Abstract
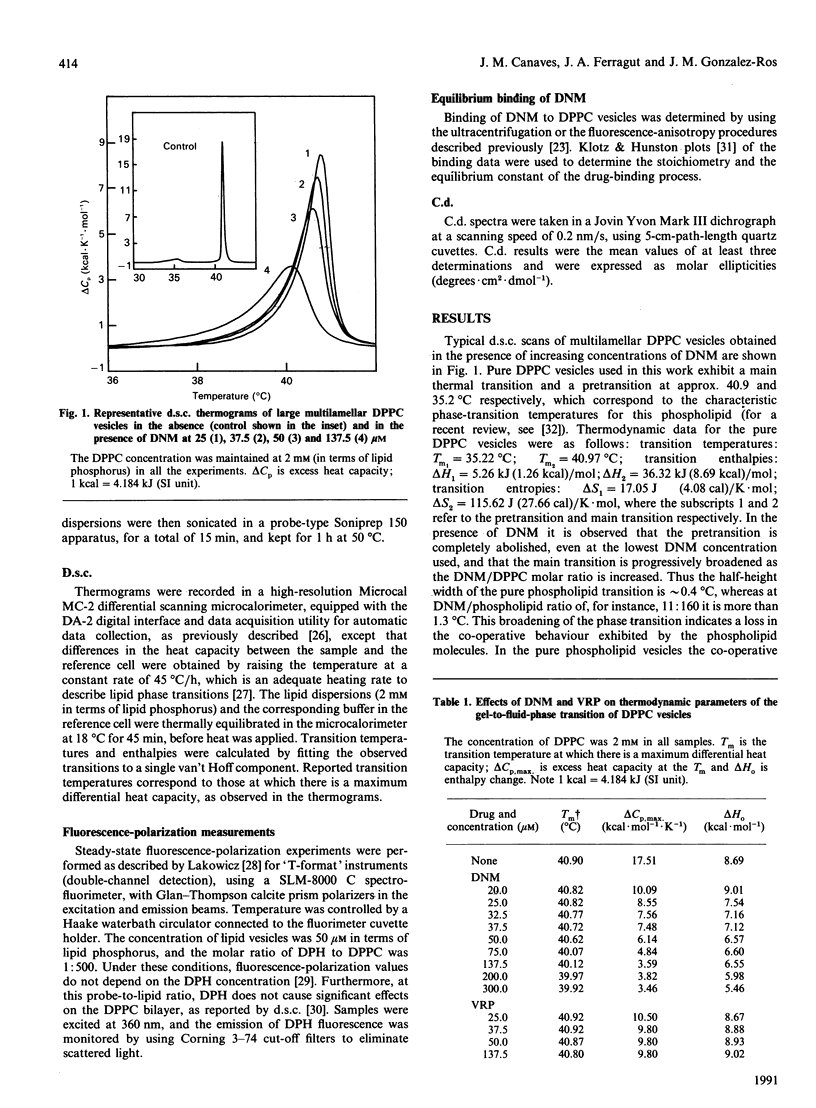
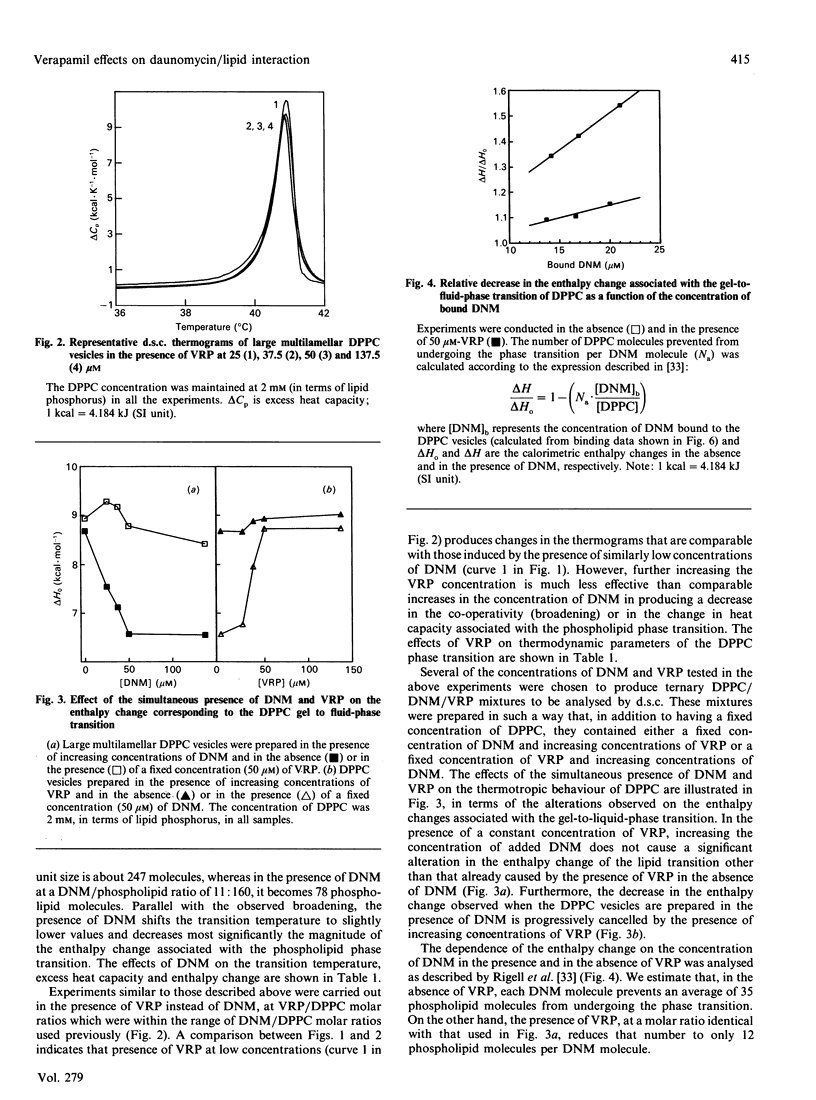
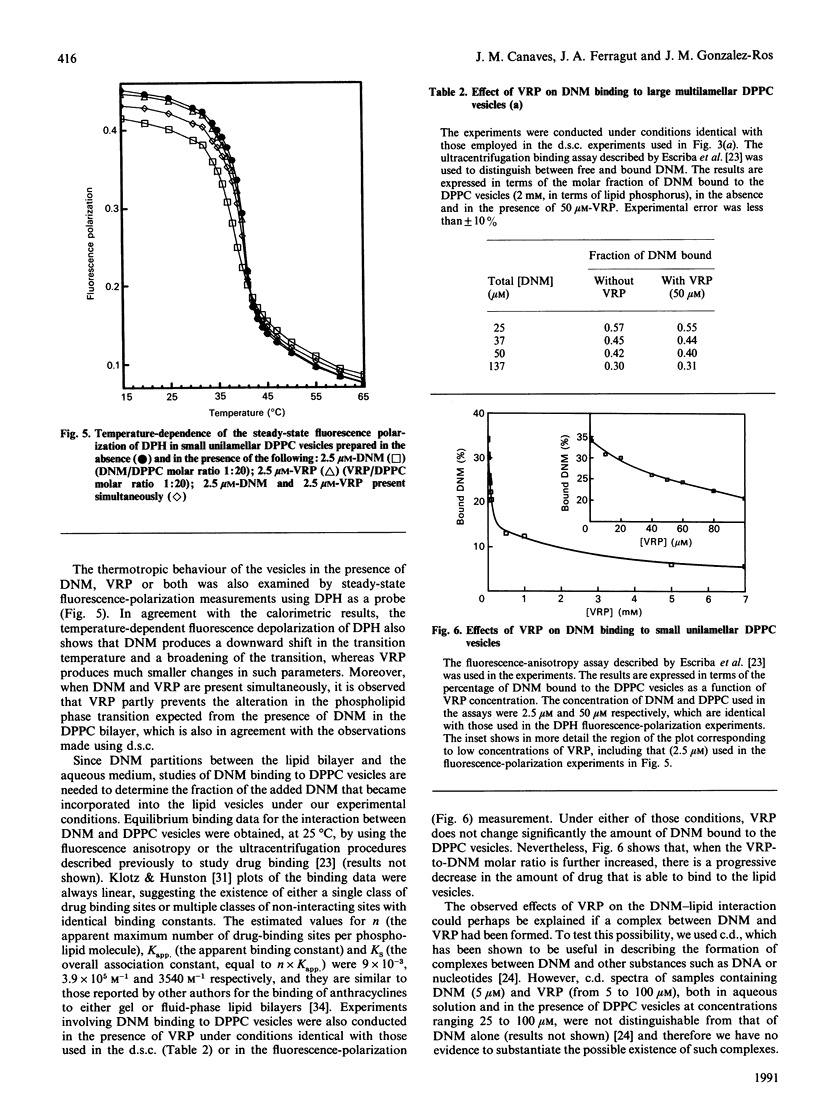
High-sensitivity differential scanning calorimetry and fluorescence-depolarization techniques were used to study how the presence of daunomycin and/or verapamil affect the thermotropic behaviour of dipalmitoyl phosphatidylcholine (DPPC) vesicles. Daunomycin, a potent anti-cancer agent, perturbs the thermodynamic parameters associated with the lipid phase transition: it decreases the enthalpy change, lowers the transition temperature and reduces the co-operative behavior of the phospholipid molecules. Verapamil, on the other hand, produces smaller alterations in the lipid phase transition. However, when daunomycin and verapamil are present simultaneously in the DPPC vesicles, it is observed that verapamil prevents, in a concentration-dependent manner, the alteration in the phospholipid phase transition expected from the presence of daunomycin in the bilayer. Furthermore, drug-binding studies suggest that the observed interference of verapamil in the daunomycin/phospholipid interaction occurs without a decrease in the amount of daunomycin bound to the lipid bilayer and without the formation of a daunomycin-verapamil complex. Because of the importance of drug-membrane interactions in anthracycline cytotoxicity, we speculate that the lipid bilayer of biological membranes may provide appropriate sites at which the presence of verapamil influences the activity of daunomycin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S., Cornwell M. M., Kuwano M., Pastan I., Gottesman M. M. Most drugs that reverse multidrug resistance also inhibit photoaffinity labeling of P-glycoprotein by a vinblastine analog. Mol Pharmacol. 1988 Feb;33(2):144–147. [PubMed] [Google Scholar]
- Bach D., Raz A., Goldman R. The effect of hashish compounds on pphospholipid phase transition. Biochim Biophys Acta. 1976 Jul 15;436(4):889–894. doi: 10.1016/0005-2736(76)90420-x. [DOI] [PubMed] [Google Scholar]
- Barcelo F., Barcelo I., Gavilanes F., Ferragut J. A., Yanovich S., Gonzalez-Ros J. M. Interaction of anthracyclines with nucleotides and related compounds studied by spectroscopy. Biochim Biophys Acta. 1986 Oct 29;884(1):172–181. doi: 10.1016/0304-4165(86)90241-2. [DOI] [PubMed] [Google Scholar]
- Bell D. R., Gerlach J. H., Kartner N., Buick R. N., Ling V. Detection of P-glycoprotein in ovarian cancer: a molecular marker associated with multidrug resistance. J Clin Oncol. 1985 Mar;3(3):311–315. doi: 10.1200/JCO.1985.3.3.311. [DOI] [PubMed] [Google Scholar]
- Bradley G., Juranka P. F., Ling V. Mechanism of multidrug resistance. Biochim Biophys Acta. 1988 Aug 3;948(1):87–128. doi: 10.1016/0304-419x(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Bruggemann E. P., Germann U. A., Gottesman M. M., Pastan I. Two different regions of P-glycoprotein [corrected] are photoaffinity-labeled by azidopine. J Biol Chem. 1989 Sep 15;264(26):15483–15488. [PubMed] [Google Scholar]
- Bruggemann E. P., Melchior D. L. Alterations in the organization of phosphatidylcholine/cholesterol bilayers by tetrahydrocannabinol. J Biol Chem. 1983 Jul 10;258(13):8298–8303. [PubMed] [Google Scholar]
- Burke T. G., Tritton T. R. Structural basis of anthracycline selectivity for unilamellar phosphatidylcholine vesicles: an equilibrium binding study. Biochemistry. 1985 Mar 26;24(7):1768–1776. doi: 10.1021/bi00328a030. [DOI] [PubMed] [Google Scholar]
- Cheetham J. J., Wachtel E., Bach D., Epand R. M. Role of the stereochemistry of the hydroxyl group of cholesterol and the formation of nonbilayer structures in phosphatidylethanolamines. Biochemistry. 1989 Oct 31;28(22):8928–8934. doi: 10.1021/bi00448a036. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Downes H. F., Slovak M. L. Effect of calcium antagonists on the chemosensitivity of two multidrug-resistant human tumour cell lines which do not overexpress P-glycoprotein. Br J Cancer. 1989 Jan;59(1):42–46. doi: 10.1038/bjc.1989.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides P. P., Inouchi N., Tritton T. R., Sartorelli A. C., Sturtevant J. M. A scanning calorimetric study of the interaction of anthracyclines with neutral and acidic phospholipids alone and in binary mixtures. J Biol Chem. 1986 Aug 5;261(22):10196–10203. [PubMed] [Google Scholar]
- Cornwell M. M., Pastan I., Gottesman M. M. Certain calcium channel blockers bind specifically to multidrug-resistant human KB carcinoma membrane vesicles and inhibit drug binding to P-glycoprotein. J Biol Chem. 1987 Feb 15;262(5):2166–2170. [PubMed] [Google Scholar]
- Danks M. K., Yalowich J. C., Beck W. T. Atypical multiple drug resistance in a human leukemic cell line selected for resistance to teniposide (VM-26). Cancer Res. 1987 Mar 1;47(5):1297–1301. [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Escriba P. V., Ferrer-Montiel A. V., Ferragut J. A., Gonzalez-Ros J. M. Role of membrane lipids in the interaction of daunomycin with plasma membranes from tumor cells: implications in drug-resistance phenomena. Biochemistry. 1990 Aug 7;29(31):7275–7282. doi: 10.1021/bi00483a017. [DOI] [PubMed] [Google Scholar]
- Ferrer-Montiel A. V., Gonzalez-Ros J. M., Ferragut J. A. Association of daunomycin to membrane domains studied by fluorescence resonance energy transfer. Biochim Biophys Acta. 1988 Jan 22;937(2):379–386. doi: 10.1016/0005-2736(88)90260-x. [DOI] [PubMed] [Google Scholar]
- Fojo A. T., Ueda K., Slamon D. J., Poplack D. G., Gottesman M. M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Segura L. M., Ferragut J. A., Ferrer-Montiel A. V., Escriba P. V., Gonzalez-Ros J. M. Ultrastructural alterations in plasma membranes from drug-resistant P388 murine leukemia cells. Biochim Biophys Acta. 1990 Nov 2;1029(1):191–195. doi: 10.1016/0005-2736(90)90454-v. [DOI] [PubMed] [Google Scholar]
- Goldman R., Facchinetti T., Bach D., Raz A., Shinitzky M. A differential interaction of daunomycin, adriamycin and their derivatives with human erythrocytes and phospholipid bilayers. Biochim Biophys Acta. 1978 Sep 22;512(2):254–269. doi: 10.1016/0005-2736(78)90251-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Ros J. M., Llanillo M., Paraschos A., Martinez-Carrion M. Lipid environment of acetylcholine receptor from Torpedo californica. Biochemistry. 1982 Jul 6;21(14):3467–3474. doi: 10.1021/bi00257a033. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. The multidrug transporter, a double-edged sword. J Biol Chem. 1988 Sep 5;263(25):12163–12166. [PubMed] [Google Scholar]
- Griffin E. A., Vanderkooi J. M., Maniara G., Erecińska M. Anthracycline binding to synthetic and natural membranes. A study using resonance energy transfer. Biochemistry. 1986 Dec 2;25(24):7875–7880. doi: 10.1021/bi00372a013. [DOI] [PubMed] [Google Scholar]
- Hindenburg A. A., Baker M. A., Gleyzer E., Stewart V. J., Case N., Taub R. N. Effect of verapamil and other agents on the distribution of anthracyclines and on reversal of drug resistance. Cancer Res. 1987 Mar 1;47(5):1421–1425. [PubMed] [Google Scholar]
- Kamiwatari M., Nagata Y., Kikuchi H., Yoshimura A., Sumizawa T., Shudo N., Sakoda R., Seto K., Akiyama S. Correlation between reversing of multidrug resistance and inhibiting of [3H]azidopine photolabeling of P-glycoprotein by newly synthesized dihydropyridine analogues in a human cell line. Cancer Res. 1989 Jun 15;49(12):3190–3195. [PubMed] [Google Scholar]
- Klotz I. M., Hunston D. L. Properties of graphical representations of multiple classes of binding sites. Biochemistry. 1971 Aug 3;10(16):3065–3069. doi: 10.1021/bi00792a013. [DOI] [PubMed] [Google Scholar]
- Kursch B., Lüllmann H., Mohr K. Influence of various cationic amphiphilic drugs on the phase-transition temperature of phosphatidylcholine liposomes. Biochem Pharmacol. 1983 Sep 1;32(17):2589–2594. doi: 10.1016/0006-2952(83)90023-0. [DOI] [PubMed] [Google Scholar]
- Kyaw A., Maung-U K., Toe T. Determination of inorganic phosphate with molybdate and Triton X-100 without reduction. Anal Biochem. 1985 Mar;145(2):230–234. doi: 10.1016/0003-2697(85)90354-9. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., Freire E., Biltonen R. L. Fluorescence and calorimetric studies of phase transitions in phosphatidylcholine multilayers: kinetics of the pretransition. Biochemistry. 1978 Oct 17;17(21):4475–4480. doi: 10.1021/bi00614a018. [DOI] [PubMed] [Google Scholar]
- McGrath T., Center M. S. Adriamycin resistance in HL60 cells in the absence of detectable P-glycoprotein. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1171–1176. doi: 10.1016/0006-291x(87)91560-9. [DOI] [PubMed] [Google Scholar]
- Naito M., Tsuruo T. Competitive inhibition by verapamil of ATP-dependent high affinity vincristine binding to the plasma membrane of multidrug-resistant K562 cells without calcium ion involvement. Cancer Res. 1989 Mar 15;49(6):1452–1455. [PubMed] [Google Scholar]
- Nicolay K., Sautereau A. M., Tocanne J. F., Brasseur R., Huart P., Ruysschaert J. M., de Kruijff B. A comparative model membrane study on structural effects of membrane-active positively charged anti-tumor drugs. Biochim Biophys Acta. 1988 May 24;940(2):197–208. doi: 10.1016/0005-2736(88)90195-2. [DOI] [PubMed] [Google Scholar]
- Norris M. D., Haber M., King M., Davey R. A. Atypical multidrug resistance in CCRF-CEM cells selected for high level methotrexate resistance: reactivity to monoclonal antibody C219 in the absence of P-glycoprotein expression. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1435–1441. doi: 10.1016/0006-291x(89)92764-2. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Jacobson K., Poste G., Shepherd G. Effects of local anesthetics on membrane properties. I. Changes in the fluidity of phospholipid bilayers. Biochim Biophys Acta. 1975 Jul 18;394(4):504–519. doi: 10.1016/0005-2736(75)90137-6. [DOI] [PubMed] [Google Scholar]
- Qian X. D., Beck W. T. Binding of an optically pure photoaffinity analogue of verapamil, LU-49888, to P-glycoprotein from multidrug-resistant human leukemic cell lines. Cancer Res. 1990 Feb 15;50(4):1132–1137. [PubMed] [Google Scholar]
- Rigell C. W., de Saussure C., Freire E. Protein and lipid structural transitions in cytochrome c oxidase-dimyristoylphosphatidylcholine reconstitutions. Biochemistry. 1985 Sep 24;24(20):5638–5646. doi: 10.1021/bi00341a053. [DOI] [PubMed] [Google Scholar]
- Safa A. R., Glover C. J., Sewell J. L., Meyers M. B., Biedler J. L., Felsted R. L. Identification of the multidrug resistance-related membrane glycoprotein as an acceptor for calcium channel blockers. J Biol Chem. 1987 Jun 5;262(16):7884–7888. [PubMed] [Google Scholar]
- Safa A. R. Photoaffinity labeling of the multidrug-resistance-related P-glycoprotein with photoactive analogs of verapamil. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7187–7191. doi: 10.1073/pnas.85.19.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar M. T., Artigues A., Ferragut J. A., Gonzalez-Ros J. M. Phospholipase A2 hydrolysis of membrane phospholipids causes structural alteration of the nicotinic acetylcholine receptor. Biochim Biophys Acta. 1988 Feb 8;938(1):35–43. doi: 10.1016/0005-2736(88)90119-8. [DOI] [PubMed] [Google Scholar]
- Zamora J. M., Pearce H. L., Beck W. T. Physical-chemical properties shared by compounds that modulate multidrug resistance in human leukemic cells. Mol Pharmacol. 1988 Apr;33(4):454–462. [PubMed] [Google Scholar]


