and harvesting. In temperate forests, 13% carbon stock is stored in deadwood and litter [142]. Decomposition of organic matter can result in the release of CH4, particularly in waterlogged or otherwise anaerobic environments. Despite this, the few studies that have explored CH4 emissions from litter and deadwood indicate emissions are negligible, or less than that from other plant types.
For leaf litter, Schipper, Harfoot [143] reported that CH4 release from decaying fresh and senescent radiata pine needles was detectable when incubated under anaerobic conditions favouring CH4 production, however the extent of CH4 production was substantially less than that associated with decomposition of biomass from other plants. In this regard, the lability of the plant-associated carbon was hypothesised as being more important than amount of carbon added per se. Gritsch, Egger [144] explored CH4 emissions from coniferous and deciduous leaf litter under a range of conditions and determined that emissions were essentially negligible. Peichl, Brodeur [145] reported a similar outcome from a study conducted in temperate pine forests, determining the presence of tree litter had no impact on CH4 emissions over multiple sampling dates. The presence of litter is comparable to a ‘blanket’ above the soil providing a buffer to temperature and moisture changes, as well as providing a range of oxic to anoxic conditions, so although litter has been shown to not emit or uptake CH4 directly, litter on the forest floor can indirectly modify CH4 flux of forest soils. This has been reviewed by Walkiewicz, Rafalska [146]. In summary, the litter layer may influence soil CH4 uptake in opposing ways by: (1) decreasing uptake by acting as a physical barrier to gas diffusion and reduced aeration due to faster litter decomposition in wet conditions, (2) increasing uptake through maintenance of soil gas diffusivity under wetter/high rainfall condition, (3) influencing the capability of the soil for oxidising CH4, (4) providing source of nutrients for methanotrophs, (5) improving formation of macro-aggregates, which facilitates CH4 transport for methanotrophs and (6) the production monoterpenes during needle decomposition that can act to reduce CH4 uptake.
For deadwood, a study incorporating a specific focus on CH4 dynamics in coarse woody debris in an upland temperate North American forest found that fresh woody debris could produce small amounts of CH4, whereas for more decayed debris variable outcomes were observed, ranging from a small amount of CH4 emissions through to substantial CH4 uptake [123]. Gorgolewski [147] also found course woody debris was a CH4 source in early stages of decay and a sink when it was more decayed in a temperate forest in Ontario. CH4 concentrations within deadwood were also found to be greatest in the least decayed wood [148]. In a temperate deciduous forest in the Upper Midwest USA a range of CH4 fluxes from woody debris were also measured (-3.73–22.8 mg CH4 kg downed woody debris− 1 s− 1) [149] and a recent study by Kipping, Gossner [150] also found that deadwood in early stages of decay was a source of CH4 with tree species as an important driver. As deadwood decomposes, it becomes closer in physical and chemical properties to soil, until it eventually becomes organic matter in the soil. Therefore, it seems reasonable that CH4 flux of deadwood would become more similar to that of soil as it decomposes. In contrast, Lagomarsino, De Meo [151] found that CH4 emissions increased as black pine deadwood decayed. Deadwood also influences soil CH4 flux. Perreault, Forrester [149] observed greater CH4 uptake in the soil near highly decayed woody debris, possibly from increased labile carbon in the soil from log decomposition.
The proposed mechanism for CH4 emissions from deadwood is through methanogenic bacteria and archaea that are active in the anaerobic conditions inside decaying wood, or that the tree stems, once cut, serve as a conduit to release CH4 stored from the soil, in the stem [152]. Methane emissions from decomposing wood under aerobic conditions have also been shown by wood-decomposing fungi in association with archaea [153]. The mechanism for CH4 uptake in deadwood is through methanotrophic bacteria inhabiting the material; Mäkipää, Leppänen [154] found the highest number of methanotrophs in Norway spruce logs in late stages of decay.
What determines if deadwood is a CH4 source, or a sink is not well understood. It appears to be a combination of tree species [150], microbial community [154] and level of decay [123]. However, there is still considerable unexplained variability in measured deadwood CH4 flux that needs to be further investigated.
For future research on litter/deadwood fluxes, field-based measurements are critical as they can account for how varying environmental moisture and temperature influence CH4 production, moving away from assumptions related to steady state conditions and reaction kinetics. This will be challenging given variability in ecosystems and difficulty partitioning CH4 from litter and deadwood from other sources, particularly given the extent in overlap in δ13-C-CH4 values among plants and litter decomposers [155]. However, bottom-up modelling of litter and deadwood CH4 emissions based on laboratory incubation studies cannot account for the variability in the field and leads to overestimation of CH4 fluxes. Sources of variability that need to be considered in temperate planted forests include tree species, age and management practices. These factors influence the amount and composition of deadwood/litter entering the system. The more complex the forest the more complex the inputs of litter and deadwood. There is also spatial and temporal variability which will also influence CH4 flux.
Although CH4 uptake/emissions from litter and deadwood are small, given the size of deadwood/litter pool in forests, and the range of types of plant carbon entering the forest system at different times/seasons etc., and the scale of the temperate forests globally, these fluxes should not be overlooked in forest CH4 budgets.
The atmospheric chemistry of methane in relation to the VOC/hydroxyl radicals
Within the atmosphere CH4 is predominantly degraded through oxidisation by OH [156, 157]. This reaction is limited by the abundance of OH, which are depleted through various reactions, including reactions with VOCs. It is therefore possible that an increase in VOC emissions could lead to a reduction in OH and a subsequent increase in the lifetime of CH4. Due to this VOCs have been listed in IPCC reports as species affecting the CH4 sink via OH oxidation [158]. Volatile organic compounds are emitted from a range of temperate tree species. Aydin, Yaman [159] found that both conifers and broad-leaved tree species emitted VOCs, with isoprene the predominant compound for broad-leaved tree species and monoterpenes the predominant compound emitted by conifers. Volatile organic compound emissions from temperate forests could therefore be influencing OH CH4 sinks. However, it is uncertain how large this effect is. In New Zealand, monoterpene emissions from planted pine forests could be influencing OH CH4 sinks but VOC emissions from pine trees have not previously been measured in New Zealand.
There is some evidence to suggest this VOC/CH4 effect could be occurring. VOC reactions have been shown to act as an OH sink in boreal forests, with monoterpenes having the greatest reactivity [160, 161] and declining global OH levels have been shown to be partially responsible for past increases in CH4 levels [156]. However, the magnitude of this effect alongside increasing atmospheric CH4 is uncertain [7].
One aspect that is unclear is how much this effect is happening over temperate forests. Since monoterpenes and isoprene, the most common VOCs emitted by forests, have a lifetime of minutes to hours, and OH has a lifetime around 1 s [162], any effect will be occurring locally. Currently, there is little measurement of CH4 OH oxidation over temperate forests, and studies tend to use global modelling [156, 163, 164].
It is also unclear if the VOC oxidation occurs at a significant magnitude to affect CH4 oxidation. Many aspects effect the abundance of VOCs and OH in the atmosphere. Biogenic VOC emissions from forests can vary with changing environmental conditions [165] and are oxidised by O3 as well as OH [162] so the abundance of this could be important. OH radicals are produced via photolysis of O3 and concentrations are temporally and spatially variable with local concentrations depending on highly variable factors such as temperature, cloud cover and surface albedo [157, 166]. OH is highly reactive and is depleted through multiple atmospheric reactions. Concentrations of carbon monoxide and NOx have been shown to be important for changes in the OH oxidation of CH4 as well as concentrations of VOCs [158].
In NOx enriched environments an increase in OH sinks such as VOCs make less difference to overall OH levels, while in NOx depleted environments an increase in OH sinks is thought to be more important [167]. There is still much uncertainty around this theory, however, due to the complexities of the environment, and measurements do not always match what is expected. While global atmospheric models show a strong OH depletion caused by VOC emissions, Lelieveld, Butler [168] measured high OH concentrations over the pristine Amazon forest. They suggest that VOC oxidation efficiently recycles OH in low NOx environments, and OH depletion over forests is not as strong as previously thought.
Overall OH is an important CH4 sink and OH depletion through VOC oxidation could be significant for increasing CH4 lifetimes. The effect of this should be considered in relation to the high VOC emissions from forestry. However, more information is needed to determine the magnitude of the impact of VOCs on CH4 lifetimes, particularly actual measurements taken in or above planted forests.
Conclusions
Temperate forests and methane fluxes
Features unique to temperate forests that influence CH4 cycling are strong seasonal cycles of temperature and moisture, and the extent of human interaction. These unique elements must be considered when accounting for CH4 flux in temperate forests.
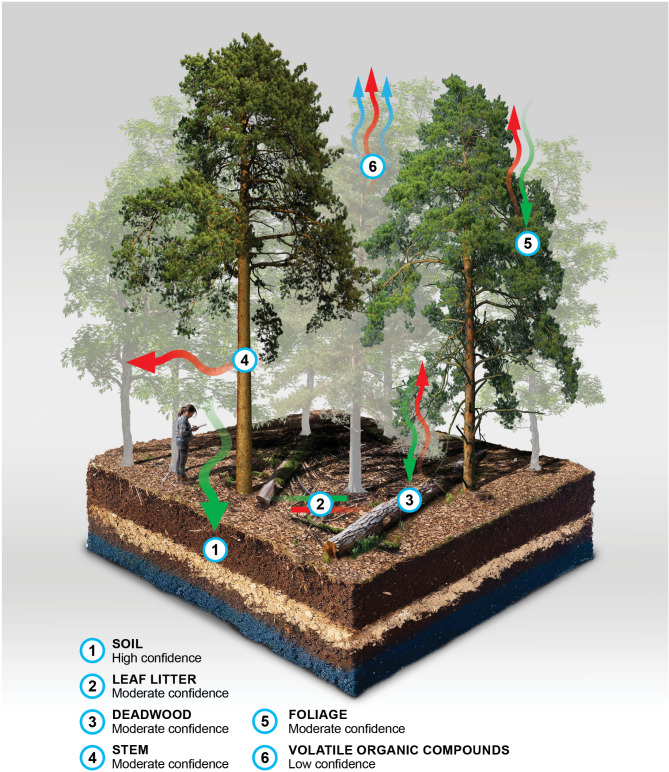
Methane cycling in temperate forests is a complex process with different components acting as either sinks or sources. Figure 3 summarises the current state of knowledge of CH4 cycling in temperate forests based on the findings of this review. The magnitude and direction of CH4 flux for each component is variable and is influenced by many factors. Some components have been studied more than others (e.g. soils, ∼ 46% of references in this review were focused on soils) but overall, more research is required into the processes that drive CH4 cycling, the sources of variability (spatial and temporal) and the factors that affect CH4 cycling for all components of temperate forests. The specific findings for each component and recommendations for further research to accurately account for CH4 flux in temperate forests are summarised in the bullet point list below.
Fig. 3.
Summary of the main sinks and sources of CH4 in temperate forests. Arrow colour and direction indicates potential of each compartment to act as a source (red) or sink (green). Size of arrows indicates the estimated size of the source or sink. The text below the forest compartment labels indicate confidence in this assessment based on the literature.
Soils
Temperate forest soils are significant CH4 sinks (Fig. 3 (Soil)). However, there are many factors that affect the magnitude and direction of CH4 flux in soil. The literature often showed contrasting responses to different factors in different environments. This highlights the importance of local/site specific measurements of CH4 flux rather than trying to extrapolate from global data.
The spatial and temporal variability of soils and climate within a temperate forest leads to changes in CH4 fluxes with hotspots occurring where conditions may favour CH4 emissions. This shows the need for high resolution of sampling within a forest to get a true measure of soil CH4 flux.
Methane flux in temperate forest soil is far more complex biogeochemically than previously thought. For example, CH4 production and consumption has been measured under conditions not previously known to support these processes. There is a need for more research into the mechanisms and microbial community responsible for the changes to CH4 flux.
In planted temperate forests there is an opportunity to influence and encourage CH4 uptake in forest soils through forest management, for example by reducing fertiliser use or selecting tree species based on the site. However, before these recommendations can be made more site-specific research needs to be done.
Tree stems
Tree stems in temperate forests are a small source of CH4 (Fig. 3 (Stem)).
The processes and microbial communities that regulate CH4 flux from trees are the least understood component of the global CH4 cycle.
There is a large amount of variability in stem CH4 flux arising from multiple factors including tree species, age, tissue type, site characteristics and environmental conditions. This variation is not well characterized nor is it included when scaling up per stem emissions to a stand or global level. As a result, CH4 emissions estimates at a global, stand or even tree level, calculated from per stem measurements, are likely overestimates.
Understanding the processes, microbial community and sources of variation involved in stem CH4 flux is needed to improve estimates of stem emissions from temperate forests for CH4 budgeting.
Foliage
The potential for tree foliage to play a role in CH4 fluxes in temperate forests is uncertain. Studies have shown conflicting evidence of CH4 fluxes from the foliage of trees in temperate forests (Fig. 3 (Foliage)). Further research is needed to confirm fluxes and mechanisms.
Litter/deadwood
Emissions from litter are negligible (Fig. 3 (Leaf litter)). However, the litter layer can influence soil CH4 uptake. Emissions from deadwood are small but variable depending on factors such as tree species, level of decay and microbial community present (Fig. 3 (Deadwood)).
Most studies on litter/deadwood CH4 flux are laboratory based. These studies do not take into account variability in the field and leads to overestimation of CH4 fluxes. Forest based studies are needed for more accurate litter/deadwood CH4 flux measurements for CH4 budgeting.
Volatile organic compounds
OH is an important CH4 sink and OH depletion through VOC oxidation could be significant for increasing CH4 lifetimes (Fig. 3 (Volatile organic compounds)). As forests emit high levels of VOCs it is possible that temperate planted forests are contributing to the depletion of OH and therefore increasing CH4 lifetime in the atmosphere. However, measurements taken in or above planted forests are needed to determine the magnitude in which forests impact CH4 in the atmosphere and if it is significant or not.
Recommendations to improve methane budgets for temperate forests
Further research into mechanisms and microbial community involved in temperate forest CH4 cycling is needed for all sources and sinks in temperate forests.
There is huge variability in CH4 flux from all components of the forest that is not well understood, nor is this variability taken into account in global CH4 budgets and models. This means most estimates of CH4 fluxes are likely inaccurate. High resolution (spatially and temporally) of sampling in forests (not laboratories) is needed to better understand variability.
Methane budgets and modelling of CH4 flux need to incorporate all sources and sinks CH4 in temperate forests (i.e. not just soils). All sources of variability also need to be considered to ensure the most accurate estimates of CH4.
A roadmap for sampling and scaling should be developed similar to the one developed for biological nitrogen fixation [169]. This will ensure that global research efforts into CH4 cycling in temperate planted forests is focused, consistent, robust and fit for purpose in order to accurately quantify the contribution of temperate planted forests to the global CH4 cycle. Being able to accurately quantify CH4 flux in temperate forests will aid in mitigating CH4 emissions from other sources and allow better global greenhouse gas emissions budgeting.
Once there is a better understanding of CH4 cycling in temperate forests the next steps will be to investigate the effects of climate change on these processes and how it will influence global CH4 budgets.
Acknowledgements
The authors would like to acknowledge Nick Lambert and Dale Corbett for creating Figs. 1, 2 and 3.
Abbreviations
- BP
Before present
- CH4
Methane
- C:N
Carbon to nitrogen
- CO2
Carbon dioxide
- Eh
Redox potential
- GHG
Greenhouse gas
- GWP
Global warming potential
- IPCC
Intergovernmental Panel on Climate Change
- N
Nitrogen
- NOx
Nitrogen oxides
- O3
Ozone
- OH
Hydroxyl radicals
- P
Phosphorus
- VOCs
Volatile organic compounds
Author contributions
K.W and S.A.W wrote the majority of the manuscript together. S.J.S and C.A contributed to the first draft of the manuscript. N.M.R contributed to the writing of the manuscript. All authors contributed to the literature search. All authors read, edited, and approved the final manuscript.
Funding
This work was funded by the New Zealand Ministry of Primary Industries through the Greenhouse gas inventory research fund.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics and Consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Myhre G, Shindell D, Bréon F, Collins W, Fuglestvedt J, Huang J, et al. Anthropogenic and natural anthropogenic and natural radiative forcing. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, et al. editors. Climate change 2013: the physical science basis contribution of working group to the fifth assessment report of the intergovernmental panel on climate change. Volume 8. Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press; 2013. [Google Scholar]
- 2.Forster P, Ramaswamy V, Artaxo P, Berntsen T, Betts R, Fahey DW, et al. Changes in atmospheric constituents and in radiative forcing. In: Solomon S, Qin Q, Manning M, Chen Z, Marquis M, Averyt KB, et al. editors. Climate Change 2007: the Physical Science Basis Contribution of Working Group I to the 4th Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press; 2007. [Google Scholar]
- 3.IPCC. Climate change 2022: impacts, adaptation and vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK and New York, NY, USA: Cambridge University Press; 2022. [Google Scholar]
- 4.Saunois M, Stavert AR, Poulter B, Bousquet P, Canadell JG, Jackson RB, et al. The global methane budget 2000–2017. Earth Syst Sci data. 2020;12(3):1561–623. [Google Scholar]
- 5.West JJ, Fiore AM. Management of tropospheric ozone by reducing methane emissions. Environ Sci Technol. 2005;39(13):4685–91. [DOI] [PubMed] [Google Scholar]
- 6.Pachauri RK, Allen MR, Barros VR, Broome J, Cramer W, Christ R, et al. Chapter 2: Drivers of Climate Change. Section 2.2.3: Greenhouse Gas Forcing and Its Role in Climate Change. In: Pachauri RK, Meyer LA, editors. Climate change 2014: synthesis report contribution of Working groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change. Geneva, Switzerland: IPCC; 2014. pp. 76–80. Core Writing Team. [Google Scholar]
- 7.Nisbet EG, Manning M, Dlugokencky E, Fisher R, Lowry D, Michel S, et al. Very strong atmospheric methane growth in the 4 years 2014–2017: implications for the Paris Agreement. Glob Biogeochem Cycles. 2019;33(3):318–42. [Google Scholar]
- 8.Forster P, Storelvmo T, Armour K, Collins W, Dufresne J-L, Frame D, et al. The Earth’s energy budget, climate feedbacks, and climate sensitivity. In: Masson-Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S, et al. editors. Climate Change 2021: the Physical Science Basis Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press; 2021. [Google Scholar]
- 9.Shindell D, Kuylenstierna JC, Vignati E, van Dingenen R, Amann M, Klimont Z, et al. Simultaneously mitigating near-term climate change and improving human health and food security. Science. 2012;335(6065):183–9. [DOI] [PubMed] [Google Scholar]
- 10.Ganesan AL, Schwietzke S, Poulter B, Arnold T, Lan X, Rigby M, et al. Advancing scientific understanding of the global methane budget in support of the Paris Agreement. Glob Biogeochem Cycles. 2019;33(12):1475–512. [Google Scholar]
- 11.Knief C. Diversity of methane-cycling microorganisms in soils and their relation to oxygen. Curr Issues Mol Biol. 2019;33(1):23–56. [DOI] [PubMed] [Google Scholar]
- 12.Conrad R. The global methane cycle: recent advances in understanding the microbial processes involved. Environ Microbiol Rep. 2009;1(5):285–92. [DOI] [PubMed] [Google Scholar]
- 13.Lenhart K, Bunge M, Ratering S, Neu TR, Schüttmann I, Greule M, et al. Evidence for methane production by saprotrophic fungi. Nat Commun. 2012;3(1):1046. [DOI] [PubMed] [Google Scholar]
- 14.Bižić M, Klintzsch T, Ionescu D, Hindiyeh M, Günthel M, Muro-Pastor AM, et al. Aquatic and terrestrial cyanobacteria produce methane. Sci Adv. 2020;6(3):eaax5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L-Y, Xie G-J, Ding J, Liu B-F, Xing D-F, Ren N-Q, et al. Microbial methane emissions from the non-methanogenesis processes: a critical review. Sci Total Environ. 2022;806:151362. [DOI] [PubMed] [Google Scholar]
- 16.Putkinen A, Siljanen HM, Laihonen A, Paasisalo I, Porkka K, Tiirola M, et al. New insight to the role of microbes in the methane exchange in trees: evidence from metagenomic sequencing. New Phytol. 2021;231(2):524–36. [DOI] [PubMed] [Google Scholar]
- 17.Ehhalt D. The atmospheric cycle of methane. Tellus. 1974;26(1–2):58–70. [Google Scholar]
- 18.Le Mer J, Roger P. Production, oxidation, emission and consumption of methane by soils: a review. Eur J Soil Biol. 2001;37(1):25–50. [Google Scholar]
- 19.Dutaur L, Verchot LV. A global inventory of the soil CH4 sink. Glob Biogeochem Cycles. 2007;21(4).
- 20.Hanson RS, Hanson TE. Methanotrophic bacteria. Microbiol Rev. 1996;60(2):439–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith GJ, Wrighton KC. Metagenomic approaches unearth methanotroph phylogenetic and metabolic diversity. Curr Issues Mol Biol. 2019;33(1):57–84. [DOI] [PubMed] [Google Scholar]
- 22.Saggar S, Tate K, Giltrap D, Singh J. Soil-atmosphere exchange of nitrous oxide and methane in New Zealand terrestrial ecosystems and their mitigation options: a review. Plant Soil. 2008;309:25–42. [Google Scholar]
- 23.Tate KR, Ross D, Saggar S, Hedley C, Dando J, Singh BK, et al. Methane uptake in soils from Pinus radiata plantations, a reverting shrubland and adjacent pastures: effects of land-use change, and soil texture, water and mineral nitrogen. Soil Biol Biochem. 2007;39(7):1437–49. [Google Scholar]
- 24.Singh BK, Tate KR, Kolipaka G, Hedley CB, Macdonald CA, Millard P, et al. Effect of afforestation and reforestation of pastures on the activity and population dynamics of methanotrophic bacteria. Appl Environ Microbiol. 2007;73(16):5153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng H, Guo J, Peng C, Ma X, Kneeshaw D, Chen H, et al. Global estimates of forest soil methane flux identify a temperate and tropical forest methane sink. Geoderma. 2023;429:116239. [Google Scholar]
- 26.Feng H, Guo J, Han M, Wang W, Peng C, Jin J, et al. A review of the mechanisms and controlling factors of methane dynamics in forest ecosystems. For Ecol Manag. 2020;455:117702. [Google Scholar]
- 27.FOA, Facts. and Fig. 2015/2016: New Zealand plantation forest industry. Wellington: FOA; 2016. [Google Scholar]
- 28.Bölöni J, Aszalós R, Frank T, Odor P. Forest type matters: global review about the structure of oak dominated old-growth temperate forests. For Ecol Manag. 2021;500:119629. [Google Scholar]
- 29.FAO. Global Forest Resources Assessment 2020: Main report. Rome. 2020.
- 30.Gilliam FS. Forest ecosystems of temperate climatic regions: from ancient use to climate change. New Phytol. 2016;212(4):871–87. [DOI] [PubMed] [Google Scholar]
- 31.de Gouvenain R, Silander J. Temperate Forests. Reference Module in Life Sciences. 2017.
- 32.Jandl R, Lindner M, Vesterdal L, Bauwens B, Baritz R, Hagedorn F, et al. How strongly can forest management influence soil carbon sequestration? Geoderma. 2007;137(3–4):253–68. [Google Scholar]
- 33.Mukul SA, Halim MA, Herbohn J. Forest carbon stock and fluxes: Distribution, biogeochemical cycles, and measurement techniques. Life on land, encyclopedia of the UN sustainable development goals. 2021:365 – 80.
- 34.Currie WS, Bergen KM. Temperate Forest. In: Jørgensen SE, Fath BD, editors. Encyclopedia of Ecology. Volume 5. Academic; 2008. pp. 3494–503.
- 35.Stevens CJ, David TI, Storkey J. Atmospheric nitrogen deposition in terrestrial ecosystems: its impact on plant communities and consequences across trophic levels. Funct Ecol. 2018;32(7):1757–69. [Google Scholar]
- 36.Ellis EC, Ramankutty N. Putting people in the map: anthropogenic biomes of the world. Front Ecol Environ. 2008;6(8):439–47. [Google Scholar]
- 37.Lewis SL, Maslin MA. Defining the anthropocene. Nature. 2015;519(7542):171–80. [DOI] [PubMed] [Google Scholar]
- 38.Holt RD. Bringing the Hutchinsonian niche into the 21st century: ecological and evolutionary perspectives. Proc Natl Acad Sci. 2009;106(supplement_2):19659–65. [DOI] [PMC free article] [PubMed]
- 39.Hill AP, Nolan CJ, Hemes KS, Cambron TW, Field CB. Low-elevation conifers in California’s Sierra Nevada are out of equilibrium with climate. PNAS Nexus. 2023;2(2):pgad004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Girisha GK. Effects of litter and woody debris quality on decomposition and nutrient release in exotic forests in New Zealand. Lincoln University; 2001.
- 41.Will GM. Nutrient return in litter and rainfall under some exotic conifer stands in New Zealand. New Z J Agricultural Res. 1959;2(4):719–34. [Google Scholar]
- 42.Smith-Hall C, Chamberlain J. Environmental products: a definition, a typology, and a goodbye to non-timber forest products. Int Forestry Rev. 2023;25(4):491–502. [Google Scholar]
- 43.Covey KR, Megonigal JP. Methane production and emissions in trees and forests. New Phytol. 2019;222(1):35–51. [DOI] [PubMed] [Google Scholar]
- 44.Maurer D, Kolb S, Haumaier L, Borken W. Inhibition of atmospheric methane oxidation by monoterpenes in Norway spruce and European beech soils. Soil Biol Biochem. 2008;40(12):3014–20. [Google Scholar]
- 45.Fehsenfeld F, Calvert J, Fall R, Goldan P, Guenther AB, Hewitt CN, et al. Emissions of volatile organic compounds from vegetation and the implications for atmospheric chemistry. Glob Biogeochem Cycles. 1992;6(4):389–430. [Google Scholar]
- 46.Dalal RC, Allen DE. Greenhouse gas fluxes from natural ecosystems. Aust J Bot. 2008;56(5):369–407. [Google Scholar]
- 47.Dalal R, Allen D, Livesley S, Richards G. Magnitude and biophysical regulators of methane emission and consumption in the Australian agricultural, forest, and submerged landscapes: a review. Plant Soil. 2008;309:43–76. [Google Scholar]
- 48.Fest BJ, Livesley SJ, Drösler M, van Gorsel E, Arndt SK. Soil–atmosphere greenhouse gas exchange in a cool, temperate Eucalyptus delegatensis forest in south-eastern Australia. Agric for Meteorol. 2009;149(3–4):393–406. [Google Scholar]
- 49.Rowlings D, Grace P, Kiese R, Weier K. Environmental factors controlling temporal and spatial variability in the soil-atmosphere exchange of CO2, CH4 and N2O from an Australian subtropical rainforest. Glob Change Biol. 2012;18(2):726–38. [Google Scholar]
- 50.Maier M, Weber TK, Fiedler J, Fuss R, Glatzel S, Huth V, et al. Introduction of a guideline for measurements of greenhouse gas fluxes from soils using non-steady‐state chambers. J Plant Nutr Soil Sci. 2022;185(4):447–61. [Google Scholar]
- 51.Wang J, Murphy J, Geddes J, Winsborough C, Basiliko N, Thomas S. Methane fluxes measured by eddy covariance and static chamber techniques at a temperate forest in central Ontario, Canada. Biogeosciences. 2013;10(6):4371–82. [Google Scholar]
- 52.Clement R, Verma S, Verry E. Relating chamber measurements to eddy correlation measurements of methane flux. J Geophys Research: Atmos. 1995;100(D10):21047–56. [Google Scholar]
- 53.Denmead O. Approaches to measure fluxes of trace gases between landscapes and atmosphere. Plant Soil Special Issue S26–Non-CO2 flux research. 2007.
- 54.Hartmann AA, Buchmann N, Niklaus PA. A study of soil methane sink regulation in two grasslands exposed to drought and N fertilization. Plant Soil. 2011;342:265–75. [Google Scholar]
- 55.Kruse C, Moldrup P, Iversen N. Modeling diffusion and reaction in soils: II. Atmospheric methane diffusion and consumption in a forest soil. Soil Sci. 1996;161(6):355–65. [Google Scholar]
- 56.Kolb S. The quest for atmospheric methane oxidizers in forest soils. Environ Microbiol Rep. 2009;1(5):336–46. [DOI] [PubMed] [Google Scholar]
- 57.Price SJ, Sherlock RR, Kelliher FM, McSeveny TM, Tate KR, Condron LM. Pristine New Zealand forest soil is a strong methane sink. Glob Change Biol. 2004;10(1):16–26. [Google Scholar]
- 58.Adamsen A, King G. Methane consumption in temperate and subarctic forest soils: rates, vertical zonation, and responses to water and nitrogen. Appl Environ Microbiol. 1993;59(2):485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Price SJ, Kelliher FM, Sherlock RR, Tate KR, Condron LM. Environmental and chemical factors regulating methane oxidation in a New Zealand forest soil. Soil Res. 2004;42(7):767–76. [Google Scholar]
- 60.Bender M, Conrad R. Effect of CH4 concentrations and soil conditions on the induction of CH4 oxidation activity. Soil Biol Biochem. 1995;27(12):1517–27. [Google Scholar]
- 61.Fest B, Hinko-Najera N, von Fischer JC, Livesley SJ, Arndt SK. Soil methane uptake increases under continuous throughfall reduction in a temperate evergreen, broadleaved eucalypt forest. Ecosystems. 2017;20:368–79. [Google Scholar]
- 62.Tate K, Saggar S, Hedley C, Dando J, Price S, Rys G. Does afforestation of pastures with pine trees reduce net emissions of methane in New Zealand. Non-CO2 Greenh Gases 2005:601–8.
- 63.Zhou X, Dong H, Chen C, Smaill SJ, Clinton PW. Ethylene rather than dissolved organic carbon controls methane uptake in upland soils. Glob Change Biol. 2014;20(8):2379–80. [DOI] [PubMed] [Google Scholar]
- 64.Gundersen P, Christiansen J, Alberti G, Brüggemann N, Castaldi S, Gasche R, et al. The response of methane and nitrous oxide fluxes to forest change in Europe. Biogeosciences. 2012;9(10):3999–4012. [Google Scholar]
- 65.Liu L, Estiarte M, Peñuelas J. Soil moisture as the key factor of atmospheric CH4 uptake in forest soils under environmental change. Geoderma. 2019;355:113920. [Google Scholar]
- 66.Shen Y, Feng J, Zhou D, He K, Zhu B. Impacts of aboveground litter and belowground roots on soil greenhouse gas emissions: evidence from a DIRT experiment in a pine plantation. Agric for Meteorol. 2023;343:109792. [Google Scholar]
- 67.Ni X, Groffman PM. Declines in methane uptake in forest soils. Proceedings of the National Academy of Sciences. 2018;115(34):8587-90. [DOI] [PMC free article] [PubMed]
- 68.Dörr H, Katruff L, Levin I. Soil texture parameterization of the methane uptake in aerated soils. Chemosphere. 1993;26(1–4):697–713. [Google Scholar]
- 69.Smith K, Dobbie K, Ball B, Bakken L, Sitaula B, Hansen S, et al. Oxidation of atmospheric methane in northern European soils, comparison with other ecosystems, and uncertainties in the global terrestrial sink. Glob Change Biol. 2000;6(7):791–803. [Google Scholar]
- 70.Hu R, Hirano T, Sakaguchi K, Yamashita S, Cui R, Sun L, et al. Spatiotemporal variation in soil methane uptake in a cool-temperate immature deciduous forest. Soil Biol Biochem. 2023;184:109094. [Google Scholar]
- 71.Oni OE, Friedrich MW. Metal oxide reduction linked to anaerobic methane oxidation. Trends Microbiol. 2017;25(2):88–90. [DOI] [PubMed] [Google Scholar]
- 72.Lacroix EM, Aeppli M, Boye K, Brodie E, Fendorf S, Keiluweit M, et al. Consider the anoxic microsite: acknowledging and appreciating Spatiotemporal Redox Heterogeneity in soils and sediments. ACS Earth Space Chem. 2023;7(9):1592–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang J, Zhao Y, Zhou M, Hu J, Hu B. Aerobic and denitrifying methanotrophs: dual wheels driving soil methane emission reduction. Sci Total Environ. 2023;867:161437. [DOI] [PubMed] [Google Scholar]
- 74.Angle JC, Morin TH, Solden LM, Narrowe AB, Smith GJ, Borton MA, et al. Methanogenesis in oxygenated soils is a substantial fraction of wetland methane emissions. Nat Commun. 2017;8(1):1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McClain ME, Boyer EW, Dent CL, Gergel SE, Grimm NB, Groffman PM et al. Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems. 2003:301 – 12.
- 76.Topp E, Pattey E. Soils as sources and sinks for atmospheric methane. Can J Soil Sci. 1997;77(2):167–77. [Google Scholar]
- 77.Mohanty SR, Bodelier PL, Conrad R. Effect of temperature on composition of the methanotrophic community in rice field and forest soil. FEMS Microbiol Ecol. 2007;62(1):24–31. [DOI] [PubMed] [Google Scholar]
- 78.Castro MS, Steudler PA, Melillo JM, Aber JD, Bowden RD. Factors controlling atmospheric methane consumption by temperate forest soils. Glob Biogeochem Cycles. 1995;9(1):1–10. [Google Scholar]
- 79.Borken W, Xu YJ, Beese F. Conversion of hardwood forests to spruce and pine plantations strongly reduced soil methane sink in Germany. Glob Change Biol. 2003;9(6):956–66. [Google Scholar]
- 80.Gatica G, Fernández ME, Juliarena MP, Gyenge J. Environmental and anthropogenic drivers of soil methane fluxes in forests: global patterns and among-biomes differences. Glob Change Biol. 2020;26(11):6604–15. [DOI] [PubMed] [Google Scholar]
- 81.Von Fischer JC, Hedin LO. Controls on soil methane fluxes: tests of biophysical mechanisms using stable isotope tracers. Glob Biogeochem Cycles. 2007;21(2).
- 82.Lee J, Oh Y, Lee ST, Seo YO, Yun J, Yang Y, et al. Soil organic carbon is a key determinant of CH4 sink in global forest soils. Nat Commun. 2023;14(1):3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weslien P, Kasimir Klemedtsson Å, Börjesson G, Klemedtsson L. Strong pH influence on N2O and CH4 fluxes from forested organic soils. Eur J Soil Sci. 2009;60(3):311–20. [Google Scholar]
- 84.Oertel C, Matschullat J, Zurba K, Zimmermann F, Erasmi S. Greenhouse gas emissions from soils—A review. Geochemistry. 2016;76(3):327–52. [Google Scholar]
- 85.Cai Y-J, Liu Z-A, Zhang S, Liu H, Nicol GW, Chen Z. Microbial community structure is stratified at the millimeter-scale across the soil–water interface. ISME Commun. 2022;2(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krause SM, Johnson T, Samadhi Karunaratne Y, Fu Y, Beck DA, Chistoserdova L et al. Lanthanide-dependent cross-feeding of methane-derived carbon is linked by microbial community interactions. Proceedings of the National Academy of Sciences. 2017;114(2):358 – 63. [DOI] [PMC free article] [PubMed]
- 87.Yu Z, Chistoserdova L. Communal metabolism of methane and the rare earth element switch. J Bacteriol. 2017;199(22). 10.1128/jb.00328-17. [DOI] [PMC free article] [PubMed]
- 88.Steudler P, Bowden R, Melillo J, Aber J. Influence of nitrogen fertilization on methane uptake in temperate forest soils. Nature. 1989;341(6240):314–6. [Google Scholar]
- 89.Bodelier PL, Laanbroek HJ. Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol Ecol. 2004;47(3):265–77. [DOI] [PubMed] [Google Scholar]
- 90.Wang Z-P, Ineson P. Methane oxidation in a temperate coniferous forest soil: effects of inorganic N. Soil Biol Biochem. 2003;35(3):427–33. [Google Scholar]
- 91.Castro MS, Peterjohn WT, Melillo JM, Steudler PA, Gholz HL, Lewis D. Effects of nitrogen fertilization on the fluxes of N2O, CH4, and CO2 from soils in a Florida slash pine plantation. Can J for Res. 1994;24(1):9–13. [Google Scholar]
- 92.Wu J, Cheng X, Xing W, Liu G. Soil-atmosphere exchange of CH4 in response to nitrogen addition in diverse upland and wetland ecosystems: a meta-analysis. Soil Biol Biochem. 2022;164:108467. [Google Scholar]
- 93.Gulledge J, Hrywna Y, Cavanaugh C, Steudler PA. Effects of long-term nitrogen fertilization on the uptake kinetics of atmospheric methane in temperate forest soils. FEMS Microbiol Ecol. 2004;49(3):389–400. [DOI] [PubMed] [Google Scholar]
- 94.Xia N, Du E, Wu X, Tang Y, Wang Y, de Vries W. Effects of nitrogen addition on soil methane uptake in global forest biomes. Environ Pollut. 2020;264:114751. [DOI] [PubMed] [Google Scholar]
- 95.Steinkamp R, Butterbach-Bahl K, Papen H. Methane oxidation by soils of an N limited and N fertilized spruce forest in the Black Forest. Ger Soil Biology Biochem. 2001;33(2):145–53. [Google Scholar]
- 96.Börjesson G, Nohrstedt H-Ö. Fast recovery of atmospheric methane consumption in a Swedish forest soil after single-shot N-fertilization. For Ecol Manag. 2000;134(1–3):83–8. [Google Scholar]
- 97.Rigler E, Zechmeister-Boltenstern S. Oxidation of ethylene and methane in forest soils—effect of CO2 and mineral nitrogen. Geoderma. 1999;90(1–2):147–59. [Google Scholar]
- 98.Papen H, Daum M, Steinkamp R, Butterbach-Bahl K. N2O and CH4-fluxes from soils of a N-limited and N-fertilized spruce forest ecosystem of the temperate zone. 2001.
- 99.Hütsch BW, Webster CP, Powlson DS. Long-term effects of nitrogen fertilization on methane oxidation in soil of the Broadbalk wheat experiment. Soil Biol Biochem. 1993;25(10):1307–15. [Google Scholar]
- 100.Xu C, Xu X, Ju C, Chen HY, Wilsey BJ, Luo Y, et al. Long-term, amplified responses of soil organic carbon to nitrogen addition worldwide. Glob Change Biol. 2021;27(6):1170–80. [DOI] [PubMed] [Google Scholar]
- 101.Mohanty SR, Bodelier PL, Floris V, Conrad R. Differential effects of nitrogenous fertilizers on methane-consuming microbes in rice field and forest soils. Appl Environ Microbiol. 2006;72(2):1346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maxfield P, Hornibrook E, Evershed R. Acute impact of agriculture on high-affinity methanotrophic bacterial populations. Environ Microbiol. 2008;10(7):1917–24. [DOI] [PubMed] [Google Scholar]
- 103.Kuypers MM, Marchant HK, Kartal B. The microbial nitrogen-cycling network. Nat Rev Microbiol. 2018;16(5):263–76. [DOI] [PubMed] [Google Scholar]
- 104.Winsborough CL, Thomas SC, Basiliko N. Soil responses to non-nitrogenous amendments in a nitrogen-saturated temperate forest: an unexpected decrease in methane oxidation after phosphorus and lime addition. Can J Soil Sci. 2017;97(4):796–800. [Google Scholar]
- 105.Burke DJ, Smemo KA, López-Gutiérrez JC, DeForest JL. Soil fungi influence the distribution of microbial functional groups that mediate forest greenhouse gas emissions. Soil Biol Biochem. 2012;53:112–9. [Google Scholar]
- 106.Veraart AJ, Steenbergh AK, Ho A, Kim SY, Bodelier PL. Beyond nitrogen: the importance of phosphorus for CH4 oxidation in soils and sediments. Geoderma. 2015;259:337–46. [Google Scholar]
- 107.Smethurst PJ. Forest fertilization: trends in knowledge and practice compared to agriculture. Plant Soil. 2010;335(1–2):83–100. [Google Scholar]
- 108.Scion. Fertiliser Use. New Zealand planted forests environmental facts. 2019.
- 109.Gatica G, Fernández ME, Juliarena MP, Gyenge J. Does forest management affect the magnitude and direction of the afforestation effect on soil methane fluxes? A meta-analysis. For Ecol Manag. 2022;507:120009. [Google Scholar]
- 110.Nazaries L, Tate KR, Ross DJ, Singh J, Dando J, Saggar S, et al. Response of methanotrophic communities to afforestation and reforestation in New Zealand. ISME J. 2011;5(11):1832–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hiltbrunner D, Zimmermann S, Karbin S, Hagedorn F, Niklaus PA. Increasing soil methane sink along a 120-year afforestation chronosequence is driven by soil moisture. Glob Change Biol. 2012;18(12):3664–71. [Google Scholar]
- 112.De Bernardi M, Priano ME, Fusé VS, Fernández ME, Gyenge J, Guzmán SA, et al. High methane uptake from soils of low and high density radiata pine afforestations compared to herbaceous systems. J Sustainable Forestry. 2021;40(1):99–109. [Google Scholar]
- 113.Sullivan B, Kolb T, Hart S, Kaye J, Dore S, Montes-Helu M. Thinning reduces soil carbon dioxide but not methane flux from southwestern USA ponderosa pine forests. For Ecol Manag. 2008;255(12):4047–55. [Google Scholar]
- 114.Reay D, Nedwell D, McNamara N, Ineson P. Effect of tree species on methane and ammonium oxidation capacity in forest soils. Soil Biol Biochem. 2005;37(4):719–30. [Google Scholar]
- 115.Borken W, Beese F. Methane and nitrous oxide fluxes of soils in pure and mixed stands of European beech and Norway spruce. Eur J Soil Sci. 2006;57(5):617–25. [Google Scholar]
- 116.Mazza G, Agnelli AE, Lagomarsino A. The effect of tree species composition on soil C and N pools and greenhouse gas fluxes in a Mediterranean reforestation. J Soil Sci Plant Nutr. 2021;21(2):1339–52. [Google Scholar]
- 117.Christiansen J, Gundersen P. Stand age and tree species affect N2O and CH4 exchange from afforested soils. Biogeosciences. 2011;8(9):2535–46. [Google Scholar]
- 118.Cambi M, Certini G, Neri F, Marchi E. The impact of heavy traffic on forest soils: a review. For Ecol Manag. 2015;338:124–38. [Google Scholar]
- 119.Teepe R, Brumme R, Beese F, Ludwig B. Nitrous oxide emission and methane consumption following compaction of forest soils. Soil Sci Soc Am J. 2004;68(2):605–11. [Google Scholar]
- 120.Frey B, Niklaus PA, Kremer J, Lüscher P, Zimmermann S. Heavy-machinery traffic impacts methane emissions as well as methanogen abundance and community structure in oxic forest soils. Appl Environ Microbiol. 2011;77(17):6060–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pitz S, Megonigal JP. Temperate forest methane sink diminished by tree emissions. New Phytol. 2017;214(4):1432–9. [DOI] [PubMed] [Google Scholar]
- 122.Gauci V, Pangala SR, Shenkin A, Barba J, Bastviken D, Figueiredo V, et al. Global atmospheric methane uptake by upland tree woody surfaces. Nature. 2024;631(8022):796–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Warner DL, Villarreal S, McWilliams K, Inamdar S, Vargas R. Carbon dioxide and methane fluxes from tree stems, coarse woody debris, and soils in an upland temperate forest. Ecosystems. 2017;20:1205–16. [Google Scholar]
- 124.Wang ZP, Gu Q, Deng FD, Huang JH, Megonigal JP, Yu Q, et al. Methane emissions from the trunks of living trees on upland soils. New Phytol. 2016;211(2):429–39. [DOI] [PubMed] [Google Scholar]
- 125.Yip DZ, Veach AM, Yang ZK, Cregger MA, Schadt CW. Methanogenic Archaea dominate mature heartwood habitats of Eastern Cottonwood (Populus deltoides). New Phytol. 2019;222(1):115–21. [DOI] [PubMed] [Google Scholar]
- 126.Jeffrey LC, Maher DT, Chiri E, Leung PM, Nauer PA, Arndt SK, et al. Bark-dwelling methanotrophic bacteria decrease methane emissions from trees. Nat Commun. 2021;12(1):2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Epron D, Mochidome T, Tanabe T, Dannoura M, Sakabe A. Variability in stem methane emissions and wood methane production of different tree species in a cold temperate mountain forest. Ecosystems. 2023;26(4):784–99. [Google Scholar]
- 128.Covey KR, Wood SA, Warren RJ, Lee X, Bradford MA. Elevated methane concentrations in trees of an upland forest. Geophys Res Lett. 2012;39(15).
- 129.Pitz SL, Megonigal JP, Chang C-H, Szlavecz K. Methane fluxes from tree stems and soils along a habitat gradient. Biogeochemistry. 2018;137:307–20. [Google Scholar]
- 130.Barba J, Bradford MA, Brewer PE, Bruhn D, Covey K, van Haren J, et al. Methane emissions from tree stems: a new frontier in the global carbon cycle. New Phytol. 2019;222(1):18–28. [DOI] [PubMed] [Google Scholar]
- 131.Barba J, Poyatos R, Vargas R. Automated measurements of greenhouse gases fluxes from tree stems and soils: magnitudes, patterns and drivers. Sci Rep. 2019;9(1):4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang ZP, Han SJ, Li HL, Deng FD, Zheng YH, Liu HF, et al. Methane production explained largely by water content in the heartwood of living trees in upland forests. J Geophys Research: Biogeosciences. 2017;122(10):2479–89. [Google Scholar]
- 133.Plain C, Ndiaye FK, Bonnaud P, Ranger J, Epron D. Impact of vegetation on the methane budget of a temperate forest. New Phytol. 2019;221(3):1447–56. [DOI] [PubMed] [Google Scholar]
- 134.Maier M, Machacova K, Lang F, Svobodova K, Urban O. Combining soil and tree-stem flux measurements and soil gas profiles to understand CH4 pathways in Fagus sylvatica forests. J Plant Nutr Soil Sci. 2018;181(1):31–5. [Google Scholar]
- 135.Sundqvist E, Crill P, Mölder M, Vestin P, Lindroth A. Atmospheric methane removal by boreal plants. Geophys Res Lett. 2012;39(21).
- 136.Kohl L, Koskinen M, Polvinen T, Tenhovirta S, Rissanen K, Patama M, et al. An automated system for trace gas flux measurements from plant foliage and other plant compartments. Atmos Meas Tech. 2021;14(6):4445–60. [Google Scholar]
- 137.Machacova K, Bäck J, Vanhatalo A, Halmeenmäki E, Kolari P, Mammarella I, et al. Pinus sylvestris as a missing source of nitrous oxide and methane in boreal forest. Sci Rep. 2016;6(1):23410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gorgolewski AS, Caspersen JP, Vantellingen J, Thomas SC. Tree foliage is a methane sink in upland temperate forests. Ecosystems. 2023;26(1):174–86. [Google Scholar]
- 139.Keppler F, Hamilton JT, Braß M, Röckmann T. Methane emissions from terrestrial plants under aerobic conditions. Nature. 2006;439(7073):187–91. [DOI] [PubMed] [Google Scholar]
- 140.Bruhn D, Møller IM, Mikkelsen TN, Ambus P. Terrestrial plant methane production and emission. Physiol Plant. 2012;144(3):201–9. [DOI] [PubMed] [Google Scholar]
- 141.Kirschbaum MU, Bruhn D, Etheridge DM, Evans JR, Farquhar GD, Gifford RM, et al. A comment on the quantitative significance of aerobic methane release by plants. Funct Plant Biol. 2006;33(6):521–30. [DOI] [PubMed] [Google Scholar]
- 142.Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, Kurz WA, et al. A large and persistent carbon sink in the world’s forests. Science. 2011;333(6045):988–93. [DOI] [PubMed] [Google Scholar]
- 143.Schipper L, Harfoot C, McFarlane P, Cooper A. Anaerobic decomposition and denitrification during plant decomposition in an organic soil. J Environ Qual. 1994;23(5):923–8. [DOI] [PubMed] [Google Scholar]
- 144.Gritsch C, Egger F, Zehetner F, Zechmeister-Boltenstern S. The effect of temperature and moisture on trace gas emissions from deciduous and coniferous leaf litter. J Geophys Research: Biogeosciences. 2016;121(5):1339–51. [Google Scholar]
- 145.Peichl M, Brodeur JJ, Khomik M, Arain MA. Biometric and eddy-covariance based estimates of carbon fluxes in an age-sequence of temperate pine forests. Agric for Meteorol. 2010;150(7–8):952–65. [Google Scholar]
- 146.Walkiewicz A, Rafalska A, Bulak P, Bieganowski A, Osborne B. How can litter modify the fluxes of CO2 and CH4 from forest soils? A mini-review. Forests. 2021;12(9):1276. [Google Scholar]
- 147.Gorgolewski AS. Methane fluxes from living and dead trees in a temperate forest. Canada: University of Toronto; 2022. [Google Scholar]
- 148.Covey K, de Mesquita CB, Oberle B, Maynard D, Bettigole C, Crowther T, et al. Greenhouse trace gases in deadwood. Biogeochemistry. 2016;130:215–26. [Google Scholar]
- 149.Perreault L, Forrester JA, Mladenoff DJ, Gower ST. Linking deadwood and soil GHG fluxes in a second growth north temperate deciduous forest (Upper Midwest USA). Biogeochemistry. 2021;156(2):177–94. [Google Scholar]
- 150.Kipping L, Gossner MM, Koschorreck M, Muszynski S, Maurer F, Weisser WW, et al. Emission of CO2 and CH4 from 13 deadwood tree species is linked to tree species identity and management intensity in forest and grassland habitats. Glob Biogeochem Cycles. 2022;36(5):e2021GB007143. [Google Scholar]
- 151.Lagomarsino A, De Meo I, Agnelli AE, Paletto A, Mazza G, Bianchetto E, et al. Decomposition of black pine (Pinus nigra JF Arnold) deadwood and its impact on forest soil components. Sci Total Environ. 2021;754:142039. [DOI] [PubMed] [Google Scholar]
- 152.Mukhortova L, Pashenova N, Meteleva M, Krivobokov L, Guggenberger G. Temperature sensitivity of CO2 and CH4 fluxes from coarse woody debris in Northern boreal forests. Forests. 2021;12(5):624. [Google Scholar]
- 153.Mukhin V, Voronin PY. Methanogenic activity of woody debris. Russian J Ecol. 2009;40:149–53. [Google Scholar]
- 154.Mäkipää R, Leppänen SM, Munoz SS, Smolander A, Tiirola M, Tuomivirta T, et al. Methanotrophs are core members of the diazotroph community in decaying Norway spruce logs. Soil Biol Biochem. 2018;120:230–2. [Google Scholar]
- 155.Schroll M, Keppler F, Greule M, Eckhardt C, Zorn H, Lenhart K. The stable carbon isotope signature of methane produced by saprotrophic fungi. Biogeosciences. 2020;17(14):3891–901. [Google Scholar]
- 156.Rigby M, Montzka SA, Prinn RG, White JW, Young D, O’doherty S, et al. Role of atmospheric oxidation in recent methane growth. Proc Natl Acad Sci. 2017;114(21):5373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wuebbles DJ, Hayhoe K. Atmospheric methane and global change. Earth Sci Rev. 2002;57(3–4):177–210. [Google Scholar]
- 158.Prather M, Ehhalt D, Dentener F, Derwent R, Dlugokencky E, Holland E et al. Atmospheric chemistry and greenhouse gases. 2001.
- 159.Aydin YM, Yaman B, Koca H, Dasdemir O, Kara M, Altiok H, et al. Biogenic volatile organic compound (BVOC) emissions from forested areas in Turkey: determination of specific emission rates for thirty-one tree species. Sci Total Environ. 2014;490:239–53. [DOI] [PubMed] [Google Scholar]
- 160.Ramasamy S, Ida A, Jones C, Kato S, Tsurumaru H, Kishimoto I, et al. Total OH reactivity measurement in a BVOC dominated temperate forest during a summer campaign, 2014. Atmos Environ. 2016;131:41–54. [Google Scholar]
- 161.Sinha V, Williams J, Lelieveld J, Ruuskanen T, Kajos M, Patokoski J, et al. OH reactivity measurements within a boreal forest: evidence for unknown reactive emissions. Environ Sci Technol. 2010;44(17):6614–20. [DOI] [PubMed] [Google Scholar]
- 162.Zannoni N, Gros V, Lanza M, Sarda R, Bonsang B, Kalogridis C, et al. OH reactivity and concentrations of biogenic volatile organic compounds in a Mediterranean forest of downy oak trees. Atmos Chem Phys. 2016;16(3):1619–36. [Google Scholar]
- 163.Zhang Y, Jacob DJ, Maasakkers JD, Sulprizio MP, Sheng J-X, Gautam R, et al. Monitoring global tropospheric OH concentrations using satellite observations of atmospheric methane. Atmos Chem Phys. 2018;18(21):15959–73. [Google Scholar]
- 164.Zhao Y, Saunois M, Bousquet P, Lin X, Berchet A, Hegglin MI, et al. Inter-model comparison of global hydroxyl radical (OH) distributions and their impact on atmospheric methane over the 2000–2016 period. Atmos Chem Phys. 2019;19(21):13701–23. [Google Scholar]
- 165.Chen J, Tang J, Yu X. Environmental and physiological controls on diurnal and seasonal patterns of biogenic volatile organic compound emissions from five dominant woody species under field conditions. Environ Pollut. 2020;259:113955. [DOI] [PubMed] [Google Scholar]
- 166.Holmes CD, Prather MJ, Søvde O, Myhre G. Future methane, hydroxyl, and their uncertainties: key climate and emission parameters for future predictions. Atmos Chem Phys. 2013;13(1):285–302. [Google Scholar]
- 167.Lelieveld J, Dentener F, Peters W, Krol M. On the role of hydroxyl radicals in the self-cleansing capacity of the Troposphere. Atmos Chem Phys. 2004;4(9/10):2337–44. [Google Scholar]
- 168.Lelieveld Ja, Butler T, Crowley J, Dillon T, Fischer H, Ganzeveld L, et al. Atmospheric oxidation capacity sustained by a tropical forest. Nature. 2008;452(7188):737–40. [DOI] [PubMed] [Google Scholar]
- 169.Soper FM, Taylor BN, Winbourne JB, Wong MY, Dynarski KA, Reis CR, et al. A roadmap for sampling and scaling biological nitrogen fixation in terrestrial ecosystems. Methods Ecol Evol. 2021;12(6):1122–37. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.