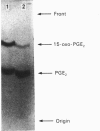
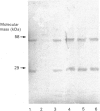
Abstract
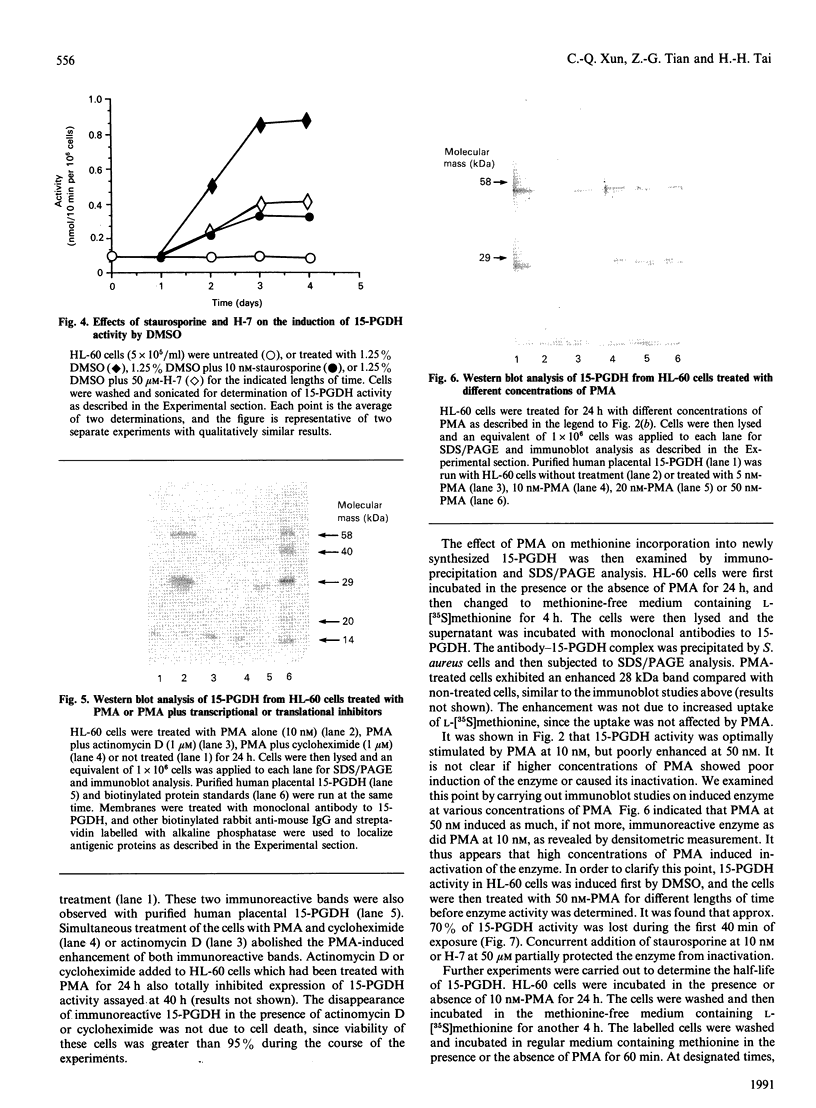
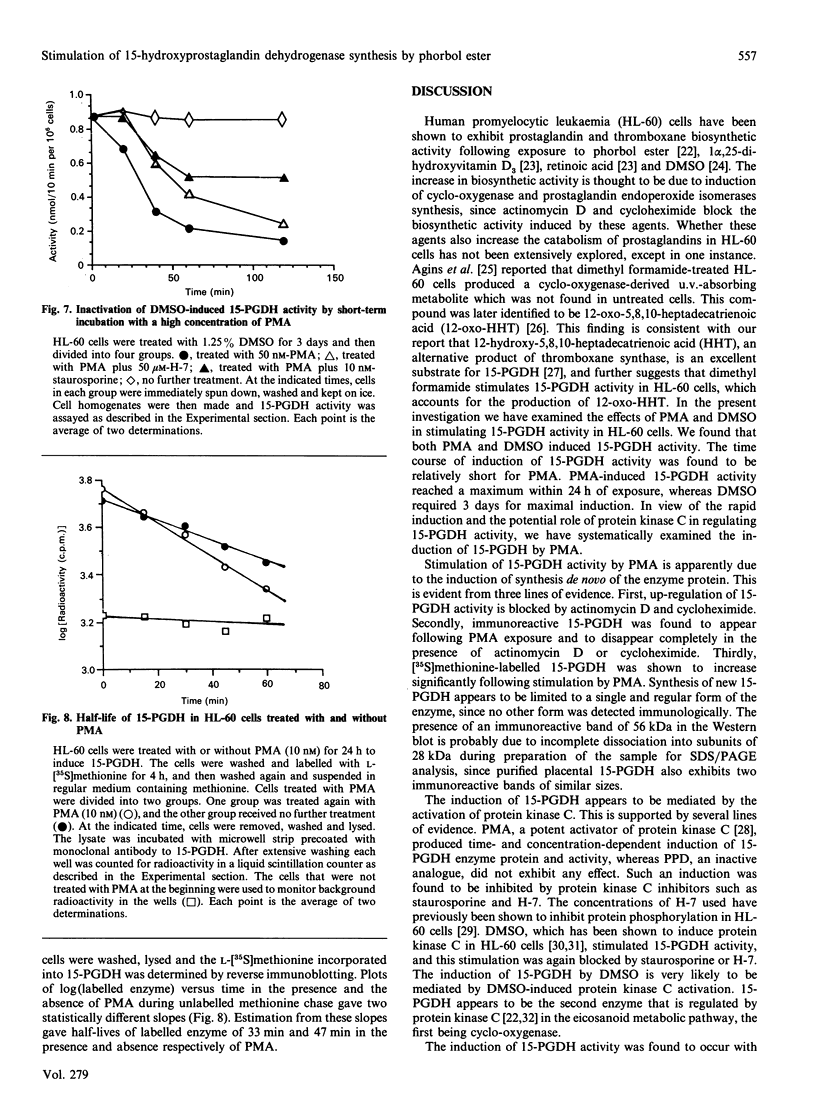
Human promyelocytic leukaemia (HL-60) cells were employed to study the induction of NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase (15-PGDH), the key enzyme in controlling prostaglandin inactivation. Phorbol 12-myristate 13-acetate (PMA) stimulated 15-PGDH activity in a time- and concentration-dependent manner. Dimethyl sulphoxide (DMSO) also stimulated the enzyme activity, although a much delayed stimulation was observed. Western blot studies indicated that PMA increased significantly a 28 kDa immunoreactive protein characteristic of 15-PGDH. L-[35S]Methionine labelling of the PMA-treated cells showed a similar enhancement over the control cells. These studies indicate that PMA induced synthesis of 15-PGDH. Stimulation of 15-PGDH activity by PMA or DMSO appears to be mediated by protein kinase C activation, since an inactive analogue of PMA failed to induce the effect, and both staurosporine and H-7 blocked the stimulation. Stimulation by PMA was optimal at 10 nM and less effective at higher concentrations. Western blot studies indicated that a similar, if not greater, amount of enzyme protein was induced at high concentrations of PMA, suggesting that enzyme inactivation might be occurring. Possible enzyme inactivation by protein kinase C activation was further examined by incubating DMSO-treated cells with a high concentration of PMA (50 nM). Time-dependent inactivation of 15-PGDH within the first 1 h was observed and this inactivation was partially blocked by staurosporine and H-7. Pulse-chase experiments indicated that 15-PGDH had a rapid turnover rate (t 1/2 = 47 min), and PMA shortened the half-life of the enzyme (t 1/2 = 33 min), suggesting that PMA might have an additional effect on 15-PGDH degradation. The rapid turnover of 15-PGDH indicates that the enzyme activity depends on continued enzyme synthesis, and this could be susceptible to hormone and drug control mechanisms.
Full text
PDF
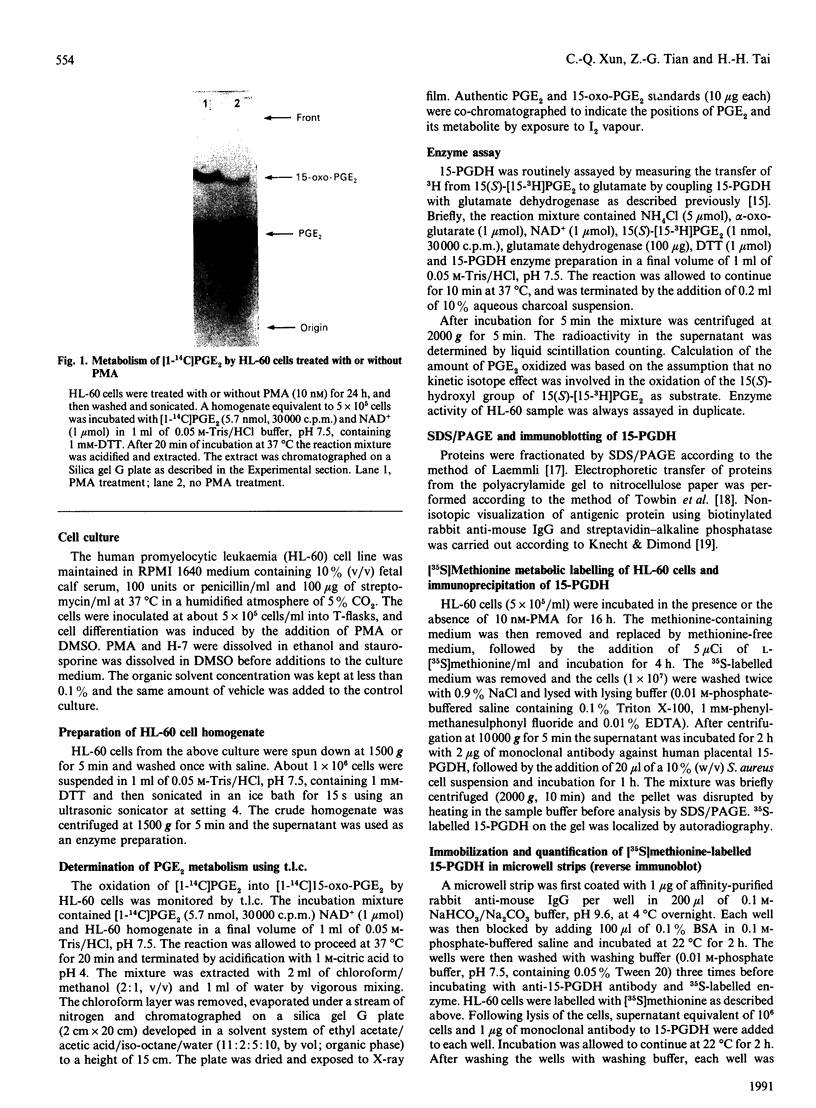

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agins A. P., Hollmann A. B., Agarwal K. C., Wiemann M. C. Detection of a novel cyclooxygenase metabolite produced by human promyelocytic leukemia (HL-60) cells. Biochem Biophys Res Commun. 1985 Jan 16;126(1):143–149. doi: 10.1016/0006-291x(85)90583-2. [DOI] [PubMed] [Google Scholar]
- Agins A. P., Thomas M. J., Edmonds C. G., McCloskey J. A. Identification of 12-keto-5,8,10-heptadecatrienoic acid as an arachidonic acid metabolite produced by human HL-60 leukemia cells. Biochem Pharmacol. 1987 Jun 1;36(11):1799–1805. doi: 10.1016/0006-2952(87)90241-3. [DOI] [PubMed] [Google Scholar]
- Alam N. A., Russell P. T., Tabor M. W., Moulton B. C. Progesterone and estrogen control of uterine prostaglandin dehydrogenase activity during deciduomal growth. Endocrinology. 1976 Apr;98(4):859–863. doi: 10.1210/endo-98-4-859. [DOI] [PubMed] [Google Scholar]
- Anggård E., Larsson C., Samuelsson B. The distribution of 15-hydroxy prostaglandin dehydrogenase and prostaglandin-delta 13-reductase in tissues of the swine. Acta Physiol Scand. 1971 Mar;81(3):396–404. doi: 10.1111/j.1748-1716.1971.tb04914.x. [DOI] [PubMed] [Google Scholar]
- Anggård E. The biological activities of three metabolites of prostaglandin E 1. Acta Physiol Scand. 1966 Apr;66(4):509–510. doi: 10.1111/j.1748-1716.1966.tb03231.x. [DOI] [PubMed] [Google Scholar]
- Bedwani J. R., Marley P. B. Enhanced inactivation of prostaglandin E2 by the rabbit lung during pregnancy or progesterone treatment. Br J Pharmacol. 1975 Apr;53(4):547–554. doi: 10.1111/j.1476-5381.1975.tb07393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C. N., Hoult J. R., Peers S. H., Agback H. Inhibition of prostaglandin 15-hydroxydehydrogenase by sulphasalazine and a novel series of potent analogues. Biochem Pharmacol. 1983 Oct 1;32(19):2863–2871. doi: 10.1016/0006-2952(83)90390-8. [DOI] [PubMed] [Google Scholar]
- Blackwell G. J., Flower R. J., Vane J. R. Rapid reduction of prostaglandin 15-hydroxy dehydrogenase activity in rat tissues after treatment with protein synthesis inhibitors. Br J Pharmacol. 1975 Oct;55(2):233–238. doi: 10.1111/j.1476-5381.1975.tb07633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W. C., Fukuda S., Tai H. H. Pulmonary NAD+-linked 15-hydroxyprostaglandin dehydrogenase activity is decreased by cigarette smoking. Life Sci. 1984 Mar 26;34(13):1261–1268. doi: 10.1016/0024-3205(84)90549-6. [DOI] [PubMed] [Google Scholar]
- Ensor C. M., Yang J. Y., Okita R. T., Tai H. H. Cloning and sequence analysis of the cDNA for human placental NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase. J Biol Chem. 1990 Sep 5;265(25):14888–14891. [PubMed] [Google Scholar]
- Goerig M., Habenicht A. J., Heitz R., Zeh W., Katus H., Kommerell B., Ziegler R., Glomset J. A. sn-1,2-Diacylglycerols and phorbol diesters stimulate thromboxane synthesis by de novo synthesis of prostaglandin H synthase in human promyelocytic leukemia cells. J Clin Invest. 1987 Mar;79(3):903–911. doi: 10.1172/JCI112900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. On the mechanism of the biosynthesis of prostaglandins E-1 and F-1-alpha. J Biol Chem. 1967 Nov 25;242(22):5336–5343. [PubMed] [Google Scholar]
- Hansen H. S. Inhibition by indomethacin and aspirin of 15-hydroxyprostaglandin dehydrogenase in vitro. Prostaglandins. 1974 Oct 25;8(2):95–105. doi: 10.1016/0090-6980(74)90072-0. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Honda A., Morita I., Murota S., Mori Y. Appearance of the arachidonic acid metabolic pathway in human promyelocytic leukemia (HL-60) cells during monocytic differentiation: enhancement of thromboxane synthesis by 1 alpha,25-dihydroxyvitamin D-3. Biochim Biophys Acta. 1986 Jul 18;877(3):423–432. doi: 10.1016/0005-2760(86)90208-0. [DOI] [PubMed] [Google Scholar]
- Hsin-Hsiung-Tai, Tai C. L., Hollander C. S. Regulation of prostaglandin metabolism: inhibition of 15-hydroxyprostaglandin dehydrogenase by thyroid hormones. Biochem Biophys Res Commun. 1974 Mar 25;57(2):457–462. doi: 10.1016/0006-291x(74)90953-x. [DOI] [PubMed] [Google Scholar]
- Iijima Y., Kawakita N., Yamazaki M. Inhibition of 15-hydroxyprostaglandin dehydrogenase by antiallergic agents. Biochem Biophys Res Commun. 1980 Apr 14;93(3):912–918. doi: 10.1016/0006-291x(80)91162-6. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Tanaka Y., Miyake R., Nishizuka Y. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J Biol Chem. 1983 Oct 10;258(19):11442–11445. [PubMed] [Google Scholar]
- Knecht D. A., Dimond R. L. Visualization of antigenic proteins on Western blots. Anal Biochem. 1984 Jan;136(1):180–184. doi: 10.1016/0003-2697(84)90321-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu Y., Tai H. H. Inactivation of pulmonary NAD+-dependent 15-hydroxyprostaglandin dehydrogenase by acrolein. Biochem Pharmacol. 1985 Dec 15;34(24):4275–4278. doi: 10.1016/0006-2952(85)90284-9. [DOI] [PubMed] [Google Scholar]
- Liu Y., Yoden K., Shen R. F., Tai H. H. 12-L-hydroxy-5,8,10-heptadecatrienoic acid (HHT) is an excellent substrate for NAD+-dependent 15-hydroxyprostaglandin dehydrogenase. Biochem Biophys Res Commun. 1985 May 31;129(1):268–274. doi: 10.1016/0006-291x(85)91432-9. [DOI] [PubMed] [Google Scholar]
- Makowske M., Ballester R., Cayre Y., Rosen O. M. Immunochemical evidence that three protein kinase C isozymes increase in abundance during HL-60 differentiation induced by dimethyl sulfoxide and retinoic acid. J Biol Chem. 1988 Mar 5;263(7):3402–3410. [PubMed] [Google Scholar]
- Moore P. K., Hoult J. R. Anti-inflammatory steroids reduce tissue PG synthetase activity and enhance PG breakdown. Nature. 1980 Nov 20;288(5788):269–270. doi: 10.1038/288269a0. [DOI] [PubMed] [Google Scholar]
- Nakano J., Prancan A. V. Metabolic degradation of prostaglandin E1 in the lung and kidney of rats in endotoxin shock. Proc Soc Exp Biol Med. 1973 Nov;144(2):506–508. doi: 10.3181/00379727-144-37623. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Uemura Y., Hidaka H., Shirakawa S. 1-(5-Isoquinolinesulfonyl)-2-methylpiperazine(H-7), a potent inhibitor of protein kinases, inhibits the differentiation of HL-60 cells induced by phorbol diester. Life Sci. 1986 Sep 22;39(12):1101–1107. doi: 10.1016/0024-3205(86)90202-x. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak C. Activity profiles of prostaglandin 15- and 9-hydroxydehydrogenase and 13-reductase in the developing rat kidney. J Biol Chem. 1975 Apr 25;250(8):2795–2800. [PubMed] [Google Scholar]
- Tai C. L., Mak O. T., Arai T., Tai H. H. Monoclonal antibodies that inhibit the enzyme activity of NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase. Biochem J. 1990 Apr 1;267(1):75–78. doi: 10.1042/bj2670075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai H. H. Enzymatic synthesis of (15s)-[15-3h]prostaglandins and their use in the development of a simple and sensitive assay for 15-hydroxyprostaglandin dehydrogenase. Biochemistry. 1976 Oct 19;15(21):4586–4592. doi: 10.1021/bi00666a007. [DOI] [PubMed] [Google Scholar]
- Tai H. H., Hollander C. S. Regulation of prostaglandin metabolism: activation of 15-hydroxyprostaglandin dehydrogenase by chlorpromazine and imipramine related drugs. Biochem Biophys Res Commun. 1976 Feb 9;68(3):814–820. doi: 10.1016/0006-291x(76)91218-3. [DOI] [PubMed] [Google Scholar]
- Tai H. H., Yuan B., Sun M. Metabolism of prostaglandins in spontaneously hypertensive rats: NAD"-dependent 15-hydroxyprostaglandin dehydrogenase activity is decreased in kidney and increased in lung. Life Sci. 1979 Apr 2;24(14):1275–1280. doi: 10.1016/0024-3205(79)90146-2. [DOI] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K. K., Hatzakis H., Lo S. S., Seong D. C., Sanduja S. K., Tai H. H. Stimulation of de novo synthesis of prostaglandin G/H synthase in human endothelial cells by phorbol ester. J Biol Chem. 1988 Dec 15;263(35):19043–19047. [PubMed] [Google Scholar]
- Zylber-Katz E., Glazer R. I. Phospholipid- and Ca2+-dependent protein kinase activity and protein phosphorylation patterns in the differentiation of human promyelocytic leukemia cell line HL-60. Cancer Res. 1985 Oct;45(10):5159–5164. [PubMed] [Google Scholar]