Abstract
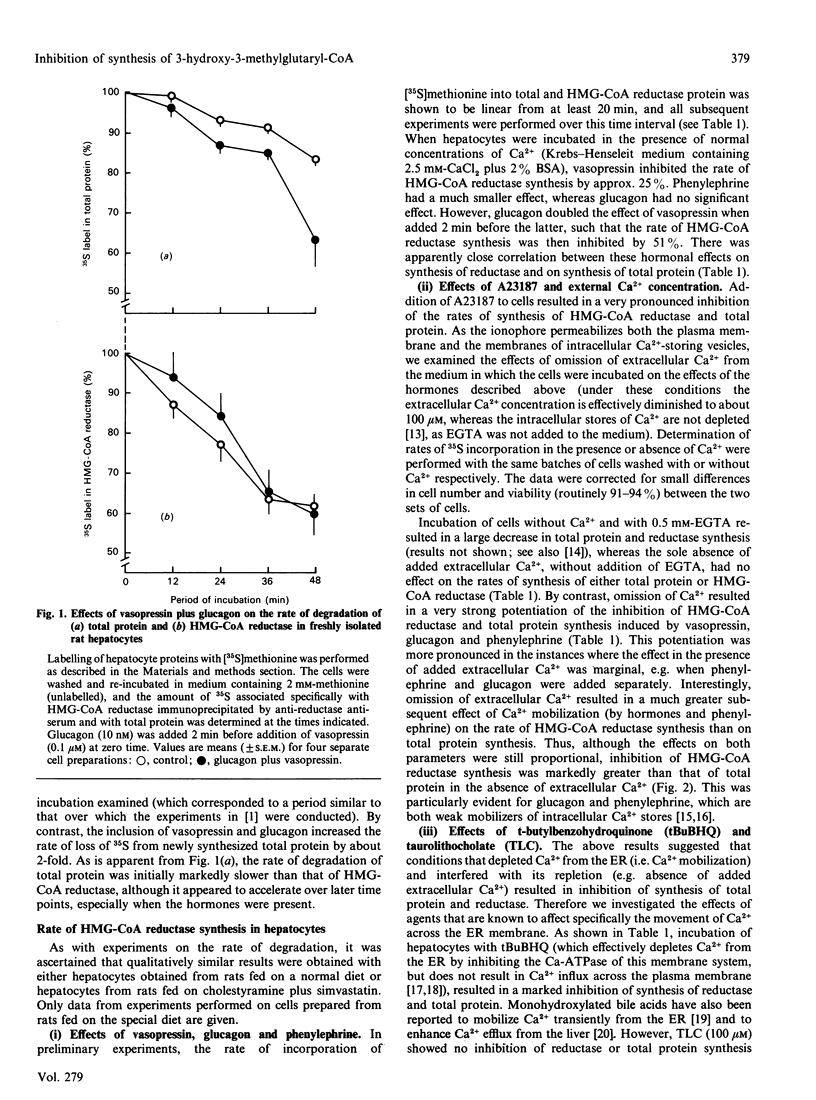
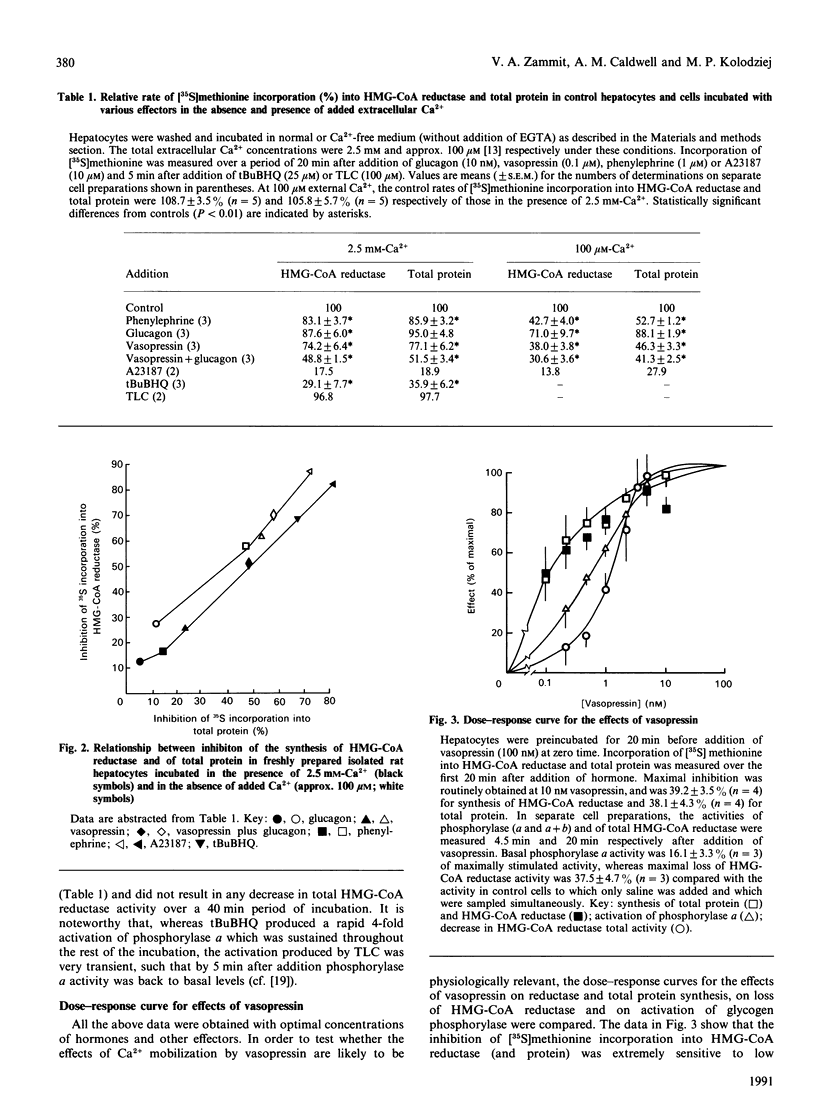
The mechanisms through which Ca2+ mobilization in rat hepatocytes results in the loss of total activity of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase [Zammit & Caldwell (1990) Biochem. J. 269, 373-379] were investigated. The loss of total activity was shown to be paralleled by an equal loss of immunoreactive HMG-CoA reductase protein after exposure of hepatocytes to optimal concentrations of vasopressin plus glucagon for 40 min. This loss of enzyme protein was due to an inhibition of enzyme synthesis; the rate of degradation was unaffected. Other Ca(2+)-mobilizing conditions (phenylephrine, glucagon, vasopressin added singly and A23187) also resulted in graded inhibition of synthesis of HMG-CoA reductase. These effects were accentuated by omission of Ca2+ from the cell incubation medium, suggesting that it is the depletion of an intracellular InsP3-sensitive pool of Ca2+ to which synthesis of HMG-CoA reductase is sensitive. In agreement with this we found that t-butylhydroxybenzoquinone, which inhibits the activity of the Ca(2+)-ATPase of the endoplasmic-reticular membrane, mimicked the action of Ca(2+)-mobilizing hormones. However, taurolithocholate, which transiently mobilizes Ca2+ from the same pool, was ineffective. All these effects on HMG-CoA reductase were accompanied by parallel inhibition of 35S incorporation from [35S]methionine into total protein, suggesting that inhibition of reductase synthesis formed part of a generalized response of the hepatocyte to Ca2+ mobilization. Inhibition of the rate of synthesis of HMG-CoA reductase was, however, more responsive to Ca2+ mobilization in the absence of added Ca2+ from the extracellular medium. The concentrations of vasopressin required to elicit the inhibition of synthesis of HMG-CoA reductase were of the same order as those that elicited activation of glycogen phosphorylase in hepatocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altin J. G., Bygrave F. L. Second messengers and the regulation of Ca2+ fluxes by Ca2+-mobilizing agonists in rat liver. Biol Rev Camb Philos Soc. 1988 Nov;63(4):551–611. doi: 10.1111/j.1469-185x.1988.tb00670.x. [DOI] [PubMed] [Google Scholar]
- Anwer M. S., Engelking L. R., Nolan K., Sullivan D., Zimniak P., Lester R. Hepatotoxic bile acids increase cytosolic Ca++ activity of isolated rat hepatocytes. Hepatology. 1988 Jul-Aug;8(4):887–891. doi: 10.1002/hep.1840080430. [DOI] [PubMed] [Google Scholar]
- Anwer M. S., Little J. M., Oelberg D. G., Zimniak P., Lester R. Effect of bile acids on calcium efflux from isolated rat hepatocytes and perfused rat livers. Proc Soc Exp Biol Med. 1989 Jun;191(2):147–152. doi: 10.3181/00379727-191-42900. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Strickland W. G., Bocckino S. B., Exton J. H. Mechanism of hepatic glycogen synthase inactivation induced by Ca2+-mobilizing hormones. Studies using phospholipase C and phorbol myristate acetate. Biochem J. 1986 Jul 1;237(1):235–242. doi: 10.1042/bj2370235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brostrom C. O., Bocckino S. B., Brostrom M. A., Galuska E. M. Regulation of protein synthesis in isolated hepatocytes by calcium-mobilizing hormones. Mol Pharmacol. 1986 Jan;29(1):104–111. [PubMed] [Google Scholar]
- Brostrom C. O., Brostrom M. A. Calcium-dependent regulation of protein synthesis in intact mammalian cells. Annu Rev Physiol. 1990;52:577–590. doi: 10.1146/annurev.ph.52.030190.003045. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Simoni R. D. Biogenesis of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, an integral glycoprotein of the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1674–1678. doi: 10.1073/pnas.81.6.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T. M., Exton J. H. alpha-Adrenergic-mediated accumulation of adenosine 3':5' monophosphate in calcium-depleted hepatocytes. J Biol Chem. 1977 Dec 10;252(23):8645–8651. [PubMed] [Google Scholar]
- Chin K. V., Cade C., Brostrom C. O., Galuska E. M., Brostrom M. A. Calcium-dependent regulation of protein synthesis at translational initiation in eukaryotic cells. J Biol Chem. 1987 Dec 5;262(34):16509–16514. [PubMed] [Google Scholar]
- Chin K. V., Cade C., Brostrom M. A., Brostrom C. O. Regulation of protein synthesis in intact rat liver by calcium mobilizing agents. Int J Biochem. 1988;20(11):1313–1319. doi: 10.1016/0020-711x(88)90236-4. [DOI] [PubMed] [Google Scholar]
- Chun K. T., Bar-Nun S., Simoni R. D. The regulated degradation of 3-hydroxy-3-methylglutaryl-CoA reductase requires a short-lived protein and occurs in the endoplasmic reticulum. J Biol Chem. 1990 Dec 15;265(35):22004–22010. [PubMed] [Google Scholar]
- Clarke P. R., Hardie D. G. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 1990 Aug;9(8):2439–2446. doi: 10.1002/j.1460-2075.1990.tb07420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combettes L., Berthon B., Doucet E., Erlinger S., Claret M. Bile acids mobilise internal Ca2+ independently of external Ca2+ in rat hepatocytes. Eur J Biochem. 1990 Jul 5;190(3):619–623. doi: 10.1111/j.1432-1033.1990.tb15617.x. [DOI] [PubMed] [Google Scholar]
- Combettes L., Berthon B., Doucet E., Erlinger S., Claret M. Characteristics of bile acid-mediated Ca2+ release from permeabilized liver cells and liver microsomes. J Biol Chem. 1989 Jan 5;264(1):157–167. [PubMed] [Google Scholar]
- Combettes L., Dumont M., Berthon B., Erlinger S., Claret M. Release of calcium from the endoplasmic reticulum by bile acids in rat liver cells. J Biol Chem. 1988 Feb 15;263(5):2299–2303. [PubMed] [Google Scholar]
- Edwards P. A., Lan S. F., Fogelman A. M. Alterations in the rates of synthesis and degradation of rat liver 3-hydroxy-3-methylglutaryl coenzyme A reductase produced by cholestyramine and mevinolin. J Biol Chem. 1983 Sep 10;258(17):10219–10222. [PubMed] [Google Scholar]
- Edwards P. A., Lan S. F., Fogelman A. M. The effect of glucagon on the synthesis and degradation of 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Lipid Res. 1986 Apr;27(4):398–403. [PubMed] [Google Scholar]
- Farrell G. C., Duddy S. K., Kass G. E., Llopis J., Gahm A., Orrenius S. Release of Ca2+ from the endoplasmic reticulum is not the mechanism for bile acid-induced cholestasis and hepatotoxicity in the intact rat liver. J Clin Invest. 1990 Apr;85(4):1255–1259. doi: 10.1172/JCI114561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gibson D. M., Parker R. A., Stewart C. S., Evenson K. J. Short-term regulation of hydroxymethylglutaryl coenzyme A reductase by reversible phosphorylation: modulation of reductase phosphatase in rat hepatocytes. Adv Enzyme Regul. 1982;20:263–283. doi: 10.1016/0065-2571(82)90020-6. [DOI] [PubMed] [Google Scholar]
- Haro D., Marrero P. F., Ayté J., Hegardt F. G. Identification of a cholesterol-regulated 180-kDa microsomal protein in rat hepatocytes. Eur J Biochem. 1990 Feb 22;188(1):123–129. doi: 10.1111/j.1432-1033.1990.tb15379.x. [DOI] [PubMed] [Google Scholar]
- Kass G. E., Duddy S. K., Moore G. A., Orrenius S. 2,5-Di-(tert-butyl)-1,4-benzohydroquinone rapidly elevates cytosolic Ca2+ concentration by mobilizing the inositol 1,4,5-trisphosphate-sensitive Ca2+ pool. J Biol Chem. 1989 Sep 15;264(26):15192–15198. [PubMed] [Google Scholar]
- Kimball S. R., Jefferson L. S. Mechanism of the inhibition of protein synthesis by vasopressin in rat liver. J Biol Chem. 1990 Oct 5;265(28):16794–16798. [PubMed] [Google Scholar]
- Kumar R. V., Wolfman A., Panniers R., Henshaw E. C. Mechanism of inhibition of polypeptide chain initiation in calcium-depleted Ehrlich ascites tumor cells. J Cell Biol. 1989 Jun;108(6):2107–2115. doi: 10.1083/jcb.108.6.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero P. F., Haro D., Hegardt F. G. Phosphorylation of HMG-CoA reductase induced by mevalonate accelerates its rate of degradation in isolated rat hepatocytes. FEBS Lett. 1986 Mar 3;197(1-2):183–186. doi: 10.1016/0014-5793(86)80323-4. [DOI] [PubMed] [Google Scholar]
- Meldolesi J., Madeddu L., Pozzan T. Intracellular Ca2+ storage organelles in non-muscle cells: heterogeneity and functional assignment. Biochim Biophys Acta. 1990 Nov 12;1055(2):130–140. doi: 10.1016/0167-4889(90)90113-r. [DOI] [PubMed] [Google Scholar]
- Menaya J., Parrilla R., Ayuso M. S. Action of phenylephrine on protein synthesis in liver cells. Biochem J. 1987 Dec 15;248(3):903–909. doi: 10.1042/bj2480903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G. A., McConkey D. J., Kass G. E., O'Brien P. J., Orrenius S. 2,5-Di(tert-butyl)-1,4-benzohydroquinone--a novel inhibitor of liver microsomal Ca2+ sequestration. FEBS Lett. 1987 Nov 30;224(2):331–336. doi: 10.1016/0014-5793(87)80479-9. [DOI] [PubMed] [Google Scholar]
- Pandak W. M., Heuman D. M., Hylemon P. B., Vlahcevic Z. R. Regulation of bile acid synthesis. IV. Interrelationship between cholesterol and bile acid biosynthesis pathways. J Lipid Res. 1990 Jan;31(1):79–90. [PubMed] [Google Scholar]
- Parker R. A., Miller S. J., Gibson D. M. Phosphorylation of microsomal HMG CoA reductase increases susceptibility to proteolytic degradation in vitro. Biochem Biophys Res Commun. 1984 Dec 14;125(2):629–635. doi: 10.1016/0006-291x(84)90585-0. [DOI] [PubMed] [Google Scholar]
- Parker R. A., Miller S. J., Gibson D. M. Phosphorylation of native 97-kDa 3-hydroxy-3-methylglutaryl-coenzyme A reductase from rat liver. Impact on activity and degradation of the enzyme. J Biol Chem. 1989 Mar 25;264(9):4877–4887. [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Wileman T., Kane L. P., Carson G. R., Terhorst C. Depletion of cellular calcium accelerates protein degradation in the endoplasmic reticulum. J Biol Chem. 1991 Mar 5;266(7):4500–4507. [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Joseph S. K., Thomas A. P. Inositol trisphosphate and diacylglycerol as intracellular second messengers in liver. Am J Physiol. 1985 Mar;248(3 Pt 1):C203–C216. doi: 10.1152/ajpcell.1985.248.3.C203. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Agonist-induced oscillations in cytoplasmic free calcium concentration in single rat hepatocytes. Cell Calcium. 1987 Feb;8(1):79–100. doi: 10.1016/0143-4160(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Dixon C. J., Cuthbertson K. S., Cobbold P. H. Modulation of free Ca oscillations in single hepatocytes by changes in extracellular K+, Na+ and Ca2+. Cell Calcium. 1990 May;11(5):353–360. doi: 10.1016/0143-4160(90)90038-v. [DOI] [PubMed] [Google Scholar]
- Zammit V. A., Caldwell A. M. Conditions that result in the mobilization and influx of Ca2+ into rat hepatocytes induce the rapid loss of 3-hydroxy-3-methylglutaryl-CoA reductase activity that is not reversed by phosphatase treatment. Biochem J. 1990 Jul 15;269(2):373–379. doi: 10.1042/bj2690373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A., Caldwell A. M. The roles of different protein kinases and of calmodulin in the effects of Ca2+ mobilization on 3-hydroxy-3-methylglutaryl-CoA reductase activity in isolated rat hepatocytes. Biochem J. 1991 Jan 15;273(Pt 2):485–488. doi: 10.1042/bj2730485. [DOI] [PMC free article] [PubMed] [Google Scholar]


