Abstract
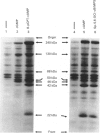
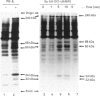
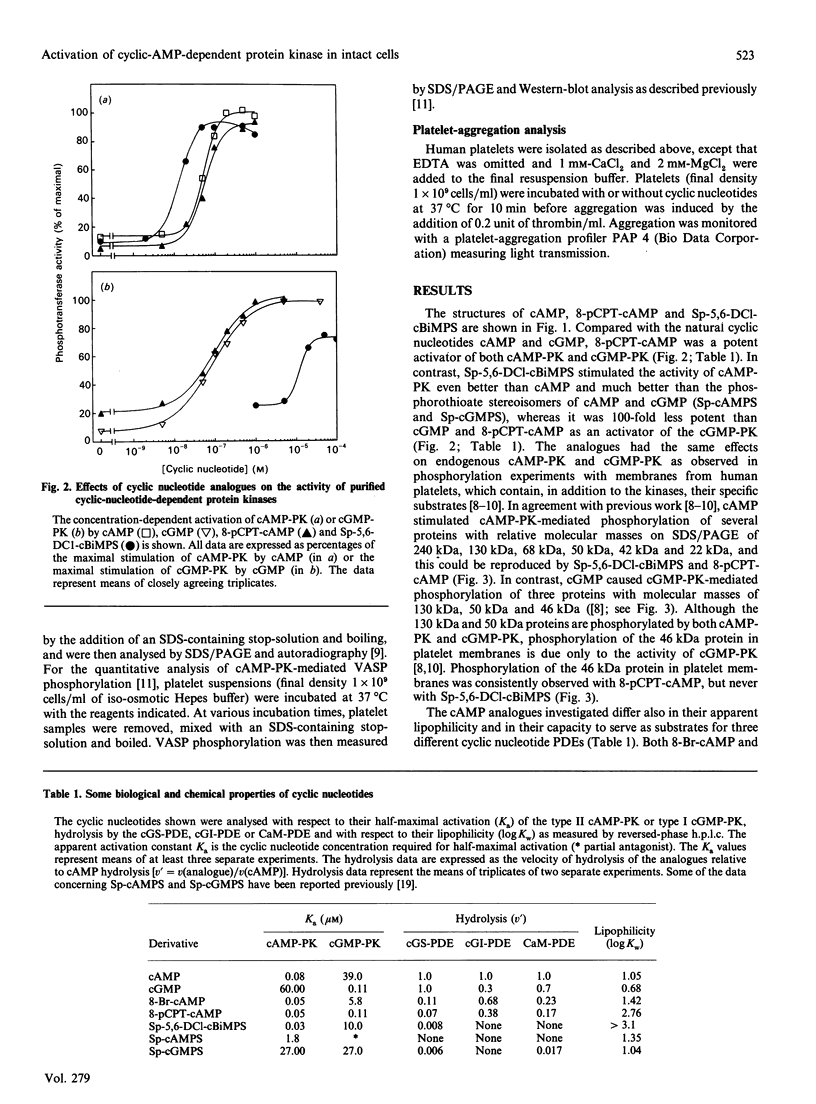
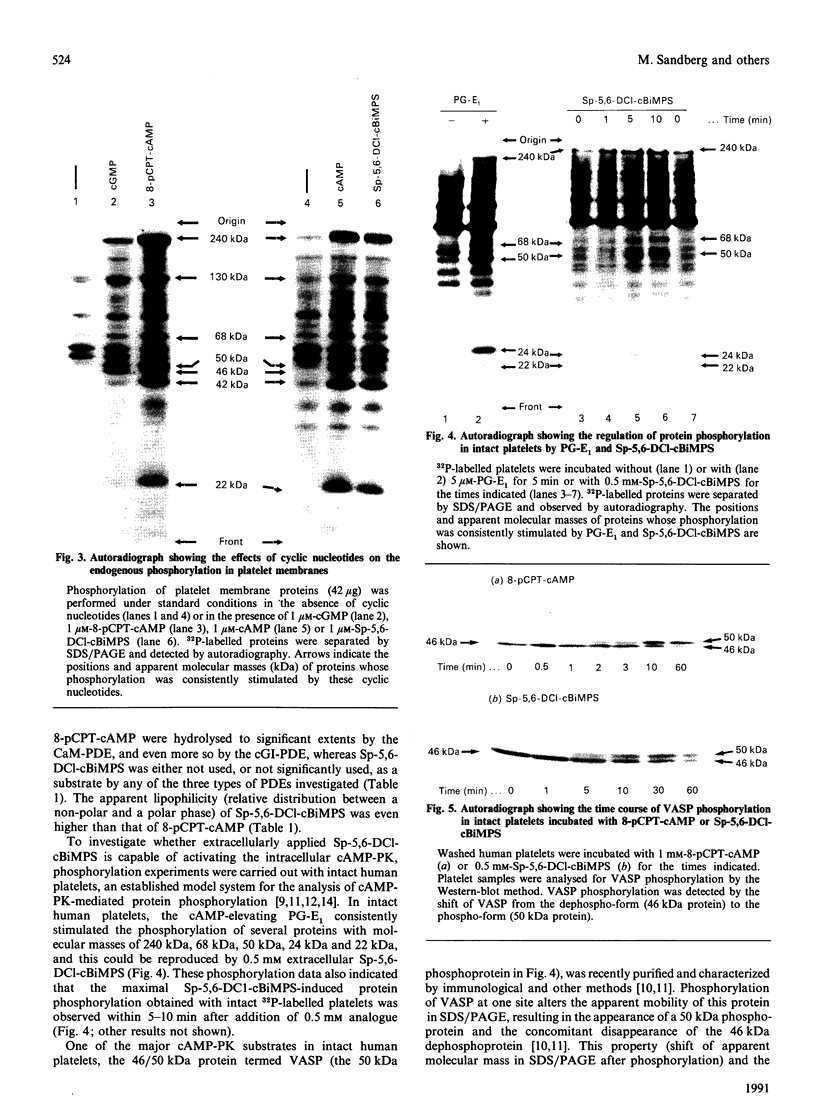
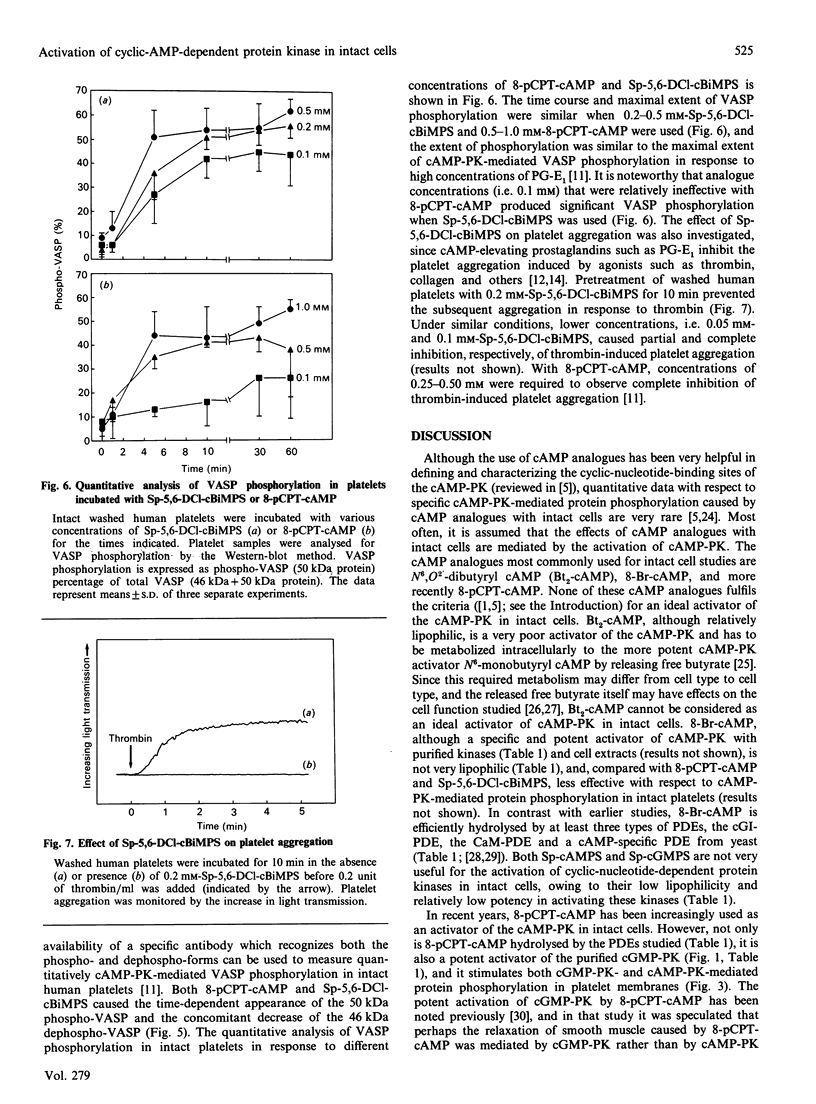
A newly designed cyclic AMP (cAMP) analogue, Sp-5,6-dichloro-1-beta-D- ribofuranosylbenzimidazole-3',5'-monophosphorothioate (Sp-5,6-DCl-cBiMPS), and 8-(p-chlorophenylthio)-cAMP (8-pCPT-cAMP) were compared with respect to their chemical and biological properties in order to assess their potential as activators of the cAMP-dependent protein kinases (cAMP-PK) in intact cells. Sp-5,6-DCl-cBiMPS was shown to be both a potent and specific activator of purified cAMP-PK and of cAMP-PK in platelet membranes, whereas 8-pCPT-cAMP proved to be a potent activator of cAMP-PK and cyclic-GMP-dependent protein kinase (cGMP-PK) both as purified enzymes and in platelet membranes. Sp-5,6-DCl-cBiMPS was not significantly hydrolysed by three types of cyclic nucleotide phosphodiesterases, whereas 8-pCPT-cAMP (and 8-bromo-cAMP) was hydrolysed to a significant extent by the Ca2+/calmodulin-dependent phosphodiesterase and by the cGMP-inhibited phosphodiesterase. The apparent lipophilicity, a measure of potential cell-membrane permeability, of Sp-5,6-DCl-cBiMPS was higher than that of 8-pCPT-cAMP. Extracellular application of Sp-5,6-DCl-cBiMPS to intact human platelets reproduced the pattern of protein phosphorylation induced by prostaglandin E1, a cAMP-increasing inhibitor of platelet activation. In intact platelets, Sp-5,6- DCl-cBiMPS was also more effective than 8-pCPT-cAMP in inducing quantitative phosphorylation of the 46/50 kDa vasodilator-stimulated phosphoprotein (VASP), a major substrate of cAMP-PK in platelets. As observed with prostaglandin E1, pretreatment of human platelets with Sp-5,6-DCl-cBiMPS prevented the aggregation induced by thrombin. The results suggest that Sp-5,6-DCl-cBiMPS is a very potent and specific activator of cAMP-PK in cell extracts and intact cells and, in this respect, is superior to any other cAMP analogue used for intact-cell studies. In contrast with 8-pCPT-cAMP, Sp-5,6-DCl-cBiMPS can be used to distinguish the signal-transduction pathways mediated by cAMP-PK and cGMP-PK.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990 Apr;11(4):150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- Butt E., van Bemmelen M., Fischer L., Walter U., Jastorff B. Inhibition of cGMP-dependent protein kinase by (Rp)-guanosine 3',5'-monophosphorothioates. FEBS Lett. 1990 Apr 9;263(1):47–50. doi: 10.1016/0014-5793(90)80702-k. [DOI] [PubMed] [Google Scholar]
- Chodosh L. A., Fire A., Samuels M., Sharp P. A. 5,6-Dichloro-1-beta-D-ribofuranosylbenzimidazole inhibits transcription elongation by RNA polymerase II in vitro. J Biol Chem. 1989 Feb 5;264(4):2250–2257. [PubMed] [Google Scholar]
- Dostmann W. R., Taylor S. S., Genieser H. G., Jastorff B., Døskeland S. O., Ogreid D. Probing the cyclic nucleotide binding sites of cAMP-dependent protein kinases I and II with analogs of adenosine 3',5'-cyclic phosphorothioates. J Biol Chem. 1990 Jun 25;265(18):10484–10491. [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Francis S. H., Noblett B. D., Todd B. W., Wells J. N., Corbin J. D. Relaxation of vascular and tracheal smooth muscle by cyclic nucleotide analogs that preferentially activate purified cGMP-dependent protein kinase. Mol Pharmacol. 1988 Oct;34(4):506–517. [PubMed] [Google Scholar]
- Gillespie P. G., Beavo J. A. Inhibition and stimulation of photoreceptor phosphodiesterases by dipyridamole and M&B 22,948. Mol Pharmacol. 1989 Nov;36(5):773–781. [PubMed] [Google Scholar]
- Halbrügge M., Friedrich C., Eigenthaler M., Schanzenbächer P., Walter U. Stoichiometric and reversible phosphorylation of a 46-kDa protein in human platelets in response to cGMP- and cAMP-elevating vasodilators. J Biol Chem. 1990 Feb 25;265(6):3088–3093. [PubMed] [Google Scholar]
- Halbrügge M., Walter U. Purification of a vasodilator-regulated phosphoprotein from human platelets. Eur J Biochem. 1989 Oct 20;185(1):41–50. doi: 10.1111/j.1432-1033.1989.tb15079.x. [DOI] [PubMed] [Google Scholar]
- Harrison S. A., Reifsnyder D. H., Gallis B., Cadd G. G., Beavo J. A. Isolation and characterization of bovine cardiac muscle cGMP-inhibited phosphodiesterase: a receptor for new cardiotonic drugs. Mol Pharmacol. 1986 May;29(5):506–514. [PubMed] [Google Scholar]
- Hofmann F., Bechtel P. J., Krebs E. G. Concentrations of cyclic AMP-dependent protein kinase subunits in various tissues. J Biol Chem. 1977 Feb 25;252(4):1441–1447. [PubMed] [Google Scholar]
- Kaczmarek L. K., Jennings K. R., Strumwasser F., Nairn A. C., Walter U., Wilson F. D., Greengard P. Microinjection of catalytic subunit of cyclic AMP-dependent protein kinase enhances calcium action potentials of bag cell neurons in cell culture. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7487–7491. doi: 10.1073/pnas.77.12.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaukel E., Hilz H. Permeation of dibutyryl cAMP into HeLa cells and its convesion to monobutyryl cAMP. Biochem Biophys Res Commun. 1972 Jan 31;46(2):1011–1018. doi: 10.1016/s0006-291x(72)80242-0. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Kruh J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biochem. 1982 Feb 5;42(2):65–82. doi: 10.1007/BF00222695. [DOI] [PubMed] [Google Scholar]
- Lohmann S. M., Walter U. Regulation of the cellular and subcellular concentrations and distribution of cyclic nucleotide-dependent protein kinases. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:63–117. [PubMed] [Google Scholar]
- MacFarland R. T., Zelus B. D., Beavo J. A. High concentrations of a cGMP-stimulated phosphodiesterase mediate ANP-induced decreases in cAMP and steroidogenesis in adrenal glomerulosa cells. J Biol Chem. 1991 Jan 5;266(1):136–142. [PubMed] [Google Scholar]
- Méry P. F., Lohmann S. M., Walter U., Fischmeister R. Ca2+ current is regulated by cyclic GMP-dependent protein kinase in mammalian cardiac myocytes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1197–1201. doi: 10.1073/pnas.88.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R., Jr Assays of protein kinase. Methods Enzymol. 1983;99:3–6. doi: 10.1016/0076-6879(83)99034-1. [DOI] [PubMed] [Google Scholar]
- Sutherland E. W. Studies on the mechanism of hormone action. Science. 1972 Aug 4;177(4047):401–408. doi: 10.1126/science.177.4047.401. [DOI] [PubMed] [Google Scholar]
- Van Lookeren Campagne M. M., Diaz F. V., Jastorff B., Winkler E., Genieser H. G., Kessin R. H. Characterization of the yeast low Km cAMP-phosphodiesterase with cAMP analogues. Applications in mammalian cells that express the yeast PDE2 gene. J Biol Chem. 1990 Apr 5;265(10):5847–5854. [PubMed] [Google Scholar]
- Waldmann R., Bauer S., Göbel C., Hofmann F., Jakobs K. H., Walter U. Demonstration of cGMP-dependent protein kinase and cGMP-dependent phosphorylation in cell-free extracts of platelets. Eur J Biochem. 1986 Jul 1;158(1):203–210. doi: 10.1111/j.1432-1033.1986.tb09739.x. [DOI] [PubMed] [Google Scholar]
- Waldmann R., Nieberding M., Walter U. Vasodilator-stimulated protein phosphorylation in platelets is mediated by cAMP- and cGMP-dependent protein kinases. Eur J Biochem. 1987 Sep 15;167(3):441–448. doi: 10.1111/j.1432-1033.1987.tb13357.x. [DOI] [PubMed] [Google Scholar]
- Walter U., Miller P., Wilson F., Menkes D., Greengard P. Immunological distinction between guanosine 3':5'-monophosphate-dependent and adenosine 3':5'-monophosphate-dependent protein kinases. J Biol Chem. 1980 Apr 25;255(8):3757–3762. [PubMed] [Google Scholar]
- Walter U. Physiological role of cGMP and cGMP-dependent protein kinase in the cardiovascular system. Rev Physiol Biochem Pharmacol. 1989;113:41–88. doi: 10.1007/BFb0032675. [DOI] [PubMed] [Google Scholar]
- Yusta B., Ortiz-Caro J., Pascual A., Aranda A. Comparison of the effects of forskolin and dibutyryl cyclic AMP in neuroblastoma cells: evidence that some of the actions of dibutyryl cyclic AMP are mediated by butyrate. J Neurochem. 1988 Dec;51(6):1808–1818. doi: 10.1111/j.1471-4159.1988.tb01162.x. [DOI] [PubMed] [Google Scholar]
- de Wit R. J., Hekstra D., Jastorff B., Stec W. J., Baraniak J., Van Driel R., Van Haastert P. J. Inhibitory action of certain cyclophosphate derivatives of cAMP on cAMP-dependent protein kinases. Eur J Biochem. 1984 Jul 16;142(2):255–260. doi: 10.1111/j.1432-1033.1984.tb08279.x. [DOI] [PubMed] [Google Scholar]
- de Wit R. J., Hoppe J., Stec W. J., Baraniak J., Jastorff B. Interaction of cAMP derivatives with the 'stable' cAMP-binding site in the cAMP-dependent protein kinase type I. Eur J Biochem. 1982 Feb;122(1):95–99. doi: 10.1111/j.1432-1033.1982.tb05852.x. [DOI] [PubMed] [Google Scholar]