ABSTRACT
Patients with complete carotid occlusion and recent ischemic symptoms are at high risk for subsequent stroke, particularly those with evidence of severe hemodynamic impairment due to poor collateral flow. Treatment options for these patients include direct extracranial to intracranial arterial bypass, or interventions aimed at improving collateral sources of flow such as endarterectomy or angioplasty and stenting of the ipsilateral external carotid artery, the contralateral carotid artery, or the vertebral arteries. The evidence supporting the use of these procedures for patients with complete occlusion of the carotid artery will be the focus of this article. The use of physiologic imaging to select subgroups of patients at high risk due to hemodynamic factors will also be discussed.
Keywords: Atherosclerosis, carotid artery, bypass, hemodynamic impairment
EPIDEMIOLOGY
Complete occlusion of the carotid artery is common in patients presenting with transient ischemic attack (TIA) or stroke. Mead and associates1 studied 380 consecutive patients presenting to seven hospitals in Manchester, England, over a 1-year period and found complete occlusion in 50. Other investigators2,3,4 have reported a similar frequency of occlusion in stroke and TIA populations. The prevalence of asymptomatic occlusion is not known. Patients with symptomatic occlusions are at high risk for subsequent stroke. The annual risk for any stroke after diagnosis of a complete occlusion is between 5% and 7% and the annual risk for stroke ipsilateral to the carotid occlusion is between 2% and 6% per year.5,6,7
The risk for future stroke is probably much lower for patients who have no symptoms of ischemia. Four prospective studies have been reported to date with conflicting results. Three of the four found a very low risk of stroke with one minor stroke occurring in 72 patients followed for at least 2 years.8,9,10 In the fourth study, seven of 49 patients followed for an average of 31.2 months suffered an ipsilateral stroke.11
ASSESSMENT OF HEMODYNAMIC FACTORS
Severe atherosclerotic disease of the carotid and vertebral arteries or their intracranial branches may lead to reduced perfusion pressure in the distal cerebral circulation, depending on the adequacy of collateral sources of blood flow. When perfusion pressure is reduced, reflex changes of the cerebrovasculature occur to maintain the normal delivery of oxygen to the brain and consequently, normal neurologic function. These responses include autoregulatory vasodilation and increasing the fraction of oxygen extracted from the blood (oxygen extraction fraction or OEF) as it passes through the cerebral circulation. A number of different methods of physiological imaging have been developed to identify the presence of these compensatory mechanisms. Several studies have shown conclusively that severe hemodynamic impairment is a powerful predictor of subsequent stroke in patients with carotid artery occlusion.10,12,13,14
The presence of complete arterial occlusion does not reliably predict hemodynamic impairment in individual patients.15 More than half of the 81 symptomatic patients and 31 of 36 asymptomatic patients with complete carotid occlusion enrolled in the St. Louis Carotid Occlusion Study, a prospective study of cerebral hemodynamics and stroke risk, had normal OEF.7,8 Certain patterns of collateral flow have been correlated with hemodynamic impairment, but the ability of these findings to identify individual patients with hemodynamic impairment has been poor.16,17 Structural imaging, such as angiography, demonstrates the pathways of blood flow, but not the amount of blood delivered.
There are specific clinical symptoms that are associated with hemodynamic mechanisms and hemodynamic impairment. These include limb-shaking or orthostatic TIAs.18 While these symptoms are strongly associated with hemodynamic impairment (high specificity), most patients with hemodynamic impairment do not have these symptoms (low sensitivity).
Similarly, the finding of a linear pattern of white matter infarctions in the white matter of the centrum semiovale or corona radiata ipsilateral to the occluded carotid artery is very specific for hemodynamic impairment in that cerebral hemisphere19,20 (Fig. 1). This finding is not very sensitive, however.20
Figure 1.
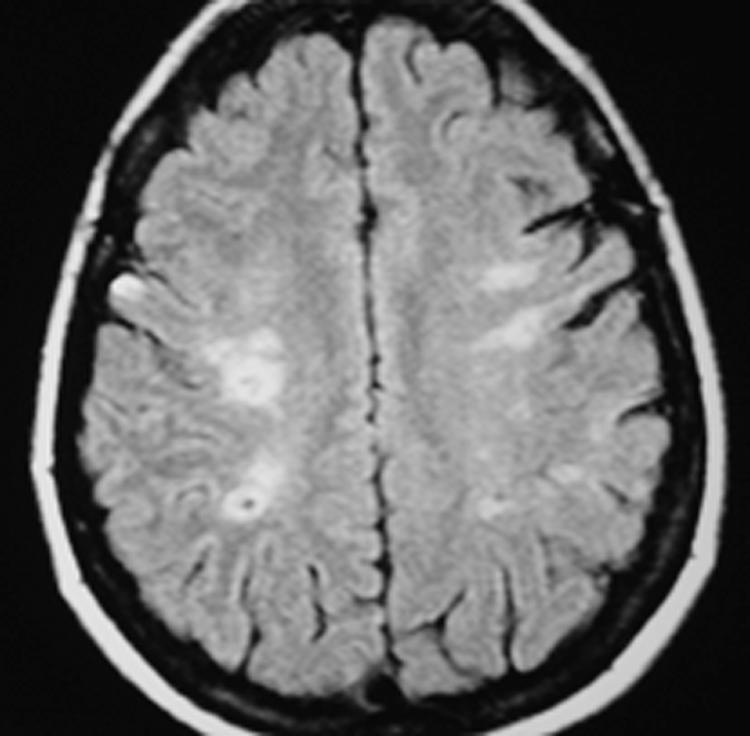
Fluid attenuated inversion recovery (FLAIR) magnetic resonance (MR) image of a 35-year-old woman with moyamoya disease demonstrating bilateral centrum semiovale white matter infarctions. This pattern of infarction is associated with hemodynamic impairment. A small right motor cortex infarction is also present, corresponding to a minor stroke affecting her left hand.
Single measurements of cerebral blood flow (CBF) alone do not adequately assess cerebral hemodynamic status. First, normal values may be found when perfusion pressure is reduced, but CBF is maintained by autoregulatory vasodilation. Second, CBF may be low when perfusion pressure is normal. This can occur when the metabolic demands of the tissue are low. Reduced flow due to reduced metabolic demand may not cause confusion when low regional CBF is measured in areas of frank tissue infarction. However, blood flow can also be reduced in normal, uninfarcted tissue due to the destruction of normal afferent or efferent fibers by a remote lesion as well.21
As a consequence of these issues, three basic strategies of hemodynamic assessment have been developed, all based on the assumption that the chronic, regional reductions in perfusion pressure in humans lead to the same compensatory mechanisms as have been observed in animal and human studies of acute and global reductions in perfusion pressure.22 These methods are indirect—the presence of hemodynamic impairment is inferred when a test is abnormal.
The first strategy relies on paired blood flow measurements with the initial measurement obtained at rest and the second measurement obtained following a cerebral vasodilatory stimulus. Hypercapnia, acetazolamide, and physiologic tasks such as hand movement have been used as vasodilatory stimuli. Normally, each will result in a robust increase in CBF. If the CBF response is muted or absent, pre-existing autoregulatory cerebral vasodilation due to reduced cerebral perfusion pressure is inferred. Quantitative or qualitative (relative) measurements of CBF can be made using a variety of methods, including 133Xenon by inhalation or intravenous injection, single photon emission computed tomography, stable xenon computed tomography, positron emission tomography (PET), and magnetic resonance imaging (MR). Changes in the velocity of blood in the middle cerebral artery trunk or internal carotid artery can be measured with transcranial Doppler and MR. The blood flow or blood velocity responses to these vasodilatory stimuli have been categorized into several grades of hemodynamic impairment: (1) reduced augmentation (relative to the contralateral hemisphere or normal controls); (2) absent augmentation (same value as baseline); and (3) paradoxical reduction in regional blood flow compared with baseline measurement. This final category, also called the “steal” phenonenon, can only be identified with quantitative on CBF techniques.23
The second strategy uses either the measurement of regional cerebral blood volume (CBV) alone or in combination with measurements of CBF in the resting brain to detect reduced perfusion pressure. The CBV/CBF ratio (or, inversely, the CBF/CBV ratio), mathematically equivalent to the vascular mean transit time, may be more sensitive than CBV alone for the identification of autoregulatory vasodilation. It may be less specific, however. The CBV/CBF ratio may increase in low-flow conditions with normal perfusion pressure, such as hypocapnia. Quantitative regional measurements of CBV and CBF can be made with PET or single photon emission computed tomography. MR techniques for the quantitative measurement of CBV have been developed. Patients are identified as abnormal with these techniques based on comparison of absolute quantitative values or hemispheric ratios of quantitative values to the range observed in normal control subjects. One issue that remains unresolved is to what extent autoregulatory vasodilation of arterioles gives rise to measurable increases in the CBV. Experimental data have produced conflicting results.24,25,26 While increases in CBV almost certainly indicate autoregulatory vasodilation, the significance of normal CBV in patients with increased OEF—a frequent finding—is unclear.27 Thus, the sensitivity and specificity of CBV measurements for detecting reduced CPP is not known.
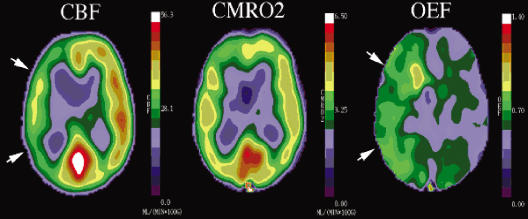
The third strategy relies on direct measurements of OEF to identify patients with increased oxygen extraction (Fig. 2). At present, regional measurements of OEF can be made only with PET using O-15 labeled radiotracers. Both absolute values and side-to-side ratios of quantitative and relative OEF have been used for the determination of abnormal from normal. MR measurements using pulse sequences sensitive to deoxy-hemoglobin (which is increased in regions with increased oxygen extraction) are being developed to provide similar information.28
Figure 2.
Compensatory increase in OEF as identified by PET. These images are from a neurologically normal patient with a unilateral carotid occlusion due to atherosclerosis. CBF is reduced on the left image (CBF, arrows). Oxygen metabolism is normal, however (CMRO2, middle image), owing to a compensatory increase in oxygen extraction (OEF, right image, arrows). OEF, oxygen extraction fraction; PET, positron emission tomography; CBF, cerebral blood flow.
ROLE OF HEMODYNAMICS IN STROKE RISK
As these methods are indirect and inferential, the association between each of these methods and stroke risk must be empirically proven. The measurement of increased OEF in the hemisphere distal to an occluded or stenotic carotid artery has been proven as a powerful predictor of subsequent stroke.7,13 At present the best evidence for an association between hemodynamic impairment and stroke risk is for PET measurements of OEF and breath-holding transcranial Doppler.10,22,29
Yamauchi and colleagues13 reported 52 patients with high-grade stenoses and occlusions of the internal carotid and middle cerebral arteries. OEF was measured on study entry. Twelve patients were censored due to interval surgical revascularization. The presence of increased OEF, defined by absolute regional OEF above the 95th percentile from the mean in normal controls (53.3%), was strongly associated with a stroke during study follow-up.
Grubb and coworkers7 reported the outcome of 81 patients with symptomatic carotid occlusion, the results of the STLCOS. This was a blinded, prospective study of 81 patients with symptomatic carotid occlusion that also specifically assessed the impact of other risk factors. The risk of all stroke and ipsilateral ischemic stroke in symptomatic subjects with increased OEF was significantly higher than in those with normal OEF (log rank p = 0.005 and p = 0.004m respectively). Univariate and multivariate analysis of 17 baseline stroke risk factors confirmed the independence of this relationship. The age-adjusted relative risk conferred by increased OEF was 6.0 (95% CI 1.7 to 21.6) for all stroke and 7.3 (95% CI 1.6 to 33.4) for ipsilateral ischemic stroke. An increase OEF was identified by a hemispheric ratio above the normal range.
What is the mechanism of stroke in these patients? Human and animal studies suggest a powerful synergy between hemodynamic and embolic mechanisms. Clinically silent emboli are commonly identified in vessels distal to symptomatic carotid stenosis.30 Animal studies have shown that for a given embolic event, the size of infarction is markedly increased if there is pre-existing hemodynamic impairment.31 Furthermore, there is some evidence from animal studies that increased baseline levels of CBF are protective against ischemic injury.32 In humans, we have proof that the presence of hemodynamic impairment, identified by some, but not all, imaging methods, is a powerful and independent risk factor for stroke in patients with carotid atherosclerotic occlusive disease.10,12,13,14 It is likely that the majority of strokes in these patients occur due to embolic material and that the presence of hemodynamic impairment increases the chances that an embolic event will result in an ischemic stroke.
IMPROVEMENT IN HEMODYNAMICS OVER TIME
It is possible for collateral sources of blood flow to improve over time in patients with atherosclerotic carotid artery occlusion.33,34,35 As part of the STLCOS, we repeated PET measurements of cerebral hemodynamics in ten patients with increased OEF and no interval stroke.35 We found that OEF can improve over time, presumably due to increased CBF through collateral channels. The duration between initial and follow-up studies ranged from 12 to 59 months. The initial ipsilateral-to-contralateral ratio of OEF for the ten patients was 1.164 (mean absolute ipsilateral OEF was 0.478). The upper limit of ipsilateral-to-contralateral OEF ratios observed in 17 normal subjects was 1.085. The mean OEF ratio fell to 1.076 on follow-up examination (p = 0.022). Individually, the OEF ratio fell in eight patients and rose slightly in two. The follow-up OEF ratio was within the normal range in five of the eight patients. The improvement in the OEF ratio was a function of time: the longer the duration between studies, the greater the improvement in OEF (p = 0.02, r = 0.706). Examination of other variables revealed a parallel increase in the mean ratio of ipsilateral to contralateral CBF from 0.806 to 0.847 (p = 0.021).
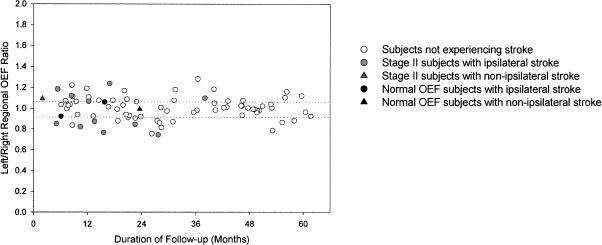
In addition, it appears that stroke risk may improve over time in this patient population. The cumulative hazard curve in both medical and surgical groups in the Extracranial to Intracranial (EC/IC) Bypass Trial flattened considerably after the first 2 years.36 We observed a similar phenomenon in the STLCOS (Fig. 3).
Figure 3.
The frequency of stroke may be higher during the first 2 years of follow-up for the 81 symptomatic patients with atherosclerotic carotid artery occlusion enrolled in the St. Louis Carotid Occlusion Study. The left axis is the left-to-right hemispheric ratio of OEF. The dotted lines indication the normal range—values above or below the line indicate increased OEF beyond the normal range. The x-axis indicates the duration of follow-up after enrollment for patients without stroke and the time of stroke after enrollment for patients suffering stroke during the follow-up period. OEF, oxygen extraction fraction.
DIRECT REVASCULARIZATION PROCEDURES
Extracranial to Intracranial Arterial Bypass
The EC/IC Bypass Trial was a large, international, randomized controlled trial which showed no benefit for superficial temporal artery to middle cerebral artery (STA-MCA) bypass over medical therapy in 808 patients with symptomatic carotid occlusion.36 Several criticisms of the trial were raised after publication of this study. These included the fact that the superficial temporal artery was a relatively low-flow donor artery and the possibility that many of the highest-risk patients may have been operated on outside of the trial and not enrolled.37,38,39 The first criticism is probably baseless, as several studies have documented that STA-MCA bypass is sufficient to reverse abnormally elevated OEF.40,41,42,43
One valid criticism was the inability to identify patients at high risk for stroke due to hemodynamic factors.44,45 At the time of the study there was no proven method to identify these patients. It is now established that many patients with complete carotid artery occlusion and normal cerebral hemodynamics have a low risk of subsequent stroke, and therefore little to gain from EC/IC bypass. The failure of the EC/IC Bypass Trial to demonstrate efficacy may have been due to the inclusion of many such patients.
The Carotid Occlusion Surgery Study is a randomized trial of STA-MCA bypass for selected symptomatic patients with increased OEF by PET.46 This trial is designed to test the hypothesis that STA-MCA bypass, when combined with the best medical therapy, can reduce by 40%, despite perioperative stroke and death, subsequent ipsilateral ischemic stroke at 2 years. Patients must have recent cerebral ischemic symptoms and increased OEF as measured by PET. Approximately 930 clinically eligible patients will be enrolled to yield 372 patients with increased OEF. Randomization is 1:1. This will provide 90% power to detect a 40% reduction in primary outcome in favor of the surgery arm with a 5% level two-sided test. This study has been funded by the National Institutes of Health and is under way (NS39526).
In summary, the benefit of EC/IC bypass is unproven. Given the results of the EC/IC Bypass Trial, the only present indication for direct EC/IC bypass in North American patients with symptomatic atherosclerotic carotid artery occlusion is within the Carotid Occlusion Surgery Study.
Direct Endovascular Recanalization
Recanalization of chronically occluded arteries in the periphery may be technically feasible, particularly with the advent of covered stent-grafts.47,48 Recanalization of a chronic carotid occlusion has not been reported. Angioplasty and stenting for acute carotid occlusions in the setting of intra-arterial stroke treatment has been performed.49,50
Indirect Procedures
Interventions aimed at improving collateral flow in patients with complete carotid occlusion include endarterectomy or angioplasty and stenting of ipsilateral common and external carotid arteries, contralateral carotid arteries, and vertebral arteries, depending on the sources of collateral flow. The best level of evidence for any of these interventions is for carotid endarterectomy. This operation has been proven to reduce stroke risk in symptomatic and asymptomatic populations, when compared with best medical therapy.51,52,53 Angioplasty is a reasonable alternative for patients who are not good surgical candidates.54 One caveat for angioplasty and stenting is for lesions at the vertebral origin. These lesions often restenose or occlude. In one significant prospective study > 50% stenosis or occlusion occurred in four of six patients treated.55 Drug-eluting stents may improve outcome at this location.
SUMMARY
The current indications for revascularization procedures for patients with carotid occlusion are limited. There are no randomized trials proving benefit of intervention in this population. The only trial to date showed no benefit of EC/IC bypass. Nevertheless, the data are compelling that hemodynamic factors play an important role in the risk for future stroke, particularly in symptomatic patients. The ongoing Carotid Occlusion Surgery Study will provide critical information regarding the benefit of this procedure. Interventions aimed at improving collateral flow for symptomatic patients with evidence of severe hemodynamic impairment make intuitive sense, but there are no data from randomized trials to support this practice. Physiological studies aimed at determining hemodynamic status will likely become common clinical tools to determine prognosis and optimal therapy.
REFERENCES
- Mead G E, Murray H, Farrell A, O'Neill P A, McColllum C M. Pilot study of carotid surgery for acute stroke. Br J Surg. 1997;84:990–992. doi: 10.1002/bjs.1800840723. [DOI] [PubMed] [Google Scholar]
- Pessin M S, Duncan G W, Mohr J P, Poskanzer D C. Clinical and angiographic features of carotid transient ischemic attacks. N Engl J Med. 1977;296:358–362. doi: 10.1056/NEJM197702172960703. [DOI] [PubMed] [Google Scholar]
- Balow J, Alter M, Resch J A. Cerebral thromboembolism: an appraisal of 100 cases. Neurology. 1966;16:559–564. doi: 10.1212/wnl.16.6.559. [DOI] [PubMed] [Google Scholar]
- Thiele B L, Young J V, Chikos P M, Hirsch J H, Strandness D E., Jr Correlation of arteriographic findings and symptoms in cerebrovascular disease. Neurology. 1980;30:1041–1046. doi: 10.1212/wnl.30.10.1041. [DOI] [PubMed] [Google Scholar]
- Klijn C JM, Kappelle L J, Tulleken C A, Gijn J van. Symptomatic carotid artery occlusion: a reappraisal of hemodynamic factors. Stroke. 1997;28:2084–2093. doi: 10.1161/01.str.28.10.2084. [DOI] [PubMed] [Google Scholar]
- Hankey G J. Prognosis of symptomatic carotid artery occlusion. Cerebrovasc Dis. 1991;1:245–256. [Google Scholar]
- Grubb R L, Jr, Derdeyn C P, Fritsch S M, et al. The importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA. 1998;280:1055–1060. doi: 10.1001/jama.280.12.1055. [DOI] [PubMed] [Google Scholar]
- Powers W J, Derdeyn C P, Fritsch S M, et al. Benign course of never-symptomatic carotid occlusion. Neurology. 2000;54:878–882. doi: 10.1212/wnl.54.4.878. [DOI] [PubMed] [Google Scholar]
- Bornstein N M, Norris J W. Benign outcome of carotid occlusion. Neurology. 1989;39:6–8. doi: 10.1212/wnl.39.1.6. [DOI] [PubMed] [Google Scholar]
- Vernieri F, Pasqualetti P, Passarelli F, et al. Outcome of carotid artery occlusion is predicted by cerebrovascular reactivity. Stroke. 1999;30:593–598. doi: 10.1161/01.str.30.3.593. [DOI] [PubMed] [Google Scholar]
- Hennerici M, Hulsbomer H B, Rautenberg W, Hefter H. Spontaneous history of asymptomatic internal carotid occlusion. Stroke. 1986;17:718–722. doi: 10.1161/01.str.17.4.718. [DOI] [PubMed] [Google Scholar]
- Grubb R L, Jr, Derdeyn C P, Fritsch S M, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA. 1998;280:1055–1060. doi: 10.1001/jama.280.12.1055. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Fukuyama H, Nagahama Y, et al. Significance of increased oxygen extraction fraction in five-year prognosis of major cerebral arterial occlusive diseases. J Nucl Med. 1999;40:1992–1998. [PubMed] [Google Scholar]
- Ogasawara K, Ogawa A, Yoshimoto T. Cerebrovascular reactivity to acetazolamide and outcome in patients with symptomatic internal carotid or middle cerebral artery occlusion: a xenon-133 single-photon emission computed tomography study. Stroke. 2002;33:1857–1862. doi: 10.1161/01.str.0000019511.81583.a8. [DOI] [PubMed] [Google Scholar]
- Powers W J, Press G A, Grubb R L, Jr, Gado M, Raichle M E. The effect of hemodynamically significant carotid artery disease on the hemodynamic status of the cerebral circulation. Ann Intern Med. 1987;106:27–35. doi: 10.7326/0003-4819-106-1-27. [DOI] [PubMed] [Google Scholar]
- Derdeyn C P, Shaibani A, Moran C J, Cross D T, 3rd, Grubb R L, Jr, Powers W J. Lack of correlation between pattern of collateralization and misery perfusion in patients with carotid occlusion. Stroke. 1999;30:1025–1032. doi: 10.1161/01.str.30.5.1025. [DOI] [PubMed] [Google Scholar]
- Everdingen K J van, Visser G H, Klijn C J, Kappelle L J, Grond J van der. Role of collateral flow on cerebral hemodynamics in patients with unilateral internal carotid artery occlusion. Ann Neurol. 1998;44:167–176. doi: 10.1002/ana.410440206. [DOI] [PubMed] [Google Scholar]
- Levine R L, Lagreze H L, Dobkin J A, et al. Cerebral vasocapacitance and TIAs. Neurology. 1989;39:25–29. doi: 10.1212/wnl.39.1.25. [DOI] [PubMed] [Google Scholar]
- Waterston J A, Brown M M, Butler P, Swash M. Small deep cerebral infarcts associated with occlusive internal carotid artery disease. A hemodynamic phenomenon? Arch Neurol. 1990;47:953–957. doi: 10.1001/archneur.1990.00530090023007. [DOI] [PubMed] [Google Scholar]
- Derdeyn C P, Khosla A, Videen T O, et al. Severe hemodynamic impairment and border zone—region infarction. Radiology. 2001;220:195–201. doi: 10.1148/radiology.220.1.r01jl09195. [DOI] [PubMed] [Google Scholar]
- Feeney D M, Baron J C. Diaschisis. Stroke. 1986;17:817–830. doi: 10.1161/01.str.17.5.817. [DOI] [PubMed] [Google Scholar]
- Derdeyn C P, Grubb R L, Jr, Powers W J. Cerebral hemodynamic impairment: methods of measurement and association with stroke risk. Neurology. 1999;53:251–259. doi: 10.1212/wnl.53.2.251. [DOI] [PubMed] [Google Scholar]
- Yonas H, Darby J M, Marks E C, Durham S T, Maxwell C. CBF measured by Xe-CT: approach to analysis and normal values. J Cereb Blood Flow Metab. 1991;11:716–725. doi: 10.1038/jcbfm.1991.128. [DOI] [PubMed] [Google Scholar]
- Tomita M. In: Tomita M, Sawada T, Naritomi H, Heiss W-D, editor. Cerebral Hyperemia and Ischemia: From the Standpoint of Cerebral Blood Volume. Amsterdam, The Netherlands: Elsevier Science Publishers BV; 1988. Significance of cerebral blood volume. pp. 3–31.
- Ferrari M, Wilson D A, Hanley D F, Traystmen R J. Effects of graded hypotension on cerebral blood flow, blood volume, and mean transit time in dogs. Am J Physiol. 1992;262:H1908–H1914. doi: 10.1152/ajpheart.1992.262.6.H1908. [DOI] [PubMed] [Google Scholar]
- Zaharchuk G, Mandeville J B, Bogdanov A A, Jr, Weissleder R, Rosen B R, Marota J J. Cerebrovascular dynamics of autoregulation and hypoperfusion: an MRI study of CBF and changes in total and microvascular cerebral blood volume during hemorrhagic hypotension. Stroke. 1999;30:2197–2205. doi: 10.1161/01.str.30.10.2197. [DOI] [PubMed] [Google Scholar]
- Derdeyn C P, Videen T O, Yundt K D, et al. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain. 2002;125:595–607. doi: 10.1093/brain/awf047. [DOI] [PubMed] [Google Scholar]
- An H, Lin W. Cerebral oxygen extraction fraction and cerebral venous blood volume measurements using MRI: effects of magnetic field variation. Magn Reson Med. 2002;47:958–966. doi: 10.1002/mrm.10148. [DOI] [PubMed] [Google Scholar]
- Vernieri F, Pasqualetti P, Matteis M. Effect of collateral blood flow and cerebral vasomotor reactivity on the outcome of carotid artery occlusion. Stroke. 2001;32:1552–1558. doi: 10.1161/01.str.32.7.1552. [DOI] [PubMed] [Google Scholar]
- Molloy J, Markus H S. Asymptomatic embolization predicts stroke and TIA risk in patients with carotid artery stenosis. Stroke. 1999;30:1440–1443. doi: 10.1161/01.str.30.7.1440. [DOI] [PubMed] [Google Scholar]
- Omae T, Mayzel-Oreg O, Li F, Sotak C H, Fisher M. Inapparent hemodynamic insufficiency exacerbates ischemic damage in a rat microembolic stroke model. Stroke. 2000;31:2494–2499. doi: 10.1161/01.str.31.10.2494. [DOI] [PubMed] [Google Scholar]
- Endres M, Laufs U, Huang Z, et al. Stroke protection by 3-hydroxy-3-methylglutary (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1998;95:8880–8885. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Yamaguchi T, Tsuchiya T, Minematsu K, Nishimura T. Sequential change of hemodynamic reserve in patients with major cerebral artery occlusions or severe stenosis. Neuroradiology. 1992;34:15–21. doi: 10.1007/BF00588426. [DOI] [PubMed] [Google Scholar]
- Widder B, Kleiser B, Krapf H. Course of cerebrovascular reactivity in patients with carotid artery occlusions. Stroke. 1994;25:1963–1967. doi: 10.1161/01.str.25.10.1963. [DOI] [PubMed] [Google Scholar]
- Derdeyn C P, Videen T O, Fritsch S M, Carpenter D A, Grubb R L, Jr, Powers W J. Compensatory mechanisms for chronic cerebral hypoperfusion in patients with carotid occlusion. Stroke. 1999;30:1019–1024. doi: 10.1161/01.str.30.5.1019. [DOI] [PubMed] [Google Scholar]
- The EC/IC Bypass Study Group Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke: results of an international randomized trial. N Engl J Med. 1985;313:1191–2000. doi: 10.1056/NEJM198511073131904. [DOI] [PubMed] [Google Scholar]
- Day A L, Rhoton A L, Little J R. The Extracranial-Intracranial Bypass Study. Surg Neurol. 986;26:222–226. doi: 10.1016/0090-3019(86)90153-9. [DOI] [PubMed] [Google Scholar]
- Ausman J I, Diaz F G. Critique of the Extracranial-Intracranial Bypass Study. Surg Neurol. 1986;26:218–221. doi: 10.1016/0090-3019(86)90152-7. [DOI] [PubMed] [Google Scholar]
- Sundt T M., Jr Was the international randomized trial of extracranial-intracranial arterial bypass representative of the population at risk? N Engl J Med. 1987;316:814–816. doi: 10.1056/NEJM198703263161318. [DOI] [PubMed] [Google Scholar]
- Gibbs J M, Wise R J, Thomas D J, Mansfield A O, Russell R W. Cerebral haemodynamic changes after extracranial-intracranial bypass surgery. J Neurol Neurosurg Psychiatry. 1987;50:140–150. doi: 10.1136/jnnp.50.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron J C, Bousser M G, Rey A, Guillard A, Comer D, Castaigne P. Reversal of focal “misery perfusion syndrome” by extra-intracranial artery bypass in hemodynamic cerebral ischemia. A case study with 150 positron emission tomography. Stroke. 1981;12:454–459. doi: 10.1161/01.str.12.4.454. [DOI] [PubMed] [Google Scholar]
- Powers W J, Martin W R, Herscovitch P, Raichle M E, Grubb R L., Jr Extracranial-intracranial bypass surgery: hemodynamic and metabolic effects. Neurology. 1984;34:1168–1174. doi: 10.1212/wnl.34.9.1168. [DOI] [PubMed] [Google Scholar]
- Samson Y, Baron J C, Bousser M G, et al. Effects of extra-intracranial arterial bypass on cerebral blood flow and oxygen metabolism in humans. Stroke. 1985;16:609–615. doi: 10.1161/01.str.16.4.609. [DOI] [PubMed] [Google Scholar]
- Yonas H, Smith H A, Durham S R, Pentheny S L, Johnson D W. Increased stroke risk predicted by compromised cerebral blood flow reactivity. J Neurosurg. 1993;79:483–489. doi: 10.3171/jns.1993.79.4.0483. [DOI] [PubMed] [Google Scholar]
- Schmiedek P, Piepgras A, Leinsinger G, Kirsch S M, Einhupl K. Improvement of cerebrovascular reserve capacity by EC-IC arterial bypass in patients with ICA occlusion and hemodynamic cerebral ischemia. J Neurosurg. 1994;81:236–244. doi: 10.3171/jns.1994.81.2.0236. [DOI] [PubMed] [Google Scholar]
- Grubb R L, Jr, Powers W J, Derdeyn C P, Adams H P, Jr, Clark W R. The Carotid Occlusion Surgery Study. Neurosurgical Focus. 2003;14:1–9. doi: 10.3171/foc.2003.14.3.10. [DOI] [PubMed] [Google Scholar]
- Carnevale F C, de Blas M, Merino S, Egana J M. Percutaneous endovascular treatment of chronic iliac artery occlusion. Cardiovasc Intervent Radiol. 2004;27:447–452. doi: 10.1007/s00270-004-0086-5. [DOI] [PubMed] [Google Scholar]
- Lee P Y, Chen W H, Ng W, Lau C P. Percutaneous recanalization of chronic subclavian artery occlusion using optical coherence reflectometry-guided radiofrequency ablation guidewire. Catheter Cardiovasc Interv. 2003;60:558–561. doi: 10.1002/ccd.10695. [DOI] [PubMed] [Google Scholar]
- Du Mesnil De Rochemont R, Sitzer M, Neumann-Haefelin T, Harmjanz A, Berkfeld J. Endovascular recanalization of acute atherothrombotic carotid artery occlusion holds up progressive stroke. Neuroradiology. 2004;46:583–586. doi: 10.1007/s00234-004-1214-2. [DOI] [PubMed] [Google Scholar]
- Wang H, Lanzino G, Fraser K, Tracy P, Wang D. Urgent endovascular treatment of acute symptomatic occlusion of the cervical internal carotid artery. J Neurosurg. 2003;99:972–977. doi: 10.3171/jns.2003.99.6.0972. [DOI] [PubMed] [Google Scholar]
- North American Symptomatic Carotid Endarterectomy Trial (NASCET) Collaborators Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- European Carotid Surgery Trialists' Collaborative Group MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. Lancet. 1991;337:1235–1243. [PubMed] [Google Scholar]
- Executive Committee of the Asymptomatic Carotid Atherosclerosis Study Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- Fox D J, Jr, Moran C J, Cross D T., 3rd Long term outcome after angioplasty and stenting for symptomatic carotid stenosis in poor surgical candidates. Stroke. 2002;33:2877–2880. doi: 10.1161/01.str.0000043820.72323.23. [DOI] [PubMed] [Google Scholar]
- SSYLVIA Study Investigators Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA): study results. Stroke. 2004;35:1388–1392. doi: 10.1161/01.STR.0000128708.86762.d6. [DOI] [PubMed] [Google Scholar]