Abstract
Myelin, produced by oligodendrocytes, insulates axons to facilitate rapid and efficient action potential propagation in the central nervous system. Traditionally viewed as a stable structure, myelin is now understood to undergo dynamic modulation throughout life. This review examines these dynamics, focusing on two key aspects: (1) the turnover of myelin, involving not only the renewal of constituents but the continuous wholesale replacement of myelin membranes, and (2) the structural remodeling of pre-existing, mature myelin, a newly discovered form of neural plasticity that can be stimulated by external factors, including neuronal activity, behavioral experience, and injury. We explore the mechanisms regulating these dynamics and speculate that myelin remodeling could be driven by an asymmetry in myelin turnover or reactivation of pathways involved in myelin formation. Finally, we outline how myelin remodeling could have profound impacts on neural function, serving as an integral component of behavioral adaptation.
Introduction
Myelin consists of tightly compacted, insulating membranes concentrically wrapped around axons in discrete sheaths called internodes. Between internodes are nodes of Ranvier, specialized axonal sites enriched with voltage-sensitive channels (Fig. 1a,c). This arrangement enables saltatory conduction, wherein action potentials regenerate exclusively at nodes to dramatically increase propagation speed and reduce neuronal energy expenditure. Central nervous system (CNS) myelin is produced by oligodendrocytes, generated via terminal differentiation of oligodendrocyte precursor cells throughout life. Upon formation, new oligodendrocytes extend processes and rapidly elaborate and arrange membrane to form internodes around numerous axonal segments (Box 1).
Fig. 1 |. Myelin components and turnover.
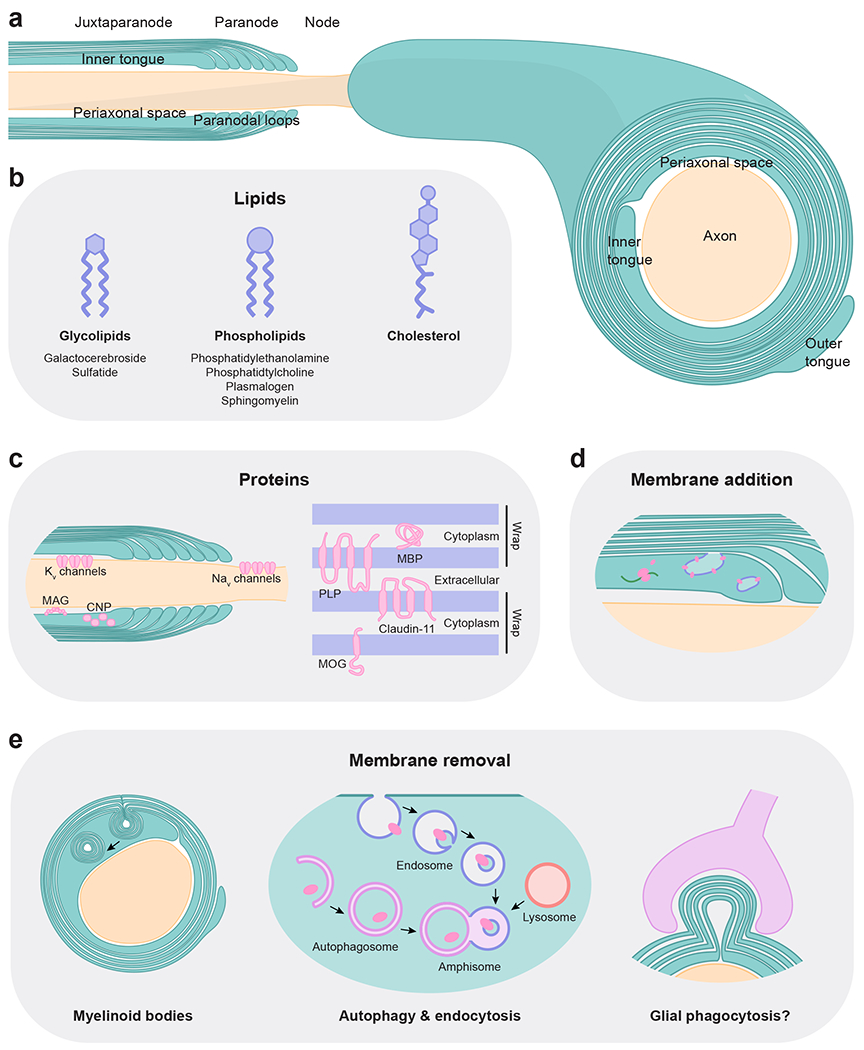
a, Schematic of myelin, a highly organized structure with well-defined compartments. b, Myelin membranes have aunique constitution of lipidsthatare turned over at different rates. c, Myelin proteins localized to compact membranes (right) are turned over more slowly than proteins in non-compact myelin or at the myelin-axon junction (left; e.g. CNP and MAG, respectively). d, Lipids and proteins are added to mature myelin at the inner tongue and paranodal loops from trafficked vesicles and local translation. e, Membranes are removed from mature myelin via myelinoid bodies and concerted autophagy and endocytosis. Microglia and astrocytes, which phagocytose myelin in other contexts, could be involved in turnover of mature myelin. How the residual myelin sheath remains structurally organized and intact after multiple layers of myelin are removed is not fully understood.
Box 1: Dynamics of Myelin Formation.
Myelin internode formation in new oligodendrocytes in zebrafish begins with a highly dynamic period of simultaneous nascent sheath initiation and removal, resulting in the net accumulation of nascent sheaths over several hours95–97. Over the next few days, most nascent sheaths are stabilized while some are removed, after which total sheath number is maintained95–97. During this period, membranes (lipids and proteins) are trafficked from the secretory pathway to the innermost layer of myelin, the inner tongue, where they incorporate into the growing sheath while other myelin proteins can be translated locally12,145. Driven by actin disassembly146,147, the number of myelin wraps increases in proportion to axonal diameter148. Myelin wraps begin to grow laterally, starting with the outermost (earliest) wrap12, which establishes the internode’s position along the axon70. Lateral extension continues from the outside-in until the inner tongue abuts against the spiraling row of lateral edges of the other myelin layers, known as the paranodal loops, which adhere to the axon12 (Fig. 1a). As paranodal adhesion matures, the assembly of the nodal region proceeds, including clustering of voltage-gated ion channels (reviewed in Rasband and Peles, 2021107). From the outside-in, the layers of myelin membrane compact, bringing the inner and outer leaflets of consecutive lipid bilayers in extremely close apposition and extruding cytoplasm and extracellular fluid12. Membrane compaction and paranodal myelin-axon sealing facilitate an electrical insulation of the axon but prevent intracellular trafficking throughout the internode. To facilitate delivery of myelin components during internode formation, some regions remain uncompacted: the inner and outer tongues and paranodal loops form a contiguous cytoplasmic perimeter around the myelin membrane, while cytoplasmic channels penetrate the center12. As myelin formation completes, the inner and outer tongues reduce in size and most cytoplasmic channels disappear12, presumably reducing – but not eliminating149 – trafficking from the soma through the internode. Concomitantly, oligodendrocytes assume a distinct, mature gene expression profile150. However, mature oligodendrocytes and myelin remain dynamic, with ongoing turnover and remodeling that resemble dynamics present during initial myelin formation and likely involve shared cellular and molecular mechanisms.
Following this dynamic period, oligodendrocytes and their internodes stabilize, mostly persisting across the lifespan1–5. Mature myelin was long considered static, with changes to CNS myelin architecture only occurring through new myelin-generating oligodendrocytes or pathological demyelination. However, recent evidence indicates mature myelin is remarkably dynamic, undergoing both continual renewal of its membrane as well as remodeling of its structure.
This review delves into these dynamics. First, we discuss the turnover of myelin, involving not only the exchange of myelin constituents but the continuous wholesale replacement of myelin membranes. Second, we review the structural remodeling of mature oligodendrocytes and internodes and its modulation by external factors. Third, we explore mechanisms regulating myelin turnover and remodeling. We propose that myelin remodeling could be driven by asymmetrical myelin turnover or reactivation of pathways involved in myelin formation. Finally, we consider the function of myelin remodeling, highlighting how this novel form of plasticity may impact neural adaptation and behavior.
Dynamics of Myelin Turnover
To produce its structure and functionality, myelin contains a unique configuration of lipids and proteins, many of which are situated within its compact structure (Fig. 1b,c). As lipid and protein turnover is typically rapid (hours-days)6–8 and is essential for cell function and viability, the turnover of seemingly inaccessible myelin components are of longstanding interest. Furthermore, the possibility that plastic changes in myelin turnover could be utilized to alter myelin structure and neural function highlights the importance of understanding these mechanisms.
Lipids
Myelin’s unique constitution of lipids (Fig. 1b) facilitates intermembrane adhesion (galactocerebroside, sphingomyelin), myelin-axon adhesion (sulfatide), and membrane rigidity (cholesterol) (reviewed in Poitelon et al., 20209). Pulse-chase dating in adult mice shows that distinct myelin lipid subclasses have radically different longevities. The half-lives of phosphatidylcholine and phosphatidylethanolamine are approximately 20 and 25 days, respectively10. By contrast, cholesterol and cerebrosides (including galactocerebroside) are long-lived, with half-lives of approximately 300 and 100 days, respectively10. Sulfatide (sulfated galactocerebroside) is similarly long-lived: inducible genetic removal of its synthesizing enzyme in mouse pre-existing oligodendrocytes reduced sulfatide levels only after three-six months11. Importantly, the half-replacement times for these lipids mirror their half-lives10; thus, there is constant turnover of lipids in mature myelin.
Lipids are presumably exchanged in uncompacted areas of mature myelin, where vesicles are observable12. Differential access to or recycling at these regions may explain the disparity in turnover rates between lipid subclasses. Interestingly, long-lived lipids like cholesterol, galactocerebroside, and sulfatide localize to membrane rafts13, structures with slowed lateral diffusion that sequester lipids14. Likely, these lipids are primarily recycled in concert with entire myelin membranes, a possibility supported by similar turnover rates (see Membranes). A recent study found lipid turnover may be modulated by learning, which induced sequential increases in sphingomyelin and galactocerebroside in subcortical white matter of young adult mice15. Whether these changes were independent of broader synthesis or removal of myelin membranes is unclear, but these findings advance the possibility that lipid turnover and composition may be a variable feature of mature myelin.
What are the consequences of perturbing myelin lipid turnover? Preventing new sulfatide formation in pre-existing oligodendrocytes in ten-week-old mice caused myelin thinning and abnormalities, mislocalization of nodal/paranodal/juxtaparanodal proteins, axonal degeneration, and motor deficits progressing over eleven months11. Broader disruption of myelin lipid synthesis through inducible deletion of quaking (Qk) from mature oligodendrocytes in eight-week-old mice caused myelin thinning within one week, followed by outright demyelination without oligodendrocyte loss, slowed axonal conduction, severe motor deficits, and death by seven weeks16. Partial rescue occurred with a high fat diet or activators of lipid metabolism16. Given the rapid deterioration, this phenotype was likely caused by deficits in production of high turnover lipids, though the responsible subtypes were not determined. These findings indicate that ongoing lipid synthesis is essential for maintaining mature myelin and present the possibility that myelin remodeling could involve modulation of this process.
Proteins
The protein makeup of myelin is unique and complex, facilitating its structure and function (reviewed in Stadelmann et al., 201917) (Fig. 1c). Pulse-chase dating of proteins found in compact myelin (MBP, PLP, Claudin-11) shows they are among the longest lived in the mouse brain, with half-lives of approximately 100 days6,7,18,19. These findings are corroborated by inducible gene deletions in mice, which estimated the half-lives of PLP and MBP through measuring residual protein to be 180 days20 and 77 days21, respectively. Alignment between these metrics is important as lifetime estimates are complicated by new protein synthesis from ongoing oligodendrogenesis, pulse-chased isotype recycling, and possible disruptions to myelin turnover with gene deletions. Myelin proteins localized to non-compact regions, like CNP (half-life 55 days), are still relatively very long-lived7,18, while proteins at myelin-axon junctions, like MAG, turn over moderately slower than typical brain proteins7. Similar to long-lived lipids, long-lived proteins in compact myelin have comparable turnover rates to myelin membrane, suggesting these proteins may turn over with the entire membrane (see Membranes).
Disruption to protein turnover in young adult mice perturbs myelin structure, although over a slower timescale than lipid turnover. Reducing protein synthesis by 25% in mature oligodendrocytes had minimal effect over three weeks22. However, over six months, halving expression of several myelin proteins via inducible Erk1/2 deletion from mature oligodendrocytes resulted in progressively thinner myelin, myelin loss, axonal degeneration, and motor deficits23. Dramatic myelin thinning and loss progressing over months without oligodendrocyte loss also resulted from inducible deletion of Mbp (MBP) in mature oligodendrocytes21. A more subtle decrease in the number of myelinated axons was observed ten months post-deletion of Plp1 (PLP) from mature oligodendrocytes; however, the remaining myelin presented with numerous ultrastructural abnormalities20. Likewise, impairing the removal of myelin proteins has structural impacts. Two recent studies found that myelin proteins are degraded via (macro)autophagy24,25. Blocking autophagy in oligodendrocytes caused myelin protein accumulation24,25. Over months, mice experienced progressive myelin thickening and other ultrastructural disruptions, resulting in motor deficits and precocious death24,25. These studies indicate that myelin protein turnover occurs over months and maintaining balance in this process is essential for the long-term maintenance of intact myelin. Intriguingly, altering the balance of protein turnover may be a means by which myelin structure could be remodeled to adapt myelin function.
Membranes
Myelin lipids and proteins are regularly replaced, but whether this occurs through the turnover of the entire myelin membrane structure or simply through the exchange of its constituents is unresolved. However, recent studies suggest there is indeed turnover of myelin membranes, and this is how at least some myelin constituents are renewed. Removing Mbp from mature oligodendrocytes in mice resulted in large, uncompacted membrane tubules originating in the inner tongue and paranodes21. These tubules were devoid of MBP but expressed PLP and were relatively newer than compact myelin21, consistent with the possibility that new myelin membranes, containing lipids and proteins, are constantly incorporated at the inner tongue and paranodal regions of mature sheaths (Fig. 1d), reminiscent of growth during sheath formation12. Without MBP, essential for wrapping and compaction26, these membranes could not incorporate to replace compact myelin that otherwise progressively thinned over six months until it was eventually lost21. Remarkably, even six months post-tamoxifen, the abundance of MBP in the remaining compact myelin was unchanged21, suggesting recycling of MBP – and likely other long-lived myelin proteins and lipids – occurs in concert with removal of compact membranes.
How, then, are compact membranes removed? First, myelinoid bodies – large, rounded, multi-lamellated structures present in cytoplasmic regions of myelin – are long considered to be a site of myelin recycling (Fig. 1e) (reviewed in Hildebrand et al., 199327). They are thought to bud from compact myelin and contain myelin degradation products27. Consistent with a source-product relationship, these structures incorporated pulsed stable isotope-labeled precursor to the same extent as compact myelin21. Future studies manipulating the formation of myelinoid bodies will provide further insights into their role in myelin membrane removal. Second, removal of lipids and proteins that constitute compact myelin can occur through autophagy and endocytosis (Fig. 1e). In blocking autophagy, concomitant with myelin thickening, both cytosolic (MBP) and integral membrane proteins (PLP, MOG) accumulated and, under normal conditions, these proteins could be found within autophagosomes24,25. Given that autophagy per se digests only cytoplasmic material, the presence of integral membrane proteins suggests the formation of amphisomes, the fusion of endosomes with autophagosomes prior to lysosomal degradation, which was indeed visualized in the cytoplasm of cultured oligodendrocyte myelin24. Internalization and lysosomal digestion of myelin membranes by oligodendrocytes is also observed in zebrafish, where new oligodendrocytes remove some of their nascent sheaths28. Though, lysosomal digestion may not be the only endpoint: oligodendrocyte release of myelin protein-containing exosomes, caused by the fusion of late endosomes or amphisomes with the plasma membrane, has been observed in vitro29. Lastly, membrane removal could be executed by microglia and astrocytes (Fig. 1e). These cells actively phagocytose aberrant myelin from sheaths during development and following myelin structural remodeling during metamorphosis28,30. Additionally, microglia are implicated in phagocytosing entire nascent sheaths in developing zebrafish31, though not all studies agree28. Whether glia remove myelin membranes in the healthy adult CNS is unknown but is interesting grounds for future study.
These data indicate that mature myelin undergoes continual renewal of not only its constituents but of entire membranes. Amazingly, myelin turnover is well-balanced to maintain intact internodes throughout life despite the range in turnover frequency between myelin components, different translation sites for myelin proteins, and the apparent mechanistic separation between myelin synthesis and removal. Intriguingly, asymmetry in myelin turnover may underlie structural changes in aging32 or, on the other hand, may be harnessed to plastically modulate myelin structure to fine tune its function.
Dynamics of Myelin Remodeling
Myelin turnover could proceed without any net gain or loss, resulting in a stable myelin structure throughout life. However, recent work shows several features of mature myelin, including myelin thickness, periaxonal space width, and the length of internodes and nodes, can undergo structural modification in the adult CNS (Fig. 2). Furthermore, entire internodes of mature oligodendrocytes may be generated or lost (Fig. 2). Myelin remodeling is an exciting new facet of neuroplasticity, providing a means by which pre-existing myelin could modify neural function and behavior.
Fig. 2 |. Myelin remodeling.
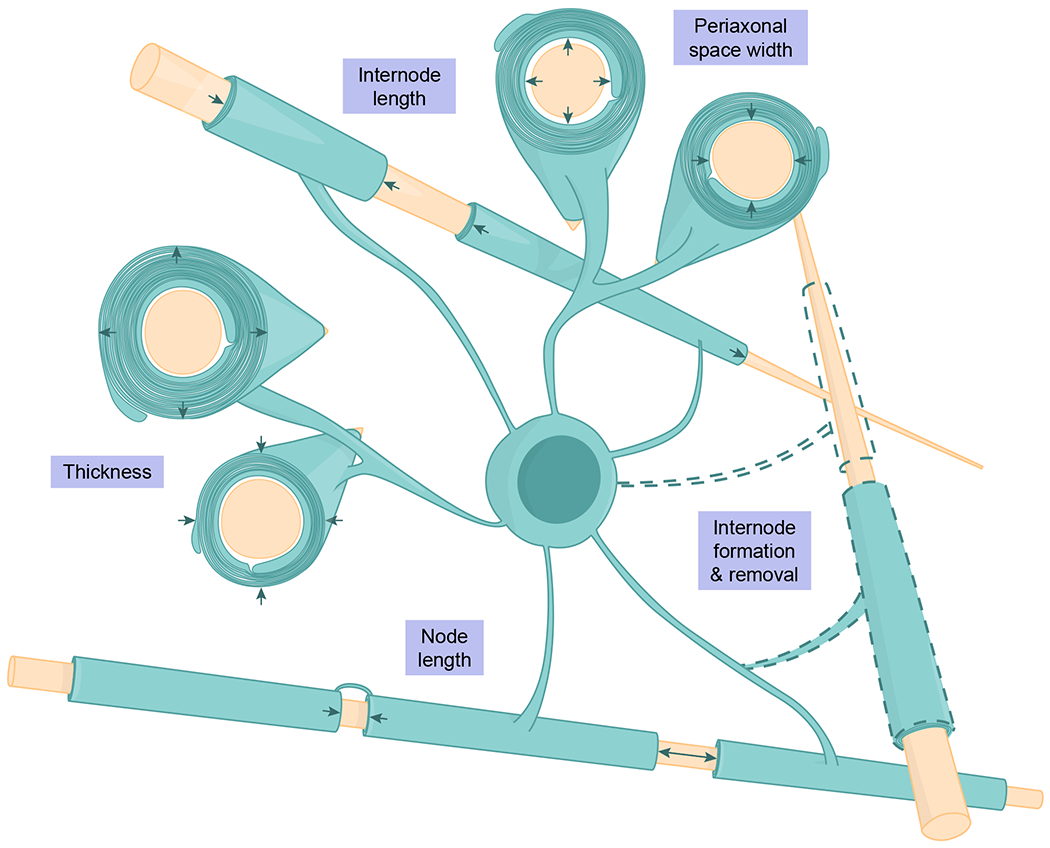
Numerous structural parameters of mature oligodendrocytes and myelin undergo remodeling over time or in response to neuronal activity, behavioral experience, injury, or genetic manipulation. The dynamics of each parameter are shown in isolation for clarity; however, remodeling of one parameter may impact another (e.g., internode and node length) and an individual sheath may undergo multiple structural changes.
Myelin Thickness
Across the lifespan, a prolonged, marginal increase in the average number of myelin wraps enlarges the thickness of sheaths (Box 2) in several mouse and primate CNS regions1,33–38 (Fig. 2). This ongoing myelin thickening typically occurs independent of changes in axonal diameter33–37, but in mouse optic nerve the radial growth of myelin and axons occurs in concert1. This prolonged radial growth of myelin implies that, on average, there exists an asymmetric addition and removal of myelin membranes. However, the temporal and spatial dynamics of this growth are unknown. Do wraps accumulate within a sheath unidirectionally and linearly? Do all mature sheaths undergo the same changes or do different oligodendrocytes or axons adjust their myelin differently?
Box 2: Radial Myelin Measurements.
Electron microscopy is the gold standard for radial measurements of myelin compartments. Myelin thickness is often measured as a g ratio, calculated as axon diameter / (axon + myelin diameter), to account for the positive correlation between myelin thickness and axon diameter148. In contrast to peripheral myelinated axons, this ratio is not constant across axon diameter in the CNS – myelin is proportionally thicker in thinner axons148. Therefore, g ratios of CNS axons are frequently evaluated within binned ranges of axon diameter. Changes in g ratio are typically considered to reflect changes in the number of myelin wraps. However, these measurements usually include the uncompacted inner tongue and the periaxonal space between the inner tongue and the axon. Changes to these compartments or in the level of myelin compaction could also alter myelin thickness measurements and should be properly considered.
Additionally, myelin thickness in young adult rodents can be altered by neuronal activity or behavioral experience. Prolonged optogenetic or chemogenetic activation of cortical projection neurons induced thicker myelin in corpus callosum39,40. Amazingly, after chemogenetic activation, this thicker myelin was found specifically around those axons with elevated activity40. In a genetic model of absence seizures, myelin was thicker in corpus callosum following seizure onset41. Social isolation or chronic social defeat stress reduced myelin thickness in medial prefrontal cortex42–44, while environmental enrichment or partial hearing deprivation increased myelin thickness in corpus callosum and trapezoid body fibers, respectively45,46.
However, it remains unclear whether these changes were mediated by pre-existing, mature sheaths adding additional wraps and/or by newly-generated oligodendrocytes augmenting wrapping during sheath formation. These manipulations were performed in young adults, which have abundant ongoing myelin formation, and analogous manipulations at earlier developmental timepoints show similar – and often greater – impacts on myelin thickness40,41,46–50, suggesting the radial growth of newly-generated sheaths is sensitive to these factors. Furthermore, in several studies, changes to myelin thickness were abated with manipulations that influence new oligodendrocyte formation39,41,42,47. In contrast, enhancing radial growth of only new myelin formed after two months of age was not sufficient to alter overall myelin thickness measurements51. Tracking myelin thickness in bone fide mature sheaths will resolve whether mature myelin thickness is in fact sensitive to neuronal activity or behavioral experience. Similarly, ongoing myelin thickening over the lifespan could be accounted for by adult-born oligodendrocytes forming thicker myelin than those born during development. However, the initial myelin formed by adult-born oligodendrocytes is actually thinner than that formed by developmentally-born oligodendrocytes1 or than surrounding mature myelin52, strongly supporting the concept of ongoing radial growth of mature sheaths.
Which pathways regulate myelin thickness in mature sheaths? Similar to myelin formation, the MAPK-ERK and PI3K-Akt-mTOR pathways are essential for ongoing thickening of mature sheaths. Genetic removal of pathway components from pre-existing oligodendrocytes in young adult mice resulted in thinner myelin six-twelve months later23,53, while pathway hyperactivation in pre-existing oligodendrocytes increased myelin thickness as early as three weeks later12,51,54,55. Upstream of ERK1/2, signaling through oligodendrocyte FGFR2 appears to drive this ongoing myelin thickening36,56. These pathways promote expression of oligodendrocyte transcription factors, myelin proteins, and lipid synthesis enzymes51, and increase the presence of cytoplasmic channels12 and the size of the inner tongue54, indicating a role in myelin growth rather than removal. Recently, signaling between microglia and oligodendrocytes via TGFβ/TGFβR1 was implicated in mediating myelin thickness in early adulthood in mice34. Blocking this pathway resulted in thicker myelin and an enlarged inner tongue34, indicative of elevated myelin growth12, suggesting that microglial TGFβ normally suppresses radial myelin growth.
Is myelin removal also subject to exogenous regulation to affect myelin thickness? A recent study in mice found that reducing oligodendrocyte glucose uptake induced myelin thinning (Fig. 2) and elevated oligodendrocyte autophagosome formation57, suggesting that autophagy-dependent removal of myelin membranes can be modulated. Whether changing rates of autophagy underlie dynamics of myelin thickness under more physiological conditions is an interesting future question.
Periaxonal Space Width
The approximately 12 nm space between the myelin inner tongue and the axon membrane, known as the periaxonal space (Box 2; Fig. 1a), serves as a longitudinal conducting pathway during saltatory conduction58 and may facilitate depolarization-dependent signaling between myelin and the internodal axon58,59. Recently, changes to the width of the periaxonal space in young adult mice have been observed60. Low intensity repetitive transcranial magnetic stimulation induced periaxonal space widening in corpus callosum, while spatial learning induced periaxonal space shrinking in hippocampal fimbria60 (Fig. 2). Changes in width averaged a few nanometers and were predicted to impact conduction velocity by five percent or more60, though individual internodes may undergo larger modulation. Importantly, whether these findings represent remodeling of mature sheaths or alterations in wrapping by newly-generated oligodendrocytes is still unknown.
Relatively little is known about the mechanisms that determine or modulate periaxonal space width. Myelin-associated glycoprotein (MAG), an oligodendrocyte transmembrane protein that spans the periaxonal space, is implicated in ensuring normal periaxonal space width61,62, although not all studies agree63. Structural analyses of MAG led to the intriguing hypothesis that periaxonal space width could be determined by MAG’s conformation and binding partners64. Another possibility is that maintaining ion and fluid homeostasis alters periaxonal space width. Potassium channels, transporters, and connexins redistribute potassium released into the periaxonal space during neuronal firing65–69. Without some of these molecules, neuronal firing causes severe swelling of this compartment66,69, provoking the compelling possibility that subtler changes to periaxonal space width could occur from neuronal firing under physiological conditions. With much more to uncover, we anticipate an exciting future for this emerging topic.
Internode Length
Compared to radial myelin structures, the ability to visualize changes in internode length using fluorescent longitudinal in vivo imaging has accelerated our understanding of mature internode length dynamics over the lifetime and in response to exogenous manipulation (Fig. 2).
Following rapid extension to establish their positions, internodes in the embryonic zebrafish spinal cord continue to increase in length, while maintaining their relative positions, to compensate for ongoing axonal growth70. The inverse phenomenon occurs during xenopus metamorphosis: as optic nerve axons decrease in length, so too do myelin internodes30. After brain size is established, 15-20% of pre-existing cortical internodes in young adult mice exhibited changes in length over about a month3,71–73. Extension (increasing length) or retraction (decreasing length) both occurred, even in internodes made by the same oligodendrocyte3, though their relative proportions differed between regions3,71–73 and myelinated axonal subtype72. Changes in length were typically small (5-10 microns), but some internodes changed more than 20 microns3,71,72. Intriguingly, isolated internodes lacking neighboring internodes were responsible for the overwhelming majority of dynamics3,73. By inducibly fluorescently-labeling oligodendrocytes, these dynamics were confirmed in sheaths at least a month old3.
As mice reach middle age, internode length dynamics precipitously decrease, with only 1% of pre-existing cortical internodes exhibiting changes over 50 days74. The vast majority were retractions averaging approximately 15 microns and, unlike in younger mice, dynamics were not biased to isolated internodes74. By old age, retractions are substantially elevated again: almost 20% of pre-existing cortical internodes underwent retraction over 60 days in two-year-old mice32. Many internodes at this timepoint also exhibited abnormal morphologies and sometimes were entirely removed, perhaps implicating these age-dependent retractions in a broader myelin pathology32. Bias towards retraction with aging may explain why average internode length is shorter in older animals75 and why fully myelinated tracts continue to have naked axonal regions for myelination by new oligodendrocytes1.
Mature internode lengths are sensitive to behavioral experience. Forelimb reach training induced pre-existing internodes in young adult mouse motor cortex to retract approximately 25 microns over a few weeks71,73. The increased retractions were biased to internodes in more continuously myelinated regions and often of sequential internodes along the same axon73, suggesting the involvement of specific neurons in this plasticity. Indeed, the axons experiencing internode retraction were those active during motor learning73. Axon-specific changes to internode length were also observed with monocular deprivation in young adult mice: sequential periods of elevated extension and retraction occurred in internodes on layer 2/3 visual cortical parvalbumin interneurons but not on cortical projection neurons72. This axon-specificity of internode length remodeling raises the intriguing possibility of axonal bias in remodeling of other myelin features.
Injury can also induce internode length remodeling. In developing zebrafish spinal cord, internodes can reinitiate rapid lengthening in response to ablation of an adjacent internode70. In most cases, lengthening internodes were “pushed back” by new sheaths forming in the vacated space, but in a few cases, new sheaths did not form, and the neighboring internodes extended to fully close the gap70. By contrast, in young adult mouse cortex, internode loss did not induce remodeling of adjacent uninjured internodes76. Nevertheless, injured oligodendrocytes themselves can alter the length of their pre-existing internodes. Internodes surviving inflammatory injury in young adult mouse cortex retracted, shrinking approximately 30 microns76. A subset then reinitiated extension, growing to partially restore their lost length, though some extended into new regions76. Similarly, in ex vivo rodent spinal cord, excessive neuronal activity or excitotoxic glutamate induced a subtle, reversible retraction of internodes77,78. Whether internode length remodeling can be targeted to prevent or repair damage in injury and disease is an important topic for future investigations.
Which mechanisms regulate remodeling of mature internode length? In developing sheaths, internode length is influenced by myelin-axon adhesion molecules79–81, mTOR signaling82, microtubule nucleation83, ion channels84, neuronal activity and vesicular release85–91, and calcium signaling89,92,93. Might these mechanisms be involved in remodeling mature internode lengths? While observable sheath calcium transients precipitously decrease as internodes mature94, the specificity of internode remodeling to activated axons following motor learning raises the intriguing possibility that activity-dependent calcium signaling could reinitiate in sheaths along specific axons to direct their remodeling. Consistent with this possibility, blocking calcium signaling prevented internode retraction following high frequency stimulation and glutamate application in ex vivo rodent spinal cord77,78. Exploring whether this mechanism applies in more physiological conditions and with cell-type specific manipulations will be of great interest.
How myelin-axon adhesion complexes and myelin membranes are modified to facilitate internode length remodeling remains unclear. Paranodal loops, normally adhered in place to the axon, would need to reinitiate movement along the axon as during sheath formation. Furthermore, myelin membranes would need to adjust, by either (1) rapidly adding or removing membrane (see Membranes) or (2) redistributing membrane into outfoldings or more or fewer wraps. In metamorphosizing xenopus optic nerve, retracting internodes remain compact but undergo dramatic outfolding and detachment from axons, which is resolved by astrocyte phagocytosis30. Dynamic internodes in adult mouse brain are also thought to remain compact3 but sheath dysmorphia is difficult to assess using fluorescence microscopy. Future correlated ultrastructural analyses will provide invaluable insights into the changes in myelin-axon adhesion and membrane remodeling that facilitate adjustments in internode length.
Internode Formation and Loss
Following the highly dynamic period of nascent sheath formation and removal by newly-generated oligodendrocytes95–97, mature oligodendrocytes appear to maintain these internodes across the lifespan3. So far, the formation or loss of an internode by mature oligodendrocytes has not been observed under baseline conditions but can occur in injury, aging, and in response to behavioral experience (Fig. 2).
Oligodendrocytes that survive a demyelinating injury involving pathological internode removal have been observed via longitudinal in vivo imaging to generate new internodes71,76,98. Only a few new internodes were formed per oligodendrocyte71,76,98 but this prevalence increased with forelimb reach training71. Some internodes grew from pre-existing processes, while others utilized de novo process growth from the oligodendrocyte soma98. Similar to internodes from newly-generated oligodendrocytes, these internodes extended over a few days and established typical internode lengths98, but other ultrastructural parameters are yet to be determined. In mouse cortex, new internodes from surviving oligodendrocytes were primarily observed in the same positions as lost internodes71,76, while in developing zebrafish spinal cord, surviving oligodendrocytes frequently mistargeted their new internodes to wrap neuronal cell bodies98. Other approaches in larger mammals, including humans, have found results consistent with new internode formation by surviving oligodendrocytes99,100, a start to validating these findings with parallel techniques.
Can new internode formation and loss occur without injury? While motor learning did not induce new internode formation in mature oligodendrocytes in the intact mouse71, visual deprivation was observed to induce both formation of new internodes and loss of pre-existing internodes from mature oligodendrocytes in young adult mouse visual cortex72. In aged mouse cortex, internode loss was common, with approximately 10% of pre-existing internodes being lost over two months32. Oligodendrocyte cell loss also occurred at this stage3,32; thus, future studies should determine whether internode loss during aging is restricted to these degenerating oligodendrocytes or is more widespread. In fact, whether internode loss from mature oligodendrocytes in any condition is a novel form of myelin plasticity or simply a pathological response to damage to or loss of axons or oligodendrocytes/myelin remains to be determined.
Which mechanisms regulate internode formation and loss in mature oligodendrocytes? One study in mice found that surviving oligodendrocytes with constitutive ERK1/2 activation had myelin within a demyelinating lesion following remyelination, perhaps indicative of new internode formation in this region51. Possibly, signaling that governs nascent sheath formation and removal in newly-formed oligodendrocytes becomes reactivated to initiate these processes in mature oligodendrocytes. This may include molecular factors, such as endothelin receptor B-PKC epsilon101, NRG196, ErbB receptors48,96,102, Fyn95, Rab597, laminin103, beta1 integrin104, Nogo A105, and Ephrin A1-EphA4-RhoA-ROCK98,106, and cellular mechanisms of sheath removal (see Membranes). Understanding these mechanisms may have important implications for preventing and repairing damage in aging and disease.
Node Length
The nodal/paranodal/juxtaparanodal region contains a unique array of anchoring and adhesion proteins and ion channels that facilitate myelin-axon adhesion and action potential regeneration (reviewed in Rasband and Peles, 2021107). Nodes highly resemble the axon initial segment, the site of action potential initiation, which is capable of adjusting its length and positioning in response to changes in neuronal activity108,109, supporting the possibility that nodes are equipped with similar capabilities. Indeed, node lengths can undergo modulation in adults, including in response to changes in neuronal activity or experience (Fig 2).
Nodal dynamics often occur along with modulation of other myelin features. In continuously myelinated regions, internode retraction is intrinsically tied to the lengthening of nodes and can induce this micron-sized structure to expand dramatically73,77,78. Subtler changes in node length accompanied periaxonal space width remodeling, but whether these structures remodeled jointly or independently remains to be determined. Node length decreased and periaxonal space width increased in corpus callosum following low intensity repetitive transcranial magnetic stimulation while node length increased and periaxonal space width decreased in hippocampal fimbria following spatial learning in young adult mice60. Node length changes in both directions were small, averaging around 0.15 microns60. Crucially, by fluorescently pre-labeling mature oligodendrocytes or preventing the formation of new oligodendrocytes, these nodal changes were attributable to pre-existing rather than newly-formed nodes60. Node remodeling also accompanied changes in sheath thickness and paranode length, decreasing in length concomitantly with increases the other parameters following environmental enrichment45 or constitutive activation of ERK1/2 in pre-existing oligodendrocytes51 in adult mice. Myelin thickness and paranode length might be expected to grow in concordance, given that additional wraps form additional paranodal loops and myelin thickness and paranode length are positively, though loosely, correlated38. However, how myelin thickening would reduce node length and whether these effects occur independently is unknown.
Exploration of mechanisms underlying node length remodeling is just beginning. A recent study suggested a role for thrombin in detaching node-adjacent paranodal loops from the axon to increase node length in young adult mouse optic nerve117. However, such an effect of thrombin was only observable when astrocyte exocytosis (including of thrombin inhibitor nexin1) was blocked110, suggesting additional mechanisms are likely at play. Paranodal loops need not even detach to modify node length: instead, individual paranodal loops could alter their lengths or translocate along the axon. Another outstanding question is how axonal protein complexes reorganize following nodal remodeling given myelin’s role in assembling the nodal/paranodal/juxtaparanodal region (reviewed in Rasband and Peles, 2021107), the redistribution of axonal components following demyelination111–113, and the “pushing” of axonal nodal components by dynamic internodes during myelin formation114. Lengthened nodes following motor learning sometimes lacked sodium channels entirely while others had redistributed sodium channels along their enlarged length73. After node lengthening from high frequency stimulation or glutamate application, normally juxtaparanodal potassium channels encroached into the paranodal and nodal regions77,78, suggesting breakdown of the axonal paranodal cytoskeletal “barrier” that typically prevents reorganization. How other components adjust to node length remodeling, whether components adjust in response to submicron-level length remodeling, and the temporal dynamics of component reorganization will be fruitful grounds for further investigation and may provide insights into the functional implications of these structural modifications. Finally, given the various ion channel subtypes present across the lifespan and in disease, an intriguing possibility is that nodes may dynamically adjust their ion channel composition independent of changes in length107.
Overall, these data provide compelling evidence that mature oligodendrocytes and myelin undergo a diversity of structural modifications throughout life and in response to external stimuli (Fig. 2). Which mechanisms underlie myelin remodeling is the subject of ongoing investigation, but asymmetries in myelin turnover and reactivation of pathways involved in myelin formation are likely major players. In future work, it will be important to determine how remodeling is managed at the level of the oligodendrocyte, whether remodeling of sheaths impacts others from the same cell, and if not, how oligodendrocytes target remodeling to subsets of sheaths.
Function of Myelin Remodeling
Neuroplasticity is considered to form the basis of neural adaptation, allowing the brain to alter (learning), maintain (homeostasis), or restore (recovery from injury) its function in response to changing circumstances. Traditionally considered to involve changes to neuronal connectivity, neural adaptation is increasingly understood to also depend on non-neuronal cells, including oligodendrocytes.
Myelin remodeling could influence neuronal adaptation through modulating action potential conduction (Fig. 3a,b). Myelin dramatically increases action potential propagation speed, but the specific structural parameters of myelin determine its precise impact. Myelin thickness positively correlates with conduction velocity46,58,102,115,116, with the greatest marginal impact from the first few wraps58,115, while periaxonal space width is negatively correlated58,60,115. The relationship between node length and conduction speed is concave when nodal ion channel density remains stable, whereas when ion channel number remains stable, node length and conduction speed are negatively correlated117. In both cases, lengthening nodes are predicted to slow conduction and even cause conduction failure73,117. For internode length, in fully myelinated regions, there exists an optimal length for conduction velocity85,117,118. However, myelination along axons is not always continuous: large unmyelinated regions exist intermittently in regions like cerebral cortex in adult mice3,119. In such regions, myelin coverage, which may be modulated bidirectionally through internode extension, formation, retraction, or removal, positively correlates with conduction velocity73,120, with the largest marginal effect through creating or eliminating continuous stretches of myelin73. Experimentally-observed myelin remodeling was predicted to change conduction velocity by 20% or more, including causing the outright failure of conduction60,73. Intriguingly, this modulation was bidirectional60,73, suggesting myelin remodeling may serve to fine tune – rather than merely enhance – conduction velocity.
Fig. 3 |. Potential functions of myelin remodeling.
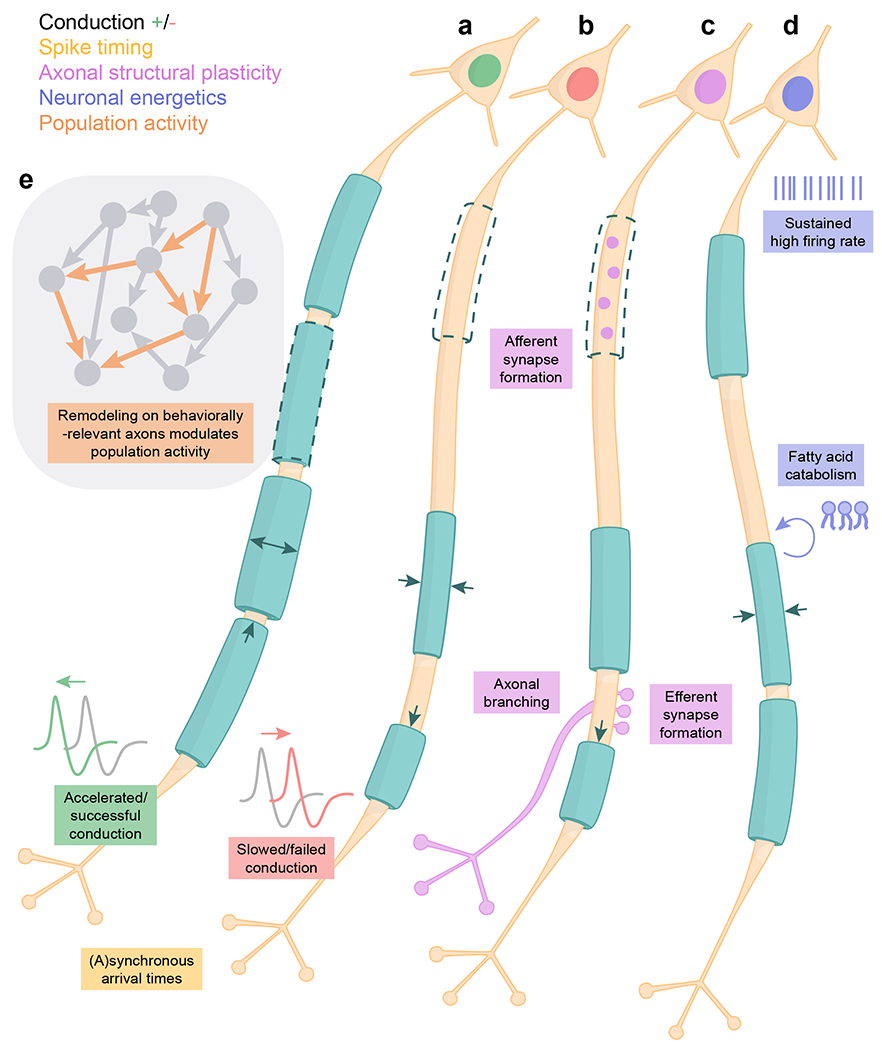
a-d, Potential functional implications of myelin remodeling. a, Anaxon experiences internode formation, myelin thickening, and internode extension, causing accelerated or successful action potential conduction. b, An axon experiences internode removal, myelin thinning, and internode retraction, causing slowed or failed action potential conduction. Changes in conduction in a and b alter the synchrony of action potential arrival times. c, An axon experiences internode removal and retraction, enabling axonal remodeling. d, An axon experiences myelin thinning. Myelin lipidsare used for energy to support neuronal firing. e, Myelin remodeling occurs on axons involved in a specific behavior to modulate neuronal population activity.
Altering conduction shifts the postsynaptic arrival time of action potentials (Fig. 3a,b). This, in turn, impacts spike timing-dependent plasticity and neuronal synchrony. Previous work has implicated oligodendrocytes and myelin, though not specifically their remodeling, in establishing neuronal synchrony, while direct evidence of an impact on spike timing-dependent plasticity is still outstanding. Using dysmyelinated rodent models, researchers showed that normal myelin is required for establishing a uniform conduction time between regions116,121,122. In the cerebellar cortex, this myelin-induced spike timing uniformity was essential for establishing synchronous spontaneous firing of Purkinje cells121. Several studies have also established a role for adaptive myelination in promoting synchrony. Following contextual fear conditioning in mice, the coupling of sharp wave ripple and spindle oscillations between hippocampal regions, thought to contribute to memory consolidation, was prevented by a gene deletion in oligodendrocyte precursor cells that disrupted experience-dependent oligodendrogenesis123. Likewise, during epilepsy development in rodents, seizure-induced myelination contributed to inter-regional synchrony and epilepsy progression41. Computational modeling also supports a role for myelin in promoting synchrony. Modulating myelin in an activity-dependent manner caused network synchronization and increased firing rates124. Another computational model specifically modulated pre-existing myelin coverage in response to oligodendrocyte integration of conduction timing along an axon bundle and found that this synchronized correlated spikes125. Importantly, myelin-induced synchrony is implicated in enhancing motor and spatial learning122,123. These data evoke the exciting possibility that remodeling of mature myelin could serve a similar role of fine-tuning conduction to facilitate neuronal synchrony and enable learning. Consistent with this idea, learning success was highly correlated with the extent of internode retraction and consequent node lengthening during learning73, though future studies should determine the causality of this relationship.
Modulating conduction via myelin remodeling could serve additional roles. Structural plasticity of axon initial segments, which highly resemble nodes, tempers neuronal excitability following sustained changes in neuronal activity108,109. Perhaps nodal plasticity serves a similar purpose. Modeling suggests node lengthening could cause conduction delay and failure73, potentially providing a means to counteract sustained changes in neuronal activity from learning- or injury-induced plasticity. Additionally, myelin remodeling may restore conduction following injury. Internode formation and extension following demyelination likely aides in recovering conduction in demyelinated axons by restoring myelin coverage – a role primarily achieved by newly-formed oligodendrocytes126. Future work correlating longitudinal in vivo measurements of conduction velocity and myelin remodeling will aid in determining the links between these parameters in individual axons and across neural circuits.
Along with myelin remodeling, axons undergo structural plasticity in response to neuronal activity and learning. Elaboration of axonal branches occurred in mouse cortical parvalbumin interneurons following chemogenetic activation127 and in primate visual cortex following perceptual learning128. Furthermore, learning altered the formation and elimination of cortical en passant (efferent) boutons129,130 while chemogenetic activation induced a greater number of inhibitory axo-axonic (afferent) boutons on activated cortical pyramidal neurons131. Intriguingly, myelin may also shape such axonal architecture. Myelinated segments of cortical pyramidal neurons or interneurons lack axonal branch points132–134, en passant synapses119,132, and axo-axonic synapses119 while mice with deficient myelin exhibited encroachment of en passant boutons into normally myelinated regions of parvalbumin interneurons132. Furthermore, myelin components are implicated in inhibiting axonal sprouting135 and limiting neuronal plasticity136. Might myelin remodeling affect neural adaptation through covering or exposing axonal regions to influence axonal remodeling (Fig. 3c)? Future work examining myelin and axonal remodeling in concert will shed light on this exciting possibility.
Myelin remodeling could alter the energetic support of neurons (Fig. 3d). Oligodendrocytes support neuronal health, firing, and perhaps information processing by providing metabolites116,137–139, proposed to be shuttled from internodes to their underlying axons140. This metabolic support is thought to be especially important in continuously myelinated axons given that myelin isolates axons from extracellular metabolites141. Thus, remodeling myelin coverage may alter the local supply of metabolites from myelin and extracellular sources to modify axonal energetics. Another study found that oligodendrocytes sustain neuronal firing in glucose-deprived mouse optic nerves through beta-oxidation of fatty acids57. This was proposed to be through increased autophagy and catabolism of myelin lipids, as reducing glucose uptake by oligodendrocytes increased autophagosome formation and thinned myelin57. Though, whether this process involves the increased removal of lipids from compact myelin or the diversion of lipids that would be used for myelin synthesis is uncertain. In either case, by tweaking the balance of myelin turnover to either favor formation or removal, energy could be stored as lipids when resources are abundant and utilized in periods of deprivation, enabling stable neural function across conditions. Perhaps myelin thickening over the lifetime serves to accumulate fatty acids while internode retraction in aging enables the use of these fatty acids for energy. Leveraging emerging technologies for in vivo measurement of local metabolites will facilitate further dissection of whether myelin remodeling influences neuronal energetics and the spatial dimensions of these effects.
Another outstanding question is the scale and distribution of myelin remodeling required to impact behavior. Only limited subsets of neurons (<20%) are involved in given task-specific memories (reviewed in Rao-Ruiz et al., 2019142) and single cortical “hub” interneurons can influence population activity and synchrony143. By focusing myelin remodeling to these axonal populations72,73, behavioral impacts could be realized with relatively little remodeling (Fig. 3e). As mechanisms regulating myelin remodeling are discovered, manipulation of specific structural changes to myelin will help to elucidate the local impact of remodeling on individual axons. Furthermore, manipulation of remodeling in specific axonal subtypes or circuits will clarify the role of remodeling in broader neural function and behavior. Population-wide assessments of neuronal responses to myelin remodeling will be an important tool in this endeavor (reviewed in Shenoy and Kao, 2021144). We anticipate these studies will provide critical insights into how the CNS adapts across the lifespan and in response to a changing environment.
Conclusion
Research has established the dynamic nature of mature oligodendrocytes and myelin, but there is much more to uncover. The field is just beginning to define the scope of myelin dynamics, and will be greatly propelled by technological progress to enable in vivo longitudinal monitoring of more features like myelin thickness or myelin lipids and proteins. These investigations will likely find additional parameters subject to dynamic modulation, such as lipid composition or the composition and distribution of nodal ion channels. But even for established myelin dynamics, many outstanding questions remain. The mechanisms regulating myelin remodeling are almost entirely unknown, though they likely involve the machinery of myelin formation or turnover. Defining these mechanisms will be critical to enable manipulation of remodeling and exploration of its function. Given the scale and specificity of myelin remodeling, this form of plasticity could have profound impacts on neural function, serving as an integral component of behavioral adaptation. We anticipate an exciting future for this nascent field that has continued to redefine the nature and function of myelin.
Acknowledgements
We thank the members of the Hughes laboratory and the reviewers for their helpful comments. Funding was provided by the National Multiple Sclerosis Society (FG-2208-40305) and the Department of Defense Congressionally Directed Medical Research Programs (MS220187) to L.A.O. Funding was provided by the University of Colorado Department of Cell and Developmental Biology Pilot grant, the Whitehall Foundation, the National Multiple Sclerosis Society (RG-1701–26733), and National Institute of Neurological Disorders and Stroke (NS115975, NS125230 and NS132859) to E.G.H.
Footnotes
Competing Interests
The authors declare no competing interests.
References
- 1.Young KM et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–885 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tripathi RB et al. Remarkable Stability of Myelinating Oligodendrocytes in Mice. Cell Rep. 21, 316–323 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill RA, Li AM & Grutzendler J Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat. Neurosci 21, 683–695 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang F et al. Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory. Nat. Neurosci 23, 481–486 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeung MS et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 159, 766–774 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Price JC, Guan S, Burlingame A, Prusiner SB & Ghaemmaghami S Analysis of proteome dynamics in the mouse brain. Proc. Natl. Acad. Sci 107, 14508–14513 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]; Landmark study finding myelin proteins are some of the longest lived in the body.
- 7.Fornasiero EF et al. Precisely measured protein lifetimes in the mouse brain reveal differences across tissues and subcellular fractions. Nat. Commun 9, 4230 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goh B, Kim J, Seo S & Kim T-Y High-Throughput Measurement of Lipid Turnover Rates Using Partial Metabolic Heavy Water Labeling. Anal. Chem 90, 6509–6518 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Poitelon Y, Kopec AM & Belin S Myelin Fat Facts: An Overview of Lipids and Fatty Acid Metabolism. Cells 9, 812 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ando S, Tanaka Y, Toyoda Y & Kon K Turnover of Myelin Lipids in Aging Brain. Neurochem. Res 28, 5–13 (2003). [DOI] [PubMed] [Google Scholar]; Demonstration of variable lifetimes of myelin lipids.
- 11.Dustin E et al. Compromised Myelin and Axonal Molecular Organization Following Adult-Onset Sulfatide Depletion. Biomedicines 11, 1431 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snaidero N et al. Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell 156, 277–290 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simons M, Krämer E-M, Thiele C, Stoffel W & Trotter J Assembly of Myelin by Association of Proteolipid Protein with Cholesterol- and Galactosylceramide-Rich Membrane Domains. J. Cell Biol 151, 143–154 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pralle A, Keller P, Florin E-L, Simons K & Hörber JKH Sphingolipid–Cholesterol Rafts Diffuse as Small Entities in the Plasma Membrane of Mammalian Cells. J. Cell Biol 148, 997–1008 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato D et al. Regulation of lipid synthesis in myelin modulates neural activity and is required for motor learning. Glia 71, 2591–2608 (2023). [DOI] [PubMed] [Google Scholar]
- 16.Zhou X et al. Mature myelin maintenance requires Qki to coactivate PPARβ-RXRα–mediated lipid metabolism. J. Clin. Investig 130, 2220–2236 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stadelmann C, Timmler S, Barrantes-Freer A & Simons M Myelin in the Central Nervous System: Structure, Function, and Pathology. Physiol. Rev 99, 1381–1431 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Toyama BH et al. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell 154, 971–982 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savas JN, Toyama BH, Xu T, Yates JR & Hetzer MW Extremely long-lived nuclear pore proteins in the rat brain. Science 335, 942 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lüders KA et al. Maintenance of high proteolipid protein level in adult central nervous system myelin is required to preserve the integrity of myelin and axons. Glia 67, 634–649 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Meschkat M et al. White matter integrity in mice requires continuous myelin synthesis at the inner tongue. Nat. Commun 13, 1163 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; Landmark observations on the continuous turnover of entire myelin membranes.
- 22.Lin Y et al. Impaired Eukaryotic Translation Initiation Factor 2B Activity Specifically in Oligodendrocytes Reproduces the Pathology of Vanishing White Matter Disease in Mice. J. Neurosci 34, 12182–12191 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishii A, Furusho M, Dupree JL & Bansal R Role of ERK1/2 MAPK Signaling in the Maintenance of Myelin and Axonal Integrity in the Adult CNS. J. Neurosci 34, 16031–16045 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aber ER et al. Oligodendroglial macroautophagy is essential for myelin sheath turnover to prevent neurodegeneration and death. Cell Rep. 41, 111480 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ktena N et al. Autophagic degradation of CNS myelin maintains axon integrity. Cell Stress 6, 93–107 (2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Readhead C et al. Expression of a myelin basic protein gene in transgenic shiverer mice: Correction of the dysmyelinating phenotype. Cell 48, 703–712 (1987). [DOI] [PubMed] [Google Scholar]
- 27.Hildebrand C, Remahl S, Persson H & Bjartmar C Myelinated nerve fibres in the CNS. Prog. Neurobiol 40, 319–384 (1993). [DOI] [PubMed] [Google Scholar]
- 28.Djannatian M et al. Myelination generates aberrant ultrastructure that is resolved by microglia. J. Cell Biol 222, e202204010 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krämer‐Albers E-M et al. Oligodendrocytes secrete exosomes containing major myelin and stress‐protective proteins: Trophic support for axons? Proteom. Clin. Appl 1, 1446–1461. [DOI] [PubMed] [Google Scholar]
- 30.Mills EA et al. Astrocytes phagocytose focal dystrophies from shortening myelin segments in the optic nerve of Xenopus laevis at metamorphosis. Proc. Natl. Acad. Sci 112, 10509–10514 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes AN & Appel B Microglia phagocytose myelin sheaths to modify developmental myelination. Nat. Neurosci 23, 1055–1066 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chapman TW, Olveda GE, Bame X, Pereira E & Hill RA Oligodendrocyte death initiates synchronous remyelination to restore cortical myelin patterns in mice. Nat. Neurosci 26, 555–569 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sturrock RR Myelination of the mouse corpus callosum. Neuropathol. Appl. Neurobiol 6, 415–420 (1980). [DOI] [PubMed] [Google Scholar]
- 34.McNamara NB et al. Microglia regulate central nervous system myelin growth and integrity. Nature 613, 120–129 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters A, Sethares C & Killiany RJ Effects of age on the thickness of myelin sheaths in monkey primary visual cortex. J. Comp. Neurol 435, 241–248 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Furusho M, Dupree JL, Nave K-A & Bansal R Fibroblast growth factor receptor signaling in oligodendrocytes regulates myelin sheath thickness. J. Neurosci 32, 6631–41 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blakemore WF Pattern of remyelination in the CNS. Nature 249, 577–578 (1974). [DOI] [PubMed] [Google Scholar]
- 38.Shepherd MN, Pomicter AD, Velazco CS, Henderson SC & Dupree JL Paranodal reorganization results in the depletion of transverse bands in the aged central nervous system. Neurobiol. Aging 33, 203.e13–203.e24 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geraghty AC et al. Loss of Adaptive Myelination Contributes to Methotrexate Chemotherapy-Related Cognitive Impairment. Neuron 103, 250–265.e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitew S et al. Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat. Commun 9, 306 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knowles JK et al. Maladaptive myelination promotes generalized epilepsy progression. Nat. Neurosci 25, 596–606 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J et al. Clemastine Enhances Myelination in the Prefrontal Cortex and Rescues Behavioral Changes in Socially Isolated Mice. J. Neurosci 36, 957–962 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci 15, 1621–1623 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonnefil V et al. Region-specific myelin differences define behavioral consequences of chronic social defeat stress in mice. eLife 8, e40855 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicholson M et al. Remodelling of myelinated axons and oligodendrocyte differentiation is stimulated by environmental enrichment in the young adult brain. Eur. J. Neurosci 56, 6099–6114 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sinclair JL et al. Sound-Evoked Activity Influences Myelination of Brainstem Axons in the Trapezoid Body. J. Neurosci 37, 8239–8255 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gibson EM et al. Neuronal Activity Promotes Oligodendrogenesis and Adaptive Myelination in the Mammalian Brain. Science 344, 1252304 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Makinodan M, Rosen KM, Ito S & Corfas G A Critical Period for Social Experience–Dependent Oligodendrocyte Maturation and Myelination. Science 337, 1357–1360 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y et al. Neonatal Maternal Separation Impairs Prefrontal Cortical Myelination and Cognitive Functions in Rats Through Activation of Wnt Signaling. Cereb. Cortex 27, 2871–2884 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Osanai Y et al. Dark Rearing in the Visual Critical Period Causes Structural Changes in Myelinated Axons in the Adult Mouse Visual Pathway. Neurochem. Res 47, 2815–2825 (2022). [DOI] [PubMed] [Google Scholar]
- 51.Jeffries MA et al. ERK1/2 Activation in Preexisting Oligodendrocytes of Adult Mice Drives New Myelin Synthesis and Enhanced CNS Function. J. Neurosci 36, 9186–9200 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snaidero N et al. Myelin replacement triggered by single-cell demyelination in mouse cortex. Nat. Commun 11, 4901 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lebrun-Julien F et al. Balanced mTORC1 activity in oligodendrocytes is required for accurate CNS myelination. J. Neurosci 34, 8432–8448 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishii A, Furusho M, Dupree JL & Bansal R Strength of ERK1/2 MAPK Activation Determines Its Effect on Myelin and Axonal Integrity in the Adult CNS. J. Neurosci 36, 6471–6487 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goebbels S et al. Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J. Neurosci 30, 8953–8964 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrated that mature sheaths can reinitiate rapid radial growth.
- 56.Furusho M, Ishii A & Bansal R Signaling by FGF Receptor 2, Not FGF Receptor 1, Regulates Myelin Thickness through Activation of ERK1/2–MAPK, Which Promotes mTORC1 Activity in an Akt-Independent Manner. J. Neurosci 37, 2931–2946 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asadollahi E et al. Myelin lipids as nervous system energy reserves. bioRxiv (2022) doi: 10.1101/2022.02.24.481621. [DOI] [Google Scholar]; Found the beta-oxidation of myelin lipids is used to support neuronal energetics.
- 58.Cohen CHC et al. Saltatory Conduction along Myelinated Axons Involves a Periaxonal Nanocircuit. Cell 180, 311–322.e15 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saab AS et al. Oligodendroglial NMDA Receptors Regulate Glucose Import and Axonal Energy Metabolism. Neuron 91, 119–132 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cullen CL et al. Periaxonal and nodal plasticities modulate action potential conduction in the adult mouse brain. Cell Rep. 34, 108641 (2021). [DOI] [PubMed] [Google Scholar]
- 61.Marcus J, Dupree JL & Popko B Myelin-associated glycoprotein and myelin galactolipids stabilize developing axo-glial interactions. J. Cell Biol 156, 567–577 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li C et al. Myelination in the absence of myelin-associated glycoprotein. Nature 369, 747–750 (1994). [DOI] [PubMed] [Google Scholar]
- 63.Montag D et al. Mice deficient for the glycoprotein show subtle abnormalities in myelin. Neuron 13, 229–246 (1994).. [DOI] [PubMed] [Google Scholar]
- 64.Pronker MF et al. Structural basis of myelin-associated glycoprotein adhesion and signalling. Nat. Commun 7, 13584 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Menichella DM, Goodenough DA, Sirkowski E, Scherer SS & Paul DL Connexins Are Critical for Normal Myelination in the CNS. J. Neurosci 23, 5963–5973 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Menichella DM et al. Genetic and physiological evidence that oligodendrocyte gap junctions contribute to spatial buffering of potassium released during neuronal activity. J. Neurosci 26, 10984–10991 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Larson VA et al. Oligodendrocytes control potassium accumulation in white matter and seizure susceptibility. eLife 7, e34829 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schirmer L et al. Oligodendrocyte-encoded Kir4.1 function is required for axonal integrity. eLife 7, e36428 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marshall-Phelps KLH et al. Neuronal activity disrupts myelinated axon integrity in the absence of NKCC1b. J. Cell Biol 219, e201909022 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Auer F, Vagionitis S & Czopka T Evidence for Myelin Sheath Remodeling in the CNS Revealed by In Vivo Imaging. Curr. Biol 28, 549–559.e3 (2018). [DOI] [PubMed] [Google Scholar]
- 71.Bacmeister CM et al. Motor learning promotes remyelination via new and surviving oligodendrocytes. Nat. Neurosci 23, 819–831 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang SM, Michel K, Jokhi V, Nedivi E & Arlotta P Neuron class-specific responses govern adaptive myelin remodeling in the neocortex. Science 370, 2109 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bacmeister CM et al. Motor learning drives dynamic patterns of intermittent myelination on learning-activated axons. Nat. Neurosci 25, 1300–1313 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; Found internode retraction along learning-activated axons.
- 74.Hughes EG, Orthmann-Murphy JL, Langseth AJ & Bergles DE Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci 21, 696–706 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lasiene J, Matsui A, Sawa Y, Wong F & Horner PJ Age related myelin dynamics revealed by increased oligodendrogenesis and short internodes. Aging Cell 8, 201–213 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mezydlo A et al. Remyelination by surviving oligodendrocytes is inefficient in the inflamed mammalian cortex. Neuron 111, 1748–1759.e8 (2023). [DOI] [PubMed] [Google Scholar]
- 77.Fu Y, Sun W, Shi Y, Shi R & Cheng J-X Glutamate Excitotoxicity Inflicts Paranodal Myelin Splitting and Retraction. PLoS ONE 4, e6705 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huff TB et al. Real-Time CARS Imaging Reveals a Calpain-Dependent Pathway for Paranodal Myelin Retraction during High-Frequency Stimulation. PLoS One 6, e17176 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hughes AN & Appel B Oligodendrocytes express synaptic proteins that modulate myelin sheath formation. Nat. Commun 10, 4125 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elazar N et al. Axoglial Adhesion by Cadm4 Regulates CNS Myelination. Neuron 101, 224–231 5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Djannatian M et al. Two adhesive systems cooperatively regulate axon ensheathment and myelin growth in the CNS. Nat. Commun 10, 4794 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kearns CA, Ravanelli AM, Cooper K & Appel B Fbxw7 Limits Myelination by Inhibiting mTOR Signaling. J. Neurosci 35, 14861–14871 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fu M-MM et al. The Golgi Outpost Protein TPPP Nucleates Microtubules and Is Critical for Myelination. Cell 179, 132–146.e14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swire M et al. Oligodendrocyte HCN2 Channels Regulate Myelin Sheath Length. J. Neurosci 41, 7954–7964 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Etxeberria A et al. Dynamic Modulation of Myelination in Response to Visual Stimuli Alters Optic Nerve Conduction Velocity. J. Neurosci 36, 6937–6948 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Osanai Y et al. Length of myelin internodes of individual oligodendrocytes is controlled by microenvironment influenced by normal and input‐deprived axonal activities in sensory deprived mouse models. Glia 66, 2514–2525 (2018). [DOI] [PubMed] [Google Scholar]
- 87.Hines JH, Ravanelli AM, Schwindt R, Scott EK & Appel B Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci 18, 683–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koudelka S et al. Individual Neuronal Subtypes Exhibit Diversity in CNS Myelination Mediated by Synaptic Vesicle Release. Curr. Biol 26, 1447–1455 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krasnow AM, Ford MC, Valdivia LE, Wilson SW & Attwell D Regulation of developing myelin sheath elongation by oligodendrocyte calcium transients in vivo. Nat. Neurosci 21, 24–28 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Almeida RG et al. Myelination induces axonal hotspots of synaptic vesicle fusion that promote sheath growth. Curr. Biol 31, 3743–3754.e5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wake H et al. Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat. Commun 6, 7844 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baraban M, Koudelka S & Lyons DA Ca 2+ activity signatures of myelin sheath formation and growth in vivo. Nat. Neurosci 21, 19–23 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iyer M et al. Oligodendrocyte calcium signaling promotes actin-dependent myelin sheath extension. Nat. Commun 15, 265 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Battefeld A, Popovic MA, Vries S. I. de & Kole MHP High-Frequency Microdomain Ca2+ Transients and Waves during Early Myelin Internode Remodeling. Cell Rep. 26, 182–191.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Czopka T, Ffrench-Constant C & Lyons DA Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev. Cell 25, 599–609 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu P, Du J & He C Developmental pruning of early-stage myelin segments during CNS myelination in vivo. Cell Res. 23, 962–964 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Almeida AR & Macklin WB Early myelination involves the dynamic and repetitive ensheathment of axons which resolves through a low and consistent stabilization rate. eLife 12, e82111 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Neely SA et al. New oligodendrocytes exhibit more abundant and accurate myelin regeneration than those that survive demyelination. Nat. Neurosci 25, 415–420 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Duncan ID et al. The adult oligodendrocyte can participate in remyelination. Proc. Natl. Acad. Sci 115, 11807–11816 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yeung MSY et al. Dynamics of oligodendrocyte generation in multiple sclerosis. Nature 566, 538–542 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Swire M, Kotelevtsev Y, Webb DJ, Lyons DA & Ffrench-Constant C Endothelin signalling mediates experience-dependent myelination in the CNS. eLife 8, e49493 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roy K et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc. Natl. Acad. Sci 104, 8131–8136 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bechler ME, Byrne L & ffrench-Constant C CNS Myelin Sheath Lengths Are an Intrinsic Property of Oligodendrocytes. Curr. Biol 25, 2411–2416 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Câmara J et al. Integrin-mediated axoglial interactions initiate myelination in the central nervous system. J. Cell Biol 185, 699–712 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chong SY et al. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc. Natl. Acad. Sci 109, 1299–1304 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Harboe M, Torvund‐Jensen J, Kjaer‐Sorensen K & Laursen LS Ephrin‐A1‐EphA4 signaling negatively regulates myelination in the central nervous system. Glia 66, 934–950 (2018). [DOI] [PubMed] [Google Scholar]
- 107.Rasband MN & Peles E Mechanisms of node of Ranvier assembly. Nat. Rev. Neurosci 22, 7–20 (2021). [DOI] [PubMed] [Google Scholar]
- 108.Kuba H, Oichi Y & Ohmori H Presynaptic activity regulates Na+ channel distribution at the axon initial segment. Nature 465, 1075–1078 (2010). [DOI] [PubMed] [Google Scholar]
- 109.Grubb MS & Burrone J Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature 465, 1070–1074 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dutta DJ et al. Regulation of myelin structure and conduction velocity by perinodal astrocytes. Proc. Natl. Acad. Sci 115, 11832–11837 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Orthmann-Murphy J et al. Remyelination alters the pattern of myelin in the cerebral cortex. eLife 9, e56621 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Craner MJ, Lo AC, Black JA & Waxman SG Abnormal sodium channel distribution in optic nerve axons in a model of inflammatory demyelination. Brain 126, 1552–1561 (2003). [DOI] [PubMed] [Google Scholar]
- 113.Coman I et al. Nodal, paranodal and juxtaparanodal axonal proteins during demyelination and remyelination in multiple sclerosis. Brain 129, 3186–3195 (2006). [DOI] [PubMed] [Google Scholar]
- 114.Vagionitis S et al. Clusters of neuronal neurofascin prefigure the position of a subset of nodes of Ranvier along individual central nervous system axons in vivo. Cell Rep. 38, 110366 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Young RG, Castelfranco AM & Hartline DK The “Lillie Transition”: models of the onset of saltatory conduction in myelinating axons. J. Comput. Neurosci 34, 533–546 (2013). [DOI] [PubMed] [Google Scholar]
- 116.Moore S et al. A role of oligodendrocytes in information processing. Nat. Commun 11, 5497 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Arancibia-Cárcamo IL et al. Node of Ranvier length as a potential regulator of myelinated axon conduction speed. eLife 6, e23329 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ford MC et al. Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nat. Commun 6, 8073 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tomassy G et al. Distinct Profiles of Myelin Distribution Along Single Axons of Pyramidal Neurons in the Neocortex. Science 344, 319–324 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Micheva KD, Kiraly M, Perez MM & Madison DV Conduction Velocity Along the Local Axons of Parvalbumin Interneurons Correlates With the Degree of Axonal Myelination. Cereb. Cortex 31, 3374–3392 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lang EJ & Rosenbluth J Role of myelination in the development of a uniform olivocerebellar conduction time. J. Neurophysiol 89, 2259–2270 (2003). [DOI] [PubMed] [Google Scholar]
- 122.Kato D et al. Motor learning requires myelination to reduce asynchrony and spontaneity in neural activity. Glia 68, 193–210 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Steadman PE et al. Disruption of Oligodendrogenesis Impairs Memory Consolidation in Adult Mice. Neuron 105, 150–164.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; Identified a role for new oligodendrocytes in promoting neuronal synchrony.
- 124.Talidou A, Frankland PW, Mabbott D & Lefebvre J Homeostatic coordination and up-regulation of neural activity by activity-dependent myelination. Nat. Comput. Sci 2, 665–676 (2022). [DOI] [PubMed] [Google Scholar]
- 125.Pajevic S, Plenz D, Basser PJ & Fields RD Oligodendrocyte-mediated myelin plasticity and its role in neural synchronization. eLife 12, e81982 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cordano C et al. Validating visual evoked potentials as a preclinical, quantitative biomarker for remyelination efficacy. Brain 145, 3943–3952 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stedehouder J, Brizee D, Shpak G & Kushner SA Activity-Dependent Myelination of Parvalbumin Interneurons Mediated by Axonal Morphological Plasticity. J. Neurosci 38, 3631–3642 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kerkoerle T. van, Marik SA, Borgloh S. M. zum A. & Gilbert CD Axonal plasticity associated with perceptual learning in adult macaque primary visual cortex. Proc. Natl. Acad. Sci 115, 10464–10469 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Johnson CM, Peckler H, Tai L-H & Wilbrecht L Rule learning enhances structural plasticity of long-range axons in frontal cortex. Nat. Commun 7, 10785 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hasegawa R, Ebina T, Tanaka YR, Kobayashi K & Matsuzaki M Structural dynamics and stability of corticocortical and thalamocortical axon terminals during motor learning. PLoS One 15, e0234930 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pan-Vazquez A, Wefelmeyer W, Sabater VG, Neves G & Burrone J Activity-Dependent Plasticity of Axo-axonic Synapses at the Axon Initial Segment. Neuron 106, 265–276.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stedehouder J et al. Fast-spiking Parvalbumin Interneurons are Frequently Myelinated in the Cerebral Cortex of Mice and Humans. Cereb. Cortex 27, 5001–5013 (2017). [DOI] [PubMed] [Google Scholar]
- 133.Stedehouder J et al. Local axonal morphology guides the topography of interneuron myelination in mouse and human neocortex. eLife 8, e48615 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Benamer N, Vidal M, Balia M & Angulo MC Myelination of parvalbumin interneurons shapes the function of cortical sensory inhibitory circuits. Nat. Commun 11, 5151 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang KC et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature 417, 941–944 (2002). [DOI] [PubMed] [Google Scholar]
- 136.McGee AW, Yang Y, Fischer QS, Daw NW & Strittmatter SM Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science 309, 2222–2226 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee Y et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Meyer N, Richter N, Fan Z, Siemonsmeier G & Pivneva T Oligodendrocytes in the Mouse Corpus Callosum Maintain Axonal Function by Delivery of Glucose. Cell Rep. 22, 2383–2394 (2018). [DOI] [PubMed] [Google Scholar]
- 139.Fünfschilling U et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Saab AS et al. Oligodendroglial NMDA Receptors Regulate Glucose Import and Axonal Energy Metabolism. Neuron 91, 119–132 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nave K-A Myelination and the trophic support of long axons. Nat. Rev. Neurosci 11, 275–283 (2010). [DOI] [PubMed] [Google Scholar]
- 142.Rao-Ruiz P, Yu J, Kushner SA & Josselyn SA Neuronal competition: microcircuit mechanisms define the sparsity of the engram. Curr. Opin. Neurobiol 54, 163–170 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bollmann Y et al. Prominent in vivo influence of single interneurons in the developing barrel cortex. Nat. Neurosci 26, 1555–1565 (2023). [DOI] [PubMed] [Google Scholar]
- 144.Shenoy KV & Kao JC Measurement, manipulation and modeling of brain-wide neural population dynamics. Nat. Commun 12, 633 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Colman DR, Kreibich G, Frey AB & Sabatini DD Synthesis and incorporation of myelin polypeptides into CNS myelin. J. Cell Biol 95, 598–608 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zuchero JB et al. CNS myelin wrapping is driven by actin disassembly. Dev. Cell 34, 152–167 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nawaz S et al. Actin filament turnover drives leading edge growth during myelin sheath formation in the central nervous system. Dev. Cell 34, 139–151 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bishop GH, Clare MH & Landau WM The relation of axon sheath thickness to fiber size in the central nervous system of vertebrates. Int. J. Neurosci 2, 69–77 (1971). [DOI] [PubMed] [Google Scholar]
- 149.Velumian AA, Samoilova M & Fehlings MG Visualization of cytoplasmic diffusion within living myelin sheaths of CNS white matter axons using microinjection of the fluorescent dye Lucifer Yellow. Neuroimage 56, 27–34 (2011). [DOI] [PubMed] [Google Scholar]
- 150.Marques S et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352, 1326–1329 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


