Abstract
Phorbol 12-myristate 13-acetate-induced luminol chemiluminescence in rat Kupffer cells was doubled by the addition of L-arginine and significantly (up to 70%) inhibited by NG-nitro-L-arginine and NG-monomethyl-L-arginine, competitive inhibitors of L-arginine-dependent nitric oxide (NO) formation. The release of superoxide anion (O2-) by NADPH oxidase was neither affected by L-arginine nor by the inhibitors. Only very slight luminol chemiluminescence was detectable in lipopolysaccharide-pretreated Kupffer cells, a condition in which significant amounts of NO were formed but no O2-. In a cell-free system, significant luminol chemiluminescence only occurred when both authentic NO and the O2-/H2O2- generating system xanthine/xanthine oxidase were present. The results indicate that luminol chemiluminescence in phorbol-ester-activated Kupffer cells largely depends on L-arginine metabolism by NO synthase, requiring the concurrent formation of NO and O2-/H2O2.
Full text
PDF
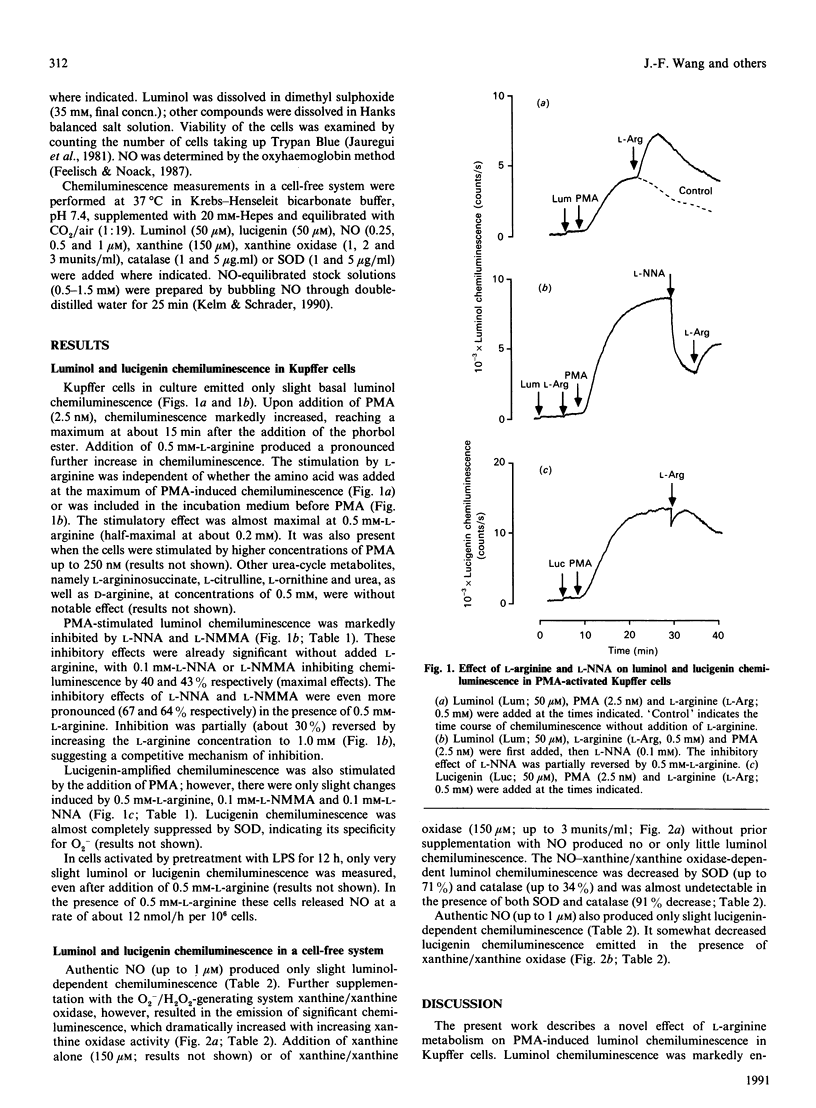
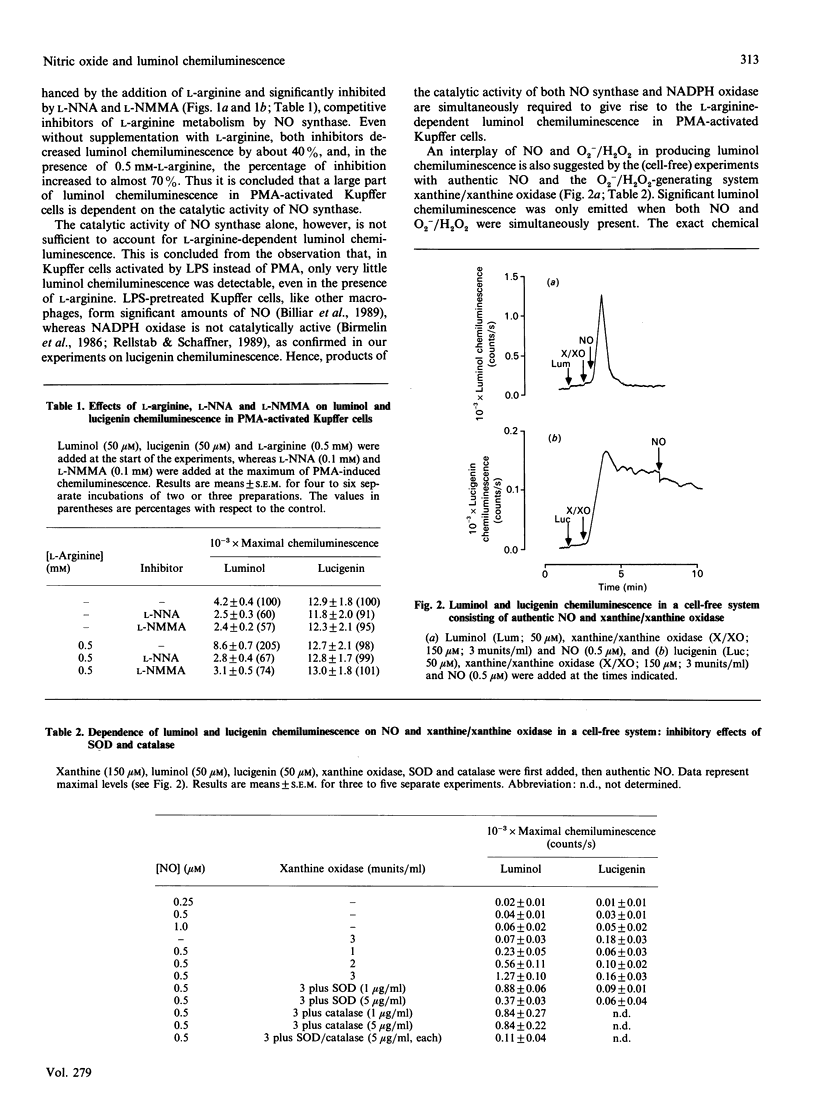

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., West M. A., Hofmann K., Simmons R. L. Kupffer cell cytotoxicity to hepatocytes in coculture requires L-arginine. Arch Surg. 1989 Dec;124(12):1416–1421. doi: 10.1001/archsurg.1989.01410120062013. [DOI] [PubMed] [Google Scholar]
- Brestel E. P. Co-oxidation of luminol by hypochlorite and hydrogen peroxide implications for neutrophil chemiluminescence. Biochem Biophys Res Commun. 1985 Jan 16;126(1):482–488. doi: 10.1016/0006-291x(85)90631-x. [DOI] [PubMed] [Google Scholar]
- Cadenas E., Sies H. Low-level chemiluminescence as an indicator of singlet molecular oxygen in biological systems. Methods Enzymol. 1984;105:221–231. doi: 10.1016/s0076-6879(84)05029-1. [DOI] [PubMed] [Google Scholar]
- Dahlgren C., Aniansson H., Magnusson K. E. Pattern of formylmethionyl-leucyl-phenylalanine-induced luminol- and lucigenin-dependent chemiluminescence in human neutrophils. Infect Immun. 1985 Jan;47(1):326–328. doi: 10.1128/iai.47.1.326-328.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feelisch M., Noack E. A. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur J Pharmacol. 1987 Jul 2;139(1):19–30. doi: 10.1016/0014-2999(87)90493-6. [DOI] [PubMed] [Google Scholar]
- Gyllenhammar H. Lucigenin chemiluminescence in the assessment of neutrophil superoxide production. J Immunol Methods. 1987 Mar 12;97(2):209–213. doi: 10.1016/0022-1759(87)90461-3. [DOI] [PubMed] [Google Scholar]
- Gyllenhammar H. Mechanisms for luminol-augmented chemiluminescence from neutrophils induced by leukotriene B4 and N-formyl-methionyl-leucyl-phenylalanine. Photochem Photobiol. 1989 Feb;49(2):217–223. doi: 10.1111/j.1751-1097.1989.tb04099.x. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Jauregui H. O., Hayner N. T., Driscoll J. L., Williams-Holland R., Lipsky M. H., Galletti P. M. Trypan blue dye uptake and lactate dehydrogenase in adult rat hepatocytes--freshly isolated cells, cell suspensions, and primary monolayer cultures. In Vitro. 1981 Dec;17(12):1100–1110. doi: 10.1007/BF02618612. [DOI] [PubMed] [Google Scholar]
- Jungi T. W., Peterhans E. Change in the chemiluminescence reactivity pattern during in vitro differentiation of human monocytes to macrophages. Blut. 1988 May;56(5):213–220. doi: 10.1007/BF00320108. [DOI] [PubMed] [Google Scholar]
- Kelm M., Schrader J. Control of coronary vascular tone by nitric oxide. Circ Res. 1990 Jun;66(6):1561–1575. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- McCall T. B., Boughton-Smith N. K., Palmer R. M., Whittle B. J., Moncada S. Synthesis of nitric oxide from L-arginine by neutrophils. Release and interaction with superoxide anion. Biochem J. 1989 Jul 1;261(1):293–296. doi: 10.1042/bj2610293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Peddinghaus R. In vitro determination of phagocyte activity by luminol- and lucigenin-amplified chemiluminescence. Int J Immunopharmacol. 1984;6(5):455–466. doi: 10.1016/0192-0561(84)90084-5. [DOI] [PubMed] [Google Scholar]
- Rellstab P., Schaffner A. Endotoxin suppresses the generation of O2- and H2O2 by "resting" and lymphokine-activated human blood-derived macrophages. J Immunol. 1989 Apr 15;142(8):2813–2820. [PubMed] [Google Scholar]
- Rymsa B., Becker H. D., Lauchart W., de Groot H. Hypoxia/reoxygenation injury in liver: Kupffer cells are much more vulnerable to reoxygenation than to hypoxia. Res Commun Chem Pathol Pharmacol. 1990 May;68(2):263–266. [PubMed] [Google Scholar]
- Stuehr D. J., Gross S. S., Sakuma I., Levi R., Nathan C. F. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J Exp Med. 1989 Mar 1;169(3):1011–1020. doi: 10.1084/jem.169.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]


