ABSTRACT
Moyamoya disease is a disorder characterized by bilateral progressive steno-occlusion of the terminal internal carotid arteries with associated development of a fragile network of basal collateral vessels. It most commonly presents in children, but is also frequently seen in adults, especially in the third or fourth decade of life. Adults afflicted with this disease have very different clinical characteristics as compared with children. For example, adults more commonly present with hemorrhage than cerebral ischemia, while children present with cerebral ischemia nearly 75% of the time and very rarely present with hemorrhage. This significantly impacts treatment considerations for the adult-onset moyamoya patient, as cerebral revascularization, though well accepted in the context of cerebral ischemia, is relatively controversial for the prevention of rehemorrhage. The purpose of this article is to review the pertinent general features of moyamoya disease, examine the clinical characteristics associated with the adult-onset form of this disease, and provide a detailed discussion regarding the indications, operative techniques, and outcomes of direct and indirect revascularization surgical procedures.
Keywords: Moyamoya disease, hemorrhage, ischemia, revascularization, adult
Moyamoya disease is a chronic occlusive vasculopathy of unknown etiology that was first described by Takeuchi and Shimizu in 1957. It is characterized by the spontaneous progressive occlusion of the supraclinoid internal carotid arteries and the circle of Willis, leading to the formation of extensive arterial collaterals at the base of the brain. The term moyamoya—Japanese for “puff of smoke”—was coined by Suzuki and Takaku1 in 1969 in reference to the angiographic appearance of these fine basal collaterals, and has since become the accepted term to define the disease throughout the world.
Moyamoya disease, which is most common in Asian populations, is characterized clinically by the onset of one or more cerebral ischemic events (particularly in children) and/or the development of one or more cerebral hemorrhages (particularly in adults). Though many medical strategies for the treatment of this disease have been investigated, none has proven effective. Currently, the mainstay of treatment involves the use of direct and/or indirect surgical techniques for cerebral revascularization. The purpose of this article is to review the pertinent clinical characteristics of patients with adult-onset moyamoya disease (including diagnostic criteria, pathology, epidemiology, clinical presentation, and neuroimaging), and to provide a detailed discussion regarding the indication, operative technique, and outcome of the various surgical revascularization techniques utilized for the treatment of these patients.
DIAGNOSTIC CRITERIA
Strict guidelines for the diagnosis of moyamoya disease have been established by the Research Committee on Moyamoya Disease of the Ministry of Health and Welfare in Japan.2 Definite diagnosis of moyamoya disease requires the following: bilateral steno-occlusive changes in the terminal internal carotid artery (ICA) and/or proximal portions of the anterior or middle cerebral arteries, abnormal vascular networks in the vicinity of the steno-occlusive disease, and no accompanying systemic disorders. Probable diagnosis of moyamoya disease is established if the steno-occlusive disease and associated abnormal vascular network are noted unilaterally, with progression to definite moyamoya disease (i.e., bilateral lesions) seen in 7 to 50% of patients.3,4 Finally, the term quasi-moyamoya disease or moyamoya syndrome is typically used to refer to patients who display steno-occlusive lesions and associated collaterals in other areas of the brain or who have systemic disorders that account for the observed vasculopathy (e.g., arteriosclerosis, autoimmune disease, Down syndrome, von Recklinghausen's disease, previous head irradiation, or meningitis).
PATHOLOGY
Autopsy studies of moyamoya disease reveal that the terminal ICA and proximal anterior and middle cerebral arteries are severely narrowed or occluded by an extensive fibrocellular intimal thickening.5,6,7 The intima is typically a laminated structure with duplication or triplication of the internal elastic lamina. Other associated findings include frequent mural thrombi and occasional lipid deposits, but inflammatory cells are conspicuously absent. Accumulating evidence suggests that smooth muscle cell proliferation and phenotypic modulation may underlie these observed vessel changes.7
The basal moyamoya collaterals themselves have unique pathological characteristics. Some are thin-walled and dilated, while others are thick-walled and stenotic.6 The presence of the latter indicates that the arterial obstructive changes seen in moyamoya patients are not limited to the circle of Willis. Even extracranial vessels have been shown to have some degree of intimal thickening. Those basal collaterals that are thin and dilated are often associated with significant vessel fibrosis and marked attenuation of the media, changes that may predispose moyamoya patients to microaneurysm formation. The presence of microaneurysms, increased hemodynamic stress, and vessel wall necrosis are all considered likely etiologies for the cerebral hemorrhages that often occur in adult-onset moyamoya disease.8,9 Common locations for such hemorrhages include the basal ganglia, thalamus, and ventricular system, all of which have great proximity to these fragile basal collateral vessels.
EPIDEMIOLOGY
Moyamoya disease was initially thought to exclusively involve Japanese and Korean populations,2,10,11 but its presence within non-Asian populations has been increasingly recognized in recent years.12,13 Overall, the incidence of moyamoya disease is considerably lower than that of other common cerebrovascular diseases (e.g., lacunar infarctions in adults). In Japan, an annual incidence of 0.35 per 100,000 population and a prevalence of 3.16 per 100,000 population have been reported.2,10 In the United States, these statistics are not available but are felt to be much lower.11,13
A bimodal age distribution is seen in most series, with a large peak in the first decade of life and a second smaller peak in the fourth decade of life.14,15,16,17 A female predominance occurs throughout all age groups, with most reporting a female:male ratio of ∼3:2.10,15 Finally, although no specific pattern of inheritance has been determined thus far, familial occurrence appears relatively common. For example, in Japan ∼8 to 10% of moyamoya patients have affected family members.10,18,19,20 In the United States, however, familial occurrence appears less common (∼2%).13
CLINICAL PRESENTATION
The clinical presentation of moyamoya disease differs considerably between pediatric and adult patients. In children, by far the most common presentation is cerebral ischemia. Approximately 40% of those under 10 years of age will present with a transient ischemic attack (TIA), while nearly 30% will present with cerebral infarction.21 Other common pediatric presentations include headaches and seizures, while cerebral hemorrhage is distinctly uncommon in children.
By contrast, cerebral hemorrhage is the most common clinical presentation in adults. Up to 66% of adult-onset moyamoya patients present with hemorrhage, the majority of which are intraventricular or periventricular in location.21 Such hemorrhages are often repetitive in nature, with an annual rate of rebleeding of ∼7%.22 The morbidity and mortality associated with these hemorrhages has been substantial. In one series, only 45% of patients made a good neurological recovery following their presenting hemorrhage, and 7% died from their initial bleed.22 Repeat cerebral hemorrhage, which often occurs apart from the original bleeding site, carried an even graver prognosis, with only 20% of patients making a good neurological recovery and nearly 30% expiring due to their rebleed.22
Adult-onset moyamoya disease can also present in a nonhemorrhagic fashion, cerebral ischemia (either TIA or infarction) being the next most common presentation for an adult. Precipitating factors may include pregnancy, hypertension, or degenerative changes within collateral vessels. The most common locations for infarction are within the “watershed” territories between the anterior, middle, and posterior cerebral arteries. Some have noted that the majority of adults who present with cerebral ischemia are relatively young (less than 30 years old), while older patients (greater than 30 years old) present almost exclusively with cerebral hemorrhage.23 These investigators concluded that young adults may represent a moyamoya population in transition (i.e., they are equally predisposed to cerebral ischemia and hemorrhage).23
These trends regarding the presentation of adult-onset moyamoya disease have come predominantly from the Asian literature. Some early studies examining non-Asian patient populations suggest that differences in presentation may occur based on ethnicity. For example, Chiu and colleagues12 reviewed their series of 32 patients with moyamoya disease, only 6% of whom were of Asian descent. In the 26 adult patients from this series, only 3 presented with cerebral hemorrhage and 23 presented with cerebral ischemia. Definitive conclusions regarding the effect of ethnicity, however, must await larger-scale analysis.
NEUROIMAGING
Cerebral angiography is the principal imaging study utilized for the diagnosis of moyamoya disease. The characteristic angiographic findings are traditionally described in stages, as the disease progresses toward total ICA occlusion and complete cerebral perfusion through external carotid and vertebral collateralizations.24 Known as the six-stage classification of Suzuki (see Table 1), angiographic progression is detailed from early carotid narrowing, to the formation of basal moyamoya vessels, to the disappearance of these collateral vessels and maintenance of the cerebrum by the external carotid and vertebral systems. Vault moyamoya vessels can also be seen, with dural and pial collaterals developing secondary to reduced blood flow.
Table 1.
Suzuki Stages of Moyamoya Disease24
| Suzuki Stage | Angiographic Finding |
|---|---|
| I | Narrowing of carotid arteries |
| II | Initial appearance of moyamoya vessels |
| III | Intensification of moyamoya vessels |
| IV | Minimization of moyamoya vessels |
| V | Reduction of moyamoya vessels |
| VI | Disappearance of moyamoya vessels |
Noninvasive imaging techniques have also proven useful in the diagnosis of moyamoya disease. Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) often demonstrate the characteristic cerebrovascular findings of steno-occlusive carotid disease and basal moyamoya vessels.25 When these findings are identified bilaterally on MRI or MRA, a cerebral angiogram is not required to establish the definite diagnosis of moyamoya disease.
Once the diagnosis is confirmed, other imaging modalities may provide additional useful information. For example, studies such as xenon-enhanced computed tomography, single photon emission tomography, and positron emission tomography can be utilized to measure regional cerebral blood flow and metabolic distribution. These measurements are used to detect regional perfusion instability, which may suggest likelihood for further clinical progression. They may also be used postoperatively to measure the effect of surgical revascularization.
TREATMENT
Medical Therapy
Various medical regimens have been investigated for the treatment of moyamoya disease, including aspirin, steroids, vasodilators, mannitol, low-molecular-weight dextran, and antibiotics, all of which have thus far proven ineffective.26 Although several case reports have documented symptomatic improvement in a small number of ischemic moyamoya patients after intravenous infusion of calcium-channel blockers such as verapamil, nimodipine, or nicardipine,27,28,29 the efficacy of this approach has not been well validated to date. In general, medical therapy has been considered largely ineffective, and as a result surgical revascularization has become the mainstay of treatment for patients with moyamoya disease.
Surgical Revascularization
INDICATIONS
The efficacy of cerebral revascularization for reducing the incidence of strokes and TIAs in both pediatric and adult patients presenting with cerebral ischemia has been generally accepted for many years. Although it has never been validated in a prospective, randomized clinical trial, this assertion has been repeatedly supported by multiple large case series.30,31,32,33,34 Due to the overwhelming acceptance of the efficacy of surgical revascularization for this patient population, it is unlikely that a clinical trial will ever be initiated.
The effect of cerebral revascularization on the risk of cerebral hemorrhage in moyamoya patients, however, is not nearly as well accepted. For example, it is unclear whether childhood revascularization for moyamoya disease significantly affects the incidence of cerebral hemorrhage once such patients reach adulthood. It is also unclear whether cerebral revascularization in the context of adult-onset hemorrhagic moyamoya disease affects the risk of subsequent rebleeding. The latter is critical to many adult moyamoya patients, as hemorrhage is the most common presenting event.
Several reports examining the efficacy of direct cerebral revascularization for the treatment of hemorrhagic moyamoya disease have now been published, providing some insight into the question of surgical effectiveness for these patients (see Table 2).35,36,37,38,39,40 Three of these reports, all of which were nonrandomized and retrospective in nature, directly compared revascularization to conservative management. Two noted trends toward reduced rate of rebleeding following surgical intervention,37,39 and one documented a statistically significant reduction in rebleeding for patients treated with superficial temporal artery to middle cerebral artery (STA-MCA) bypass.40 These encouraging but inconclusive results have provided the necessary foundation for the initiation of the Japan Adult Moyamoya Trial, which was designed as a multicenter, prospective randomized trial of extracranial-intracranial bypass for the treatment of adult moyamoya patients who present with cerebral hemorrhage.41 It is hoped that results from this study will ultimately provide guidance to the appropriate therapy for adult-onset hemorrhagic moyamoya patients. In the interim, recommendations are left to the individual practitioner.
Table 2.
Literature Evaluating the Efficacy of Revascularization for the Treatment of Hemorrhagic Moyamoya Disease
| Report | Patient # | Method | F/U (mean) | Result | Conclusion |
|---|---|---|---|---|---|
| Houkin et al35 (1996) | 24 | Single institution; All underwent STA-MCA bypass + EDAMS | 6.4 years | 25% decrease in moyamoya vessels; 12.5% rebleed | Rebleed risk may be reduced by surgery |
| Iwama et al36 (1997) | 45 | Single institution; Bypass: 43, No bypass: 2 | 3.7 years | 60% decrease in moyamoya vessels; 20% rebleed (postop) | Rebleed risk may be reduced by surgery |
| Fujii et al37 (1997) | 290 | Multicenter retrospective study; Bypass: 152, No bypass: 138 | ? | Bypass: 19.1% rebleed No bypass: 28.3% rebleed | Rebleed risk may be reduced by surgery |
| Okada et al38 (1998) | 15 | Single institution; All underwent STA-MCA bypass | 7.8 years | 60% decrease in moyamoya vessels; 6.7% perioperative bleed; 20% rebleed | Rebleed risk may be reduced by surgery |
| Yoshida et al39 (1999) | 28 | Single institution; Revascularization: 10, No revascularization: 18 | 14.2 years | Revascularization: 12.5% rebleed No revascularization: 38.5% rebleed | Rebleed risk may be reduced by surgery |
| Kawaguchi et al40 (2000) | 22 | Single institution; STA-MCA bypass: 6, EDAS: 5, No revascularization:11 | 8 years | STA-MCA bypass: 0% recurrent stroke EDAS: 60% recurrent stroke No revascularization: 54% recurrent stroke | Rebleed risk reduced by bypass (p < 0.05) |
Our criteria for cerebral revascularization in adult moyamoya patients are similar to those of Okada and associates38 and are as follows: (1) Patients who present with infarction or cerebral hemorrhage are assessed on their ability to perform activities of daily living. Surgical intervention is only recommended for those found to be in good neurological condition. (2) On computed tomography, infarctions are less than 2 cm in maximal diameter and all previous hemorrhages have completely resolved. (3) The angiographic stage of disease is Suzuki stage II to IV. (4) The timing of operation is at least 2 months after the most recent attack. Surgery for an asymptomatic patient or for an asymptomatic hemisphere is rarely offered.
ANESTHETIC CONSIDERATIONS
Anesthetic complications can be a major source of perioperative morbidity in moyamoya patients, owing primarily to the tenuous blood supply present in these individuals. Special care must therefore be taken to minimize these risks. Patients are placed under general anesthesia with a combination of inhaled and intravenous agents. Great efforts are made to minimize the cerebral metabolic oxygen consumption rate (CMRO2) and maintain adequate cerebral blood flow (CBF). Painful stimuli from sources such as intubation and surgical incision that may increase CMRO2 are kept to a minimum with adequate general anesthesia. CBF is maintained by meticulously avoiding hypotension and by assuring that partial pressure of carbon dioxide levels remain between 35 to 40 mm Hg throughout the procedure.
During direct arterial bypass procedures where temporary cerebral artery occlusion is required, certain neuroprotective measures are also taken. For example, mild hypothermia (33 to 35°C) is maintained throughout the surgical procedure. Also, barbiturate-induced burst suppression and mild hypertension during arterial cross clamping is utilized routinely. Finally, mannitol is typically avoided, as brain relaxation can cause the cortical surface to fall away from the dural edge and potentially place tension on a bypass graft.
DIRECT REVASCULARIZATION
Direct cerebral revascularization in the context of moyamoya disease generally involves the connection of the STA to the MCA, in an effort to immediately revascularize the MCA territory and eventually reduce fragile moyamoya collateral vessels in the cerebral hemisphere. Though the efficacy of direct arterial bypass for arteriosclerotic disease remains unproven,42 this approach has been an effective therapeutic modality for moyamoya disease (at least in those presenting with cerebral ischemia).30,31,33,34 In comparison to indirect revascularization procedures, the direct STA-MCA bypass has the distinct advantage of providing an immediate increase in cerebral blood flow. Yet some questions regarding its utility still exist, as indirect bypass procedures can provide effective revascularization in many patients and likely carry less risk.43
For the adult moyamoya patient, however, several lines of evidence support the notion that direct bypasses, when feasible, may be superior to indirect techniques. For example, indirect bypass procedures, though highly effective in children,35,44,45 are considerably less predictable in their ability to provide adequate cerebral collateralization in adults.35,40,46 One study by Houkin and associates45 noted that indirect revascularization through an encephaloduroarteriomyosynangiosis procedure proved effective in all pediatric patients, while many adults responded poorly to the procedure. In their analysis, indirect revascularization was found to be statistically less effective in adults as compared with children (p < 0.001, χ2 test). These authors also noted that direct arterial bypass surgery was statistically more effective in adults than children (p < 0.05, χ2 test). Finally, some authors have suggested that direct bypass procedures may reduce hemodynamic stress across moyamoya vessels to a greater degree than indirect techniques, and would therefore be more likely to reduce hemorrhage risk in the adult patient.35
Traditional uses for the STA-MCA bypass have primarily focused on using the parietal branch of the STA as the donor and the posterior temporal branch of the MCA (M3 branch) as the recipient vessel. Others advocate the use of the anterior branch of the STA as the donor vessel and an M2 branch distal to the MCA bifurcation as the recipient vessel. All patients undergoing cerebral revascularization procedures should have computed tomography of the head and four-vessel cerebral angiography with selected external carotid injections for mapping and sizing of the STA. In patients with ischemic disease, hemodynamic imaging studies can be obtained to examine the cerebrovascular reserve.
STA-MCA Bypass Technique
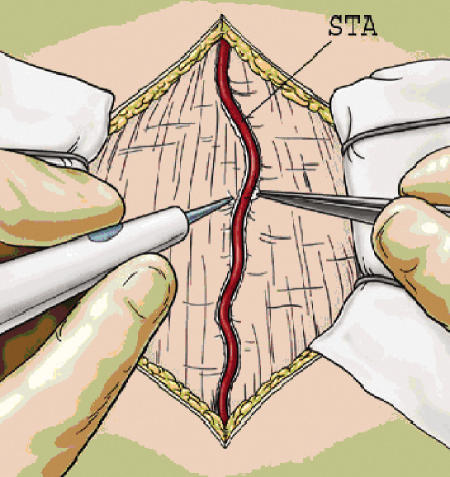
The patient is positioned supine with the head turned parallel to the operative room floor and a roll under the shoulder. The positioning of the patient should place the probable site of anastomosis at the highest point in the operative field to reduce the accumulation of cerebrospinal fluid at the anastomotic site.47 Doppler ultrasound is then used to map the course of the donor branch of the STA. For a single bypass using the parietal branch, a linear incision is made over the identified vessel from the zygoma to the superior temporal line. The use of Loupe magnification or the operative microscope for this portion of the procedure is strongly encouraged to minimize the risk of STA damage. The skin incision can be started either distally near the superior temporal line (a method we prefer, as inadvertent damage to the distal STA does not preclude its use as a donor vessel while more proximal STA damage might) or proximally over the root of the zygoma. Incision and dissection over the STA can be performed either sharply or through use of a needle point cautery (Colorado Biomedical) (Fig. 1). Regardless of the instrument used, bipolar cautery is used for the careful cauterization and ligation of the small arterial branches that emanate from the STA. Hemostasis must be meticulous, as failure to cauterize branches fully may lead to a postoperative epidural hematoma. A cuff of ∼5 mm of tissue is left on the STA to limit the potential for thermal injury from the cautery device.
Figure 1.
After opening the skin, the superficial temporal artery is dissected free along its length with a small 5-mm cuff using either sharp dissection or needle cautery.
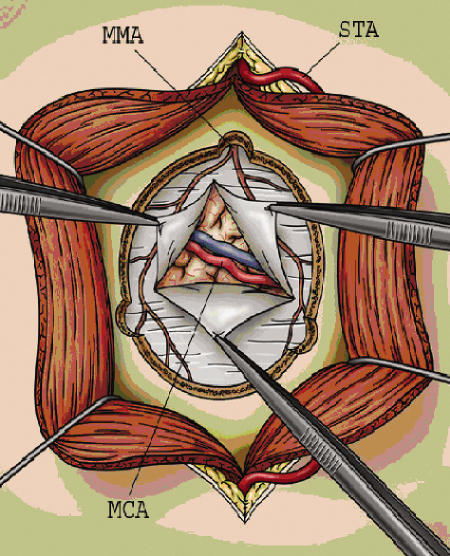
Two choices for preparation of the STA as a donor vessel exist, either early STA ligation or preservation of the vessel until the time of bypass. We prefer the latter, as it allows normal arterial flow up to the time of the anastomosis (see Fig. 2). In addition, early STA ligation obviates its use as an onlay graft (see Fig. 3) should a suitable recipient vessel not be found. A linear incision is then made in the temporalis muscle, which is elevated in a subperiosteal fashion. Care is taken to gently mobilize the intact STA out of the operative field, allowing a craniotomy to be safely performed (Fig. 2). The bone flap is centered on the sylvian fissure at a point approximately 6 cm above the external auditory canal. Burr holes are created at the root of the zygoma and at the superior temporal line, and the dura is carefully dissected free in an attempt to preserve the middle meningeal artery (MMA) (Fig. 2).
Figure 2.
Craniotomy is performed with multiple burr holes to preserve the middle meningeal artery (MMA), and the dura is opened to find a recipient branch of the middle cerebral artery (MCA). Note that the superficial temporal artery (STA) is positioned safely behind the temporalis muscle.
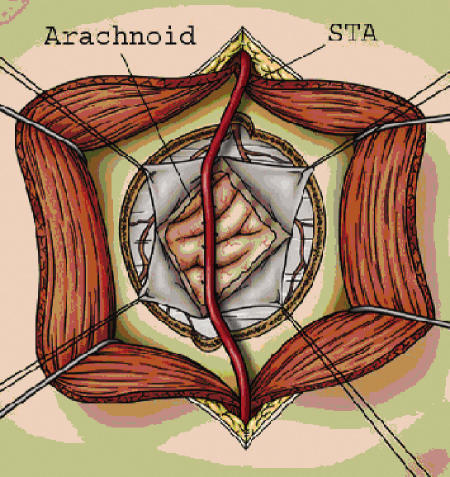
Figure 3.
“Pial synangiosis”—a modification to the traditional encephaloduroarteriosynangiosis (EDAS) procedure, as advocated by Scott and associates.54 The original EDAS procedure involved suturing the superficial temporal artery (STA) to the dural edges without opening the arachnoid. Scott and colleagues modified the EDAS procedure by widely opening the arachnoid and directly suturing the adventitia of the STA to the cortical pia, in an effort to promote maximal exposure of the cortical surface to the overlying STA.
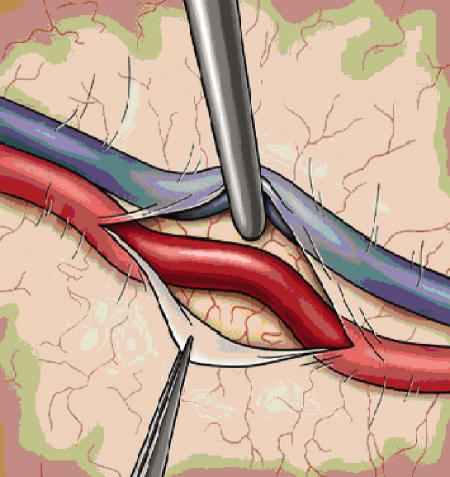
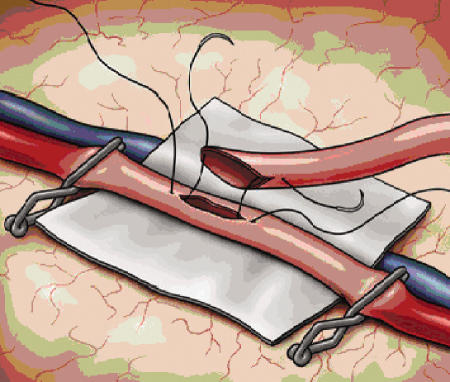
The dural opening is fashioned in such a way as to preserve the main branches of the MMA, as these vessels often provide critical collateralization to the cerebrum (Fig. 2). Once the dura is opened, the cortical surface is examined and generally the largest diameter vessel is selected. The ideal recipient is a surface cortical vessel without significant branching and a diameter of 1.5 mm.48 Some authors feel that a direct arterial bypass can be performed with good results in vessels as small as 0.5 mm, although the smaller the vessel the more difficult the procedure.33 Once the vessel is identified, the arachnoid is opened and a 1.5- to 2-cm segment of artery is dissected free (Fig. 4). Small perforating branches are coagulated and ligated, and a rubber dam is placed behind the dissected recipient vessel (Fig. 5). The donor artery is then prepared by removing the connective tissue and adventitia from the distal portion of the artery, allowing for unobstructed access to the vessel during anastomosis.
Figure 4.
The arachnoid is opened sharply and the recipient vessel is dissected free for up to 2 centimeters. Small perforators are coagulated with bipolar forceps to prepare the vessel for anastomosis.
Figure 5.
The recipient vessel is opened and a “fish-mouth” graft is anchored into place with 10–0 monofilament nylon suture at each end. Hemostatic clips are secured proximally and distally along the recipient vessel prior to arteriotomy. A rubber dam provides further stability to the anastomotic site.
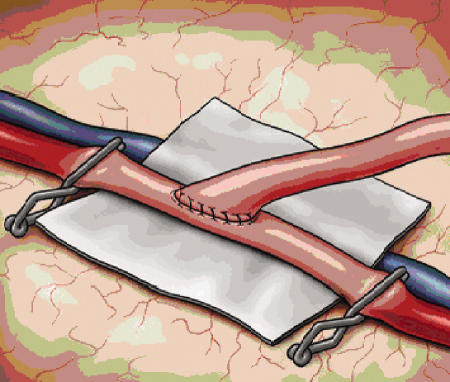
The patient is placed in burst suppression with continuous EEG monitoring and mild hypertension is instituted. A dilute papaverine solution can be placed on the STA to help dilate the artery. A temporary clip is placed across the proximal STA, which is then sharply incised at an angle and spatulated using microscissors, creating an opening that is approximately two times the diameter of the recipient vessel (see Fig. 5). Atraumatic clips are then applied to both ends of the anastomotic site and an arteriotomy is performed with an 11-blade and completed with microscissors. The vessel is immediately irrigated with heparinized saline. We prefer to stain the edges of the artery with blue marker to ease identification of the vessel wall. A 10–0 monofilament suture with a tapered needle is then used to anchor both ends of the donor and recipient vessels (Fig. 5). The anchor sutures are then continued using a running technique whereby the sutures are placed under little or no tension and then tightened after ensuring the suture does not capture the back wall of the vessel (Fig. 6). Interrupted sutures have also proven effective and remain a reasonable alternative to a running suture. Vessel flow can then be restored, with the distal MCA clip being removed first followed by the proximal MCA clip. Any sites of bleeding can usually be controlled with oxidized regenerated cellulose (Surgicel™ Ethicon), but another 10–0 suture can be placed if necessary. The temporary clip on the STA is removed last and patency is assessed with Doppler ultrasonography. Papaverine is then irrigated on the donor STA and recipient MCA to prevent vasospasm. Warm saline is used for the remainder of the case as an additional means to reduce arterial spasm. The dura is closed leaving a large durotomy for the graft that can be covered with muscle. The bone flap is fashioned so as to prevent compression or kinking of the graft. The temporalis muscle is then reapproximated, and the scalp is closed over a subgaleal drain. Dressings are applied with care to prevent compression of the graft. Postoperatively, aspirin (325 mg/d) is administered, normal blood pressure is maintained, and graft patency is serially assessed with Doppler ultrasonography.
Figure 6.
The anastomosis is performed in a running fashion utilizing the 10–0 monofilament nylon sutures used to initially anchor the donor vessel. Alternatively, multiple interrupted sutures can be utilized for the anastomosis (not pictured).
INDIRECT REVASCULARIZATION
A wide variety of indirect revascularization procedures have been devised in an attempt to increase blood supply to the moyamoya-affected brain. Most were developed when it was believed that this disease did not affect the external carotid artery. All share the common goal of providing a mechanism to promote collateral blood vessel formation in an effort to address cerebral ischemia and attempt to reduce hemodynamic stress across fragile moyamoya collaterals. They are considered “indirect” as they rely on the subsequent formation of collateral vessels to enhance blood delivery. They therefore provide no immediate revascularization.
Though the delayed nature of blood flow augmentation is a drawback, indirect revascularization procedures do offer several advantages over direct arterial bypasses. For example, in children the caliber of both the external carotid artery distal branches and the intracranial arterial trunks is often so small that a bypass is not technically feasible. Indirect procedures are perfectly suited to address this situation. In adults, anterior and posterior cerebral artery regions of hypoperfusion can be difficult to access via a direct arterial bypass, but indirect procedures can be easily designed to address such regions. In addition, indirect revascularization procedures are not limited by the distribution of a single major arterial division. This can be useful when both anterior and middle cerebral artery territories are affected or when there is holohemispheric involvement. Most indirect revascularization procedures can also be performed in less time, which may significantly reduce general anesthetic complications (a major source of perioperative morbidity in these patients). Finally, multiple indirect procedures on the same hemisphere can be performed over time if there is interval progression to new vascular territories.
As mentioned above, the major disadvantage of indirect revascularization is the inherent delay in collateralization, which is true for both pediatric and adult moyamoya patients. An additional drawback specific to adults, as noted previously, has been the inconsistent angiographic response after indirect revascularization in this patient population. Several reports have suggested that, although indirect revascularization techniques consistently lead to the development of significant collateralization in pediatric patients,35,44,45 this has not been the experience in adults.35,40,46 Despite these disadvantages, indirect revascularization remains a principal treatment option for many adult-onset moyamoya patients, especially when adequate donor and recipient arteries are unavailable for direct bypass. A variety of indirect procedures have been designed over the years, the majority of which attempt to increase the accessibility of the internal cerebral circulation to the external vasculature supplying the scalp, galea, skull, and dura. We shall consider them in approximation to their chronological development, as many of the newer strategies are simply additions to the earlier principal techniques.
Encephalomyosynangiosis (EMS) was first described in 1950 by Henschen49 and was later applied to moyamoya disease by Karasawa and associates50 in the late 1970s. This technique involves implanting the temporalis muscle on the lateral brain surface and suturing it in place to the dural edges. This takes advantage of the rich vascularity of the temporalis muscle and leads to collateral vessel formation from the deep temporal artery, but has the disadvantage of creating significant mass effect, increasing seizure incidence, and being cosmetically less appealing.
Using EMS as a starting point, and drawing on observations of spontaneous collateral vessels forming between scalp and cortex51 and dura and cortex,52 Matsushima and coworkers outlined a new method of indirect bypass—the encephaloduroarteriosynangiosis (EDAS) (Fig. 3), which remains one of the more common strategies of indirect revascularization. The procedure involves exposing one of the main scalp arteries, usually the STA, and preserving the inflow and outflow while keeping it attached to a strip of galea along its course (Fig. 1). The skull is then exposed through fascia and muscle and two burr holes are placed at the proximal and distal aspects of the scalp artery (Fig. 3). A craniotomy is performed along the vessel course and the dura beneath the bone flap exposed and opened, taking care not to divide MMA branches, which can be a significant source for arterial collateralization (Fig. 3). The artery and cuff of galea are then sutured to the dural edges and the bone flap replaced (not pictured). Adelson and Scott53 modified the EDAS procedure slightly, advocating not only opening of the dura but also wide opening of the arachnoid and suturing the adventitia of the STA to the brain surface in a “pial synangiosis” (Fig. 3). Recently, they reported the long-term results with this method on a cohort of 143 patients, finding that the majority of pediatric patients with moyamoya disease stopped having strokes and TIAs and experienced an excellent long-term prognosis.54
Another evolution in these procedures resulted in the technique known as encephaloduroarteriomyosynangiosis (EDAMS).55 This combines EDAS with EMS to allow for collateral formation from both the STA and deep temporal arteries. In addition, along the margins of the dural opening, the dura is cut into leaf-like flaps, which are then folded back under the dural margin into the epiarachnoid space so that the outer dural surface contacts the brain surface. This results in the promotion of collateral formation from the MMA as well. The temporalis muscle is then sutured down and used to cover the dural opening.
It was subsequently realized that in many cases the anterior cerebral artery (ACA) circulation was not being addressed by the various techniques described above, as these procedures had been designed to primarily improve blood flow to the MCA territory. It is known, however, that the ACA is often involved in the dynamic progression of the disease, with ischemia as common here as in the MCA territory.56 Several variations to the above-mentioned indirect techniques have been subsequently developed to help address the ACA circulation. For example, a modification to the EDAMS procedure was described in 199457 whereby a “ribbon” of galea and periosteum are dissected out and raised from the skull in a zigzag pattern and then inserted deeply into the interhemispheric fissure to lay on the medial frontal lobe. A recent comparison between this modification, referred to as a frontal encephalogaleosynangiosis and EDAS, to EDAS alone, indicated better clinical and neuroimaging outcomes, though no difference in the incidence of postoperative infarctions was shown.58
Other techniques such as the placement of multiple burr holes,59 cervical carotid sympathectomy,60 and omental transplantation61,62 have also been described. Omental tissue offers the advantage of inducing extensive collateral formation due to the production of lipid angiogenic factors and endothelial growth factors.63 However, it requires an extensive craniotomy as well as a laparotomy. The placement of burr holes was described as a treatment after it was noticed that neovascularization was occurring at the site of previous ventriculostomies.64 Although this impacts a limited area, it is technically simple, can be combined with other procedures (both direct and indirect) and can even be performed under local anesthesia. Cervical carotid sympathectomy was attempted as a means of increasing cerebral blood flow65; however, no durable improvements in blood flow have been observed.
COMBINING DIRECT AND INDIRECT REVASCULARIZATION TECHNIQUES
Some have advocated combining direct and indirect revascularization procedures in an effort to take advantage of the immediate revascularization provided by direct arterial bypass, but also to maximize the eventual cerebral revascularization to include regions outside the distribution of a single major arterial division.43,46,66,67 This approach typically involves combining an STA-MCA direct arterial bypass with an indirect procedure such as EDAS. Commonly, this approach would utilize a skin incision along the parietal branch of the STA, which is used as the arterial donor for an EDAS procedure, while the frontal branch of the STA is subsequently dissected free from the overlying skin flap and used for the direct STA-MCA bypass.
OUTCOME
As detailed above, it is generally accepted that overall outcome is improved (i.e., cerebral ischemic events are reduced) when children and adult moyamoya patients who present with cerebral ischemia are aggressively treated with surgical revascularization.30,31,32,33,34 The perioperative risk of stroke in this patient population, which has been documented to be ∼4 to 17%,12,54,58 appears to be outweighed by the long-term stroke risk reduction afforded by cerebral revascularization.
For adult moyamoya patients presenting with hemorrhage, however, it is unclear whether overall outcome is improved by surgical revascularization, although a few small case series have provided some support for this notion.37,39,40 The perioperative risk of stroke in this select group of moyamoya patients has not been well documented to date, but a risk of 0 to 7% has been reported.38,40 The hemorrhage risk reduction afforded by surgery, however, is clearly incomplete (Table 2), making it difficult to determine whether cerebral revascularization provides an overall benefit to the those moyamoya patients presenting with hemorrhage. It is hoped that the recently initiated Japan Adult Moyamoya Trial will ultimately provide the definitive answer to this question.
CONCLUSIONS
Adult-onset moyamoya disease has been shown to have distinct clinical characteristics that set this condition apart from that seen in the pediatric population. Adults are less likely to present with cerebral ischemia and much more likely to present with cerebral hemorrhage. In addition, the response of adults to direct and indirect cerebral revascularization procedures appears to differ from that of children. In adults the angiographic response to indirect surgical procedures has been relatively inconsistent, while the same procedures have proven very reliable in children. In contrast, direct arterial bypasses appear to be more effective in the adult, as compared with the pediatric, population. These differences suggest that an age-specific treatment paradigm must be adhered to when managing patients with moyamoya disease.
For those adult moyamoya patients presenting with cerebral ischemia, most advocate cerebral revascularization, and many recommend the primary use of a direct arterial bypass, possibly in conjunction with other indirect revascularization techniques. For those adult moyamoya patients presenting with cerebral hemorrhage, it is uncertain whether surgical revascularization is of clear benefit. However, we as well as many others continue to recommend surgical intervention, as the morbidity and mortality associated with repeat cerebral hemorrhage is very high and an apparent reduced risk of rebleeding after surgical revascularization has been noted in several case series. Again, most advocate the use of a direct arterial bypass if technically feasible, as indirect procedures in adults appear to be less effective. All practitioners involved in the treatment of adult patients with moyamoya disease anxiously await the results of the Japan Adult Moyamoya Trial, which, it is hoped, will provide a definitive answer to the question of optimal therapy in the adult hemorrhagic patient population.
FUNDING
No grant or industry funding was received in support of this work.
REFERENCES
- Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969;20:288–299. doi: 10.1001/archneur.1969.00480090076012. [DOI] [PubMed] [Google Scholar]
- Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (“Moyamoya” disease) Clin Neurol Neurosurg. 1997;99:S238–S240. [PubMed] [Google Scholar]
- Kawano T, Fukui M, Hashimoto N, Yonekawa Y. Follow-up study of patients with “unilateral” moyamoya disease. Neurol Med Chir (Tokyo) 1994;34:744–747. doi: 10.2176/nmc.34.744. [DOI] [PubMed] [Google Scholar]
- Hirotsune N, Meguro T, Kawada S, Nakashima H, Ohmoto T. Long-term follow-up study of patients with unilateral moyamoya disease. Clin Neurol Neurosurg. 1997;99:S178–S181. doi: 10.1016/s0303-8467(97)00043-7. [DOI] [PubMed] [Google Scholar]
- Hosoda Y. Pathology of so-called “spontaneous occusion of the circle of Willis.”. Pathol Annu. 1984;19:221–244. [PubMed] [Google Scholar]
- Yamashita M, Oka K, Tanaka K. Histopathology of the brain vascular network in moyamoya disease. Stroke. 1983;14:50–58. doi: 10.1161/01.str.14.1.50. [DOI] [PubMed] [Google Scholar]
- Masuda J, Ogata J, Yutani C. Smooth muscle cell proliferation and localization of macrophages and T cells in the occlusive intracranial major arteries in moyamoya disease. Stroke. 1993;24:1960–1967. doi: 10.1161/01.str.24.12.1960. [DOI] [PubMed] [Google Scholar]
- Mauro A J, Johnson E S, Chikos P M, Alvord E C Jr. Lipohyalinosis and miliary microaneurysms causing cerebral hemorrhage in a patient with moyamoya. A clinicopathological study. Stroke. 1980;11:405–412. doi: 10.1161/01.str.11.4.405. [DOI] [PubMed] [Google Scholar]
- Oka K, Yamashita M, Sadoshima S, Tanaka K. Cerebral haemorrhage in Moyamoya disease at autopsy. Virchows Arch A Pathol Anat Histol. 1981;392:247–261. doi: 10.1007/BF02155663. [DOI] [PubMed] [Google Scholar]
- Wakai K, Tamakoshi A, Ikezaki K, et al. Epidemiological features of moyamoya disease in Japan: findings from a nationwide survey. Clin Neurol Neurosurg. 1997;99:S1–S5. doi: 10.1016/s0303-8467(97)00031-0. [DOI] [PubMed] [Google Scholar]
- Hoffman H J. Moyamoya disease and syndrome. Clin Neurol Neurosurg. 1997;99:S39–S44. doi: 10.1016/s0303-8467(97)00057-7. [DOI] [PubMed] [Google Scholar]
- Chiu D, Shedden P, Bratina P, Grotta J C. Clinical features of moyamoya disease in the United States. Stroke. 1998;29:1347–1351. doi: 10.1161/01.str.29.7.1347. [DOI] [PubMed] [Google Scholar]
- Numaguchi Y, Gonzalez C F, Davis P C, et al. Moyamoya disease in the United States. Clin Neurol Neurosurg. 1997;99:S26–S30. doi: 10.1016/s0303-8467(97)00060-7. [DOI] [PubMed] [Google Scholar]
- Maki Y, Enomoto T. Moyamoya disease. Childs Nerv Syst. 1988;4:204–212. doi: 10.1007/BF00270916. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Kodama N. Moyamoya disease—a review. Stroke. 1983;14:104–109. doi: 10.1161/01.str.14.1.104. [DOI] [PubMed] [Google Scholar]
- Kudo T, Fukuta S. Spontaneous occlusion of the circle of Willis. Shinkei-Shimpo. 1976;20:750–757. [Google Scholar]
- Kudou T. Obstruction of circle of Willis: moyamoya disease. Int Med (Tokyo) 1977;28:465–469. [Google Scholar]
- Houkin K, Tanaka N, Takahashi A, Kamiyama H, Abe H, Kajii N. Familial occurrence of moyamoya disease. Magnetic resonance angiography as a screening test for high-risk subjects. Childs Nerv Syst. 1994;10:421–425. doi: 10.1007/BF00303605. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Houkin K, Tada M, Abe H. Familial occurrence of moyamoya disease. Clin Neurol Neurosurg. 1997;99:S162–S167. doi: 10.1016/s0303-8467(97)00054-1. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Tada M, Houkin K, et al. Linkage of familial moyamoya disease (spontaneous occlusion of the circle of Willis) to chromosome 17q25. Stroke. 2000;31:930–935. doi: 10.1161/01.str.31.4.930. [DOI] [PubMed] [Google Scholar]
- Handa H, Yonekawa Y, Goto Y, et al. In: Handa H, editor. Annual Report (1984) by Research Committee on Spontaneous Occlusion of the Circle of Willis. Japan: Ministry of Health and Welfare; 1985. Analysis of the filing data bank of 1500 cases of spontaneous occlusion of the circle of Willis and follow-up study of 200 cases for more than five years.
- Kobayashi E, Saeki N, Oishi H, Hirai S, Yamaura A. Long-term natural history of hemorrhagic moyamoya disease in 42 patients. J Neurosurg. 2000;93:976–980. doi: 10.3171/jns.2000.93.6.0976. [DOI] [PubMed] [Google Scholar]
- Han D H, Nam D H, Oh C W. Moyamoya disease in adults: characteristics of clinical presentation and outcome after encephalo-duro-arterio-synangiosis. Clin Neurol Neurosurg. 1997;99:S151–S155. doi: 10.1016/s0303-8467(97)00058-9. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Fujiwara S, Suzuki J. Long-term follow-up angiography of moyamoya disease—cases followed from childhood to adolescence [in Japanese] No Shinkei Geka. 1986;14:23–29. [PubMed] [Google Scholar]
- Houkin K, Aoki T, Takahashi A, Abe H. Diagnosis of moyamoya disease with magnetic resonance angiography. Stroke. 1994;25:2159–2164. doi: 10.1161/01.str.25.11.2159. [DOI] [PubMed] [Google Scholar]
- Suzuki J. Moyamoya Disease. Tokyo, Japan: Springer-Verlag; 1986.
- McLean M J, Gebarski S S, Spek A F van der, Goldstein G W. Response of moyamoya disease to verapamil. Lancet. 1985;1:163–164. doi: 10.1016/s0140-6736(85)91931-2. [DOI] [PubMed] [Google Scholar]
- Spittler J F, Smektala K. Pharmacotherapy in moyamoya disease. Hokkaido Igaku Zasshi. 1990;65:235–240. [PubMed] [Google Scholar]
- Hosain S A, Hughes J T, Forem S L, Wisoff J, Fish I. Use of a calcium channel blocker (nicardipine HCl) in the treatment of childhood moyamoya disease. J Child Neurol. 1994;9:378–380. doi: 10.1177/088307389400900407. [DOI] [PubMed] [Google Scholar]
- Imaizumi T, Hayashi K, Saito K, Osawa M, Fukuyama Y. Long-term outcomes of pediatric moyamoya disease monitored to adulthood. Pediatr Neurol. 1998;18:321–325. doi: 10.1016/s0887-8994(97)00209-9. [DOI] [PubMed] [Google Scholar]
- Karasawa J, Touho H, Ohnishi H, Miyamoto S, Kikuchi H. Long-term follow-up study after extracranial-intracranial bypass surgery for anterior circulation ischemia in childhood moyamoya disease. J Neurosurg. 1992;77:84–89. doi: 10.3171/jns.1992.77.1.0084. [DOI] [PubMed] [Google Scholar]
- Isono M, Ishii K, Kamida T, Inoue R, Fujiki M, Kobayashi H. Long-term outcomes of pediatric moyamoya disease treated by encephalo-duro-arterio-synangiosis. Pediatr Neurosurg. 2002;36:14–21. doi: 10.1159/000048343. [DOI] [PubMed] [Google Scholar]
- Karasawa J, Kikuchi H, Furuse S, Kawamura J, Sakaki T. Treatment of moyamoya disease with STA-MCA anastomosis. J Neurosurg. 1978;49:679–688. doi: 10.3171/jns.1978.49.5.0679. [DOI] [PubMed] [Google Scholar]
- Matsushima Y, Fukai N, Tanaka K, et al. A new surgical treatment of moyamoya disease in children: a preliminary report. Surg Neurol. 1981;15:313–320. doi: 10.1016/s0090-3019(81)80017-1. [DOI] [PubMed] [Google Scholar]
- Houkin K, Kamiyama H, Abe H, Takahashi A, Kuroda S. Surgical therapy for adult moyamoya disease. Can surgical revascularization prevent the recurrence of intracerebral hemorrhage? Stroke. 1996;27:1342–1346. doi: 10.1161/01.str.27.8.1342. [DOI] [PubMed] [Google Scholar]
- Iwama T, Hashimoto N, Murai B N, Tsukahara T, Yonekawa Y. Intracranial rebleeding in moyamoya disease. J Clin Neurosci. 1997;4:169–170. doi: 10.1016/s0967-5868(97)90068-0. [DOI] [PubMed] [Google Scholar]
- Fujii K, Ikezaki K, Irikura K, Miyasaka Y, Fukui M. The efficacy of bypass surgery for the patients with hemorrhagic moyamoya disease. Clin Neurol Neurosurg. 1997;99:S194–S195. doi: 10.1016/s0303-8467(97)00078-4. [DOI] [PubMed] [Google Scholar]
- Okada Y, Shima T, Nishida M, Yamane K, Yamada T, Yamanaka C. Effectiveness of superficial temporal artery–middle cerebral artery anastomosis in adult moyamoya disease: cerebral hemodynamics and clinical course in ischemic and hemorrhagic varieties. Stroke. 1998;29:625–630. doi: 10.1161/01.str.29.3.625. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Yoshimoto T, Shirane R, Sakurai Y. Clinical course, surgical management, and long-term outcome of moyamoya patients with rebleeding after an episode of intracerebral hemorrhage: an extensive follow-up study. Stroke. 1999;30:2272–2276. doi: 10.1161/01.str.30.11.2272. [DOI] [PubMed] [Google Scholar]
- Kawaguchi S, Okuno S, Sakaki T. Effect of direct arterial bypass on the prevention of future stroke in patients with the hemorrhagic variety of moyamoya disease. J Neurosurg. 2000;93:397–401. doi: 10.3171/jns.2000.93.3.0397. [DOI] [PubMed] [Google Scholar]
- Miyamoto S. Study design for a prospective randomized trial of extracranial-intracranial bypass surgery for adults with moyamoya disease and hemorrhagic onset—the Japan Adult Moyamoya Trial Group. Neurol Med Chir (Tokyo) 2004;44:218–219. doi: 10.2176/nmc.44.218. [DOI] [PubMed] [Google Scholar]
- Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. The EC/IC Bypass Study Group. N Engl J Med. 1985;313:1191–200. doi: 10.1056/NEJM198511073131904. [No authors listed]. [DOI] [PubMed] [Google Scholar]
- Houkin K, Ishikawa T, Yoshimoto T, Abe H. Direct and indirect revascularization for moyamoya disease surgical techniques and peri-operative complications. Clin Neurol Neurosurg. 1997;99:S142–S145. doi: 10.1016/s0303-8467(97)00075-9. [DOI] [PubMed] [Google Scholar]
- Robertson R L, Burrows P E, Barnes P D, Robson C D, Poussaint T Y, Scott R M. Angiographic changes after pial synangiosis in childhood moyamoya disease. AJNR Am J Neuroradiol. 1997;18:837–845. [PMC free article] [PubMed] [Google Scholar]
- Houkin K, Kuroda S, Abe H. Operative record using intraoperative digital data in neurosurgery. Acta Neurochir (Wien) 2000;142:913–919. doi: 10.1007/s007010070078. [DOI] [PubMed] [Google Scholar]
- Asforsa W T, West M, McClarty B. Angiography of encephalomyosynangiosis and superficial temporal artery to middle cerebral artery anastomosis in moyamoya disease. AJNR Am J Neuroradiol. 1993;14:29–30. [PMC free article] [PubMed] [Google Scholar]
- Wanebo J E, Zabramski J M, Spetzler R F. Superficial temporal artery-to-middle cerebral artery bypass grafting for cerebral revascularization. Neurosurgery. 2004;55:395–398. discussion 398–399. doi: 10.1227/01.neu.0000129549.99061.94. [DOI] [PubMed] [Google Scholar]
- Newell D W, Vilela M D. Superficial temporal artery to middle cerebral artery bypass. Neurosurgery. 2004;54:1441–1448. discussion 1448–1449. doi: 10.1227/01.neu.0000124754.84425.48. [DOI] [PubMed] [Google Scholar]
- Henschen C. Surgical revascularization of cerebral injury of circulatory origin by means of stratification of pedunculated muscle flaps [in German] Langenbecks Arch Klin Chir Ver Dtsch Z Chir. 1950;264:392–401. [PubMed] [Google Scholar]
- Karasawa J, Kikuchi H, Furuse S, Sakaki T, Yoshida Y. A surgical treatment of “moyamoya” disease “encephalo-myo synangiosis.”. Neurol Med Chir (Tokyo) 1977;17:29–37. doi: 10.2176/nmc.17pt1.29. [DOI] [PubMed] [Google Scholar]
- Ausman J I, Moore J, Chou S N. Spontaneous cerebral revascularization in a patient with STA-MCA anastomosis. Case report. J Neurosurg. 1976;44:84–87. doi: 10.3171/jns.1976.44.1.0084. [DOI] [PubMed] [Google Scholar]
- Tubokawa T, Kikuchi M, Asano S, Ito H, Urabe M. Surgical treatment for intracranial thrombosis. Case report of “durepexia” [in Japanese] Neurol Med Chir (Tokyo) 1964;6:47–48. [Google Scholar]
- Adelson P D, Scott R M. Pial synangiosis for moyamoya syndrome in children. Pediatr Neurosurg. 1995;23:26–33. doi: 10.1159/000120932. [DOI] [PubMed] [Google Scholar]
- Scott R M, Smith J L, Robertson R L, Madsen J R, Soriano S G, Rockoff M A. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg Spine. 2004;100:142–149. doi: 10.3171/ped.2004.100.2.0142. [DOI] [PubMed] [Google Scholar]
- Kinugasa K, Mandai S, Kamata I, Sugiu K, Ohmoto T. Surgical treatment of moyamoya disease: operative technique for encephalo-duro-arterio-myo-synangiosis, its follow-up, clinical results, and angiograms. Neurosurgery. 1993;32:527–531. doi: 10.1227/00006123-199304000-00006. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Matsushima Y, Takada Y, Nariai T, Wakabayashi S, Tone O. Changes in cerebral hemodynamics following encephalo-duro-arterio-synangiosis (EDAS) in young patients with moyamoya disease. Surg Neurol. 1989;31:343–349. doi: 10.1016/0090-3019(89)90065-7. [DOI] [PubMed] [Google Scholar]
- Kinugasa K, Mandai S, Tokunaga K, et al. Ribbon enchephalo-duro-arterio-myo-synangiosis for moyamoya disease. Surg Neurol. 1994;41:455–461. doi: 10.1016/0090-3019(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Kim S K, Wang K C, Kim I O, Lee D S, Cho B K. Combined encephaloduroarteriosynangiosis and bifrontal encephalogaleo(periosteal)synangiosis in pediatric moyamoya disease. Neurosurgery. 2002;50:88–96. doi: 10.1097/00006123-200201000-00016. [DOI] [PubMed] [Google Scholar]
- Kawaguchi T, Fujita S, Hosoda K, et al. Multiple burr-hole operation for adult moyamoya disease. J Neurosurg. 1996;84:468–476. doi: 10.3171/jns.1996.84.3.0468. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Takaku A, Kodama N, Sato S. An attempt to treat cerebrovascular “Moyamoya” disease in children. Childs Brain. 1975;1:193–206. doi: 10.1159/000119568. [DOI] [PubMed] [Google Scholar]
- Yasargil M G, Yonekawa Y, Denton I, Piroth D, Benes I. Experimental intracranial transplantation of autogenic omentum majus. J Neurosurg. 1974;40:213–217. doi: 10.3171/jns.1974.40.2.0213. [DOI] [PubMed] [Google Scholar]
- Karasawa J, Kikuchi H, Kawamura J, Sakai T. Intracranial transplantation of the omentum for cerebrovascular moyamoya disease: a two-year follow-up study. Surg Neurol. 1980;14:444–449. [PubMed] [Google Scholar]
- Imaizumi T, Hashi K. A basic study on omental transplantation. Vascular endothelial cell growth factor in human omentum [in Japanese] Neurol Med Chir (Tokyo) 1991;31:839–845. doi: 10.2176/nmc.31.839. [DOI] [PubMed] [Google Scholar]
- Endo M, Kawano N, Miyaska Y, Yada K. Cranial burr hole for revascularization in moyamoya disease. J Neurosurg. 1989;71:180–185. doi: 10.3171/jns.1989.71.2.0180. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Iwabuchi T, Hori S. Cervical sympathectomy for cerebral vasospasm after aneurysm rupture. Neurol Med Chir (Tokyo) 1975;15:41–50. doi: 10.2176/nmc.15pt1.41. [DOI] [PubMed] [Google Scholar]
- Matsushima T, Inoue T, Suzuki S O, Fujii K, Fukui M, Hasuo K. Surgical treatment of moyamoya disease in pediatric patients—comparison between the results of indirect and direct revascularization procedures. Neurosurgery. 1992;31:401–405. doi: 10.1227/00006123-199209000-00003. [DOI] [PubMed] [Google Scholar]
- Kim D S, Kye D K, Cho K S, Song J U, Kang J K. Combined direct and indirect reconstructive vascular surgery on the fronto-parieto-occipital region in moyamoya disease. Clin Neurol Neurosurg. 1997;99:S137–S141. doi: 10.1016/s0303-8467(97)00072-3. [DOI] [PubMed] [Google Scholar]