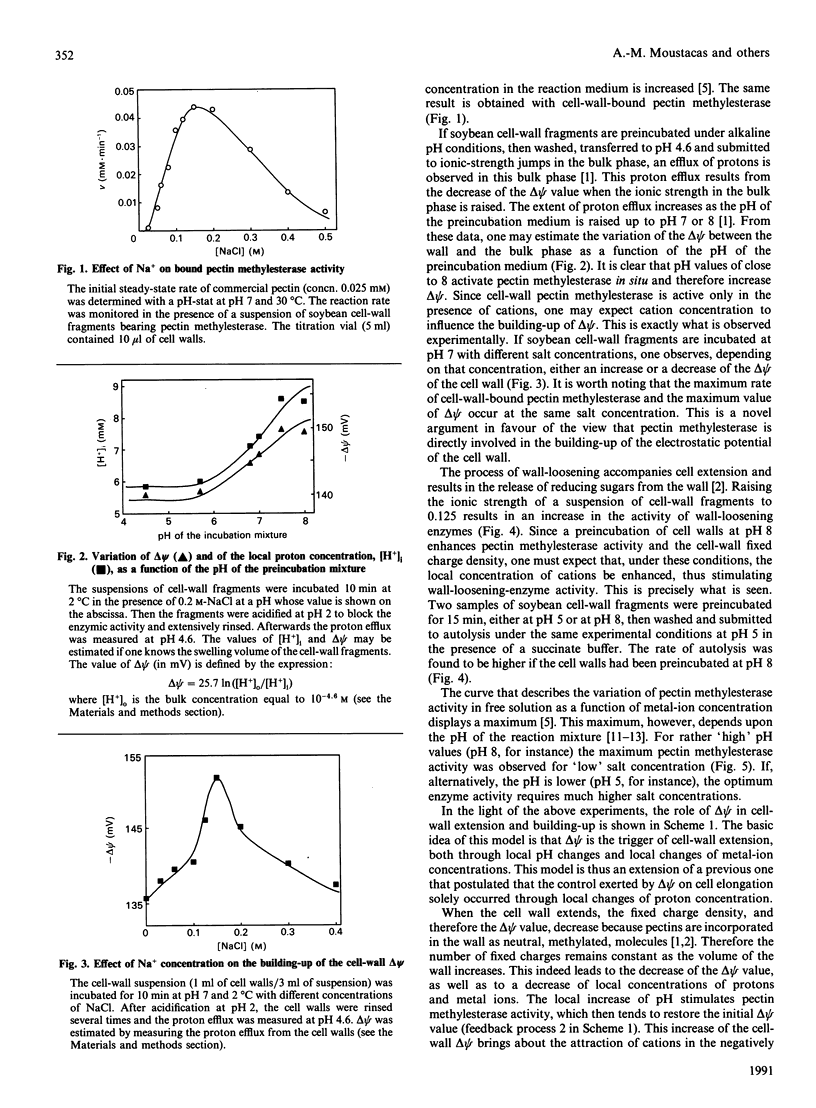
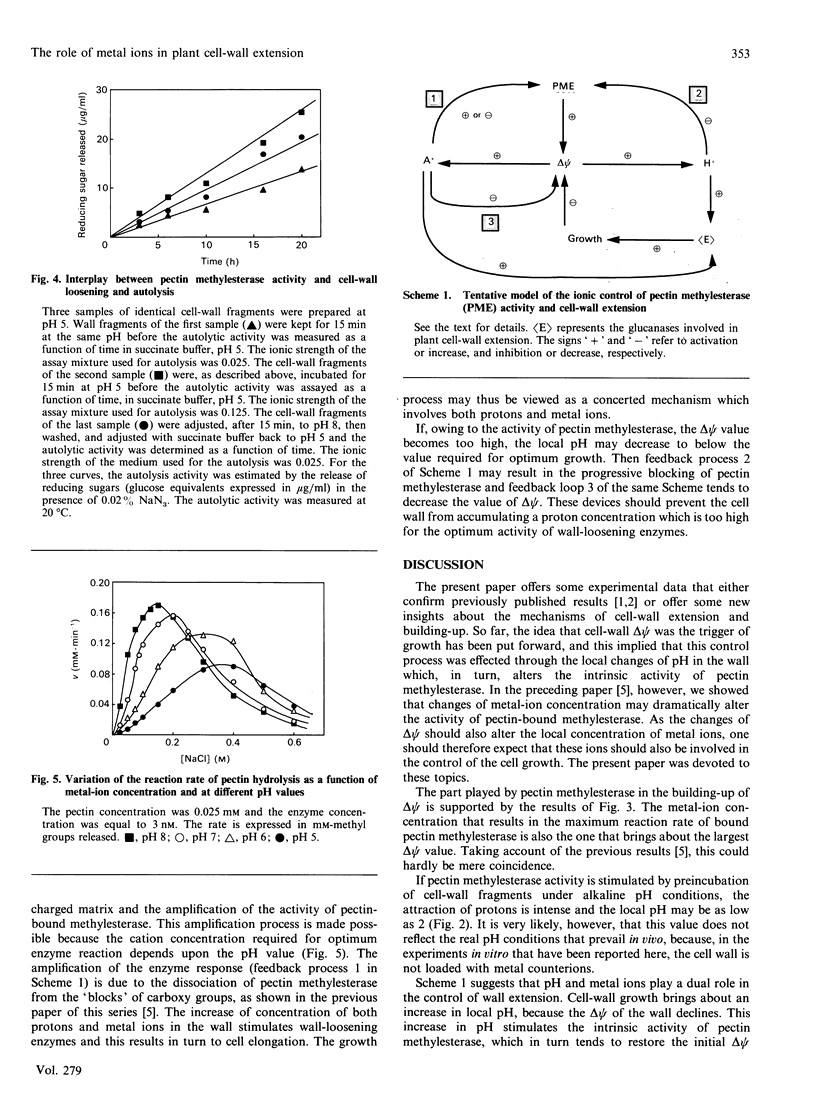
Abstract
The study of pectin methylesterase and wall-loosening enzyme activities in situ, as well as the estimation of the electrostatic potential of the cell wall, suggest a coherent picture of the role played by metal ions and pH in cell-wall extension. Cell-wall growth brings about a decrease of local proton concentration because the electrostatic potential difference (delta psi) of the wall decreases. This in turn activates pectin methylesterase, which restores the initial delta psi value. This process is amplified by the attraction of metal ions in the polyanionic cell-wall matrix. The amplification process is basically due to the release of enzyme molecules that were initially bound to 'blocks' of carboxy groups. This increase of metal-ion concentration also results in the activation of wall-loosening enzymes. Moreover, the apparent 'inhibition' of pectin methylesterase by high salt concentrations may be considered as a device which prevents the electrostatic potential from becoming too high.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crasnier M., Moustacas A. M., Ricard J. Electrostatic effects and calcium ion concentration as modulators of acid phosphatase bound to plant cell walls. Eur J Biochem. 1985 Aug 15;151(1):187–190. doi: 10.1111/j.1432-1033.1985.tb09084.x. [DOI] [PubMed] [Google Scholar]
- DEUEL H., STUTZ E. Pectic substances and pectic enzymes. Adv Enzymol Relat Subj Biochem. 1958;20:341–382. doi: 10.1002/9780470122655.ch11. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Lee M., Macmillan J. D. Mode of action of pectic enzymes. 3. Site of initial action of tomato pectinesterase on highly esterified pectin. Biochemistry. 1970 Apr 28;9(9):1930–1934. doi: 10.1021/bi00811a011. [DOI] [PubMed] [Google Scholar]
- Moustacas A. M., Nari J., Diamantidis G., Noat G., Crasnier M., Borel M., Ricard J. Electrostatic effects and the dynamics of enzyme reactions at the surface of plant cells. 2. The role of pectin methyl esterase in the modulation of electrostatic effects in soybean cell walls. Eur J Biochem. 1986 Feb 17;155(1):191–197. doi: 10.1111/j.1432-1033.1986.tb09476.x. [DOI] [PubMed] [Google Scholar]
- Nari J., Noat G., Diamantidis G., Woudstra M., Ricard J. Electrostatic effects and the dynamics of enzyme reactions at the surface of plant cells. 3. Interplay between limited cell-wall autolysis, pectin methyl esterase activity and electrostatic effects in soybean cell walls. Eur J Biochem. 1986 Feb 17;155(1):199–202. doi: 10.1111/j.1432-1033.1986.tb09477.x. [DOI] [PubMed] [Google Scholar]
- Nari J., Noat G., Ricard J. Pectin methylesterase, metal ions and plant cell-wall extension. Hydrolysis of pectin by plant cell-wall pectin methylesterase. Biochem J. 1991 Oct 15;279(Pt 2):343–350. doi: 10.1042/bj2790343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nari J., Noat G., Ricard J. pH-induced co-operative effects in hysteretic enzymes. 2. pH-induced co-operative effects in a cell-wall beta-glucosyltransferase. Eur J Biochem. 1984 Dec 3;145(2):319–322. doi: 10.1111/j.1432-1033.1984.tb08555.x. [DOI] [PubMed] [Google Scholar]
- Rexová-Benková L., Markovic O. Pectic enzymes. Adv Carbohydr Chem Biochem. 1976;33:323–385. doi: 10.1016/s0065-2318(08)60285-1. [DOI] [PubMed] [Google Scholar]
- Ricard J., Noat G. Electrostatic effects and the dynamics of enzyme reactions at the surface of plant cells. 1. A theory of the ionic control of a complex multi-enzyme system. Eur J Biochem. 1986 Feb 17;155(1):183–190. doi: 10.1111/j.1432-1033.1986.tb09475.x. [DOI] [PubMed] [Google Scholar]


