ABSTRACT
Helical computed tomographic angiography (CTA) is a relatively new noninvasive volumetric imaging technique. Since early reports in the 1990s, CTA has rapidly improved image resolution and scan volume. Cerebral arteries can be imaged clearly, which is advantageous in the diagnosis of vascular diseases such as cerebral aneurysms, arteriovenous malformations, and cerebrovascular occlusive disease. Before attacking a cerebrovascular lesion near or in the skull base, precise preoperative knowledge of anatomic relationships between the bony and neurovascular structures is critical for obtaining successful outcomes. The sensitivity of CTA for the detection of cerebral aneurysms ≤ 5 mm in diameter may be higher than that of digital subtraction angiography (DSA), with equal specificity and high interoperator reliability. With minor modification to the technique, paraclinoid vascular lesions can be depicted using CTA. We present our experience using CTA in addition to DSA to obtain important anatomic information about skull base vascular lesions that assisted in the clinical decision-making process.
Keywords: Computed tomographic angiography, cerebrovascular lesion, skull base, surgical decision making
The treatment of cerebrovascular lesions requires precise preoperative anatomic evaluation. Advances in all facets of neurosurgery, including the operating microscope, microsurgical techniques, emergence of neuroanesthesia, and skull base approaches to complex cerebrovascular structures, have improved surgical results and lowered rates of morbidity and mortality. The widely recognized advantages of skull base approaches to cerebrovascular lesions include early proximal and distal control of vessels, minimal brain retraction, shortening and widening of the operative distance, direct approach and entry into the basal cisterns, and good cosmetic outcomes.1 These advantages are achieved through extensive extradural dissection and bone removal.
Selection of the approach and technique for surgical repair of skull-base vascular lesions is primarily based on angiographic features. Preoperative evaluation of the individual configuration of the skull base and the relationship between the lesion and surrounding bony structures are important considerations during skull base surgery.
Helical computed tomographic angiography (CTA) with three-dimensional (3D) reconstruction is a contemporary technique that improves the spatial understanding of the skull base. The sensitivity of CTA for the detection of cerebral aneurysms ≤ 5 mm in diameter may be higher than that of intra-arterial digital subtraction angiography (DSA), with equal specificity and high interoperator reliability.2 We present our experience in using CTA in addition to DSA in making surgical decisions about complex cerebrovascular lesions involving the skull base.
MATERIALS AND METHODS
Two males and three females (Table 1) underwent CTA studies in a GE Light-Speed Plus multislice scanner (GE Medical Systems, Milwaukee, WI). All patients were positioned supine, with standard head immobilization techniques. The settings used were a fixed prescan delay of 13 to 16 seconds, 140 kV, and 340 mA. The scanning techniques included pitch 1.25 × 0.6-mm section collimation, a 1.25-mm reconstruction interval, coverage from the bottom of the anterior arch of C1 to the midlateral ventricles, a 512 × 512 matrix, and a 20-mm field of view (FOV). The contrast solution, Omnipaque™ 300 (NOVAPLUS, Princeton, NJ), was injected intravenously through a 20-gauge antecubital angiocatheter with a power injector at a rate of 4 cc/sec. The total volume injected was 100 cc.
Table 1.
Clinical Summary of Patients' Preoperative Findings
| Pt no | Age/Sex | Presentation | Lesion | Approach | Complications |
|---|---|---|---|---|---|
| ICA internal carotid artery; CN cranial nerve; PCA posterior cerebral artery | |||||
| 1 | 54/M | Right visual loss | Large calcified left paraclinoid ICA aneurysm | Left frontotemporal with Dolenc anterior clinoidectomy | Transient right hemiparesis |
| 2 | 54/F | Diplopia | Giant left superior cerebellar aneurysm | Left orbitozygomatic | Transient CN III palsy, ataxia |
| 3 | 62/F | Vertigo | Incidental left paraclinoid ICA aneurysm | Left frontotemporal with Dolenc anterior clinoidectomy | None |
| 4 | 43/M | Right homonymous hemianopsia | Giant partially thrombosed PCA aneurysm | Left subtemporal transzygomatic | Transient CN III palsy, receptive aphasia |
| 5 | 50/F | Right incongruent hemianopsia | Left paraclinoid ICA aneurysm | Left frontotemporal | None |
Image processing was performed using the Advantage Workstation (GE Medical Systems, Milwaukee, WI, Version AW4.0_03). Single-section two-dimensional, thick-slab 3D multiplanar reformatted and 3D volume-rendered images were used for evaluation of cerebrovascular lesions by independent neuroradiologists.
Standard four-vessel, multiple-projection DSA was performed by interventional neuroradiologists using the Seldinger technique through the right femoral artery approach.
ILLUSTRATIVE CASES
All patients had good outcomes (Table 1).
Case 1
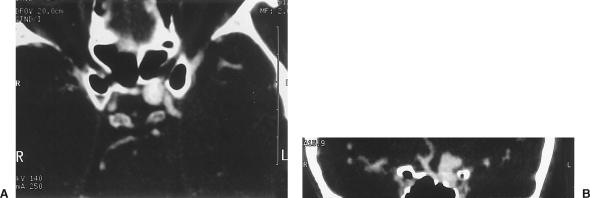
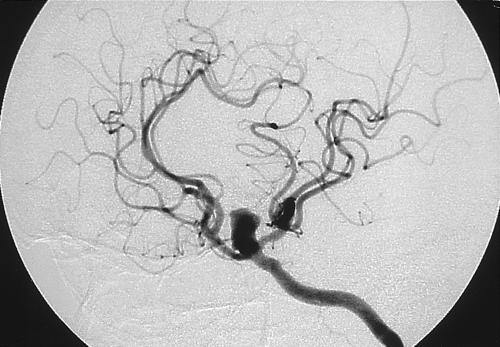
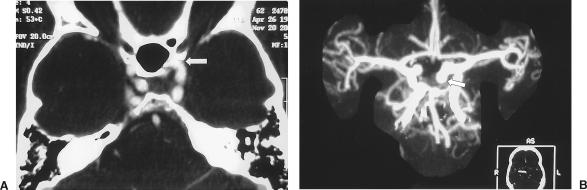
A 54-year-old man had a 4- or 5-month history of vision loss in his right eye and chronic cephalgia. CTA showed a peripherally calcified aneurysm measuring 11 mm extending from the region of the clinoid to the bifurcation of the internal carotid artery (ICA). An aberrant left A1 exited the ophthalmic portion of the ICA (Fig. 1). Cerebral angiography showed that the ICA aneurysm had a relatively wide neck (Fig. 2). Because of its proximity to the clinoid process and the mass effect exerted on the second cranial nerve, a left Dolenc procedure was performed to achieve complete proximal and distal control through the C2 and C3 portion of the ICA. The aneurysm neck was initially secured with fenestrated tip. However, intraoperative angiography showed that the aberrant A1 branching off the back side of the aneurysm had been occluded. The clips were then reapplied and microDoppler ultrasonography confirmed opening of the A2 segments. Postoperatively, the patient experienced transient memory difficulties and mild right hemiparesis, both of which subsequently resolved.
Figure 1.
(A) CTA showing a left ICA aneurysm extending from the region of the clinoid to the level of the ICA bifurcation. (B) Coronal CTA showing aberrant A1 exiting the ophthalmic segment of the ICA. CTA, computed tomographic angiography; ICA, internal carotid artery.
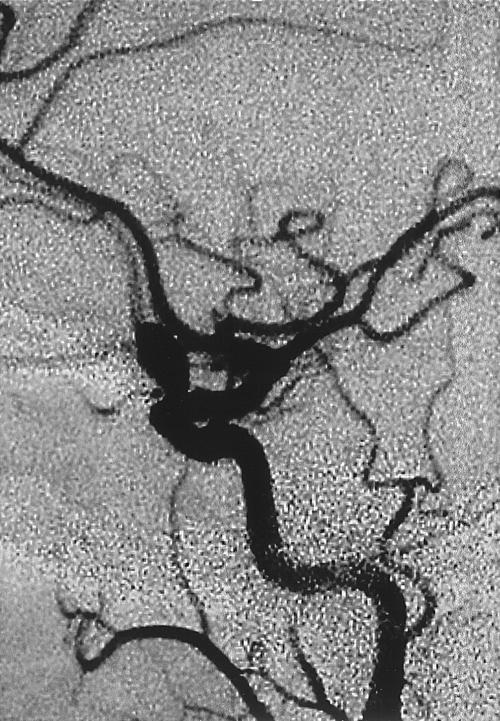
Figure 2.
Cerebral angiogram showing the left ICA aneurysm with a relatively wide neck. ICA, internal carotid artery.
Case 2
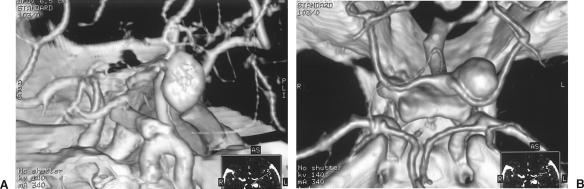
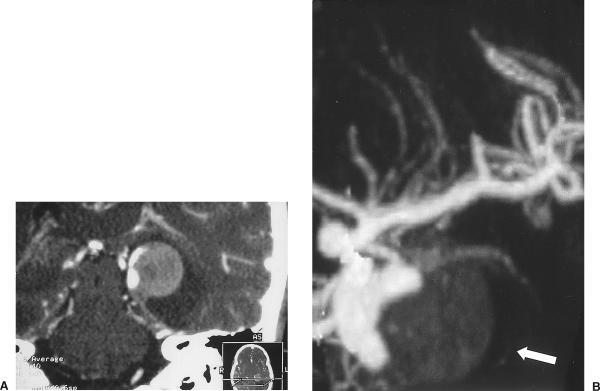
A 54-year-old woman had persistent migraine headaches and progressive diplopia. Routine magnetic resonance imaging (MRI) showed a basilar artery aneurysm in contact with the left side of the medulla oblongata. CTA revealed a 15 mm × 9 mm aneurysm arising from the distal portion of the basilar artery on the left between the origin of the left posterior cerebral artery and left superior cerebellar artery (Fig. 3). Four-vessel cerebral angiography showed that the aneurysm had a wide neck that was deemed unsuitable for coil reconstruction. Three-dimensional CTA showed that the aneurysm dome projected superiorly, laterally, and posteriorly above the posterior clinoid process (Fig. 4). This configuration permitted a left frontotemporal craniotomy with an orbitozygomatic osteotomy. The aneurysm was reached through a trans-sylvian, extended lateral approach over the tentorial incisura into the interpeduncular cistern. The aneurysm was large; therefore, temporary clips were applied to the basilar artery, to both superior cerebellar arteries, and to both posterior cerebral arteries using burst suppression and mild hypothermia. The aneurysm was aspirated, dissected free, and clipped using a bayonetted clip. Postoperatively, the patient experienced transient palsy of the left third cranial nerve and mild gait ataxia without radiographic evidence of ischemia.
Figure 3.
(A) Axial CTA showing the aneurysm arising from the distal portion of the basilar artery on the left. (B) Reconstructed coronal projection of the aneurysm with a wide neck. CTA, computed tomographic angiography.
Figure 4.
(A) Three-dimensional CTA showing the aneurysm dome projecting superiorly, laterally, and posteriorly above the posterior clinoid process. (B) Three-dimensional CTA superior-inferior view further defines the relationship of the aneurysm fundus to the left posterior cerebral artery. CTA, computed tomographic angiography.
Case 3
After undergoing a posterior fossa decompression for Chiari I malformation, a 62-year-old woman developed vertigo and blurry vision. Routine MRI showed a focal dilation of the left ICA between a vessel loop or a focal aneurysm. CTA showed an 8-mm diameter clinoidal aneurysm that projected medially (Fig. 5). The aneurysm projected medially and posteriorly at the level of the ophthalmic artery just below the level of the anterior clinoid process. The patient requested surgical intervention. A left frontotemporal craniotomy with a Dolenc procedure was required to expose the optic nerve and paraclinoid segment of the ICA. The aneurysm was secured with a 90-degree fenestrated clip, and the patient's postoperative course was uneventful.
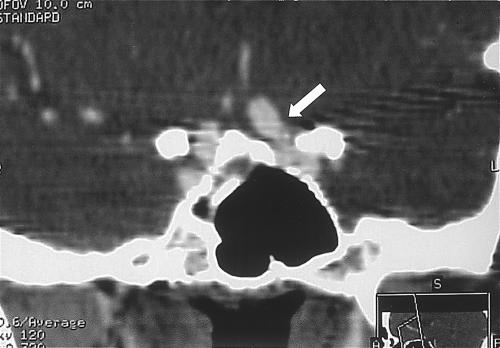
Figure 5.
(A) Axial CTA showing a medial projecting clinoidal aneurysm (arrow). (B) Reconstructed 3D maximum intensity pixel projection confirms the suspicion of a small clinoidal aneurysm (arrow). CTA, computed tomographic angiography.
Case 4
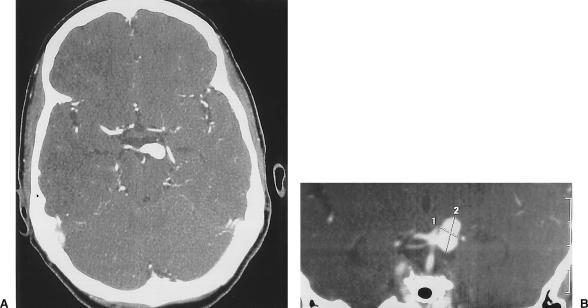
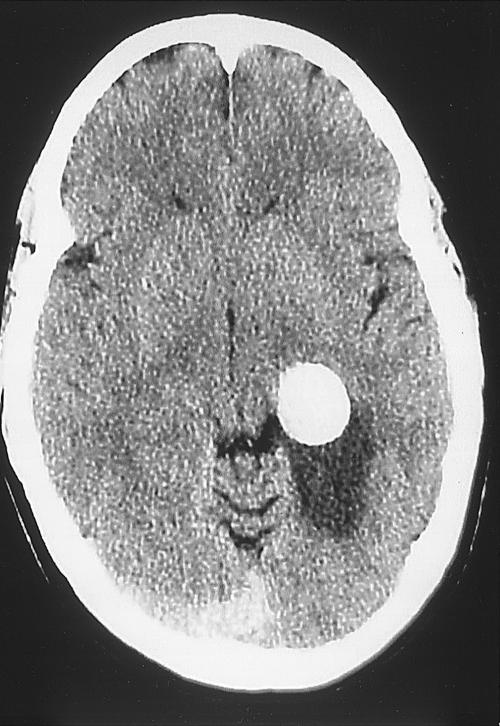
A 43-year-old man sought treatment for severe headache and blurry vision related to a right homonymous hemianopsia. CT without contrast showed a hyperdense lesion along the left medial temporal lobe (Fig. 6). CTA further showed the aneurysm, which measured 25 mm, arising from the P2-P3 segment of the left posterior cerebral artery with a wide base and dissecting thrombus (Fig. 7). In an attempt to re-establish blood flow to the left posterior circulation, the patient underwent a left subtemporal transzygomatic craniotomy. However, the dissection was found to extend into the aneurysm, which ruptured intraoperatively. The left posterior cerebral artery was sacrificed at the junction of P2-P3. Postoperatively, the patient experienced transient palsy of the left third cranial nerve and receptive aphasia. Eventually, however, he made a normal recovery with no visual deficit.
Figure 6.
CT without contrast showing a hyperdense lesion along the left medial temporal lobe. CT, computed tomography.
Figure 7.
(A) Reconstructed coronal CTA showing the aneurysm arising from the P2-P3 segment of the left posterior cerebral artery with a wide base and dissecting thrombus. (B) Reconstructed 3D maximum intensity pixel projection confirms the presence of the thrombus within the aneurysm (arrow). CTA, computed tomographic angiography.
Case 5
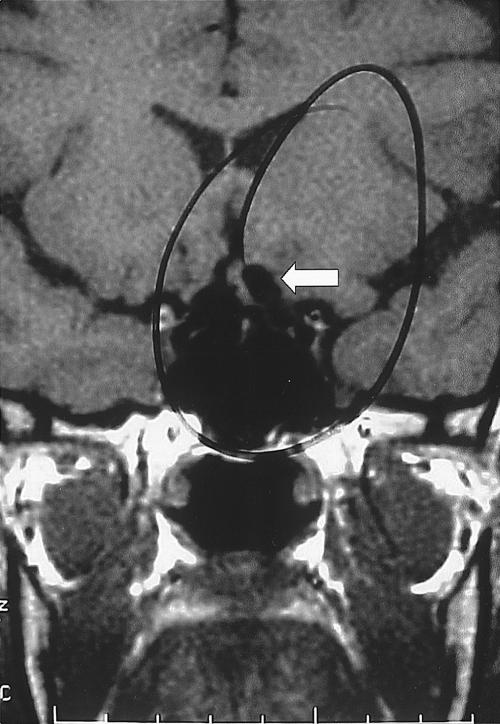
A 50-year-old woman sought treatment for blurry vision related to right incongruent hemianopsia. MRI showed a tortuosity of the supraclinoid portion of the left ICA protruding into the suprasellar cistern and contacting the left side of the optic chiasm, which was displaced slightly. No aneurysm, however, was visible (Fig. 8). Cerebral angiography showed a left dorsal paraclinoid aneurysm projecting superiorly and medially (Fig. 9). CTA confirmed the presence of the aneurysm and further defined its neck in relationship to the anterior clinoid process (Fig. 10). This configuration permitted the aneurysm to be clipped successfully through a left frontotemporal craniotomy with no need to remove the anterior clinoid process. The patient experienced no postoperative complications.
Figure 8.
Coronal MRI showing a tortuous left ICA in the supraclinoid region (arrow). MRI, magnetic resonance image; ICA, internal carotid artery.
Figure 9.
Cerebral angiogram showing a left dorsal paraclinoid aneurysm projecting superiorly and medially.
Figure 10.
CTA defined the anterior clinoid process in relationship to the neck of the aneurysm, allowing the aneurysm to be clipped without removing the clinoid process. CTA, computed tomographic angiography.
DISCUSSION
The introduction of helical CT with its rapid-volume acquisition enables vascular examinations with a wide clinical acceptance. The volumetric dataset allows retrospective multiplanar or 3D rendering of the original axial slices. Three-dimensional CTA has proven its value in intracranial vascular exploration.3,4,5
The selection of patients for treatment of cerebrovascular lesions requires precise, detailed characterization of their anatomy to determine the suitability of either surgical or endovascular treatment. The ability to achieve complete endovascular occlusion of an aneurysm is related to the density of coil packing as well as to the geometry of the aneurysm.6 Aneurysms that incorporate important arteries into the sac may not be suited for endovascular treatment. In addition, recent advances in skull base techniques allow greater accessibility to complex cranial base lesions without excessive morbidity. Accurate definition of the anatomy around the skull base is a critical factor in choosing treatment modalities and in justifying the associated risks of morbidity.
Previous experiences with CTA proved that this imaging method provides anatomical information that is equal to or more detailed than that provided by DSA.2,7 CTA can accurately describe all aspects of an aneurysm, including its branching pattern at the neck and arterial incorporations into the aneurysmal sac. It also shows the presence of mural calcium and thrombi. As our cases demonstrate, CTA also can enhance the ability to select the optimum treatment modality. This ability allowed us to achieve excellent operative results (Table 1).
Since the first published reports, CTA has contributed to significant improvements in image resolution and scan volume.4,8,9,10 As a result of using thin-slice collimation to improve spatial resolution, vascular lesions as small as 1.7 mm have been detected using contemporary methods of image acquisition.7 Smaller FOVs9,11 and the use of helical scanning to reduce scan time and motion degradation further enhance image quality. Undoubtedly, protocol optimization is leading to progressively higher quality CTA.12
Complex skull base aneurysms are ideally suited for evaluation by CTA. Specifically, paraclinoid aneurysms of the ICA are technically difficult to clip because important adjacent structures, especially the optic nerve, can easily be injured during dissection and clipping.13 Skull base approaches to this area are a vital element in the surgical treatment strategy, and CTA allows surgeons to select the best route to paraclinoid lesions. Volume rendering is now the preferred rendering technique for CTA. This technique makes available all the voxels within a volume. It thereby avoids extensive loss of information, the potentially arbitrary definition of vessel borders, difficulties with vessel-bone interface, and loss of perspective, all of which are inherent to shaded surface display and maximum intensity pixel techniques.14,15 The most important role of 3D volumetric rendering is to facilitate a thorough understanding of the shape of the aneurysmal sac and neck and of the spatial relationship of the sac to the surrounding branches and local bony anatomy.
CTA also allows visualization of the superficial temporal arteries and occipital arteries with quantitation of possible primary and branch donor sites without the need for selective catheterization of the external circulation. This information is useful when the need for an arterial bypass cannot be established at the time of catheter angiography.
Arterial spasm after subarachnoid hemorrhage can also be visualized by CTA, and the presence of aneurysm clips does not degrade the images significantly for postoperative angiography.16 In fact, the overall agreement rate between CTA and DSA for evaluating for vasospasm is 92%.17 Compared with those of DSA, the sensitivity and specificity of CTA are excellent in the proximal and distal arterial regions of interest.
CONCLUSION
With recent improvements in imaging protocols and advances in software development, CTA has proven to be an excellent complement to the complete evaluation of complex skull base cerebrovascular lesions and can be helpful in selecting the optimal skull base approach to such lesions.
REFERENCES
- Smith R R, Al-Mefty O. In: Al-Mefty O, editor. Surgery of the Cranial Base. Boston, MA: Kluwer Academic Publishers; 1989. Orbitocranial approach to complex anterior circulation aneurysms. pp. 107–118.
- Villablanca J P, Jahan R, Hooshi P, et al. Detection and characterization of very small cerebral aneurysms by using 2D and 3D helical CT angiography. AJNR Am J Neuroradiol. 2002;23:1187–1198. [PMC free article] [PubMed] [Google Scholar]
- Aoki S, Sasaki Y, Machida T, Ohkubo T, Minami M, Sasaki Y. Cerebral aneurysms: detection using 3-D CT angiography. AJNR Am J Neuroradiol. 1992;13:1115–1120. [PMC free article] [PubMed] [Google Scholar]
- Dorsch N WC, Young N Y, Kingston R J, Compton J S. Early experience with spiral CT in the diagnosis of intracranial aneurysms. Neurosurgery. 1995;36:230–238. doi: 10.1227/00006123-199501000-00037. [DOI] [PubMed] [Google Scholar]
- Harbaugh R E, Schlusselberg D S, Jeffery R, et al. Three-dimensional computed tomographic angiography in the preoperative evaluation of cerebrovascular lesions. Neurosurgery. 1995;36:320–326. doi: 10.1227/00006123-199502000-00011. [DOI] [PubMed] [Google Scholar]
- Debrun G M, Aletich V A, Kehrli P, et al. Selection of cerebral aneurysms for treatment using Gugliemi detachable coils: the preliminary University of Illinois at Chicago experience. Neurosurgery. 1998;43:1281–1297. doi: 10.1097/00006123-199812000-00011. [DOI] [PubMed] [Google Scholar]
- Villablanca J P, Martin N, Jahan R, et al. Volume-rendered helical computerized tomography angiography in the detection and characterization of intracranial aneurysms. J Neurosurg. 2000;93:254–264. doi: 10.3171/jns.2000.93.2.0254. [DOI] [PubMed] [Google Scholar]
- Hope J K, Wilson J L, Thompson F J. Three-dimensional CT angiography in the detection and characterization of intracranial berry aneurysms. AJNR Am J Neuroradiol. 1996;17:439–445. [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Okudera T, Noguchi K, et al. Cerebral aneurysms: evaluation with three-dimensional CT angiography. AJNR Am J Neuroradiol. 1996;17:447–454. [PMC free article] [PubMed] [Google Scholar]
- Velthuis B K, Rinkel G J, Ramos L MP, et al. Subarachnoid hemorrhage: aneurysm detection and preoperative evaluation with CT angiography. Radiology. 1998;208:423–430. doi: 10.1148/radiology.208.2.9680571. [DOI] [PubMed] [Google Scholar]
- Hsiang J NK, Liang E Y, Lam J MK, et al. The role of computed tomographic angiography in the diagnosis of intracranial aneurysms and emergent aneurysm clipping. Neurosurgery. 1996;38:481–487. doi: 10.1097/00006123-199603000-00011. [DOI] [PubMed] [Google Scholar]
- Velthuis B K, Leeuwen M S van, Witkamp T D, Ramos L M, Sprenkel J W van der, Rinkel G J. Computerized tomography angiography in patients with subarachnoid hemorrhage: from aneurysm detection to treatment without conventional angiography. J Neurosurg. 1999;91:761–767. doi: 10.3171/jns.1999.91.5.0761. [DOI] [PubMed] [Google Scholar]
- Day A K. Aneurysms of the ophthalmic segment: a clinical and anatomical analysis. J Neurosurg. 1990;72:677–691. doi: 10.3171/jns.1990.72.5.0677. [DOI] [PubMed] [Google Scholar]
- Kuszyk B S, Heath D G, Ney D R, et al. CT angiography with volume rendering: imaging findings. AJR Am J Roentgenol. 1995;165:445–448. doi: 10.2214/ajr.165.2.7618574. [DOI] [PubMed] [Google Scholar]
- Kuszyk B S, Heath D G, Johnson P T, Eng J, Fishman E K. CT angiography with volume rendering for quantifying vascular stenoses: in vitro validation of accuracy. AJR Am J Roentgenol. 1999;173:449–455. doi: 10.2214/ajr.173.2.10430152. [DOI] [PubMed] [Google Scholar]
- Zouaoui A, Sahel M, Marro B, et al. Three-dimensional computed tomographic angiography in detection of cerebral aneurysms in acute subarachnoid hemorrhage. Neurosurgery. 1997;41:125–130. doi: 10.1097/00006123-199707000-00026. [DOI] [PubMed] [Google Scholar]
- Otawara Y, Ogasawara K, Ogawa A, Sasaki M, Takahashi K. Evaluation of vasospasm after subarachnoid hemorrhage by use of multislice computed tomographic angiography. Neurosurgery. 2002;51:939–943. doi: 10.1097/00006123-200210000-00015. [DOI] [PubMed] [Google Scholar]