ABSTRACT
The possibility of creating a middle meningeal artery (MMA)-to-petrous internal carotid artery (ICA) bypass was investigated in six cadavers (bilaterally). Such a procedure could be used to treat patients with high cervical vascular lesions and those with tumors of the infratemporal fossa invading the high cervical ICA. After a frontotemporal craniotomy, the foramen spinosum and foramen ovale were exposed extradurally. Immediately posterior to the foramen ovale and medial to the foramen spinosum, the petrous portion of the ICA was exposed with a diamond-tipped drill. The MMA was lifted from its groove, and a sufficient length was transected to perform a bypass with the petrous ICA medially. The mean width of the MMA at the site of anastomosis was 2.3 ± 0.35 mm. The mean length of MMA from the foramen spinosum to the site of the anastomosis was 9.6 ± 1.7 mm. Based on these measurements, width and length of MMA appear to be sufficient for a bypass with petrous ICA.
Keywords: Middle meningeal artery, internal carotid artery, bypass procedure, angiography
High cervical vascular lesions or tumors that invade the high cervical internal carotid artery (ICA) may be treated with a bypass procedure. Ideally, the bypass graft should be long enough and should provide enough blood flow to approximate that of the bypassed ICA. To obtain adequate blood flow, the diameter of the donor artery must be sufficient. Miyazaki et al1 described a cervical-to-petrous ICA bypass procedure using a saphenous vein graft to reconstruct the ICA during removal of tumors. Fitzpatrick et al2 described a similar procedure for the treatment of high cervical or skull base ICA aneurysms or dissections. The patency of a bypass with a vein graft, however, is less than that of a direct arterial-to-arterial bypass.3,4 Therefore, we studied whether the caliber and length of the middle meningeal artery (MMA) would be suitable for a bypass with the petrous ICA.
METHODS
Six adult cadaveric heads were dissected bilaterally. In the supine position, the heads were turned 70 degrees from the side of dissection. A preauricular frontotemporal skin incision was used. After interfascial dissection and downward reflection of the temporal muscle, a frontotemporal craniotomy was performed.
The dura mater of the temporal lobe was carefully separated from the temporal fossa and elevated along the course of the MMA to the foramen spinosum. The exposure was continued medially and anteriorly to the foramen ovale. Just posterior to the foramen ovale and medial to the foramen spinosum, the petrous portion of the ICA was exposed with a diamond-tipped drill. The MMA lies in a groove after it emerges from the foramen spinosum. The MMA was lifted from its groove and carefully separated from the middle meningeal veins. The greater petrosal nerve was identified and sectioned.
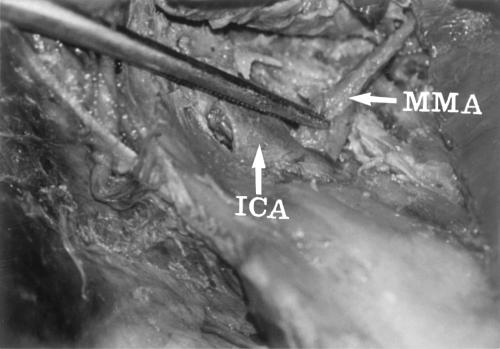
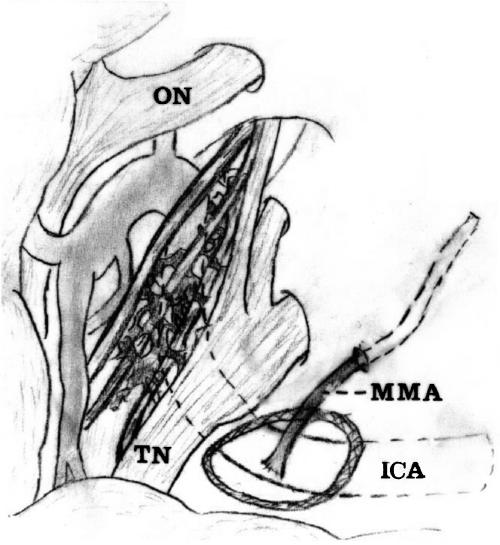
The floor of the middle fossa was drilled, and the petrous ICA was exposed from the level of the trigeminal ganglion to the vertical segment of the intrapetrous ICA. The Eustachian tube was opened to obtain unobstructed access to the ICA and to allow a vascular loop to be secured around it. Caution was taken to avoid injury to the geniculate ganglion of the facial nerve and cochlea. The carotid artery was mobilized and secured with a no. 1 nylon vascular loop passed through a small silastic tube with a flanged tip. Thus, a segment of the intrapetrous ICA, averaging 15 mm long, was exposed and used as described by Al-Mefty.5 A sufficient length of the MMA was transected to perform an end-to-side bypass with the petrous ICA using an interrupted 7-0 prolene suture medially (Figs. 1, 2).
Figure 1.
End-to-side bypass of the middle meningeal artery (MMA)-to-petrous internal carotid artery (ICA).
Figure 2.
Schematic illustration of middle meningeal artery (MMA)-to-petrous internal carotid artery (ICA) bypass. ON, optic nerve; TN, trigeminal nerve.
The diameter of the MMA at the site of anastomosis and the length of the MMA from the foramen spinosum to the site of anastomosis were measured with an electronic caliper.
RESULTS
The mean diameter ± SD of the MMA at the site of anastomosis was 2.3 ± 0.35 mm (range, 1.9 to 2.6). The mean length of the MMA from the foramen spinosum to the site of anastomosis was 9.6 ± 1.7 mm (range, 7 to 12 mm).
DISCUSSION
Numerous techniques to expose the distal cervical ICA for the direct repair of dissections or aneurysms have been described.2,6,7,8,9,10,11,12 However, all direct approaches are limited by the proximity of the lesion to the skull base.9,10,13,14
Four bypass procedures have been used to treat distal cervical/skull base ICA vascular lesions and skull base neoplasms: the superficial temporal-to-middle cerebral artery (STA-MCA), cervical-to-supraclinoid ICA, maxillary-to-supraclinoid ICA, and cervical-to-petrous ICA anastomosis.1,2,15,16 The first three procedures require intradural exposure of either the MCA or the supraclinoid ICA. An STA-MCA bypass does not isolate the damaged ICA from the circulation, and the patient is left at risk for thrombosis and ischemia from emboli. At least initially, the small diameter of the STA does not supply blood flow equivalent to the damaged ICA. The loss of blood flow may be clinically significant in patients who already suffer from ischemia caused by hypoperfusion.2 Cervical-to-supraclinoid ICA and cervical-to-petrous ICA bypasses require long vein grafts that require more time to perform because a double anastomosis is necessary. Although these are high-flow grafts, their patency rate at 18 months is only ∼76%.4
Other revascularization techniques, such as external carotid artery-to-MCA saphenous vein grafts, yield high-flow grafts but have even lower rates of patency (∼60%).3 Virionis et al12 described a new bypass graft to the supraclinoid ICA using the maxillary artery with a width of 2.2 mm at the anastomosic site. The procedure, however, is difficult and time consuming, and an extensive low temporal craniotomy with a zygomatic arch osteotomy is necessary.
A bypass between the MMA and petrous ICA has five theoretical advantages. First, it seems to be a more simple procedure than a cervical-to-supraclinoid ICA, cervical-to-petrous ICA, and maxillary-to-supraclinoid ICA anastomosis. Second, it provides more blood flow than an STA-to-MCA bypass; the mean outer diameter of the frontal branch of the STA and of the parietal branch when both are less than 2 mm long is 1.65 and 1.44 mm, respectively.17 Third, an MMA-to-petrous ICA bypass requires a single anastomosis, which reduces the length of the operation. Fourth, it has a higher patency rate than venous bypasses because it is an artery-to-artery bypass. In the cooperative extracranial-intracranial bypass study, patency rates were 96%.16,18 Finally, the length of the MMA required for the bypass is ∼1 cm. Therefore, separating the middle meningeal veins caused little difficulty.
Two technical points should be considered. The range of the diameter of the MMA varied between 1.9 and 2.6 mm in our study. Before performing such a bypass in patients, preoperative angiography to assess the diameter of MMA may be helpful. Because the clip proximal to the anastomosis site is permanent, monitoring the EEG and evoked potentials, applying awake sedation as an anesthetic technique, or both may help determine the adequacy of the bypass.
The results of this cadaveric study suggest that the MMA-to-petrous ICA bypass may be a reasonable alternative to previously described bypass procedures used to manage high cervical or skull base vascular lesions and infratemporal fossa tumors invading high cervical ICA.
REFERENCES
- Miyazaki S, Fukushima T, Fujimaki T. Resection of high-cervical paraganglioma with cervical-to-petrous internal carotid artery saphenous vein bypass. Report of two cases. J Neurosurg. 1990;73(1):141–146. doi: 10.3171/jns.1990.73.1.0141. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick B C, Spetzler R F, Ballard J L, Zimmerman R S. Cervical-to-petrous internal carotid artery bypass procedure. Technical note. J Neurosurg. 1993;79:138–141. doi: 10.3171/jns.1993.79.1.0138. [DOI] [PubMed] [Google Scholar]
- Diaz F G, Pearce J, Ausman J I. Complications of cerebral revascularization with autogenous vein grafts. Neurosurgery. 1985;17:271–276. doi: 10.1227/00006123-198508000-00004. [DOI] [PubMed] [Google Scholar]
- Sen C, Sekhar L N. Direct vein graft reconstruction of the cavernous, petrous, and upper cervical internal carotid artery: lessons learned from 30 cases. Neurosurgery. 1992;30:732–743. [PubMed] [Google Scholar]
- Al-Mefty O, Khalil N, Elwany M N, Smith R R. Shunt for bypass graft of the cavernous carotid artery: an anatomical and technical study. Neurosurgery. 1990;27(5):721–727. doi: 10.1097/00006123-199011000-00009. [DOI] [PubMed] [Google Scholar]
- Batzdorf U, Gregorius F K. Surgical exposure of the high cervical carotid artery: experimental study and review. Neurosurgery. 1983;13:657–661. doi: 10.1227/00006123-198312000-00007. [DOI] [PubMed] [Google Scholar]
- Fist U P, Moldering D J, Seining A. Surgical therapy of internal carotid artery lesions of the skull base and temporal bone. Otolaryngol Head Neck Surg. 1980;88:548–554. doi: 10.1177/019459988008800507. [DOI] [PubMed] [Google Scholar]
- Friedman W A, Day A L, Quisling R G, Sypert G W, Rhoton A L., Jr Cervical carotid dissecting aneurysms. Neurosurgery. 1980;7(3):207–214. doi: 10.1227/00006123-198009000-00001. [DOI] [PubMed] [Google Scholar]
- Sandmann W, Hennerici M, Aulich A, Kniemeyer H, Kremer K W. Progress in carotid artery surgery at the base of the skull. J Vasc Surg. 1984;1(6):734–743. [PubMed] [Google Scholar]
- Shaha A, Phillips T, Scalea T, et al. Exposure of the internal carotid artery near the skull base: the posterolateral anatomic approach. J Vasc Surg. 1988;8(5):618–622. doi: 10.1067/mva.1988.avs0080618. [DOI] [PubMed] [Google Scholar]
- Sundt T M, Jr, Pearson B W, Piepgras D G, Houser O W, Mokri B. Surgical management of aneurysms of the distal extracranial internal carotid artery. J Neurosurg. 1986;64(2):169–182. doi: 10.3171/jns.1986.64.2.0169. [DOI] [PubMed] [Google Scholar]
- Virionis F D, Cano W G, Heilman C B. Microsurgical anatomy of the infratemporal fossa as viewed laterally and superiorly. Neurosurgery. 1996;39(4):777–786. doi: 10.1097/00006123-199610000-00027. [DOI] [PubMed] [Google Scholar]
- Fisher D F, Jr, Clagett G P, Parker J I, et al. Mandibular subluxation for high carotid exposure. J Vasc Surg. 1984;1(6):727–733. [PubMed] [Google Scholar]
- Sekhar L N, Schramm V L, Jr, Jones N F, et al. Operative exposure and management of the petrous and upper cervical internal carotid artery. Neurosurgery. 1986;19(6):967–982. doi: 10.1227/00006123-198612000-00012. [DOI] [PubMed] [Google Scholar]
- Lougheed W M, Marshall B M, Hunter M, Michel E R, Sandwith-Smyth H. Common carotid to intracranial internal carotid bypass venous graft: technical note. J Neurosurg. 1971;34:114–118. doi: 10.3171/jns.1971.34.1.0114. [DOI] [PubMed] [Google Scholar]
- Onesti S T, Solomon R A, Quest D O. Cerebral revascularization: a review. Neurosurgery. 1989;25(4):618–629. [PubMed] [Google Scholar]
- Lang J. Skull Base and Related Structures. Atlas of Clinical Anatomy. Stuttgart: Schattauer; 1995. pp. 122–123.
- EC/IC Bypass Study Group Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Engl J Med. 1985;313(19):1191–1200. doi: 10.1056/NEJM198511073131904. [DOI] [PubMed] [Google Scholar]