Abstract
Aims
Some data have shown the functional connection between calpain and caspase-3. Here, we investigated the cross-talk between calpain and caspase-3 in penumbra and core during focal cerebral ischemia-reperfusion.
Methods
The activities of calpain and the levels of calpastatin, microtubule-associated protein-2 (MAP-2), and spectrin in penumbra and core at 3 or 23 h of reperfusion (R 3 h or R 23 h) after 1-h focal cerebral ischemia in rats were determined in sham- or caspase-3 inhibitor z-DEVD-CHO-treated rats.On the other hand, the determination of the activities of caspase-3 and the levels of MAP-2 and spectrin was done in sham- or calpain-inhibitor I-treated rats.
Results
z-DEVD-CHO (600 ng/rat, i.c.v.) markedly reduced the μ- and m-calpain activities in penumbra and the m-calpain activities in core at R 3 h and R 23 h, and enhanced the calpastatin levels in penumbra at R 3 h and in core at R 3 h and R 23 h significantly; however, it had no significant effects on the μ-calpain activities in core and the calpastatin levels in penumbra at R 23 h. Calpain inhibitor I (0.8 mg/rat, i.c.v.) markedly reduced the caspase-3 activities in core at R 3 h and R 23 h, but not in penumbra. Both calpain and caspase-3 inhibitors increased the levels of MAP-2 and spectrin in penumbra and core significantly after focal cerebral ischemia-reperfusion.
Conclusions
Our data provide direct evidence to demonstrate the cross-talk between calpain and caspase-3 in penumbra and core during focal cerebral ischemia-reperfusion.
Keywords: Focal cerebral ischemia-reperfusion, Calpain, Calpastatin, Caspase-3, Cross-talk
Caspases and calpains are two intracellular cysteine proteases. Caspases are specifically activated in response to apoptotic stimuli, and caspase-3 is one of the major executioners to act downstream in the apoptotic cascade (Salvesen and Dixit 1997). Recently, some reports have shown the involvement of caspase-3 in a typical necrotic death routine (Schwab et al. 2002; Berliocchi et al. 2005). Calpains are activated by calcium and autolytic processing. They are proposed to participate in the turnover of cytoskeletal proteins and regulation of kinases, transcription factors, and receptors. Ubiquitously expressed μ- and m-calpains are regulated reversibly by calcium and calpastatin, an endogenous calpain inhibitor (Goll et al. 2003). Calpain/calpastatin has been implicated in necrosis and apoptosis (Seubert et al. 1989; Porn-Ares et al. 1998; Neumar et al. 2003).
A growing body of literature has emerged, demonstrating functional connections between calpains and caspases. Common substrate proteins have been identified, such as cytoskeletal and regulatory proteins (Wang 2000). There are reports demonstrating calpain-mediated cleavage of caspases-3 and -7 as well as caspases-8, -9 and -12 (Chua et al. 2000; Nakagawa and Yuan 2000; Blomgren et al. 2001; Bizat et al. 2003). In addition to direct cleavage of caspases, calpains have also been shown to cleave several apoptosis regulatory proteins including apoptotic protease-activating factor-1, Bcl-xL, Bax, Bid, and p53 (Reimertz et al. 2001; Wood et al. 1998; Chen et al. 2001; Atencio et al. 2000; Nakagawa and Yuan 2000). Based on the available evidence, calpains have the potential to both positively and negatively modulate the caspase cascade during apoptosis. There is also evidence that caspases have the potential to indirectly up-regulate calpain activity through cleaving calpastatin, the endogenous calpain inhibitor (Wang et al. 1998; Porn-Ares et al. 1998). Moreover, some reports have demonstrated the cross-talk between calpains and caspases in vitro (Nakagawa and Yuan 2000; Neumar et al. 2003) and in vivo (Blomgren et al. 2001; Bizat et al. 2003).
The brain lesion due to focal cerebral ischemia encompasses an irreversibly injured core and a peripheral zone (penumbra), where tissue is at risk but potentially viable. The neuronal death in penumbra is predominant in apoptosis, while that in core is predominant in necrosis (Lipton 1999). During focal cerebral ischemia, calpains and caspase-3 are activated and involved in the ischemic neuronal death, which have been demonstrated through investigating the protection of calpain and caspase-3 inhibitors and the proteolysis of substrates, such as microtubule-associated protein-2 (MAP-2), spectrin, and calpastatin (Dawson and Hallenbeck 1996; Endres et al. 1998; Lipton 1999; Kambe et al. 2005; Han et al. 2006).
These above findings urge us to investigate whether both calpain and caspase-3 are together involved in both necrotic and apoptotic cell death that happened in penumbra or core during focal cerebral ischemia-reperfusion through their cross-talk. Thus, in this study, we combined the biochemical estimations of enzyme activities and their substrates with pharmacological blockade using enzyme inhibitors in the experiment, by way of such investigation, to demonstrate the above hypothesis.
Materials and Methods
Rat Model of Focal Cerebral Ischemia
All animal protocols were approved by the national institutes of health guide for the care and the use of laboratory animals. Male Sprague-Dawley rats weighing between 315 and 330 g were anesthetized with chloral hydrate (400 mg/kg, i.p.) and the right middle cerebral artery occlusion (MCAo) was produced by inversion of a 4-0-nylon filament as described previously (Schmid-Elsaesser et al. 1998). The filament was withdrawn 1 h after onset of MCAo and then reperfused. Rectal temperature was continuously monitored and maintained at 37 ± 0.5°C by a negative-feedback-controlled heating pad during the whole experiment.
Experimental Protocols
Effects of Caspase-3 Inhibition on the Activities of Calpain, and the Protein Levels of Calpastatin, MAP-2, and spectrin
z-DEVD-CHO (Calbiochem, San Diego, CA, USA), a specific and reversible inhibitor of caspase-3, has been used in in vitro studies (Huston et al. 2000). Based on the dose of caspase-3 inhibitor z-DEVD-FMK used in vitro and in vivo (Yakovlev et al. 1997; Hara et al. 1997; Stefanis et al. 1998; Yakovlev et al. 1997; Hara et al. 1997), 600 ng/rat of z-DEVD-CHO was used in the study. Animals were randomly assigned to 5 groups treated with z-DEVD-CHO or vehicle (dimethyl sulfoxide (DMSO)): (1) sham: DMSO; (2) R 3 h and R 23 h: DMSO, and (3) R 3 h + z-DEVD-CHO and R 23 h + z-DEVD-CHO: 600 ng/rat of z-DEVD-CHO. The respective agent (20 μl) was administrated intraventricularly (bergma: 1.5 mm lateral, 0.8 mm posterior, 3.5 mm deep) at 15 min before MCAo. Rats were decapitated and the tissues of penumbras and cores were dissected at 3 or 23 h of reperfusion (R 3 h or R 23 h) in the vehicle- or z-DEVD-CHO-treated rats, and the regions from the right hemispheres that corresponded to the ischemic penumbra and core were dissected 4 h after operation in sham-operated rats. The cytosolic fractions were prepared and used to determine the activities of calpain (n = 8 per group), and the levels of calpastatin protein, MAP-2, and spectrin (n = 6 per group).
Effects of Calpain Inhibition on the Activities of Caspase-3, and the Protein Levels of MAP-2 and spectrin
Calpain inhibitor I (Roche Diagnostics, GmbH, Germany) is a cell-permeable peptide aldehyde that blocks the active site of calpain (Mehdi 1991; Wang and Yuen 1997). Referring to the dose of calpain inhibitor I used in brain ischemia (Rami and Krieglstein 1993), 0.8 mg/rat of calpain inhibitor I was used to inhibit calpain activity. Animals were randomly assigned to 5 groups treated with calpain inhibitor I or vehicle (DMSO): (1) sham: DMSO; (2) R 3 h and R 23 h: DMSO, and (3) R 3 h + calpain inhibitor I and R 23 h + calpain inhibitor I: 0.8 mg/rat of calpain inhibitor I. The respective agent (20 μl) was administrated intraventricularly at 15 min before MCAo. Rats were decapitated and the tissues of penumbras and cores were dissected at R 3 h or R 23 h in the vehicle- or calpain-inhibitor I-treated rats, and the regions from the right hemispheres that corresponded to the ischemic penumbra and core were dissected 4 h after operation in sham-operated rats. The cytosolic fractions were prepared and used to determine the activities of caspase-3 (n = 8 per group), and the levels of MAP-2 and spectrin (n = 6 per group).
Sample Collection and Preparation of Cytosolic Fraction
As has been previously described by Ashwal et al. (1998), the tissues of penumbras and cores were dissected according to the experimental protocols at 4°C. Briefly, in each animal, the brain was sectioned into three sections beginning 3 mm from the anterior tip of the frontal lobe and 3 mm from the posterior tip of occipital lobe. Regions from the right hemisphere of the middle section that corresponded to the ischemic core and penumbra were dissected. We initially identified the midline between the two hemispheres and then made a longitudinal cut (from top to bottom) approximately 2 mm from the midline through right (ischemic) hemisphere, then made a transverse diagonal cut at approximately the “2 o’clock” position to separate the core (i.e., striatum and overlying cortex) from the penumbra (adjacent cortex). The tissues of penumbras and cores were homogenized in 7 volumes of homogenization buffer (25 mM HEPES, pH 7.4, 0.1% Triton X-100, 5 mM MgCl2, 2 mM DTT, 1.3 mM EDTA, 1 mM EGTA, 0.5 mM phenylmethanesulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml pepstatin A, 10 μg/ml leupeptin). The homogenates were centrifuged at 1,000 × g for 10 min at 4°C, and the supernatants were centrifuged at 17,000 × g for 90 min at 4°C again. The final supernatants (cytosolic fractions) were used to measure the activities of calpain or caspase-3, and the levels of calpastatin, MAP-2, or spectrin. The protein concentrations in supernatants were determined by the method of Bradford (1976).
Calpain Activity Assay
As has been previously described (Raser et al. 1995), 12% polyacrylamide gel containing 0.05% casein and 4% polyacrylamide gel were prepared and used as separating and stacking gels, respectively. The casein gel was pre-run at 50 V for 1 h at 4°C in a running buffer (25 mM Tris, 192 mM glycine, 1 mM EGTA, 1 mM DTT, pH 8.3). Proteins from each sample (50 μg) were loaded in each well and then given electrophoresis at 50 V, 4°C. When bromophenol blue reached the base of the gel, the gel was removed and rinsed in incubation buffer (20 mM Tris, 10 mM DTT, 3 mM CaCl2, pH 7.6 for μ-calpain and pH 7.3 for m-calpain) twice, then incubated in the same incubation buffer at 32 ± 1°C for 24 h. The gel was stained in 0.2% Commassie blue R-250 for 2 h and incubated in a destaining solution overnight. The gel was analyzed by a gel image analyzer (Aalpha Innotech Co., USA). Results were normalized as the integrated optical density (arbitrary densitometric units) of the lysed region (cleared band).
Caspase-3 Activity Assay
The caspase-3 activity was determined by the use of ac-DEVD-AFC (Calbiochem, San Diego, CA, USA), a fluorescent substrate to be used as a susceptible fluorescent substrate for caspase-3 (Benchoua et al. 2001). Ten-microliter sample was diluted in caspase-3 assay buffer (50 mM HEPES, pH 7.4, 100 mM NaCl, 1 mM EDTA, 10 mM DTT) to a final volume of 990 μl. The enzymatic reaction was started by addition of 10 μl of a 2 mM solution of ac-DEVD-AFC and incubated for 2 h at 37°C. Quantification of substrate cleavage leading to release of free AFC was determined with a spectrofluorometer set at 400 nm excitation wavelength and at 505 nm emission wavelength (Hitachi Co., Japan). Fluorescent arbitrary units were converted into micromoles of AFC released per hour and milligram of protein using a standard curve of free AFC (Sigma Co., St Louis, MO, USA).
Western Blot Analysis
Calpastatin, MAP-2, and spectrin were determined from cytosolic fraction separated by 10%, 7.5%, and 6% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), respectively. Twenty-microliter sample was mixed with 5 μl of sample buffer and then boiled for 5 min. Equal amount of proteins (30 μg) was separated by SDS-PAGE, and molecular weight markers (R&D Systems Inc., Minneapolis, MN, USA) were loaded on each gel for protein band identification. The proteins on the gel were subsequently transferred onto a nitrocellulose membrane using a semidry transfer apparatus. Blots were probed with either primary rabbit polyclonal antibody reactive with calpastatin (Sigma Co., St Louis, MO, USA, 1:1200), or primary mouse monoclonal antibody reactive with MAP-2 (Sigma Co., St Louis, MO, USA, 1:800), or primary mouse monoclonal antibody reactive with spectrin (Chemicon International Inc, Temecula, CA, USA, 1:100), and subsequently incubated with secondary anti-rabbit or mouse antibody conjugated with horseradish peroxidase (Beijing Zhongshan Golden Bridge Biotech Co., China). Finally, the color reaction was observed by incubation of membrane with 3,3′-diaminobenzidine (Beijing Zhongshan Golden Bridge Biotech Co., China). The staining results were scanned into a computer and the integrated optical densities of the protein bands were analyzed by a gel image analyzer (Aalpha Innotech Co., USA).
Data Expression and Statistical Analysis
Data were presented as mean ± SEM. Comparisons among multiple groups were statistically evaluated by one-way ANOVA with a post hoc Fisher’s test and comparisons between two groups were evaluated by Student’s t test. A probability of <0.05 was considered statistically significant.
Results
Effects of Caspase-3 Inhibition on Calpain Activity
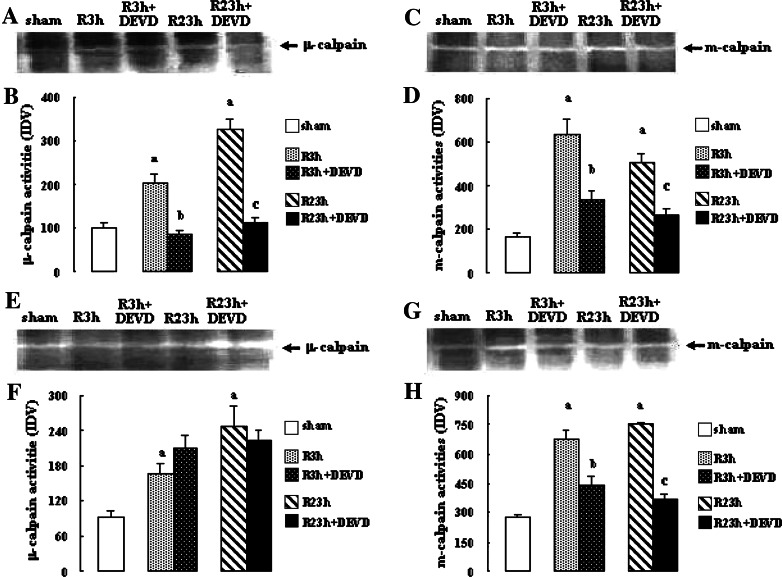
The results are shown in Fig. 1. The activities of μ- and m-calpains at R 3 h and R 23 h in penumbra and core in vehicle-treated rats increased significantly (μ-calpain and m-calpains: all P < 0.01 vs. sham-treated rats). Caspase-3 inhibition by z-DEVD-CHO markedly reduced the activities of μ- and m-calpains in penumbra at R 3 h and 23 h (μ-calpain: both P < 0.01 vs. vehicle-treated rats; m-calpain: both P < 0.05 vs. vehicle-treated rats), and the activities of m-calpain in core at R 3 h and R 23 h (both P < 0.05 vs. vehicle-treated rats). However, it had no significant effects on the activities of μ-calpain in core at R 3 h and R 23 h.
Fig. 1.
Effects of z-DEVD-CHO on the μ- and m-calpain activities in penumbra and core after transient focal cerebral ischemia. The rats received 1-h ischemia and then were reperfused. (A) Casein zymography of μ-calpain in penumbra. (B) The bar graph reflects the densitometry data from the casein zymography of μ-calpain in penumbra (mean ± SEM; n = 8; a P < 0.01 vs. sham; b P < 0.01 vs. R 3 h; c P < 0.01 vs. R 23 h). (C) Casein zymography of m-calpain in penumbra. (D) The bar graph reflects the densitometry data from the casein zymography of m-calpain in penumbra (mean ± SEM; n = 8; a P < 0.01 vs. sham; b P < 0.05 vs. R 3 h; c P < 0.05 vs. R 23 h). (E) Casein zymography of μ-calpain in core. (F) The bar graph reflects the densitometry data from the casein zymography of μ-calpain in core (mean ± SEM; n = 8; a P < 0.01 vs. sham). (G) Casein zymography of m-calpain in core. (H) The bar graph reflects the densitometry data from the casein zymography of m-calpain in core (mean ± SEM; n = 8; a P < 0.01 vs. sham; b P < 0.05 vs. R 3 h. c P < 0.05 vs. R 23 h). R: reperfusion; DEVD: z-DEVD-CHO
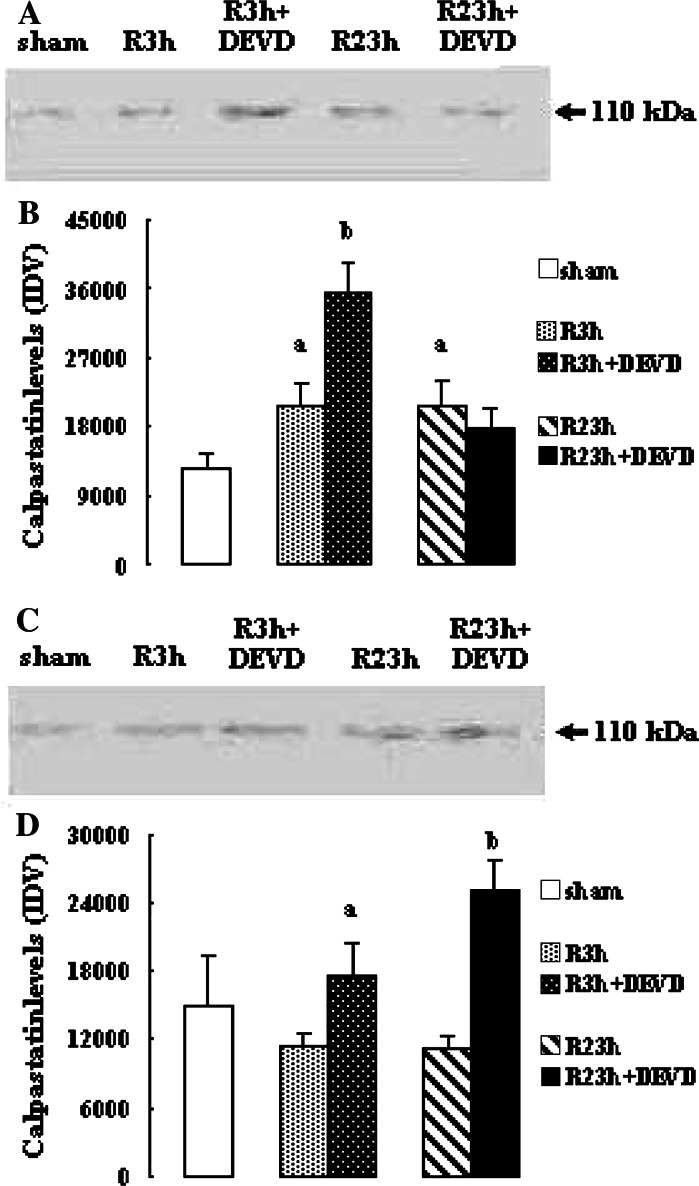
Effects of Caspase-3 Inhibition on Calpastatin Protein Levels
As illustrated in Fig. 2 the protein levels of calpastatin in penumbra at R 3 h and R 23 h in vehicle-treated rats increased significantly (both P < 0.05 vs. sham-treated rats), while the protein levels of calpastatin in core at R 3 h and R 23 h reduced, but it did not reach statistical significance. Caspase-3 inhibition by z-DEVD-CHO markedly enhanced the calpastatin protein levels in penumbra at R 3 h (P < 0.05 vs. vehicle-treated rats) and in core at R 3 h and R 23 h (both P < 0.05 vs. vehicle-treated rats); however, it had no significant effect on the calpastatin protein levels in penumbra at R 23 h.
Fig. 2.
Effects of z-DEVD-CHO on the calpastatin protein levels in penumbra and core after transient focal cerebral ischemia. The rats received 1-h ischemia and then were reperfused. (A) Western blot analysis using anti-calpastatin antibody in penumbra. (B) The bar graph reflects the densitometry data from the experiment of calpastatin western blot analysis in penumbra (mean ± SEM; n = 6; a P < 0.05 vs. sham; b P < 0.05 vs. R 3 h). (C) Western blot analysis using anti-calpastatin antibody in core. (D) The bar graph reflects the densitometry data from the experiment of calpastatin western blot analysis in core (mean ± SEM; n = 6; a P < 0.05 vs. R 3 h; b P < 0.05 vs. R 23 h). R: reperfusion; DEVD: z-DEVD-CHO
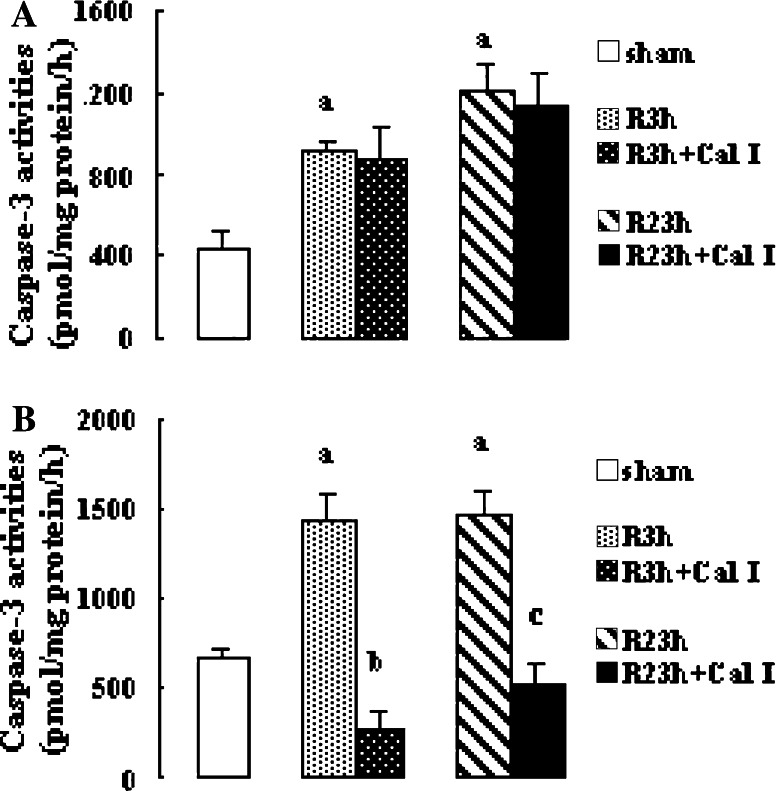
Effects of Calpain Inhibitor on Caspase-3 Activity
The activities of caspase-3 at R 3 h and R 23 h in penumbra and core increased significantly (all P < 0.01 vs. sham-treated rats). Calpain inhibitor I reduced the caspase-3 activities at R 3 h and R 23 h in core significantly (both P < 0.01 vs. vehicle-treated rats); however, it had no significant effect on the caspase-3 activities at R 3 h and R 23 h in penumbra (Fig. 3).
Fig. 3.
Effects of calpain inhibitor I on the caspase-3 activities in penumbra and core after transient focal cerebral ischemia. The rats received 1-h ischemia and then were reperfused. Fluorescent arbitrary units were converted into picomoles of AFC released per hour and milligrams of protein. (A) Caspase-3 activities in penumbra (mean ± SEM; n = 8; a P < 0.01 vs. sham). (B) Caspase-3 activities in core (mean ± SEM; n = 8; a P < 0.01 vs. sham; b P < 0.01 vs. R 3 h; c P < 0.01 vs. R 23 h). R: reperfusion; Cal I: calpain inhibitor I
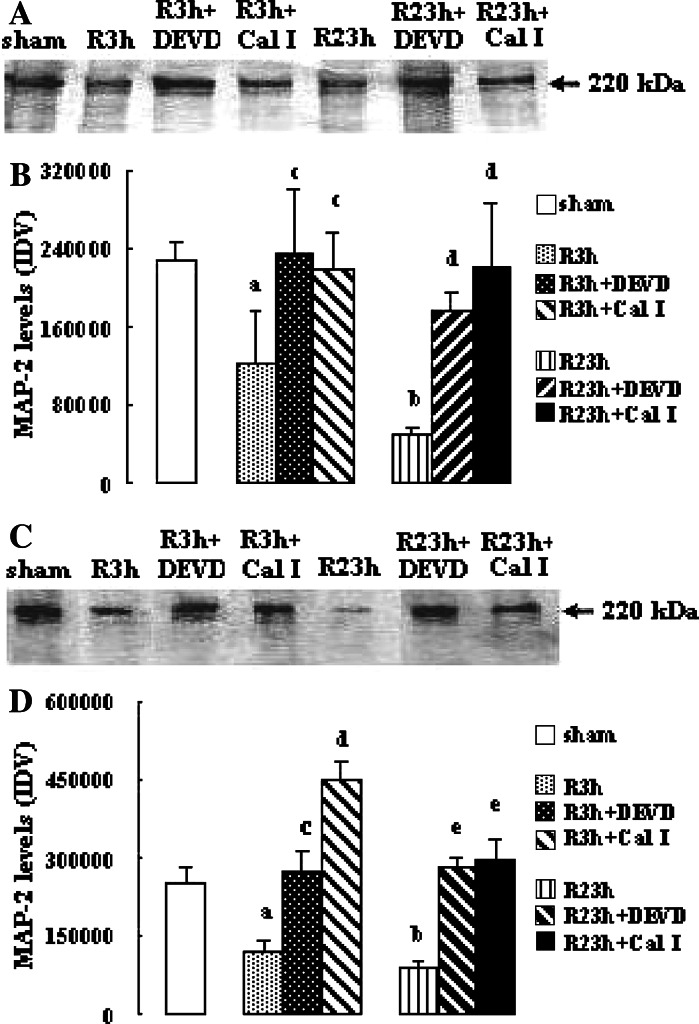
Effects of Inhibition of Calpain or Caspase-3 on the Levels of MAP-2 and Spectrin
As illustrated in Fig. 4 the levels of MAP-2 in penumbra and core in vehicle-treated rats reduced significantly at R 3 h and R 23 h (both P < 0.05 vs. sham-treated rats at R 3 h; both P < 0.01 vs. sham-treated rats at R 23 h). Both calpain inhibitor I and z-DEVD-CHO increased the levels of MAP-2 significantly at R 3 h and R 23 h in penumbra (calpain inhibitor I or z-DEVD-CHO: both P < 0.05 and 0.01 vs. vehicle-treated rats at R 3 h and R 23 h, respectively) and core (calpain inhibitor I: both P < 0.01 vs. vehicle-treated rats; z-DEVD-CHO: P < 0 .05 and 0.01 vs. vehicle-treated rats at R 3 h and R 23 h, respectively).
Fig. 4.
Effects of z-DEVD-CHO and calpain inhibitor I on the MAP-2 levels in penumbra and core after transient focal cerebral ischemia. The rats received 1-h ischemia and then were reperfused. (A) Western blot analysis using anti-MAP-2 antibody in penumbra. (B) The bar graph reflects the densitometry data from the experiment of MAP-2 Western blot analysis in penumbra (mean ± SEM; n = 6; a P < 0.05 and b P < 0.01 vs. sham; c P < 0.05 vs. R 3 h; d P < 0.01 vs. R 23 h). (C) Western blot analysis using anti-MAP-2 antibody in core. (D) The bar graph reflects the densitometry data from the experiment of MAP-2 Western blot analysis in core (mean ± SEM; n = 6; a P < 0.05 and b P < 0.01 vs. sham; c P < 0.05 vs. R 3 h; d P < 0.01 vs. R 3 h; e P < 0.01 vs. R 23 h). R: reperfusion; DEVD: z-DEVD-CHO; Cal I: calpain inhibitor I
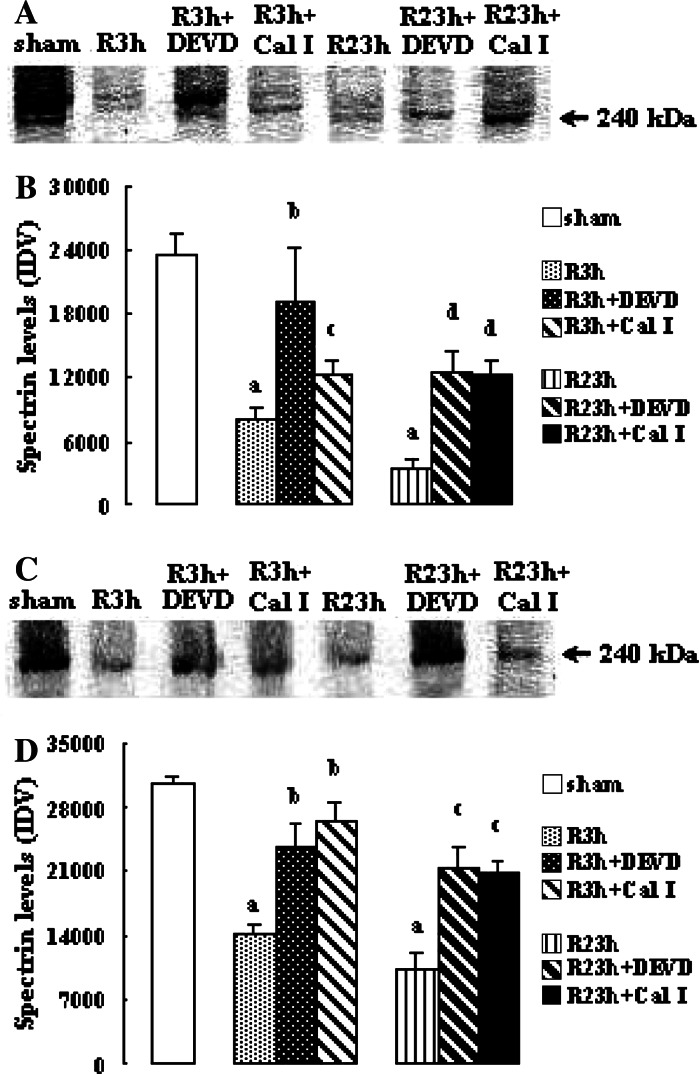
The effects of calpain and caspase-3 inhibitors on spectrin levels are shown in Fig. 5 Spectrin levels at R 3 h and R 23 h in penumbra and core in vehicle-treated rats reduced significantly (all P < 0.01 vs. sham-treated rats). Both calpain inhibitor I and z-DEVD-CHO increased the spectrin levels significantly at R 3 h and R 23 h in penumbra (calpain inhibitor I: P < 0.05 and 0.01 vs. vehicle-treated rats at R 3 h and R 23 h, respectively; z-DEVD-CHO: both P < 0.01 vs. vehicle-treated rats) and in core (calpain inhibitor I and z-DEVD-CHO: all P < 0.05 vs. vehicle-treated rats).
Fig. 5.
Effects of z-DEVD-CHO and calpain inhibitor I on the spectrin levels in penumbra and core after transient focal cerebral ischemia. The rats received 1-h ischemia and then were reperfused. (A) Western blot analysis using anti-spectrin antibody in penumbra. (B) The bar graph reflects the densitometry data from the experiment of spectrin Western blot analysis in penumbra (mean ± SEM; n = 6; a P < 0.01 vs. sham; b P < 0.01 and c P < 0.05 vs. R 3 h; d P < 0.01 vs. R 23 h). (C) Western blot analysis using anti-spectrin antibody in core. (D) The bar graph reflects the densitometry data from the experiment of spectrin Western blot analysis in core (mean ± SEM; n = 6; a P < 0.01 vs. sham; b P < 0.05 vs. R 3 h; c P < 0.05 vs. R 23 h). R: reperfusion; DEVD: z-DEVD-CHO; Cal I: calpain inhibitor I
Discussion
Caspase-3 and calpain/calpastatin play central roles in neuronal death during focal cerebral ischemia. Caspase-3 acts as an executioner of the neuronal apoptosis (Endres et al. 1998; Lipton 1999), and calpain/calpastatin has been implicated in neuronal necrosis or apoptosis after brain ischemia (Seubert et al. 1989; Lipton 1999; Kambe et al. 2005; Han et al. 2006). Recently, some reports have shown the involvement of caspase-3 in necrosis after brain ischemia (Schwab et al. 2002; Berliocchi et al. 2005). MAP-2 and spectrin, two cytoskeletal proteins, play important roles in maintenance of structural integrity, neuronal stability, normal functions, and plasticity through interaction with neuronal cytoskeleton (Ludin and Matus 1993; Goodman and Zagon 1986; Lipton 1999). Spectrin is a common substrate for calpain and caspase-3 (Wang 2000), and MAP-2 is one of the substrates for calpain (Johnson et al. 1991). Some reports demonstrate that cytoskeletal degradation, represented by the loss of MAP-2 or spectrin shown by immunochemical methods, is a sensitive indicator of neuronal damage after focal cerebral ischemia (Dawson and Hallenbeck 1996; Kambe et al. 2005).
In the present study, we find that the activities of μ- and m-calpains, and caspase-3 markedly increase; meanwhile, the levels of MAP-2 and spectrin decrease significantly, and both calpain and caspase-3 inhibition reduce the degradation of MAP-2 and spectrin in penumbra and core at 3 and 23 h of reperfusion. These data indicate that calpain and caspase-3 are activated concurrently in penumbra and core, subsequently, degrade MAP-2 and spectrin, together, leading to the loss of structural integrity and stability of neurons, resulting in neuronal death. Our data imply the existence of cross-talk between calpain and caspase-3 after focal cerebral ischemia-reperfusion. This implication is supported by some previous studies: (1) caspases may cleave calpastatin and thus regulate calpain activity during apoptosis (Wang et al. 1998; Porn-Ares et al. 1998; Neumar et al. 2003) and renal ischemia-reperfusion (Shi et al. 2000) and (2) calpain may contribute to caspase-3 activation during cerebral hypoxia-ischemia in the immature rat (Blomgren et al. 2001) and apoptosis (Wood et al. 1998; Nakagawa and Yuan 2000; Bitko and Barik 2001).
In order to further confirm the cross-talk between calpain and caspase-3 in penumbra or core during focal cerebral ischemia-reperfusion, pharmacological blockade of calpain and caspase-3 by their inhibitors was used in the experiments to study their interaction. Our results reveal that z-DEVD-CHO markedly reduces the μ- and m-calpain activities in penumbra and the m-calpain activities in core at 3 and 23 h of reperfusion, and enhances the calpastatin levels in penumbra at 3 h of reperfusion and in core at 3 and 23 h of reperfusion significantly, although it has no significant effects on the μ-calpain activities in core at 3 and 23 h of reperfusion and the calpastatin levels in penumbra at 23 h of reperfusion. Meanwhile, calpain inhibitor I markedly reduces the caspase-3 activities in core at 3 and 23 h of reperfusion, but not in penumbra. These data provide direct evidence to demonstrate the cross-talk between calpain and caspase-3 in penumbra and core during focal cerebral ischemia-reperfusion.
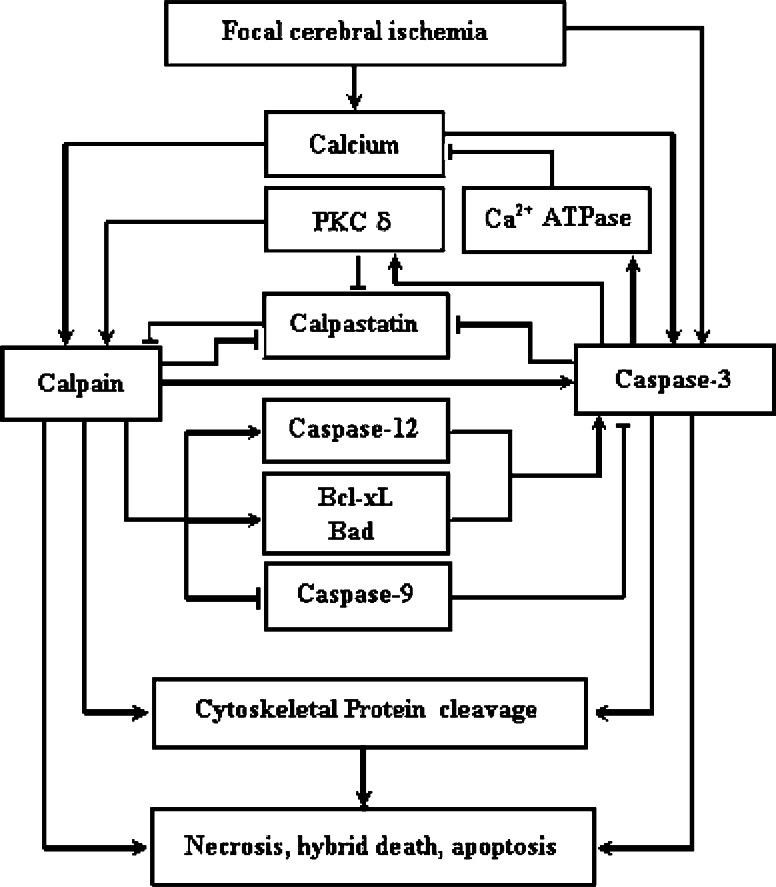
The possible mechanisms concerning the effects of caspase-3 inhibition on the calpain activities during focal cerebral ischemia-reperfusion in rats may be explained by: (1) reducing the degradation of calpastatin, maintaining the inhibitory ability of calpastatin to calpain (Wang et al. 1998; Porn-Ares et al. 1998; Shi et al. 2000), as shown in this study that caspase-3 inhibition enhances the calpastatin protein levels in penumbra at 3 h of reperfusion and in core at 3 and 23 h of reperfusion. Although caspase-3 inhibition has no significant effect on the calpastatin protein levels in penumbra at 23 h of reperfusion, it is not ruled out that caspase-3 may up-regulate calpain activities through degrading calpastatin protein, since the calpain activity in penumbra shows a small-to-moderate increase at the second hour of ischemia and a larger increase between the 6th and 24th hour after transient focal cerebral ischemia (Hong et al. 1994), and calpastatin may act as a suicide substrate for calpain to prevent the over-activation of calpain (Mellgren et al. 1986; Blomgren et al. 1999); (2) suppressing PKC activation (Ghayur et al. 1996), as PKC may phosphorylate calpain and calpastatin (Kuo et al. 1993; Brumley and Wallace 1989; Averna et al. 1999), leading to the activation of calpain and the reduction of the capacity of calpastatin to inhibit calpain. During transient focal cerebral ischemia, PKC δ mRNA is specifically induced in penumbra at 4, 12, and 24 h after ischemia, while the mRNA levels of PKC-α, -β, -γ, -δ, -ε, and -ζ are decreased in core 4 h after ischemia and lost completely 12 h after ischemia (Miettinen et al. 1996). Possibly, caspase-3 inhibition may down-regulate calpain activation in penumbra by suppressing the activation of PKC δ; and (3) depressing the cleavage of plasma membrane calcium pump, which is contributed to maintain intracellular calcium homeostasis (Schwab et al. 2002), leading to the reduction of calpain activation. Figure 6 summarizes the possible regulation of caspase-3 on calpain activation after transient focal cerebral ischemia.
Fig. 6.
Overview of the cross-talk between calpain and caspase-3 after focal cerebral ischemia. “→” indicates positive actions. “˧” indicates negative actions
However, the different effects of caspase-3 inhibition on μ-calpain activities in penumbra and core, and on the μ- and m-calpain activities in core are observed simultaneously. The former may be due to the degree of insults, i.e., penumbra suffers milder insults compared with core (Lipton 1999; Mergenthaler et al. 2004), and the latter may be related to (1) the distribution of μ- and m-calpains within brain, as μ-calpain is located primarily in neurons, whereas m-calpain is more prominent in glial cells (Hamakubo et al. 1986). It is reported that glial activation after brain ischemia contributes to neuronal death (Stoll et al. 1998), which implies that the glial cells are more resistant to ischemic damage than neurons and suggests that m-calpain are more susceptible to pharmacological intervention than μ-calpain, and (2) the calcium concentration required for μ- and m-calpain activations, since μ- and m-calpains need micromolar and millimolar calcium for activation, respectively (Goll et al. 2003), and caspase-3 may modulate intracellular calcium through cleaving plasma membrane calcium pump (Schwab et al. 2002).
Not only do caspases modulate calpain activity, they also regulate caspase activity (Fig. 6). Calpain may up- or down-regulate caspase-3 activation in different experimental system through one or more mechanisms as follows: (1) cleaving procaspase-3 directly, facilitating the caspase-3 activation (Blomgren et al. 2001); (2) cleaving caspase-12 (Bitko and Barik 2001), or the proapoptotic protein Bax and the antiapoptotic molecule Bcl-xL (Wood et al. 1998; Nakagawa and Yuan 2000), promoting the activation of caspase-3; and (3) cleaving procaspase-9, leading to the loss of the capacity of caspase-9 to activate caspase-3 (Chua et al. 2000), or cleaving active caspase-3 directly, resulting in the inactivation of caspase-3 (Bizat et al. 2003). Therefore, it is possible that calpain may exert either positive or negative effects on caspase-3 activation in penumbra or core by affecting different point of the caspase-3 apoptotic cascade after focal cerebral ischemia-reperfusion. The results that calpain inhibition does not exert significant effect on caspase-3 activation in penumbra, however, down-regulate caspase-3 activation in core, may be due to the final outcome (the net effect) of their complex cross-talk. This difference may be attributed to the diversity of insults in penumbra and core (Lipton 1999; Mergenthaler et al. 2004).
Moreover, the observation with regard to the effect of calpain and caspase-3 inhibitors on substrates of two enzymes provides further evidence to support our conclusion that there is a cross-talk between calpain and caspase-3 during focal cerebral ischemia-reperfusion. The evidences include: (1) caspase-3 inhibition reduces the MAP-2 degradation both in penumbra and core, indicating the down-regulation of calpain activation by caspase-3 inhibition, since MAP-2 can be degraded specifically by calpain (Johnson et al. 1991), and (2) the reduction of spectrin degradation by either calpain or caspase-3 inhibition may imply the coordinated actions of calpain and caspase-3, as spectrin can be cleaved by calpain and caspase-3 at different sites (Wang 2000).
In general terms, we provide the biochemical and pharmacological evidences to demonstrate the existence of the cross-talk between calpain and caspase-3 in penumbra and core during focal cerebral ischemia-reperfusion in rats. Furthermore, calpastatin, as an endogenous calpain inhibitor and a common substrate for calpain and caspase-3, may play an important role in the process of this cross-talk. Our data suggest that there are two different types of cross-talk between calpain and caspase-3 during focal cerebral ischemia-reperfusion in rats. First, in penumbra, the activated caspase-3 up-regulates calpain activation through degrading calpastatin; however, calpain has no significant effects on caspase-3 activation, suggesting that calpain may act as a downstream event to participate in the caspase-3-mediated ischemic neuronal death, whereas caspase-3 may not be involved in the calpain-mediated ischemic neuronal death. Second, in core, the activated caspase-3 up-regulates m-calpain activation through degrading calpastatin; simultaneously, calpain is contributed to the caspase-3 activation, suggesting that both calpain and caspase-3 may be involved in neuronal death in core, and the positive cross-talk between m-calpain and caspase-3 may play more important roles.
Apoptosis is an energy-dependent and regulated active process of cell death. It differs from necrosis, a toxic process in which the cell is a passive victim (Harwood et al. 2005). The mode of cell death, apoptosis or necrosis, is determined by the energy status under a stress or an insult (Nicotera et al. 1999). The interrelationship between apoptosis and necrosis is highlighted by the fact that necrotic cell death can be mediated by part of the apoptotic machinery (Wang et al. 2003), and that both death modes can share common pathways (Kim et al. 2003). Caspases and calpains, two cysteine protease families, play key roles in apoptosis and necrosis. Caspases are thought of as the essential apparatus of classical apoptosis, and caspase-3 is an execution caspase. Calpains are primarily involved in necrotic cell death (Harwood et al. 2005). Recently, some data suggest that calpain can function in apoptotic cell death. For example, the cross-talk between calpain and caspase-3 has been demonstrated in vitro and in vivo (Nakagawa and Yuan 2000; Blomgren et al. 2001; Neumar et al. 2003; Bizat et al. 2003), and μ-calpain, but not m-calpain, is transported into nuclei in an ATP-dependent fashion (Mellgren 1997), and the calpain activity in the nuclear fraction is observed (Kubbutat and Vousden 1997). It is well known that ischemic penumbra suffers milder insults compared with core. In penumbra, the caspase-3 and μ-calpain-mediated apoptotic cascades can continue as the energy metabolism is partly preserved, and the neuronal death is predominant in apoptosis. While in core, the caspase-3 and μ-calpain-mediated apoptotic cascades cannot continue due to the ATP depletion, and the neuronal death is predominant in necrosis (Lipton 1999). Moreover, some reports have shown the concomitant activation of apoptotic and necrotic death mechanisms in the same cell after focal cerebral ischemia (Unal-Cevik et al. 2004), and the morphological characteristics of ischemic neuronal death, including necrosis, apoptosis, or combination of necrosis and apoptosis (hybrid death) (Martin et al. 1998; Lipton 1999; Wei et al. 2004). Therefore, we hypothesize that the complex cross-talk between calpain and caspase-3 in penumbra or core may contribute to the hybrid morphology of neuronal death commonly observed in in vivo studies (Wei et al. 2004), since both calpain and caspase-3 are involved in the apoptosis and necrosis after brain ischemia (Endres et al. 1998; Lipton 1999; Blomgren et al. 2001; Schwab et al. 2002; Berliocchi et al. 2005; Kawamura et al. 2005). Furthermore, the complex cross-talk of these two pathways should be considered when evaluating the mechanism and efficacy of therapeutic interventions aimed at reducing injury-induced neuronal death.
In summary, the present study provides direct evidence to demonstrate the concurrent activation of calpain and caspase-3, and the interaction between calpain and caspase-3 in penumbra and core during focal cerebral ischemia-reperfusion. Further study should be performed to elucidate the detailed mechanism mediating the interaction between calpain and caspase-3 during focal cerebral ischemia-reperfusion.
Acknowledgment
This research is supported by Beijing Natural Science Foundation (No. 7002013).
References
- Ashwal S, Tone B, Tian HR, Cole DJ, Pearce WJ (1998) Core and penumbral nitric oxide synthase activity during cerebral ischemia and reperfusion. Stroke 29:1037–1047 [PubMed] [Google Scholar]
- Atencio IA, Ramachandra M, Shabram P, Demers GW (2000) Calpain inhibitor 1 activates p53-dependent apoptosis in tumor cell lines. Cell Growth Differ 11:247–253 [PubMed] [Google Scholar]
- Averna M, De Tullio R, Salamino F, Melloni E, Pontremoli S (1999) Phosphorylation of rat brain calpastatins by protein kinase C. FEBS Lett 450:13–16 [DOI] [PubMed] [Google Scholar]
- Benchoua A, Guegan C, Couriaud C, Hosseini H, Sampaio N, Morin D, Onteniente B (2001) Specific caspase pathways are activated in the two stages of cerebral infarction. J Neurosci 21:7127–7134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berliocchi L, Bano D, Nicotera P (2005) Ca2+ signals and death programmes in neurons. Phil Trans R Soc B 360:2255–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitko V, Barik S (2001) An endoplasmic reticulum-specific stress-activated caspase (caspase-12) is implicated in the apoptosis of A549 epithelial cells by respiratory syncytial virus. J Cell Biochem 80:441–454 [DOI] [PubMed] [Google Scholar]
- Bizat N, Hermel JM, Humbert S, Jacquard C, Creminon C, Escartin C, Saudou F, Krajewski S, Hantraye P, Brouillet E (2003) In vivo calpain/caspase cross-talk during 3-nitropropionic acid-induced striatal degeneration: implication of a calpain-mediated cleavage of active caspase-3. J Biol Chem 278:43245–43253 [DOI] [PubMed] [Google Scholar]
- Blomgren K, Hallin U, Andersson AL, Puka-Sundvall M, Bahr BA, McRae A, Saido TC, Kawashima S, Hagberg H (1999) Calpastatin is up-regulated in response to hypoxia and is a suicide substrate to calpain after neonatal cerebral hypoxia-ischemia. J Biol Chem 274:14046–14052 [DOI] [PubMed] [Google Scholar]
- Blomgren K, Zhu C, Wang X, Karlsson JO, Leverin AL, Bahr BA, Mallard C, Hagberg H (2001) Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: a mechanism of “pathological apoptosis”? J Biol Chem 276:10191–10198 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Brumley LM, Wallace RW (1989) Calmodulin and protein kinase C antagonists also inhibit the Ca2+-dependent protein protease, calpain I. Biochem Biophys Res Commun 159:1297–1303 [DOI] [PubMed] [Google Scholar]
- Chen M, He H, Zhan S, Krajewski S, Reed JC, Gottlieb RA (2001) Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J Biol Chem 276:30724–30728 [DOI] [PubMed] [Google Scholar]
- Chua BT, Guo K, Li P (2000) Direct cleavage by the calcium-activated protease calpain can lead to inactivation of caspases. J Biol Chem 275:5131–5135 [DOI] [PubMed] [Google Scholar]
- Dawson DA, Hallenbeck JM (1996) Acute focal ischemia-induced alterations in MAP2 immunostaining: description of temporal changes and utilization as a marker for volumetric assessment of acute brain injury. J Cereb Blood Flow Metab 16:170–174 [DOI] [PubMed] [Google Scholar]
- Endres M, Namura S, Shimizu-Sasamata M, Waeber C, Zhang L, Gomez-Isla T, Hyman BT, Moskowitz MA (1998) Attenuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family. J Cereb Blood Flow Metab 18:238–247 [DOI] [PubMed] [Google Scholar]
- Ghayur T, Hugunin M, Talanian RV, Ratnofsky S, Quinlan C, Emoto Y, Pandey P, Datta R, Huang Y, Kharbanda S, Allen H, Kamen R, Wong W, Kufe D (1996) Proteolytic activation of protein kinase C delta by an ICE/CED 3-like protease induces characteristics of apoptosis. J Exp Med 184:2399–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll DE, Thompson VF, Li H, Wei W, Cong J (2003) The calpain system. Physiol Rev 83:731–801 [DOI] [PubMed] [Google Scholar]
- Goodman SR, Zagon IS (1986) The neural cell spectrin skeleton: a review. Am J Physiol 250:C347–C360 [DOI] [PubMed] [Google Scholar]
- Hamakubo T, Kannagi R, Murachi T, Matus A (1986) Distribution of calpains I and II in rat brain. J Neurosci 6:3103–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F, Shirasaki Y, Fukunaga K (2006) 3-[2-[4-(3-Chloro-2-methylphenylmethyl)-1- piperazinyl]ethyl]-5,6-dimethoxy-1-(4-imidazolylmethyl)-1H-indazole dihydro-chloride 3.5 hydrate (DY-9760e) is neuroprotective in rat microsphere embolism: role of the cross-talk between calpain and caspase-3 through calpastatin. J Pharmacol Exp Ther 317:529–536 [DOI] [PubMed] [Google Scholar]
- Hara H, Friedlander RM, Gagliardini V, Ayata C, Fink K, Huang Z, Shimizu-Sasamata M, Yuan J, Moskowitz MA (1997) Inhibition of interleukin 1beta converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc Natl Acad Sci USA 94:2007–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood SM, Yaqoob MM, Allen DA (2005) Caspase and calpain function in cell death: bridging the gap between apoptosis and necrosis. Ann Clin Biochem 42:415–431 [DOI] [PubMed] [Google Scholar]
- Hong SC, Lanzino G, Goto Y, Kang SK, Schottler F, Kassell NF, Lee KS (1994) Calcium-activated proteolysis in rat neocortex induced by transient focal ischemia. Brain Res 661:43–50 [DOI] [PubMed] [Google Scholar]
- Huston E, Beard M, McCallum F, Pyne NJ, Vandenabeele P, Scotland G, Houslay MD (2000) The cAMP-specific phosphodiesterase PDE4A5 is cleaved downstream of its SH3 interaction domain by caspase-3. Consequences for altered intracellular distribution. J Biol Chem 275:28063–28074 [DOI] [PubMed] [Google Scholar]
- Johnson GV, Litersky JM, Jope RS (1991) Degradation of microtubule-associated protein 2 and brain spectrin by calpain: a comparative study. J Neurochem 56:1630–1638 [DOI] [PubMed] [Google Scholar]
- Kambe A, Yokota M, Saido TC, Satokata I, Fujikawa H, Tabuchi S, Kamitani H, Watanabe T (2005) Spatial resolution of calpain-catalyzed proteolysis in focal cerebral ischemia. Brain Res 1040:36–43 [DOI] [PubMed] [Google Scholar]
- Kawamura M, Nakajima W, Ishida A, Ohmura A, Miura S, Takada G (2005) Calpain inhibitor MDL 28170 protects hypoxic-ischemic brain injury in neonatal rats by inhibition of both apoptosis and necrosis. Brain Res 1037:59–69 [DOI] [PubMed] [Google Scholar]
- Kim JS, He L, Lemasters JJ (2003) Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun 304:463–470 [DOI] [PubMed] [Google Scholar]
- Kubbutat MH, Vousden KH (1997) Proteolytic cleavage of human p53 by calpain: a potential regulator of protein stability. Mol Cell Biol 17:460–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo WN, Ganesan U, Walbey DL, Davis DL, Allen K, McCall LK (1993) Modulation of the activity of calpain II by phosphorylation—changes in the proteolysis of cyclic AMP-dependent protein kinase (peak II, DEAE). Appl Theor Electrophor 3:317–320 [PubMed] [Google Scholar]
- Lipton P (1999) Ischemic cell death in brain neurons. Physiol Rev 79:1431–1568 [DOI] [PubMed] [Google Scholar]
- Ludin B, Matus A (1993) The neuronal cytoskeleton and its role in axonal and dendritic plasticity. Hippocampus 3:61–71 [PubMed] [Google Scholar]
- Martin LJ, Al-Abdulla NA, Brambrink AM, Kirsch JR, Sieber FE, Portera-Cailliau C (1998) Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: a perspective on the contributions of apoptosis and necrosis. Brain Res Bull 46:281–309 [DOI] [PubMed] [Google Scholar]
- Mehdi S (1991) Cell-penetrating inhibitors of calpain. Trends Biol Sci 16:150–153 [DOI] [PubMed] [Google Scholar]
- Mellgren RL (1997) Evidence for participation of a calpain-like cysteine protease in cell cycle progression through late G1 phase. Biochem Biophys Res Commun 236:555–558 [DOI] [PubMed] [Google Scholar]
- Mellgren RL, Mericle MT, Lane RD (1986) Proteolysis of the calcium-dependent protease inhibitor by myocardial calcium-dependent protease. Arch Biochem Biophys 246:233–239 [DOI] [PubMed] [Google Scholar]
- Mergenthaler P, Dirnagl U, Meisel A (2004) Pathophysiology of stroke: lessons from animal models. Metab Brain Dis 19:151–167 [DOI] [PubMed] [Google Scholar]
- Miettinen S, Roivainen R, Keinanen R, Hokfelt T, Koistinaho J (1996) Specific induction of protein kinase Cδ subspecies after transient middle cerebral artery occlusion in the rat brain: inhibitory by MK-801. J Neurosci 16:6236–6245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Yuan J (2000) Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol 150:887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumar RW, Xu YA, Gada H, Guttmann RP, Siman R (2003) Cross-talk between calpain and caspase proteolytic systems during neuronal apoptosis. J Biol Chem 278:14162–14167 [DOI] [PubMed] [Google Scholar]
- Nicotera P, Leist M, Ferrando-May E (1999) Apoptosis and necrosis: different execution of the same death. Biochem Soc Symp 66:69–73 [DOI] [PubMed] [Google Scholar]
- Porn-Ares MI, Samali A, Orrenius S (1998) Cleavage of the calpain inhibitor, calpastatin, during apoptosis. Cell Death Differ 5:1028–1033 [DOI] [PubMed] [Google Scholar]
- Rami A, Krieglstein J (1993) Protective effects of calpain inhibitors against neuronal damage caused by cytotoxic hypoxia in vitro and ischemia in vivo. Brain Res 609:67–70 [DOI] [PubMed] [Google Scholar]
- Raser KL, Posner A, Wang KK (1995) Casein zymography: a method to study μ-calpain, m-calpain and their inhibitory agents. Arch Biochem Biophys 319:211–216 [DOI] [PubMed] [Google Scholar]
- Reimertz C, Kogel D, Lankiewicz S, Poppe M, Prehn JH (2001) Ca2+-induced inhibition of apoptosis in human SH-SY5Y neuroblastoma cells: degradation of apoptotic protease activating factor-1 (APAF-1). J Neurochem 78:1256–1266 [DOI] [PubMed] [Google Scholar]
- Salvesen GS, Dixit VM (1997) Caspases: intracellular signaling by proteolysis. Cell 91:443–446 [DOI] [PubMed] [Google Scholar]
- Schmid-Elsaesser R, Zausinger S, Hungerhuber E, Baethmann A, Reulen HJ (1998) A critical reevaluation of the intraluminal thread model of focal cerebral ischemia. Evidence of inadvertent premature reperfusion and subarachnoid hemorrhage in rats by laser-Doppler flowmetry. Stroke 29:2162–2170 [DOI] [PubMed] [Google Scholar]
- Schwab BL, Guerini D, Didszun C, Bano D, Ferrando-May E, Fava E, Tam J, Xu D, Xanthoudakis S, Nicholson DW, Carafoli E, Nicotera P (2002) Cleavage of plasma membrane calcium pumps by caspases: a link between apoptosis and necrosis. Cell Death Differ 9:818–831 [DOI] [PubMed] [Google Scholar]
- Seubert P, Lee K, Lynch G (1989) Ischemia triggers NMDA receptor-linked cytoskeletal proteolysis in hippocampus. Brain Res 492:366–370 [DOI] [PubMed] [Google Scholar]
- Shi Y, Melnikov VY, Schrier RW, Edelstein CL (2000) Downregulation of the calpain inhibitor protein calpastatin by caspases during renal ischemia-reperfusion. Am J Physiol Renal Physiol 279:F509–F517 [DOI] [PubMed] [Google Scholar]
- Stefanis L, Troy CM, Qi H, Shelanski ML, Greene LA (1998) Caspase-2 (Nedd-2) processing and death of trophic factor-deprived PC12 cells and sympathetic neurons occur independently of caspase-3 (CPP32)-like activity. J Neurosci 18:9204–9215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M (1998) Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol 56:149–171 [DOI] [PubMed] [Google Scholar]
- Unal-Cevik I, Kilinc M, Can A, Gursoy-Ozdemir Y, Dalkara T (2004) Apoptotic and necrotic death mechanisms are concomitantly activated in the same cell after cerebral ischemia. Stroke 35:2189–2194 [DOI] [PubMed] [Google Scholar]
- Wang KK (2000) Calpain and caspase: can you tell the difference? Trends Neurosci 23:20–26 [DOI] [PubMed] [Google Scholar]
- Wang KK, Yuen PW (1997) Development and therapeutic potential of calpain inhibitors. Adv Pharmacol 37:117–152 [DOI] [PubMed] [Google Scholar]
- Wang KK, Posmantur R, Nadimpalli R, Nath R, Mohan P, Nixon RA, Talanian RV, Keegan M, Herzog L, Allen H (1998) Caspase-mediated fragmentation of calpain inhibitor protein calpastatin during apoptosis. Arch Biochem Biophys 356:187–196 [DOI] [PubMed] [Google Scholar]
- Wang X, Ryter SW, Dai C, Tang ZL, Watkins SC, Yin XM, Song R, Choi AM (2003) Necrotic cell death in response to oxidant stress involves the activation of the apoptogenic caspase-8/bid pathway. J Biol Chem 278:29184–19191 [DOI] [PubMed] [Google Scholar]
- Wei L, Ying DJ, Cui L, Langsdorf J, Yu SP (2004) Necrosis, apoptosis and hybrid death in the cortex and thalamus after barrel cortex ischemia in rats. Brain Res 1022:54–61 [DOI] [PubMed] [Google Scholar]
- Wood DE, Thomas A, Devi LA, Berman Y, Beavis RC, Reed JC, Newcomb EW (1998) Bax cleavage is mediated by calpain during drug-induced apoptosis. Oncogene 17:1069–1078 [DOI] [PubMed] [Google Scholar]
- Yakovlev AG, Knoblach SM, Fan L, Fox GB, Goodnight R, Faden AI (1997) Activation of CPP32-like caspases contributes to neuronal apoptosis and neurological dysfunction after traumatic brain injury. J Neurosci 17:7415–7424 [DOI] [PMC free article] [PubMed] [Google Scholar]