Abstract
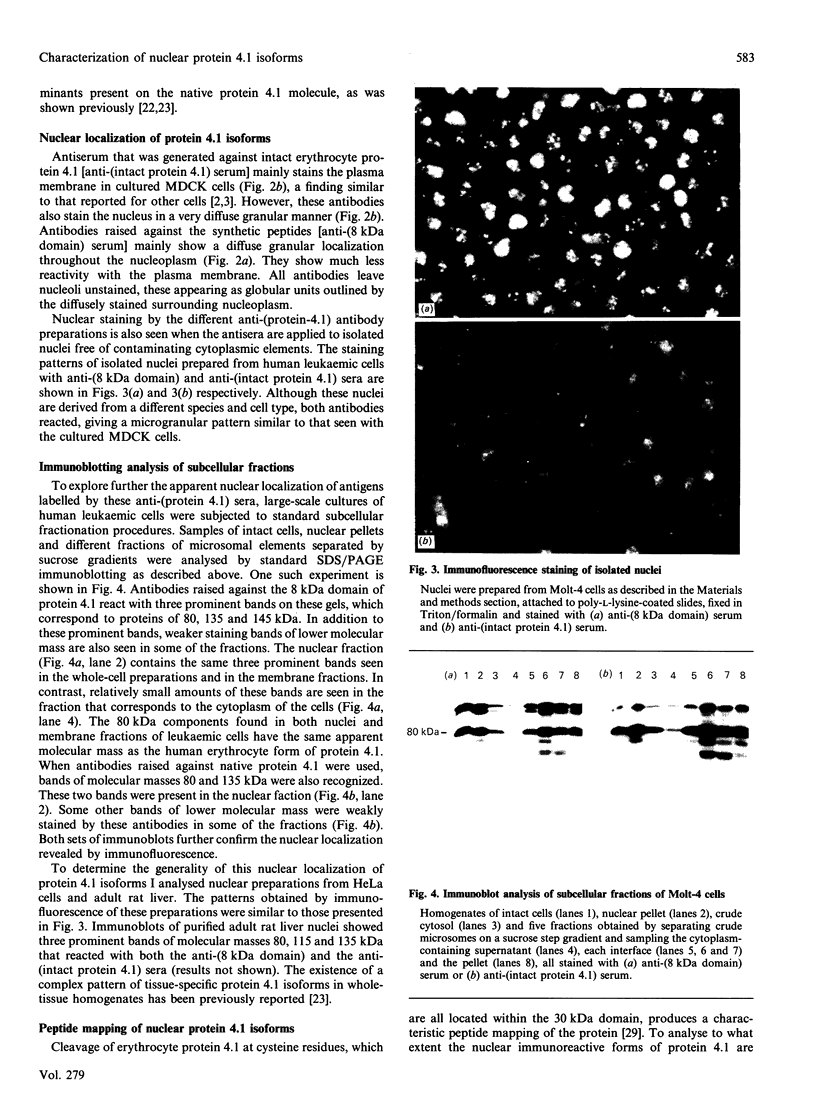
Although protein 4.1 was originally identified as an element of the erythrocyte membrane skeleton, its presence in most mammalian cell types is now well described. Antibodies raised against erythrocyte protein 4.1 or synthetic peptides corresponding to the spectrin-actin-binding domain of protein 4.1 react with plasma membranes and, unexpectedly, nuclei of different cell types. Nuclear staining was further confirmed in isolated nuclei prepared from rat liver and human leukaemic cell lines. Immunoblot analysis of subcellular fractions derived from these cells revealed three prominent proteins, of 80, 135 and 145 kDa. The structural relationship of the high-molecular-mass proteins with erythrocyte protein 4.1 was demonstrated by peptide mapping. These results indicate that mammalian nucleated cells contain several isoforms of erythrocyte protein 4.1 and that some high-molecular-mass forms may primarily reside in the nucleus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. A., Correas I., Mazzucco C., Castle J. D., Marchesi V. T. Tissue-specific analogues of erythrocyte protein 4.1 retain functional domains. J Cell Biochem. 1988 Jul;37(3):269–284. doi: 10.1002/jcb.240370303. [DOI] [PubMed] [Google Scholar]
- Aster J. C., Welsh M. J., Brewer G. J., Maisel H. Identification of spectrin and protein 4.1-like proteins in mammalian lens. Biochem Biophys Res Commun. 1984 Mar 15;119(2):726–734. doi: 10.1016/s0006-291x(84)80311-3. [DOI] [PubMed] [Google Scholar]
- Bachs O., Lanini L., Serratosa J., Coll M. J., Bastos R., Aligué R., Rius E., Carafoli E. Calmodulin-binding proteins in the nuclei of quiescent and proliferatively activated rat liver cells. J Biol Chem. 1990 Oct 25;265(30):18595–18600. [PubMed] [Google Scholar]
- Baines A. J., Bennett V. Synapsin I is a spectrin-binding protein immunologically related to erythrocyte protein 4.1. 1985 May 30-Jun 5Nature. 315(6018):410–413. doi: 10.1038/315410a0. [DOI] [PubMed] [Google Scholar]
- Bennett V. Spectrin-based membrane skeleton: a multipotential adaptor between plasma membrane and cytoplasm. Physiol Rev. 1990 Oct;70(4):1029–1065. doi: 10.1152/physrev.1990.70.4.1029. [DOI] [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. Identification and partial purification of ankyrin, the high affinity membrane attachment site for human erythrocyte spectrin. J Biol Chem. 1979 Apr 10;254(7):2533–2541. [PubMed] [Google Scholar]
- Boivin P. Role of the phosphorylation of red blood cell membrane proteins. Biochem J. 1988 Dec 15;256(3):689–695. doi: 10.1042/bj2560689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton D., Cohen C. M., Tyler J. Interaction of cytoskeletal proteins on the human erythrocyte membrane. Cell. 1981 Apr;24(1):24–32. doi: 10.1016/0092-8674(81)90497-9. [DOI] [PubMed] [Google Scholar]
- Byers T. J., Branton D. Visualization of the protein associations in the erythrocyte membrane skeleton. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6153–6157. doi: 10.1073/pnas.82.18.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark T. G., Merriam R. W. Diffusible and bound actin nuclei of Xenopus laevis oocytes. Cell. 1977 Dec;12(4):883–891. doi: 10.1016/0092-8674(77)90152-0. [DOI] [PubMed] [Google Scholar]
- Cohen C. M., Foley S. F., Korsgren C. A protein immunologically related to erythrocyte band 4.1 is found on stress fibres on non-erythroid cells. Nature. 1982 Oct 14;299(5884):648–650. doi: 10.1038/299648a0. [DOI] [PubMed] [Google Scholar]
- Conboy J. G., Chan J., Mohandas N., Kan Y. W. Multiple protein 4.1 isoforms produced by alternative splicing in human erythroid cells. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9062–9065. doi: 10.1073/pnas.85.23.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correas I., Avila J. Erythrocyte protein 4.1 associates with tubulin. Biochem J. 1988 Oct 1;255(1):217–221. [PMC free article] [PubMed] [Google Scholar]
- Correas I., Leto T. L., Speicher D. W., Marchesi V. T. Identification of the functional site of erythrocyte protein 4.1 involved in spectrin-actin associations. J Biol Chem. 1986 Mar 5;261(7):3310–3315. [PubMed] [Google Scholar]
- Correas I., Speicher D. W., Marchesi V. T. Structure of the spectrin-actin binding site of erythrocyte protein 4.1. J Biol Chem. 1986 Oct 5;261(28):13362–13366. [PubMed] [Google Scholar]
- Davies G. E., Cohen C. M. Platelets contain proteins immunologically related to red cell spectrin and protein 4.1. Blood. 1985 Jan;65(1):52–59. [PubMed] [Google Scholar]
- Díaz-Nido J., Avila J. Characterization of proteins immunologically related to brain microtubule-associated protein MAP-1B in non-neural cells. J Cell Sci. 1989 Apr;92(Pt 4):607–620. doi: 10.1242/jcs.92.4.607. [DOI] [PubMed] [Google Scholar]
- Goodman S. R., Casoria L. A., Coleman D. B., Zagon I. S. Identification and location of brain protein 4.1. Science. 1984 Jun 29;224(4656):1433–1436. doi: 10.1126/science.6374897. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Bretscher A., Esch F. S., Hunter T. cDNA cloning and sequencing of the protein-tyrosine kinase substrate, ezrin, reveals homology to band 4.1. EMBO J. 1989 Dec 20;8(13):4133–4142. doi: 10.1002/j.1460-2075.1989.tb08598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger B. L., Lazarides E. Membrane skeletal protein 4.1 of avian erythrocytes is composed of multiple variants that exhibit tissue-specific expression. Cell. 1984 Jun;37(2):595–607. doi: 10.1016/0092-8674(84)90390-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leto T. L., Marchesi V. T. A structural model of human erythrocyte protein 4.1. J Biol Chem. 1984 Apr 10;259(7):4603–4608. [PubMed] [Google Scholar]
- Leto T. L., Pratt B. M., Madri J. A. Mechanisms of cytoskeletal regulation: modulation of aortic endothelial cell protein band 4.1 by the extracellular matrix. J Cell Physiol. 1986 Jun;127(3):423–431. doi: 10.1002/jcp.1041270311. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Derick L. H., Palek J. Visualization of the hexagonal lattice in the erythrocyte membrane skeleton. J Cell Biol. 1987 Mar;104(3):527–536. doi: 10.1083/jcb.104.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T. Stabilizing infrastructure of cell membranes. Annu Rev Cell Biol. 1985;1:531–561. doi: 10.1146/annurev.cb.01.110185.002531. [DOI] [PubMed] [Google Scholar]
- Matsuzaki F., Sutoh K., Ikai A. Structural unit of the erythrocyte cytoskeleton. Isolation and electron microscopic examination. Eur J Cell Biol. 1985 Nov;39(1):153–160. [PubMed] [Google Scholar]
- Morrow J. S., Haigh W. B., Jr, Marchesi V. T. Spectrin oligomers: a structural feature of the erythrocyte cytoskeleton. J Supramol Struct Cell Biochem. 1981;17(3):275–287. doi: 10.1002/jsscb.380170308. [DOI] [PubMed] [Google Scholar]
- Nakayasu H., Ueda K. Association of actin with the nuclear matrix from bovine lymphocytes. Exp Cell Res. 1983 Jan;143(1):55–62. doi: 10.1016/0014-4827(83)90108-8. [DOI] [PubMed] [Google Scholar]
- Ngai J., Stack J. H., Moon R. T., Lazarides E. Regulated expression of multiple chicken erythroid membrane skeletal protein 4.1 variants is governed by differential RNA processing and translational control. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4432–4436. doi: 10.1073/pnas.84.13.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternack G. R., Racusen R. H. Erythrocyte protein 4.1 binds and regulates myosin. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9712–9716. doi: 10.1073/pnas.86.24.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinder J. C., Ohanian V., Gratzer W. B. Spectrin and protein 4.1 as an actin filament capping complex. FEBS Lett. 1984 Apr 24;169(2):161–164. doi: 10.1016/0014-5793(84)80310-5. [DOI] [PubMed] [Google Scholar]
- Shen B. W., Josephs R., Steck T. L. Ultrastructure of the intact skeleton of the human erythrocyte membrane. J Cell Biol. 1986 Mar;102(3):997–1006. doi: 10.1083/jcb.102.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. C., Ochs R. L., Fernandez E. A., Spector D. L. Macromolecular domains containing nuclear protein p107 and U-snRNP protein p28: further evidence for an in situ nuclear matrix. Mol Cell Biochem. 1986 May;70(2):151–168. doi: 10.1007/BF00229430. [DOI] [PubMed] [Google Scholar]
- Spiegel J. E., Beardsley D. S., Southwick F. S., Lux S. E. An analogue of the erythroid membrane skeletal protein 4.1 in nonerythroid cells. J Cell Biol. 1984 Sep;99(3):886–893. doi: 10.1083/jcb.99.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T. K., Leto T. L., Correas I., Alonso M. A., Marchesi V. T., Benz E. J., Jr Selective expression of an erythroid-specific isoform of protein 4.1. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3713–3717. doi: 10.1073/pnas.85.11.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T. K., Qin Z., Leto T., Marchesi V. T., Benz E. J., Jr Heterogeneity of mRNA and protein products arising from the protein 4.1 gene in erythroid and nonerythroid tissues. J Cell Biol. 1990 Mar;110(3):617–624. doi: 10.1083/jcb.110.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E., Bennett P. M., Calvert R., Ohanian V., Gratzer W. B. In vitro formation of a complex between cytoskeletal proteins of the human erythrocyte. Nature. 1979 Aug 30;280(5725):811–814. doi: 10.1038/280811a0. [DOI] [PubMed] [Google Scholar]
- Ungewickell E., Gratzer W. Self-association of human spectrin. A thermodynamic and kinetic study. Eur J Biochem. 1978 Aug 1;88(2):379–385. doi: 10.1111/j.1432-1033.1978.tb12459.x. [DOI] [PubMed] [Google Scholar]
- Verheijen R., Kuijpers H., Vooijs P., Van Venrooij W., Ramaekers F. Distribution of the 70K U1 RNA-associated protein during interphase and mitosis. Correlation with other U RNP particles and proteins of the nuclear matrix. J Cell Sci. 1986 Dec;86:173–190. doi: 10.1242/jcs.86.1.173. [DOI] [PubMed] [Google Scholar]
- Warner J. R. Distribution of newly formed ribosomal proteins in HeLa cell fractions. J Cell Biol. 1979 Mar;80(3):767–772. doi: 10.1083/jcb.80.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]






