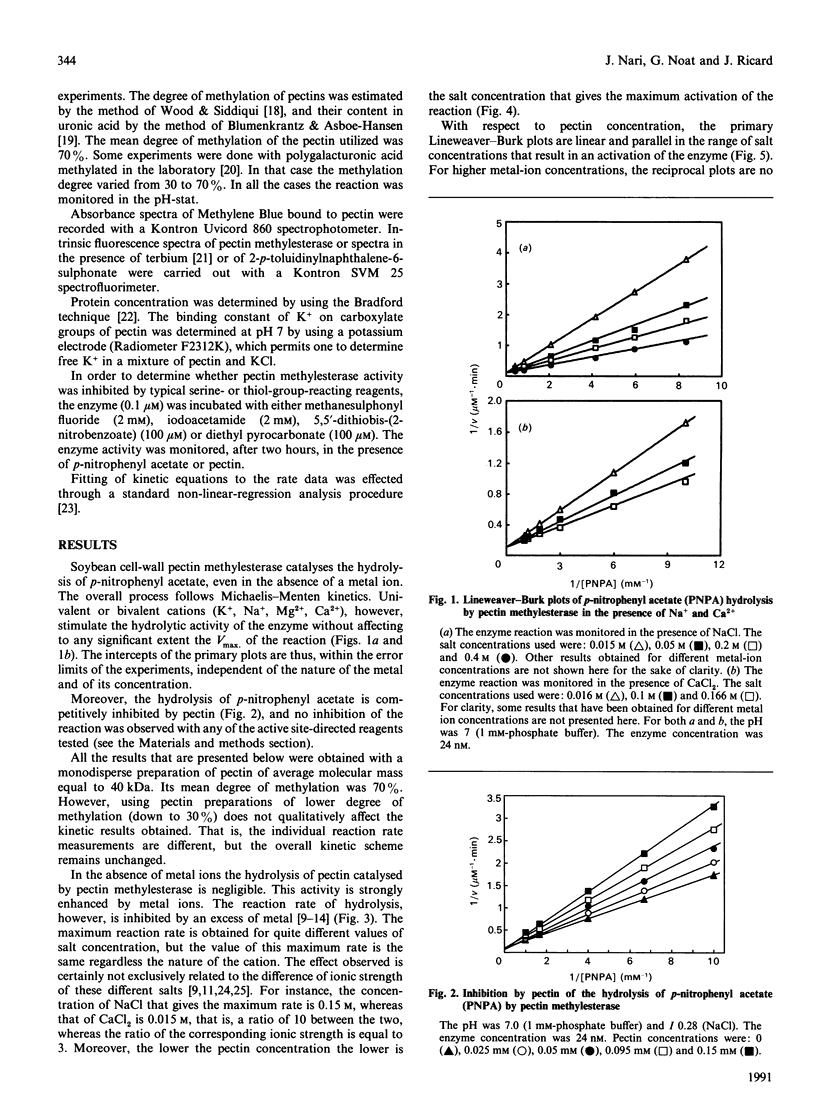
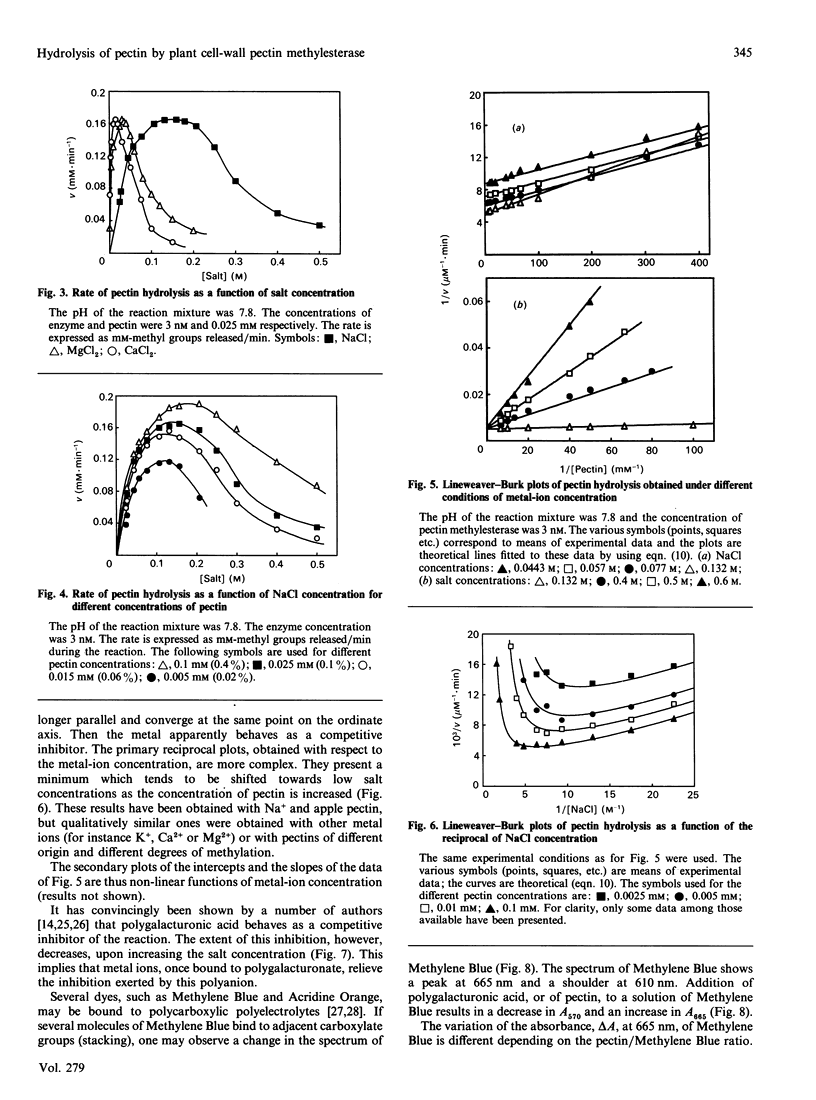
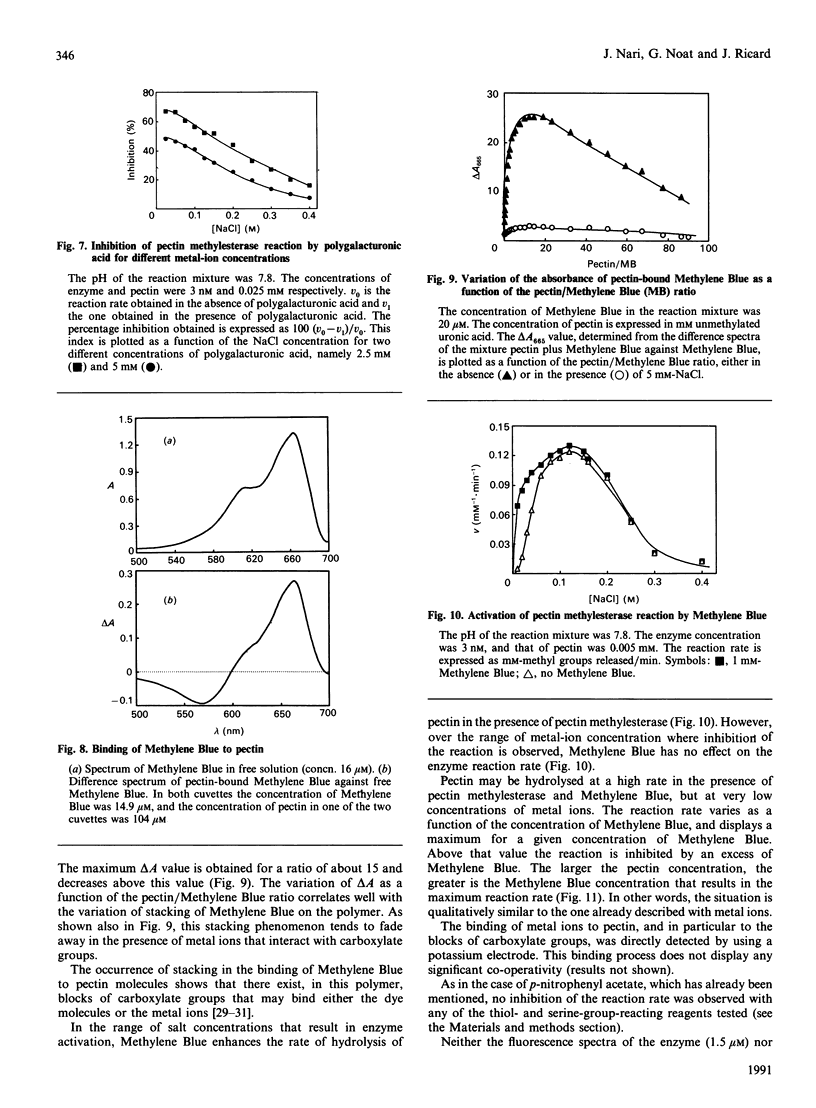
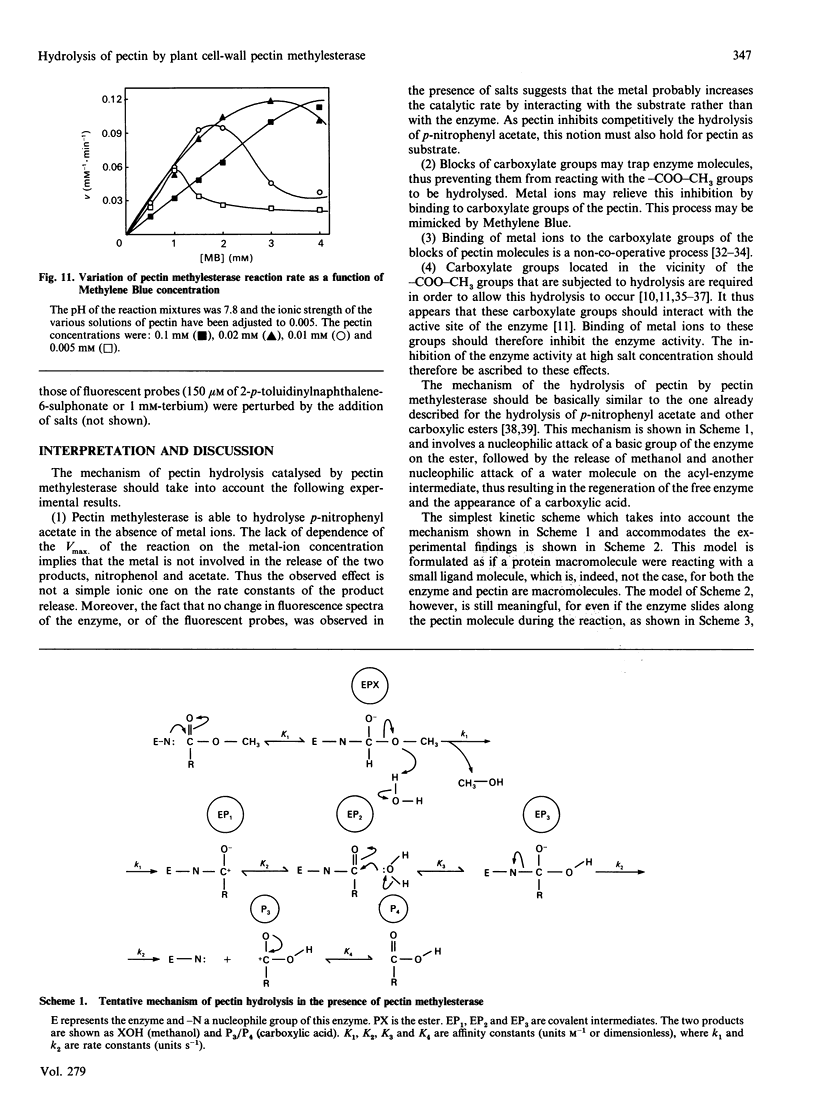
Abstract
The hydrolysis of p-nitrophenyl acetate catalysed by pectin methylesterase is competitively inhibited by pectin and does not require metal ions to occur. The results suggest that the activastion by metal ions may be explained by assuming that they interact with the substrate rather than with the enzyme. With pectin used as substrate, metal ions are required in order to allow the hydrolysis to occur in the presence of pectin methylesterase. This is explained by the existence of 'blocks' of carboxy groups on pectin that may trap enzyme molecules and thus prevent the enzyme reaction occurring. Metal ions may interact with these negatively charged groups, thus allowing the enzyme to interact with the ester bonds to be cleaved. At high concentrations, however, metal ions inhibit the enzyme reaction. This is again understandable on the basis of the view that some carboxy groups must be adjacent to the ester bond to be cleaved in order to allow the reaction to proceed. Indeed, if these groups are blocked by metal ions, the enzyme reaction cannot occur, and this is the reason for the apparent inhibition of the reaction by high concentrations of metal ions. Methylene Blue, which may be bound to pectin, may replace metal ions in the 'activation' and 'inhibition' of the enzyme reaction. A kinetic model based on these results has been proposed and fits the kinetic data very well. All the available results favour the view that metal ions do not affect the reaction through a direct interaction with enzyme, but rather with pectin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bradley D. F., Wolf M. K. AGGREGATION OF DYES BOUND TO POLYANIONS. Proc Natl Acad Sci U S A. 1959 Jul;45(7):944–952. doi: 10.1073/pnas.45.7.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha S. A simple method for derivation of rate equations for enzyme-catalyzed reactions under the rapid equilibrium assumption or combined assumptions of equilibrium and steady state. J Biol Chem. 1968 Feb 25;243(4):820–825. [PubMed] [Google Scholar]
- Crasnier M., Moustacas A. M., Ricard J. Electrostatic effects and calcium ion concentration as modulators of acid phosphatase bound to plant cell walls. Eur J Biochem. 1985 Aug 15;151(1):187–190. doi: 10.1111/j.1432-1033.1985.tb09084.x. [DOI] [PubMed] [Google Scholar]
- DEUEL H., STUTZ E. Pectic substances and pectic enzymes. Adv Enzymol Relat Subj Biochem. 1958;20:341–382. doi: 10.1002/9780470122655.ch11. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Huang C. Y. Derivation and initial velocity and isotope exchange rate equations. Methods Enzymol. 1979;63:54–84. doi: 10.1016/0076-6879(79)63006-9. [DOI] [PubMed] [Google Scholar]
- Lee M., Macmillan J. D. Mode of action of pectic enzymes. 3. Site of initial action of tomato pectinesterase on highly esterified pectin. Biochemistry. 1970 Apr 28;9(9):1930–1934. doi: 10.1021/bi00811a011. [DOI] [PubMed] [Google Scholar]
- Lee M., Macmillan J. D. Mode of action of pectic enzymes. I. Purification and certain properties of tomato pectinesterase. Biochemistry. 1968 Nov;7(11):4005–4010. doi: 10.1021/bi00851a030. [DOI] [PubMed] [Google Scholar]
- Martin R. B., Richardson F. S. Lanthanides as probes for calcium in biological systems. Q Rev Biophys. 1979 May;12(2):181–209. doi: 10.1017/s0033583500002754. [DOI] [PubMed] [Google Scholar]
- Miller L., Macmillan J. D. Purification and pattern of action of pectinesterase from Fusarium oxysporum f. sp. vasinfectum. Biochemistry. 1971 Feb 16;10(4):570–576. doi: 10.1021/bi00780a005. [DOI] [PubMed] [Google Scholar]
- Morris E. R., Powell D. A., Gidley M. J., Rees D. A. Conformations and interactions of pectins. I. Polymorphism between gel and solid states of calcium polygalacturonate. J Mol Biol. 1982 Mar 15;155(4):507–516. doi: 10.1016/0022-2836(82)90484-3. [DOI] [PubMed] [Google Scholar]
- Moustacas A. M., Nari J., Borel M., Noat G., Ricard J. Pectin methylesterase, metal ions and plant cell-wall extension. The role of metal ions in plant cell-wall extension. Biochem J. 1991 Oct 15;279(Pt 2):351–354. doi: 10.1042/bj2790351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustacas A. M., Nari J., Diamantidis G., Noat G., Crasnier M., Borel M., Ricard J. Electrostatic effects and the dynamics of enzyme reactions at the surface of plant cells. 2. The role of pectin methyl esterase in the modulation of electrostatic effects in soybean cell walls. Eur J Biochem. 1986 Feb 17;155(1):191–197. doi: 10.1111/j.1432-1033.1986.tb09476.x. [DOI] [PubMed] [Google Scholar]
- Nari J., Noat G., Diamantidis G., Woudstra M., Ricard J. Electrostatic effects and the dynamics of enzyme reactions at the surface of plant cells. 3. Interplay between limited cell-wall autolysis, pectin methyl esterase activity and electrostatic effects in soybean cell walls. Eur J Biochem. 1986 Feb 17;155(1):199–202. doi: 10.1111/j.1432-1033.1986.tb09477.x. [DOI] [PubMed] [Google Scholar]
- Powell D. A., Morris E. R., Gidley M. J., Rees D. A. Conformations and interactions of pectins. II. Influences of residue sequence on chain association in calcium pectate gels. J Mol Biol. 1982 Mar 15;155(4):517–531. doi: 10.1016/0022-2836(82)90485-5. [DOI] [PubMed] [Google Scholar]
- Rexová-Benková L., Markovic O. Pectic enzymes. Adv Carbohydr Chem Biochem. 1976;33:323–385. doi: 10.1016/s0065-2318(08)60285-1. [DOI] [PubMed] [Google Scholar]
- Ricard J., Noat G., Crasnier M., Job D. Ionic control of immobilized enzymes. Kinetics of acid phosphatase bound to plant cell walls. Biochem J. 1981 May 1;195(2):357–367. doi: 10.1042/bj1950357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard J., Noat G. Electrostatic effects and the dynamics of enzyme reactions at the surface of plant cells. 1. A theory of the ionic control of a complex multi-enzyme system. Eur J Biochem. 1986 Feb 17;155(1):183–190. doi: 10.1111/j.1432-1033.1986.tb09475.x. [DOI] [PubMed] [Google Scholar]
- Wood P. J., Siddiqui I. R. Determination of methanol and its application to measurement of pectin ester content and pectin methyl esterase activity. Anal Biochem. 1971 Feb;39(2):418–428. doi: 10.1016/0003-2697(71)90432-5. [DOI] [PubMed] [Google Scholar]
- Zimmerman R. E. A rapid assay for pectinesterase activity which can be used as a prescreen for pectinesterase inhibitors. Anal Biochem. 1978 Mar;85(1):219–223. doi: 10.1016/0003-2697(78)90292-0. [DOI] [PubMed] [Google Scholar]


