Abstract
Bmi-1 is required for the post-natal maintenance of stem cells in multiple tissues including the central nervous system (CNS) and peripheral nervous system (PNS). Deletion of Ink4a or Arf from Bmi-1-/- mice partially rescued stem cell self-renewal and stem cell frequency in the CNS and PNS, as well as forebrain proliferation and gut neurogenesis. Arf deficiency, but not Ink4a deficiency, partially rescued cerebellum development, demonstrating regional differences in the sensitivity of progenitors to p16Ink4a and p19Arf. Deletion of both Ink4a and Arf did not affect the growth or survival of Bmi-1-/- mice or completely rescue neural development. Bmi-1 thus prevents the premature senescence of neural stem cells by repressing Ink4a and Arf, but additional pathways must also function downstream of Bmi-1.
Keywords: Bmi-1, neural crest, stem cell, p16Ink4a, p19Arf, self-renewal
Stem cells must persist throughout adult life in numerous tissues in order to replace the mature cells that are lost to turnover, injury, or disease. The mechanism by which stem cells persist throughout life involves self-renewal—stem cell divisions that generate one or two daughter stem cells (Morrison et al. 1997; Molofsky et al. 2004). Stem cells self-renew post-natally in numerous tissues, including the central nervous system (CNS) (Maslov et al. 2004), peripheral nervous system (PNS) (Kruger et al. 2002), and hematopoietic system (Harrison 1979; Morrison et al. 1996).
The polycomb family transcriptional repressor Bmi-1 is required for the self-renewal and post-natal maintenance of hematopoietic stem cells (Lessard and Sauvageau 2003; Park et al. 2003) and neural stem cells from the CNS and PNS (Molofsky et al. 2003). In each tissue Bmi-1-/- stem cells form in normal numbers and appear to differentiate normally, but exhibit a post-natal self-renewal defect that leads to their depletion by early adulthood. Bmi-1 tends to be turned off as cells differentiate (Lessard et al. 1998) and is not required for the proliferation of all cells (Molofsky et al. 2003). Bmi-1 functions as part of a protein complex that maintains gene silencing by regulating chromatin structure (Valk-Lingbeek et al. 2004). Except for a mild skeletal transformation, Bmi-1-/- mice are normal in size and appearance at birth (van der Lugt et al. 1994). However, they exhibit progressive post-natal growth retardation and die by early adulthood with signs of hematopoietic failure (hypocellular bone marrow) and neurological abnormalities (seizures and ataxia) (van der Lugt et al. 1994).
Bmi-1-/- mice develop several specific neural abnormalities. The rate of proliferation in the forebrain subventricular zone (SVZ; where CNS stem cells undergo neurogenesis), is reduced by post-natal day 30 (P30) when stem cell depletion becomes severe (Molofsky et al. 2003). The cerebellum also fails to develop normally, partly because Bmi-1 is required for the proliferation of granule precursor cells (Leung et al. 2004). Finally, adult Bmi-1-/- mice exhibit fewer neurons in the myenteric plexus of the gut as the neural crest stem cells (NCSCs) in this region of the post-natal PNS become depleted (Molofsky et al. 2003). These defects indicate that the pathways regulated by Bmi-1 have important consequences for neural development.
Bmi-1 represses transcription at the Ink4a-Arf locus (Jacobs et al. 1999a,b), which encodes two inhibitors of cell proliferation (Sherr 2001). Ink4a encodes p16Ink4a, a cyclin-dependent kinase inhibitor that promotes Rb activation. Arf encodes p19Arf, which promotes p53 activation. p16Ink4a and p19Arf are induced in cultured primary cells and can cause these cells to senesce (for review, see Lowe and Sherr 2003). Bmi-1 overexpression can prevent senescence and extend the replicative lifespan of primary cells by reducing p16Ink4a and p19Arf expression (Jacobs et al. 1999a; Dimri et al. 2002; Itahana et al. 2003). Deletion of Ink4a-Arf from Bmi-1-/- mice rescues the ability of mouse embryonic fibroblasts to proliferate in culture and at least partially rescues defects in cerebellum development (Jacobs et al. 1999a). p16Ink4a expression is elevated in Bmi-1-/- neural stem cells and deletion of Ink4a from Bmi-1-/- mice partially rescues neural stem cell self-renewal in culture (Molofsky et al. 2003).
To test whether Ink4a deletion rescues stem cell frequency or neural development in Bmi-1-/- mice, and whether p19Arf also mediates part of the effect of Bmi-1 on stem cell function or neural development, we have generated compound mutant mice that lack Bmi-1 (van der Lugt et al. 1994) and/or Ink4a (Sharpless et al. 2001), Arf (Kamijo et al. 1997), or Ink4a-Arf (Serrano et al. 1996). Our results indicate that the repression of Ink4a and Arf each represent major mechanisms by which Bmi-1 promotes neural stem cell self-renewal and neural development. The post-natal maintenance of neural stem cells depends upon the repression of senescence pathways that otherwise cause the premature depletion of stem cells.
Results and Discussion
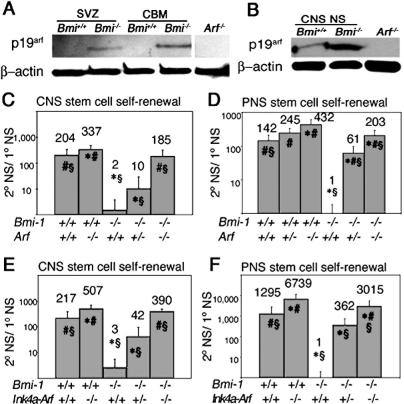
Like p16Ink4a (Molofsky et al. 2003), p19Arf expression increased in the absence of Bmi-1. p19Arf was not detected in the wild-type SVZ or cerebellum, but was detected in these tissues in the absence of Bmi-1 (Fig. 1A). p19Arf was up-regulated in Bmi-1-/- CNS neurospheres cultured from the SVZ (Fig. 1B) and Bmi-1-/- PNS neurospheres cultured from the adult gut wall (data not shown), though p19Arf was also detected at lower levels in wild-type neurospheres. The detection of both p16Ink4a (Molofsky et al. 2003) and p19Arf in cultured neurospheres despite not detecting these proteins in vivo suggests that Ink4a and Arf are induced in wild-type cells in culture, though not to the extent as in Bmi-1-/- cells. This is consistent with our failure to detect Ink4a or Arf by PCR in uncultured wild-type NCSCs isolated by flow cytometry (Molofsky et al. 2003).
Figure 1.
p19Arf is up-regulated in the absence of Bmi-1, and deletion of Arf or Ink4a-Arf significantly increased the self-renewal of neural stem cells from the SVZ and gut of Bmi-1-/- mice. (A) p19Arf was increased in uncultured SVZ and cerebellum (CBM) of 4-wk-old Bmi-1-/- mice. Western blot of neurosphere cells from Arf-/- mice is shown as a negative control. (B) p19Arf was increased in Bmi-1-/- CNS neurospheres (NS) cultured for 13 d. (C,D) Arf deficiency significantly increased self-renewal (the number of secondary neurospheres generated per subcloned primary neurosphere) within Bmi-1+/+ and Bmi-1-/- CNS neurospheres (C) and PNS neurospheres (D), as well as the percentage of cells from Bmi-1-/- (but not Bmi-1+/+) primary neurospheres that formed secondary neurospheres upon replating (Supplementary Fig. 6). CNS data represent mean ± SD for two to four mice per genotype, in four independent experiments. PNS data are from three to seven mice per genotype in four independent experiments. (E,F) Ink4a-Arf deficiency significantly increased self-renewal within Bmi-1+/+ and Bmi-1-/- CNS neurospheres (E; mean ± SD for two to four mice per genotype, four independent experiments) and PNS neurospheres (F; three to six mice per genotype, six independent experiments), and the percentage of cells from Bmi-1-/- (but not Bmi-1+/+) primary neurospheres that formed secondary neurospheres upon replating (Supplementary Fig. 6). (★) Significantly different (P < 0.05) from Bmi-1+/+Arf+/+ or Bmi-1+/+Ink4a-Arf+/+; (#) significantly different from Bmi-1-/-Arf+/+ or Bmi-1-/-Ink4a-Arf+/+; (§) significantly different from Bmi-1+/+Arf-/- or Bmi-1+/+Ink4a-Arf-/-. All experiments were performed on 4- to 8-wk-old mice.
Arf deficiency significantly increased self-renewal within both Bmi-1+/+ and Bmi-1-/- neurospheres from the CNS and PNS (Fig. 1C,D). The ability of Arf deficiency to increase the self-renewal of Bmi-1+/+ neural stem cells in culture presumably reflects the fact that primary neurospheres were subcloned after 10 d in culture, well after p19Arf was induced in these cells. Since only about half as much self-renewal was observed within Bmi-1-/-Arf-/- neurospheres as compared with Bmi-1+/+Arf-/- neurospheres in the CNS and PNS, Arf deficiency appeared to only partially rescue the self-renewal of Bmi-1-/- neurospheres. Consistent with this, Arf deficiency also significantly increased the size of neurospheres (Supplementary Fig. 1B) and the percentage of cells within Bmi-1-/- neurospheres that could form secondary neurospheres (Supplementary Fig. 6B).
Deletion of Ink4a and Arf (Ink4a-Arf-/-) significantly increased self-renewal within Bmi-1+/+ and Bmi-1-/- neurospheres from the CNS and PNS (Fig. 1E,F). The rates of self-renewal suggested that Ink4a-Arf deficiency rescued most but not all of the Bmi-1-/- CNS self-renewal defect and about half of the PNS self-renewal defect (cf. Bmi-1-/-Ink4a-Arf-/- vs. Bmi-1+/+Ink4a-Arf-/- in Fig. 1E,F). More self-renewal was observed in Figure 1F than in Figure 1D because the neurospheres were cultured longer before subcloning. Ink4a-Arf deficiency also significantly increased the size of neurospheres (Supplementary Fig. 1C,D) and the percentage of cells within Bmi-1-/- neurospheres that could form secondary neurospheres (Supplementary Fig. 6C).
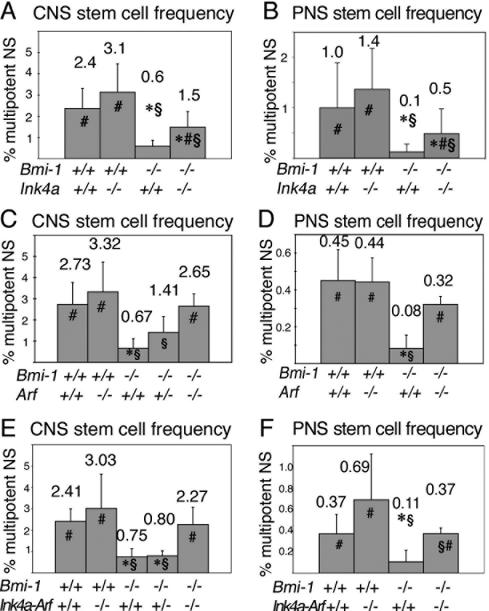
Ink4a, Arf, or Ink4a-Arf deficiency partially rescue Bmi-1-/- neural stem cell frequency
To test whether Ink4a deficiency could also rescue stem cell frequency in vivo, we cultured dissociated forebrain SVZ cells from 4- to 8-wk-old mice to determine the frequency of freshly dissociated cells that could form multipotent neurospheres. Cells were plated at clonal density, cultured for 10 d, then replated to adherent cultures for 3-5 d and stained for neurons, astrocytes, and oligodendrocytes. As observed previously, a significantly lower percentage of cells from the Bmi-1-/- SVZ formed multipotent neurospheres (Fig. 2). Ink4a deficiency significantly increased the percentage of Bmi-1-/- but not Bmi-1+/+ SVZ cells that formed multipotent neurospheres (Fig. 2A). This is consistent with p16Ink4a expression in Bmi-1-/- but not in Bmi-1+/+ SVZ cells in vivo (Molofsky et al. 2003). The magnitude of the increase suggested that Ink4a deficiency partially rescued stem cell frequency in the Bmi-1-/- SVZ.
Figure 2.
Deletion of Ink4a, Arf, or Ink4a-Arf significantly increased neural stem cell frequency in Bmi-1-/- mice. (A,B) Ink4a deficiency significantly increased the percentage of SVZ cells (A) or adult gut NCSCs (B) from Bmi-1-/- mice that formed multipotent neurospheres in culture (mean ± SD for four independent experiments). (C,D) Arf deficiency also significantly increased the percentage of Bmi-1-/- cells that formed multipotent neurospheres from the SVZ (C; three to six mice per genotype, in six independent experiments) or gut wall (D; four mice per genotype, in four independent experiments). (E,F) Ink4a-Arf deficiency significantly increased the frequency of cells from the Bmi-1-/- adult SVZ (E; two to seven mice per genotype, eight independent experiments) or gut wall (F; three to eight mice per genotype, three independent experiments) that formed multipotent neurospheres in culture. (★) Significantly different (P < 0.05 by t-test) from wild-type; (#) significantly different from Bmi-1-/-Ink4a+/+, Bmi-1-/-Arf+/+, or Bmi-1-/-Ink4a-Arf+/+; (§) significantly different from Bmi-1+/+Ink4a-/-, Bmi-1+/+Arf-/-, or Bmi-1+/+Ink4a-Arf-/-. All experiments employed 4- to 8-wk-old mice.
We also cultured dissociated cells from the outer gut wall where NCSCs persist throughout adult life (Kruger et al. 2002). Cells were plated at clonal density, cultured for 10 d, then replated to adherent cultures for 5 d and stained for neurons, glia, and myofibroblasts. Ink4a deficiency significantly increased the percentage of Bmi-1-/- but not Bmi-1+/+ cells that formed multipotent neurospheres (Fig. 2B). The magnitude of the increase again suggested that Ink4a deficiency partially rescued stem cell frequency in the Bmi-1-/- gut.
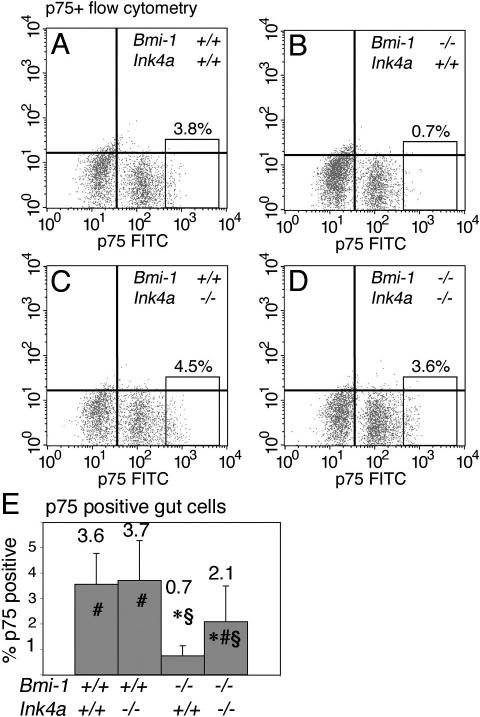
To confirm the partial rescue in neural stem cell frequency in vivo, we exploited our ability to prospectively identify uncultured NCSCs by flow cytometry based on their expression of high levels of the p75 neurotrophin receptor (p75+ cells) (Bixby et al. 2002; Kruger et al. 2002). In these experiments, 30%-50% of p75+ cells of all genotypes formed NCSC colonies in culture (data not shown), a 30- to 300-fold enrichment for NCSCs relative to unfractionated cells (0.1%-1.4% of unfractionated gut cells, depending on genotype, formed stem cell colonies in culture) (Fig. 2). The frequency of p75+ cells was significantly reduced in the absence of Bmi-1 (Fig. 3A,B). Ink4a deficiency had no effect on the frequency of p75+ cells from Bmi-1+/+ mice, but did significantly increase the frequency of p75+ cells in Bmi-1-/- mice (Fig. 3C-E). The magnitude of the increase again suggested a partial rescue of Bmi-1-/- stem cell depletion. This analysis of prospectively identified, uncultured NCSCs was thus consistent with results from the functional assays in culture in indicating that Bmi-1 promotes the post-natal maintenance of neural stem cells partly by repressing Ink4a.
Figure 3.
Ink4a deficiency partially rescued the frequency of uncultured NCSCs in the adult gut. Ink4a deficiency partially rescued the frequency of p75+ cells, which are highly enriched for NCSCs, in the adult gut wall of Bmi-1-/- mice. (A-D) Representative flow-cytometry plots from the indicated genotypes demonstrating p75+ NCSC frequency (boxed region of each plot) among freshly dissociated gut wall cells. (E) The frequency of p75+ cells was significantly reduced by Bmi-1 deficiency, and significantly increased by Ink4a deficiency. Data in panel E represent mean ± SD for 10 independent experiments using 4- to 8-wk-old mice. Statistical significance is indicated as in Figure 2.
Arf deficiency (Fig. 2C,D) and Ink4a-Arf deficiency (Fig. 2E,F) also partly rescued the depletion of CNS and PNS stem cells in Bmi-1-/- mice, significantly increasing the percentage of freshly dissociated SVZ cells and outer gut wall cells from Bmi-1-/- but not Bmi-1+/+ mice that formed multipotent neurospheres in culture. The percentages of SVZ and gut cells from Bmi-1-/-Ink4a-Arf-/- and Bmi-1+/+Ink4a-Arf-/- mice that formed multipotent neurospheres suggest that Ink4a-Arf deficiency rescued most of the CNS stem cell maintenance defect and about half of the Bmi-1-/- PNS stem cell maintenance defect. These results demonstrate that increased expression of p16Ink4a and p19Arf in the absence of Bmi-1 are responsible for much but not all of the neural stem cell self-renewal and maintenance defects observed in Bmi-1-/- mice.
Ink4a, Arf, or Ink4a-Arf deficiency partially rescue neural development in Bmi-1-/- mice
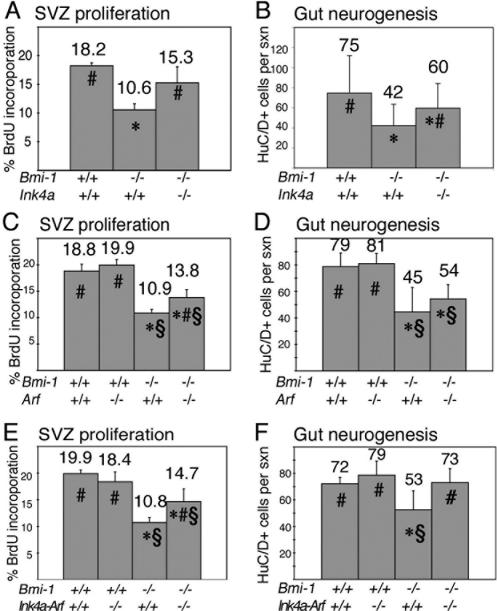
In the absence of Bmi-1 there was a significant decline in the rate of proliferation (the percentage of cells that incorporated a pulse of BrdU) in the adult SVZ, where neurogenesis occurs in the forebrain (Fig. 4A). Ink4a deficiency had no effect on proliferation in the Bmi-1+/+ forebrain (data not shown) but significantly increased proliferation in the Bmi-1-/- forebrain, suggesting a partial rescue of this phenotype (Fig. 4A). The number of myenteric plexus neurons per cross-section of the adult small intestine was significantly reduced in the absence of Bmi-1 (Fig. 4B). Ink4a deficiency significantly increased the number of neurons per section through the Bmi-1-/- intestine (Fig. 4B). Thus Ink4a deficiency partially rescued proliferation/neurogenesis in the forebrain and enteric nervous system of Bmi-1-/- mice.
Figure 4.
Deletion of Ink4a, Arf, or Ink4a-Arf partially rescued SVZ proliferation and gut neurogenesis in 3- to 9-wk-old Bmi-1-/- mice in vivo. (A,C,E) The rate of proliferation (percentage of cells that incorporate a pulse of BrdU) in the SVZ of Bmi-1-/- mice was significantly increased by Ink4a deficiency (A), Arf deficiency (C), or combined Ink4a-Arf deficiency (E). Note that deletion of these genes had no effect on SVZ proliferation in Bmi-1+/+ mice (C,E; Ink4a data not shown; six to eight sections per mouse, three to five mice per genotype). Ink4a deficiency partially rescued the number of myenteric plexus neurons per cross-section through the distal small intestine of Bmi-1-/- mice (B; mean ± SD for four to seven mice per genotype, seven to 10 sections per mouse). Arf deficiency tended to increase the number of neurons per cross-section in Bmi-1-/- mice, though the effect was not statistically significant (D; mean ± SD for four to five mice per genotype and eight to 10 sections per mouse). Ink4a-Arf deficiency significantly increased the number of myenteric plexus neurons per cross-section of Bmi-1-/- mice in a way that was consistent with a complete rescue (F; mean ± SD for five mice per genotype and six to eight sections per mouse). (★) Significantly different (P < 0.05 by t-test) from wild-type; (#) significantly different from Bmi-1-/-Ink4a+/+, Bmi-1-/-Arf+/+, or Bmi-1-/-Ink4a-Arf+/+; (§) significantly different from Bmi-1+/+Arf-/- or Bmi-1+/+Ink4a-Arf-/-.
Arf deficiency (Fig. 4C) or Ink4a-Arf deficiency (Fig. 4E) also significantly increased proliferation in the Bmi-1-/- but not Bmi-1+/+ SVZ, suggesting partial rescues of this defect. Arf deficiency appeared to increase the number of neurons per section through the Bmi-1-/- small intestine, though the effect was not statistically significant (Fig. 4D). Ink4a-Arf deficiency significantly increased the number of neurons per section through the Bmi-1-/- but not Bmi-1+/+ small intestine (Fig. 4F) to a degree consistent with a complete rescue. That some phenotypes (e.g., gut neurogenesis) appeared to be completely rescued by deleting Ink4a and Arf, while other phenotypes (e.g., SVZ proliferation) were only partially rescued indicates that the importance of other pathways downstream of Bmi-1 differs between regions of the nervous system.
Arf or Ink4a-Arf deficiency partially rescue cerebellum development in Bmi-1-/- mice
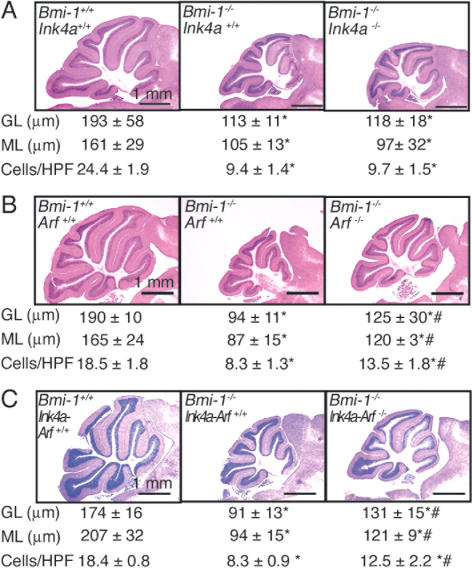
Bmi-1-/- mice exhibit morphologically smaller cerebellums, including significantly thinner granular and molecular cell layers and reduced cell density in the molecular layer (Fig. 5A-C; van der Lugt et al. 1994; Leung et al. 2004). Ink4a-Arf deficiency partially rescues cerebellum development in Bmi-1-/- mice (Jacobs et al. 1999a), though the relative importance of Ink4a and Arf in cerebellum development was not tested. Consistent with this prior report, we found that Ink4a-Arf deficiency had no effect on cerebellum development in Bmi-1+/+ mice (data not shown), but did increase the overall cerebellum size in Bmi-1-/- mice, including significantly increasing the thickness of the granular and molecular layers, as well as cell density in the molecular layer (Fig. 5C). Nonetheless, all of these parameters remained significantly less than observed in wild-type littermates, indicating a partial rescue of cerebellum growth (Fig. 5C).
Figure 5.
Deletion of Arf or Ink4a-Arf, but not Ink4a alone, partially rescues cerebellum development in Bmi-1-/- mice. Hematoxylin and eosin-stained saggital sections of adult cerebellum. Measurements of granular layer thickness (GL), molecular layer thickness (ML), and cell density in the molecular layer (cells/high power field [HPF] is 100 μm2) for each genotype are represented below the corresponding picture. (A) Ink4a deficiency did not affect cerebellum development in Bmi-1-/- mice (mean ± SD for four mice per genotype, seven to 25 measurements per mouse). (B) Arf deficiency partially rescued cerebellum growth in 4- to 8-wk-old Bmi-1-/- mice (mean ± SD for three to four mice per genotype and 10-23 measurements per mouse). (C) Ink4a-Arf deficiency also partially rescued cerebellum growth in Bmi-1-/- mice (mean ± SD for three to five mice per genotype, six to 32 measurements per mouse). Ink4a, Arf, and Ink4a-Arf deficiencies did not affect cerebellum growth in Bmi-1+/+ mice (data not shown). Statistical significance is indicated as in Figure 4.
Ink4a deficiency had no effect on cerebellum development in Bmi-1-/- mice (Fig. 5A), despite the increased expression of p16Ink4a in the Bmi-1-/- cerebellum (Supplementary Fig. 2). This suggests that progenitors from some regions of the nervous system can be insensitive to p16Ink4a expression. Arf deficiency had no effect on cerebellum development in Bmi-1+/+ mice (data not shown) but increased the overall cerebellum size in Bmi-1-/- mice, in addition to significantly increasing the thickness of the granular and molecular layers, and cell density in the molecular layer (Fig. 5B), to a similar degree as observed from Ink4a-Arf deficiency (cf. Fig. 5C). The observation that Arf deficiency had a greater effect than Ink4a deficiency on cerebellum development in Bmi-1-/- mice indicates that the relative importance of p16Ink4a and p19Arf differs between progenitor populations and regions of the nervous system.
Ink4a, Arf, or Ink4a-Arf deficiency do not rescue the growth or survival of Bmi-1-/- mice
Although Ink4a deficiency, Arf deficiency, or Ink4a-Arf deficiency each partially rescued aspects of neural stem cell function and neural development in Bmi-1-/- mice, they did not affect the overall growth of Bmi-1-/- mice, which were significantly smaller than wild-type littermates (Supplementary Fig. 3). Ink4a deficiency or Arf deficiency also did not significantly affect brain mass in Bmi-1-/- mice (Supplementary Fig. 4A,B). Ink4a-Arf deficiency, on the other hand, did significantly increase brain mass in adult Bmi-1-/- mice, though only slightly (Supplementary Fig. 4C). The ability of Ink4a deficiency, Arf deficiency, and Ink4a-Arf deficiency to substantially rescue neural stem cell function while having little or no effect on brain growth in Bmi-1-/- mice indicates that the effects of Bmi-1 on neural stem cells can be uncoupled from effects on tissue growth.
Ink4a deficiency, Arf deficiency, or Ink4a-Arf deficiency also did not rescue the survival of Bmi-1-/- mice, which usually died by P30 (Supplementary Fig. 3), despite being born in nearly expected numbers (data not shown). Ink4a-Arf deletion did not prevent Bmi-1-/- mice from exhibiting ataxia (data not shown). Abnormalities in other tissues, such as the hematopoietic system, are also not completely rescued by Ink4a-Arf deletion and may exhibit less of a rescue than observed in the nervous system (see companion paper from Bruggeman et al. [2005]). It is likely that Bmi-1-/- mice have uncharacterized defects in the maintenance of other tissues that could also affect viability. Thus the early death of these mice is likely to derive from complex combinations of defects in multiple tissues.
It is difficult to precisely estimate the fraction of each phenotype rescued by Ink4a or Arf deletion; however, the extent to which Ink4a-Arf deficiency rescued SVZ proliferation (Fig. 4E) did not appear to be greater than observed from deletion of Ink4a (Fig. 4A) or Arf alone (Fig. 4C). Similarly, the extent to which Ink4a-Arf deficiency rescued neural stem cell frequency (Fig. 2E,F) appeared to be less than the sum of the effects of Ink4a deficiency (Fig. 2A,B) and Arf deficiency (Fig. 2C,D). This suggests that there is cross-regulation between the p16Ink4a and p19Arf pathways. A number of mechanisms by which the p16Ink4a and p19Arf pathways influence each other have already been identified (Lowe and Sherr 2003). For example, since p21cip1, like p16Ink4a, promotes Rb activation, changes in p21cip1 expression affect the levels of p16Ink4a that are required to inhibit proliferation (Lowe and Sherr 2003). Consistent with this, we found that cip1 RNA levels were reduced by Arf deficiency and increased by Bmi-1 deficiency (as would be predicted based on the presumed changes in p19Arf and p53 activity in these cells) (Supplementary Fig. 4). These changes in cip1 levels offer one possible source of cross-regulation that could account for the less-than-additive effects of Ink4a and Arf deletion, particularly given that p21cip1 regulates neural stem cell self-renewal (Qiu et al. 2004; Kippin et al. 2005).
Ink4a and Arf repression represent important mechanisms by which Bmi-1 promotes post-natal stem cell self-renewal, stem cell maintenance, and development in the nervous system. Since induction of p16Ink4a and p19Arf have been associated with cellular senescence (Lowe and Sherr 2003), neural stem cells appear to undergo premature senescence in the absence of Bmi-1 and become depleted by adulthood. This suggests that the repression of these senescence pathways is a fundamental requirement for the maintenance of neural stem cells throughout life.
Materials and methods
The Bmi-1+/-, Arf+/-, and Ink4a-Arf+/- mice were backcrossed at least eight times onto a C57BL background. Initial experiments employed Ink4a+/- mice on an FvB background, but all results were subsequently confirmed using Ink4a+/- mice backcrossed five times onto a C57BL background. All mice were genotyped by PCR using primers described in the Supplemental Material.
Isolation of CNS and PNS progenitors
Adult SVZ was obtained by coronally sectioning brains in ice-cold Opti-MEM medium (Gibco). The lateral walls of the lateral ventricles were removed, minced, then dissociated for 20 min at 37°C in 0.025% trypsin/0.5 mM EDTA (Calbiochem) plus 0.001% DNase1 (Roche). Cells were quenched with staining medium (L15 medium containing 1 mg/mL BSA [Sigma A-3912], 10 mM HEPES at pH 7.4, and 1% penicillin/streptomycin [BioWhittaker]) containing 0.014% soybean trypsin inhibitor (Sigma) and 0.001% DNase1. After centrifuging, the cells were resuspended in staining medium, triturated, filtered through nylon screen (45 μm, Sefar America), counted by hemocytometer, and plated.
For PNS progenitor isolation, adult mouse guts were dissected into ice-cold PBS. Outer muscle/plexus layers were peeled free from the underlying epithelium as described (Kruger et al. 2002), then minced, and dissociated for 45 min in 0.025% trypsin/EDTA (Gibco 25300-054) plus 1 mg/mL type 4 collagenase (Worthington) in Ca, Mg-free HBSS at 37°C with agitation every 5 min. The dissociated cells were then quenched in staining medium, resuspended, and filtered as described above. Dissociated gut cells were sometimes stained with an antibody against p75 (Ab 1554; Chemicon International) as described previously (Bixby et al. 2002). The analysis and sorting of p75+ cells was performed by a FACS VantageSE flow cytometer (Becton-Dickinson).
Cell culture and self-renewal assay
Cells were plated at clonal density (2000 cells per well of a six-well plate; 1.3 cells/μL of culture medium) on ultra-low binding plates (Corning) to grow neurospheres. Culture medium was based on a 5:3 mixture of DMEM-low glucose: neurobasal medium (Gibco) supplemented with 20 ng/mL human bFGF (R&D Systems), 1% N2 supplement (Gibco), 2% B27 supplement (Gibco), 50 μM 2-mercaptoethanol, and 1% pen/strep (Biowhittaker). CNS cultures also contained 20 ng/mL EGF (R&D Systems) and 10% chick embryo extract (prepared as described [Stemple and Anderson 1992]). PNS cultures contained 15% chick embryo extract, 35 mg/mL (110 nM) retinoic acid (Sigma), and 20 ng/mL IGF1 (R&D Systems). All cultures were maintained at 37°C in 6% CO2/balance air.
To measure self-renewal, individual CNS neurospheres were dissociated by trituration, then replated at clonal density in nonadherent cultures. Secondary neurospheres were counted 5-10 d later to determine the number of secondary neurospheres formed per primary neurosphere. Individual PNS neurospheres were replated for 48 h into adherent plates to allow the spheres to spread out over the culture dish. The adherent colonies were then treated with trypsin and collagenase (four parts 0.05% trypsin-EDTA plus one part 10 mg/mL collagenase IV) for 5 min at 37°C followed by trituration. Five thousand dissociated cells were replated per well of a six-well plate and secondary neurospheres were counted 10 d later.
Western blots, qRT-PCR, and immunohistochemistry were performed as described in the Supplemental Material.
Acknowledgments
Thanks to David Adams, Anne Marie Deslaurier, and the University of Michigan Flow-Cytometry Core Facility (supported by UM-Comprehensive Cancer NIH CA46592, and UM-Multipurpose Arthritis Center NIH P60-AR20557). Thanks to Elizabeth Smith in the Hybridoma Core Facility (supported through the Michigan Diabetes Research and Training Center, NIH5P60-DK20572, and the Rheumatic Disease Center, P30 AR48310). Thanks to Kelly Yeager for tissue sectioning. Thanks to Maarten van Lohuizen for providing Bmi-1+/- mice and for sharing unpublished results, to Charles Sherr for providing Arf+/- mice, and to Ronald DePinho for providing Ink4a+/- and Ink4a-Arf+/- mice. This work was supported by the National Institutes of Health (R01 NS40750), the James S. McDonnell Foundation, and the Howard Hughes Medical Institute. A.V.M. was supported by a National Research Service Award from NIH (F30 NS048642). R.P. was supported by a post-doctoral fellowship from the Spanish Ministry of Science and Technology. S.H. was supported by a fellowship from the University of Michigan Cellular and Molecular Biology program.
Supplemental material is available at http://www.genesdev.org.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1299505.
References
- Bixby S., Kruger, G.M., Mosher, J.T., Joseph, N.M., and Morrison, S.J. 2002. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron 35: 643-656. [DOI] [PubMed] [Google Scholar]
- Bruggeman S.W.M., Valk-Lingbeek, M.E., van der Stoop, P.P.M., Jacobs, J.J.L., Kieboom, K., Tanger, E., Hulsman, D., Leung, C., Arsenijevic, Y., Marino, S., and van Lohuizen, M. 2005. Ink4a and Arf differentially affect cell proliferation and neural stem cell self-renewal in Bmi1-deficient mice. Genes & Dev. (this issue). [DOI] [PMC free article] [PubMed]
- Dimri G.P., Martinez, J.L., Jacobs, J.J., Keblusek, P., Itahana, K., van Lohuizen, M., Campisi, J., Wazer, D.E., and Band, V. 2002. The Bmi-1 oncogene induces telomerase activity and immortalizes human mammary epithelial cells. Cancer Res. 62: 4736-4745. [PubMed] [Google Scholar]
- Harrison D.E. 1979. Proliferative capacity of erythropoietic stem cell lines and aging: An overview. Mech. Ageing Dev. 9: 409-426. [DOI] [PubMed] [Google Scholar]
- Itahana K., Zou, Y., Itahana, Y., Martinez, J.-L., Beausejour, C., Jacobs, J.J.L., van Lohuizen, M., Band, V., Campisi, J., and Dimri, G.P. 2003. Control of the replicative life span of human fibroblasts by p16 and the polycomb protein Bmi-1. Mol. Cell. Biol. 23: 389-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J.J.L., Kieboom, K., Marino, S., DePinho, R.A., and van Lohuizen, M. 1999a. The oncogene and polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 397: 164-168. [DOI] [PubMed] [Google Scholar]
- Jacobs J.J.L., Scheijen, B., Voncken, J.-W., Kieboom, K., Berns, A., and van Lohuizen, M. 1999b. Bmi-1 collaborates with c-Myc in tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF. Genes & Dev. 13: 2678-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo T., Zindy, F., Roussel, M.F., Quelle, D.E., Downing, J.R., Ashmun, R.A., Grosveld, G., and Sherr, C.J. 1997. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91: 649-659. [DOI] [PubMed] [Google Scholar]
- Kippin T.E., Martens, D.J., and van der Kooy, D. 2005. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes & Dev. 19: 756-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger G.M., Mosher, J.T., Bixby, S., Joseph, N., Iwashita, T., and Morrison, S.J. 2002. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron 35: 657-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard J. and Sauvageau, G. 2003. Bmi-1 determines the proliferative capacity of normal and leukemic stem cells. Nature 423: 255-260. [DOI] [PubMed] [Google Scholar]
- Lessard J., Baban, S., and Sauvageau, G. 1998. Stage-specific expression of Polycomb Group genes in human bone marrow cells. Blood 91: 1216-1224. [PubMed] [Google Scholar]
- Leung C., Lingbeek, M., Shakhova, O., Liu, J., Tanger, E., Saremaslani, P., van Lohuizen, M., and Marino, S. 2004. Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature 428: 337-341. [DOI] [PubMed] [Google Scholar]
- Lowe S.W. and Sherr, C.J. 2003. Tumor suppression by Ink4a-Arf: Progress and puzzles. Curr. Opin. Genet. Dev. 13: 77-83. [DOI] [PubMed] [Google Scholar]
- Maslov A.Y., Barone, T.A., Plunkett, R.J., and Pruitt, S.C. 2004. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J. Neurosci. 24: 1726-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky A.V., Pardal, R., Iwashita, T., Park, I.K., Clarke, M.F., and Morrison, S.J. 2003. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 425: 962-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky A.V., Pardal, R., and Morrison, S.J. 2004. Diverse mechanisms regulate stem cell self-renewal. Curr. Opin. Cell Biol. 16: 700-707. [DOI] [PubMed] [Google Scholar]
- Morrison S.J., Wandycz, A.M., Akashi, K., Globerson, A., and Weissman, I.L. 1996. The aging of hematopoietic stem cells. Nat. Med. 2: 1011-1016. [DOI] [PubMed] [Google Scholar]
- Morrison S.J., Shah, N.M., and Anderson, D.J. 1997. Regulatory mechanisms in stem cell biology. Cell 88: 287-298. [DOI] [PubMed] [Google Scholar]
- Park I.-K., Qian, D., Kiel, M., Becker, M., Pihalja, M., Weissman, I.L., Morrison, S.J., and Clarke, M. 2003. Bmi-1 is required for the maintenance of adult self-renewing hematopoietic stem cells. Nature 423: 302-305. [DOI] [PubMed] [Google Scholar]
- Qiu J., Takagi, Y., Harada, J., Rodrigues, N., Moskowitz, M.A., Scadden, D.T., and Cheng, T. 2004. Regenerative response in ischemic brain restricted by p21cip1/waf1. J. Exp. Med. 199: 937-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Lee, H., Chin, L., Cordon-Cardo, C., Beach, D., and DePinho, R.A. 1996. Role of the INK4a locus in tumor suppression and cell mortality. Cell 85: 27-37. [DOI] [PubMed] [Google Scholar]
- Sharpless N.E., Bardeesy, N., Lee, K.-H., Carrasco, D., Castrillon, D.H., Aguirre, A.J., Wu, E.A., Horner, J.W., and DePinho, R.A. 2001. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 413: 86-91. [DOI] [PubMed] [Google Scholar]
- Sherr C.J. 2001. The INK4a/ARF network in tumour suppression. Nat. Rev. Mol. Cell Biol. 2: 731-737. [DOI] [PubMed] [Google Scholar]
- Stemple D.L. and Anderson, D.J. 1992. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell 71: 973-985. [DOI] [PubMed] [Google Scholar]
- Valk-Lingbeek M.E., Bruggeman, S.W., and van Lohuizen, M. 2004. Stem cells and cancer: The polycomb connection. Cell 118: 409-418. [DOI] [PubMed] [Google Scholar]
- van der Lugt N.M.T., Domen, J., Linders, K., van Roon, M., Robanus-Maandag, E., te Riele, H., van der Valk, M., Deschamps, J., Sofroniew, M., van Lohuizen, M., et al. 1994. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes & Dev. 8: 757-769. [DOI] [PubMed] [Google Scholar]