ABSTRACT
The primary objective of revascularization procedures in the posterior circulation is the prevention of vertebrobasilar ischemic stroke. Specific anatomical and neurophysiologic characteristics such as posterior communicating artery size affect the susceptibility to ischemia. Current indications for revascularization include symptomatic vertebrobasilar ischemia refractory to medical therapy and ischemia caused by parent vessel occlusion as treatment for complex aneurysms. Treatment options include endovascular angioplasty and stenting, surgical endarterectomy, arterial reimplantation, extracranial-to-intracranial anastomosis, and indirect bypasses. Pretreatment studies including cerebral blood flow measurements with assessment of hemodynamic reserve can affect treatment decisions. Careful blood pressure regulation, neurophysiologic monitoring, and neuroprotective measures such as mild brain hypothermia can help minimize the risks of intervention. Microscope, microinstruments and intraoperative Doppler are routinely used. The superficial temporal artery, occipital artery, and external carotid artery can be used to augment blood flow to the superior cerebellar artery, posterior cerebral artery, posterior inferior cerebellar artery, or anterior inferior cerebellar artery. Interposition venous or arterial grafts can be used to increase length. Several published series report improvement or relief of symptoms in 60 to 100% of patients with a reduction of risk of future stroke and low complication rates.
Keywords: Vertebrobasilar ischemia, angioplasty, parent vessel occlusion, bypass, revascularization
The first successful anterior circulation microvascular extracranial-to-intracranial (EC-IC) bypass surgery was performed in 19671 and over the next 20 years several reports appeared documenting the ability of the procedure to result in long-term patency with low morbidity and mortality.2,3,4,5,6,7,8,9,10 The history of diagnosis and management of posterior circulation ischemia parallels that of anterior circulation in terms of the development of pharmacological, endovascular, and surgical options. Low complication rates and excellent patency have also been reported for posterior circulation surgical revascularization,11,12,13,14,15,16,17,18,19,20,21 as well as improvement of symptoms and regional cerebral blood flow (CBF) and a reduction of the risk of future strokes.10,22 Revascularization can be used to treat occlusive cerebrovascular disease and in conjunction with treatments for complex aneurysms that involve parent vessel occlusion. Here we outline the relevant anatomy, indications, and nonsurgical treatment options, then describe various surgical revascularization techniques.
ANATOMY OF THE POSTERIOR CIRCULATION
An abundance of collaterals accounts for the fact that indications for revascularization of the posterior circulation are rarely encountered.23 There is usually excellent collateral flow to the posterior cerebral artery (PCA) distribution from leptomeninges, posterior communicating arteries (PCoA), the superior cerebellar arteries (SCA) at the quadrigeminal plate, the anterior inferior cerebellar arteries (AICA), and the posterior inferior cerebellar arteries (PICA).24,25 The different PCA territory segments also receive collateral flow from the anterior circulation through the anterior choroidal and posterior pericallosal artery and superior temporal branches of the middle cerebral artery (MCA).26
PCoAs decrease in size in adults relative to children. The term “fetal type” is used to describe persisting large PCoAs in adults that provide most of the blood flow to the PCA. This is important because ischemic injury in patients with carotid occlusion was shown to be significantly less frequent when PCoAs were equal to or larger than 1 mm,27 and patients with large PCoAs are more tolerant to occlusions of the basilar artery and PCA P1 segment.28 In vitro studies suggest that brainstem neurons may react to ischemia differently than hemispherical cortical neurons, rendering them more susceptible to ischemic damage.29 Usually the supply to the basilar bifurcation, PCA, and SCA after upper basilar artery occlusion is through the uni- or bilateral PCoAs and not through leptomeningeal sources.28
NATURAL HISTORY AND INDICATIONS FOR VERTEBROBASILAR OCCLUSIVE DISEASE AND COMPLEX ANEURYSMS
Visual changes, motor or sensory deficits, vertigo, or cranial nerve symptoms caused by atherosclerotic occlusive cerebrovascular disease refractory to medical therapy are indications for a revascularization procedure. The risk of stroke within 5 years was estimated to be ∼35%.30 Underlying problems such as hypercoagulability, cardiac arrhythmia, blood dyscrasia, and nonatherosclerotic vascular disease such as arteritis should be acknowledged and treated. Patients who are likely to benefit from posterior circulation bypass procedures include those with abnormal CBF and metabolism studies on xenon-computed tomography (CT), abnormal CBF and metabolism measurements on positron emission tomography (PET) within the PCA territory, and those with abnormal transcranial Doppler ultrasonography flow rates. Collateral flow was generally shown to be compromised on cerebral angiography in patients failing medical therapy.
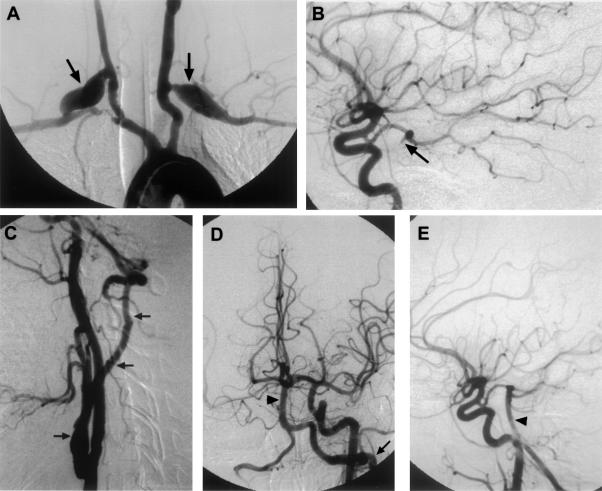
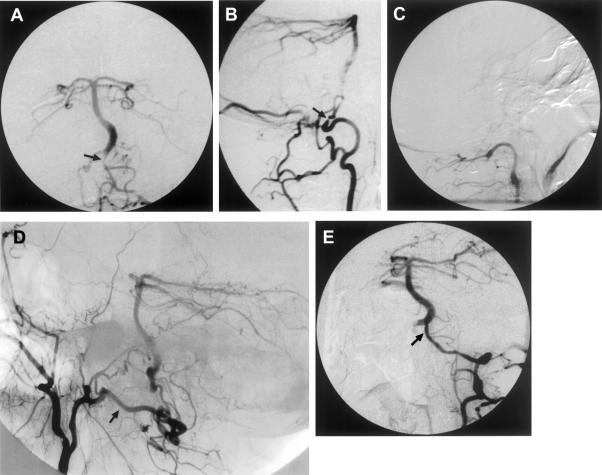
Takayasu's arteritis, a large-vessel arteritis, is characterized by mononuclear and giant cell infiltration of the adventitia and media in the acute phase, with proliferation and fibrosis of the intima and adventitia in the chronic phase causing progressive stenosis and occlusion. Medical therapeutic options include aspirin, glucosteroids, and methotrexate. Endovascular therapy was found to be effective in the majority of symptomatic patients but was associated with high long-term failure rates. Eventually the majority of patients in this study underwent surgical intervention.31 Figure 1 depicts imaging on a patient with Takayasu arteritis.
Figure 1.
This 30-year-old woman with Takayasu's arteritis underwent bilateral CCA-subclavian artery bypass graft for bilateral upper extremity ischemia. She presented later with progressive severe vertebrobasilar TIAs despite medical treatment. (A) Aortic arch angiography revealed occlusions of the left subclavian artery, right brachiocephalic artery, and both vertebral artery origins, with the bilateral CCA-subclavian artery grafts (arrows). Thyrocervical collaterals filled the cervical segment of the left vertebral artery with poor filling of the intracranial portion. (B) The posterior cerebral artery and the top part of the basilar artery (arrow) were filled retrograde through the posterior communicating artery from the left CCA. This patient underwent a left CCA-vertebral artery vein graft using an autologous saphenous vein. (C) Postoperative angiogram showed a patent graft (three arrows) with (D and E) filling of the basilar artery (arrowhead) and posterior circulation. From Stoodley et al,31 copied with permission. CCA, common carotid artery; TIAs, transcient ischemic attacks.
Moyamoya disease primarily affects the anterior circulation. The progressive stenosis of the proximal carotid and middle cerebral and anterior cerebral arteries can cause ischemic symptoms and strokes, while fragile reactive moyamoya vessels can cause hemorrhagic strokes in adults. Involvement of the posterior circulation arteries can result in vertebrobasilar ischemia. Medical and endovascular therapies have not been shown to be effective in preventing ischemic and hemorrhagic strokes. There is good evidence, however, that bypasses can decrease future risks of transient ischemic attacks (TIA) and ischemic strokes,9,32 while data suggest that bypasses may reduce the risk of recurrent hemorrhage from moyamoya vessels.33
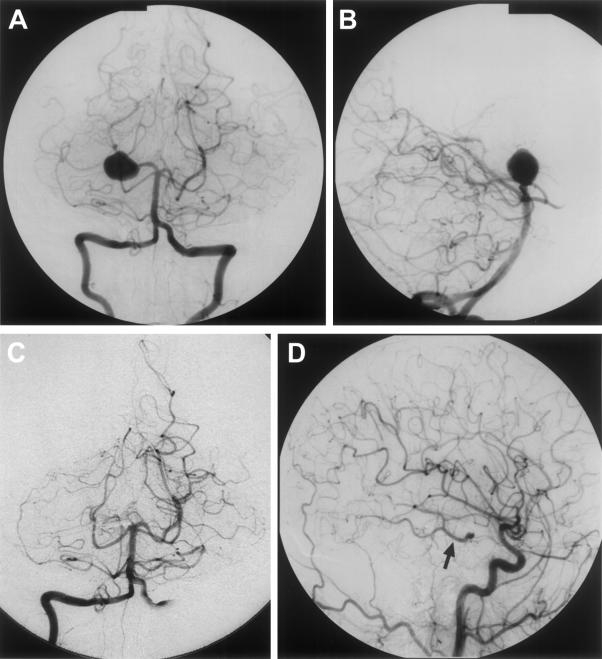
The natural history of the untreated giant and large aneurysms should be weighed against the inherent risk of permanent arterial occlusion for each patient. Spontaneous thrombosis of giant aneurysms has been reported in incidental case reports,34,35 but in general the natural history of untreated giant and fusiform aneurysms seems to be poor. In different series, 80 to 100% of patients with untreated giant posterior circulation aneurysms were severely disabled or dead within 5 years due to brainstem compression, hemorrhage, or thrombosis.36,37,38,39,40 Prior reports have shown that parent vessel occlusion can be used for aneurysms in which direct clipping or coiling is not possible.30,41 Figure 2 depicts an example of a giant P1–2 wide-based aneurysm that was treated with parent vessel occlusion. A balloon occlusion trial can be performed before treatment to determine tolerance to deliberate parent vessel occlusion.42,43 When considering proximal (Hunterian) occlusion using ligation or coiling as treatment for posterior circulation complex giant and/or fusiform aneurysms,28,44,45,46,47,48,49 it should be noted that native collaterals may already effectively provide flow to arterial territories.50 Information on collateral flow can be obtained from angiography or measures of CBF during the occlusion.51 Generally, patients that can tolerate 20 to 30 minutes of balloon occlusion without neurologic or electrophysiological changes can tolerate permanent occlusion.
Figure 2.
This is a 32-year-old woman who presented with increasing temporal headaches. (Pretreatment vertebrobasilar angiogram A and B) Imaging revealed a giant right PCA aneurysm, which was treated with a deliberate right P1 occlusion (post-treatment vertebrobasilar angiogram C and right lateral internal carotid angiogram D). Note filling of the right PCA (arrow) from the right PCoA on the carotid injection (D). At 36 months' follow-up she was neurologically intact and doing well. PCA, posterior cerebral artery; PCoA, posterior communicating artery.
Failure of a trial balloon occlusion test in cases of giant, fusiform, atherosclerotic, and partly calcified aneurysms would support a decision to perform a revascularization procedure.
Incomplete thrombosis of the aneurysm was found to increase the risk of developing late neurological complications.28 The location of the arterial occlusion was found to affect the probability of thrombosis. Occlusions closer to the aneurysm were more likely to result in complete or nearly complete thrombosis, which resulted in a change in the preferred occlusion site from extracranial (posterior triangle)28 to intracranial as close to the aneurysm as possible. Surgical occlusion may be preferable to endovascular occlusion because it allows exploration of the aneurysm neck and direct clipping, if possible. Direct visualization at surgery can guide the choice of occlusion site while avoiding perforators. Of course, endovascular treatment, which does not require craniotomy or general anesthesia, has obvious advantages. Detachable balloon occlusions had been used primarily, but currently endovascular coils are used. In a 1987 study by Fox and colleagues52 of 68 patients with unclippable aneurysms, the proximal artery was occluded using endovascular balloons. Ischemic changes occurred in 9 of 65 patients, possibly due to the inability to discern small perforating vessels on the angiogram or to balloon migration.
PRETREATMENT RADIOLOGIC EVALUATIONS
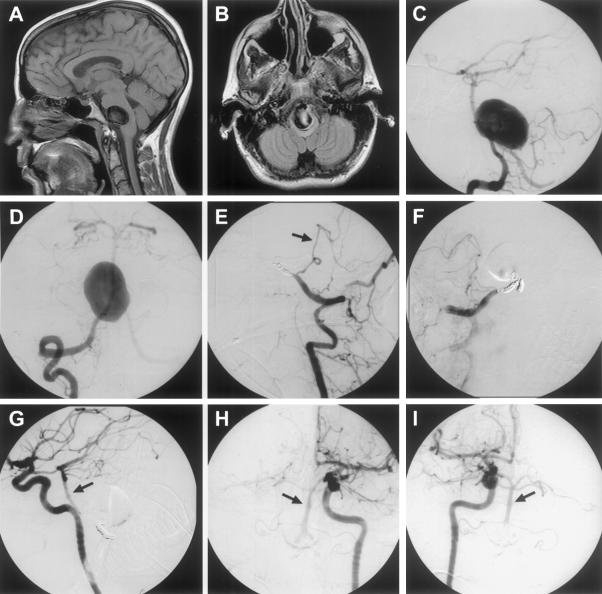
Magnetic resonance imaging, CT scans, and cerebral angiograms can reveal signs of hemodynamic insufficiency such as watershed area infarctions. Reduced collateral flow can be visualized on magnetic resonance angiography or CT angiography. Xenon CT, Xenon 133, and single photon emission computed tomography CBF studies followed by an acetazolamide challenge can identify areas of low perfusion and evaluate CBF reserve capacity. Studies focusing on the anterior circulation have shown that the optimal revascularization candidate has minimal or absent reserve.53,54,55,56 Whether these data can be extrapolated to the posterior circulation remains uncertain. Cerebral angiographic characteristics help determine eligibility for treatment modalities such as coiling (endovascular) and clipping (surgical). Angiography provides information regarding collateral flow. Figure 3 depicts an example of treatment of a giant lower basilar trunk aneurysm with bilateral vertebral artery occlusion. The Allcock test, compression of one or both carotid arteries during vertebral injection, can be used to determine the size of PCoAs.41 Additional information on the extent of thrombosis can be obtained with magnetic resonance imaging, while CT angiography can noninvasively assess the three-dimensional configuration of the aneurysm.
Figure 3.
This 37-year-old woman presented with a 2-week history of difficulty swallowing and headaches. MRI (A and B), CT angio and vertebral angiography (C and D) revealed a giant lower basilar trunk aneurysm compressing the lower pons and medulla. She tolerated trial balloon bilateral vertebral artery occlusion and underwent bilateral vertebral artery coil occlusion (lateral left vertebral artery injection E and anterior-posterior AP right vertebral artery injection F). Post-treatment carotid angiography revealed retrograde filling of: the basilar artery, bilateral anterior inferior cerebellar arteries, and both left AP carotid injection (H) and right AP carotid injection (I) distal vertebral arteries with no filling of the aneurysm (lateral left carotid injection G, left AP carotid injection H and right AP carotid injection I). The patient tolerated the bilateral permanent Vertebral artery A occlusions with no neurologic deficits. MRI, magnetic resonance imaging; CT, computed tomography; AP, anterior-posterior.
MEDICAL THERAPY
In patients with symptomatic cerebral ischemia in the posterior circulation, a search for underlying medical problems, including cardiac arrhythmia, coagulopathy, and hyperlipidemia, is essential. Current medical therapy in secondary prevention of ischemic stroke, myocardial infarction, and vascular death includes aspirin, clopidogrel (Plavix), and statins. Coumadin has been shown to be effective in reducing the risk of stroke caused by cardiogenic emboli. When symptoms are postural or exertion- or ambulation-related, indicative of a hemodynamic contribution,57 discontinuation of antihypertensives should be considered. Failure of medical therapy warrants evaluation for a possible revascularization procedure.
ENDOVASCULAR THERAPY
Endovascular techniques such as angioplasty and stenting are effective treatments of focal stenosis58 and facilitate immediate and local delivery of thrombolytic agents.27,28 Figures 4 and 5 demonstrate the angiographic results following angioplasty in patients with symptomatic vertebral and basilar artery stenosis. Absence of PCoAs bilaterally necessitated a left occipital artery (OA) to PICA bypass. The annual stroke rates under medical therapy of 8 to 11%59 were reduced to 3% by the addition of angioplasty in the Stanford series.60 Transluminal balloon angioplasty for symptomatic hemodynamically significant atherosclerotic lesions of the vertebral or basilar artery resulted in improvement of symptoms in 64 to 93% of patients.58,61 Complication rates were low for vertebral artery stenosis in the series of Higashida and colleagues (8.8% transient neurologic deficit). The small subgroup with basilar artery stenosis did worse, with one third of patients dying.58 In a mixed series with vertebral, basilar, carotid, and MCA stenosis, 17 of 27 (63%) treated patients remained symptom-free after an average of 28 months (range 1 to 56 months).62 Morphological features such as length and eccentricity of the stenosis were predictive of restenosis.63 Improved rates of procedural success in coronary arteries64 and high recurrence rates for angioplasty have led to the use of stents in conjunction with angioplasty for intracranial artery stenosis. However, a report on stent use in extracranial vertebral artery stenosis revealed an in-stent restenosis rate of 31%.65 Staging of treatment, with a delay between angioplasty and stent placement, has been proposed to reduce procedure-related risks.66 In this study, complications occurred in 3 of 8 and 1 of 5 patients treated with delayed stent placement.66
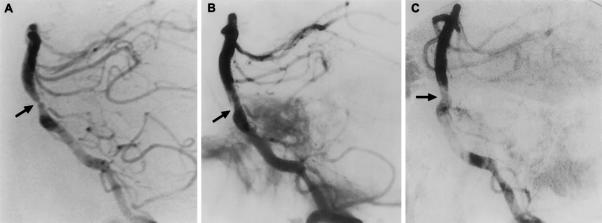
Figure 4.
This is a 63-year-old man who experienced vertebrobasilar TIAs despite therapeutic doses of warfarin. (A) The lateral projection of the right vertebral artery injection of the angiogram revealed a severe proximal basilar artery stenosis (arrow). Imaging (B) immediately after angioplasty revealed a small intimal tear which was (C) healed at 3 months with minimal residual stenosis. From Marks et al,60 copied with permission. TIAs, transient ischemic attacks.
Figure 5.
This 44-year-old man presented with persistent severe vertebrobasilar TIAs with gait unsteadiness, blackout spells, and visual disturbances despite oral anticoagulation. The cerebral angiogram revealed a high-grade left vertebral artery stenosis (arrows, A and B) with (C) the right-sided vertebral artery ending in PICA and no visible posterior communicating arteries. He underwent angioplasty of the left vertebral artery stenosis (arrow, E) and a right-sided OA (arrow) to (D) PICA revascularization procedure. His vertebrobasilar TIAs resolved. TIAs, transient ischemic attacks; PICA, posterior inferior cerebellar artery; OA, occipital artery.
SURGICAL TREATMENT
Anesthesia
Control of hemodynamics under general anesthesia during the revascularization procedure is essential in patients with already focally marginal cerebral perfusion. During the initial part of the procedure hypotension should be avoided. While the recipient vessel is clamped for anastomosis, mildly hypertensive levels are maintained to augment collateral flow. Hyperventilation and α-adrenergic agents are generally not recommended because of vasoconstrictive effects. Scalp infiltration solutions should not contain vasoconstrictive agents. The patient is positioned with head above heart level to reduce cerebral venous congestion. General management includes the prophylactic use of antibiotics, Decadron, H2 blockers and sequential compression devices. If significant brain retraction is required, lumbar cerebrospinal fluid (CSF) drainage, mannitol, and furosemide can be used. On completion of the procedure patients should be extubated and narcotics and paralytics reversed as quickly as possible to enable neurologic evaluation.
Monitoring
Electrophysiologic monitoring has been shown to be useful and effective in improving results of aneurysm clipping and vascular malformation resection.67,68,69 In revascularization procedures it permits early detection of ischemia by assessing somatosensory and motor-evoked potentials and brainstem auditory-evoked potentials. Bilateral upper and lower somatosensory-evoked potentials and brainstem-evoked potentials are also used in evaluating endovascular Hunterian ligation in posterior circulation aneurysms. Monitoring provides early detection of excessive retraction and manipulation as well as early detection of ischemia.
Hypothermia
Mild brain hypothermia was first found to protect against experimental ischemic and traumatic brain injury70 and is currently used during neurosurgical procedures.71,72 Core body temperature of 32 to 33°C can be obtained using surface methods, such as cooling blankets, or parenteral methods, such as via an endovascular catheter cooling system. A recent study comparing endovascular to surface cooling revealed more rapid cooling and warming for the endovascular group.73 Of the obese patients (body mass index > 30 kg/m2) only 5% of patients treated with surface cooling reached target temperature before clip placement, while 96% of obese patients treated with endovascular cooling did.73 Delayed warming following the surface cooling method can result in longer anesthesia recovery time74 as well as increased postoperative bleeding rates,75 surgical wound infection,76 and length of hospital stay,75 so the ability to produce a controlled, rapid increase to normal body temperature may be a valuable feature of any cooling system. Endovascular cooling to 33°C is routinely used for our intracranial surgical revascularization procedures.
Barbiturates
Multiple neuroprotective effects of barbiturates have been described, and are mediated through reduction of cerebral metabolic rate of oxygen consumption (CMRO2), scavenging of free radicals, blood flow redistribution, and membrane stabilization. We utilize barbiturate-induced burst suppression on the electroencephalogram (EEG) during temporary occlusion of major extracranial and intracranial arteries.
SURGICAL TECHNIQUES
Extracranial Reconstructions
Reimplantation (transposition) of the vertebral artery on the common carotid artery, vertebral-to-carotid bypass, and vertebral endarterectomy are extracranial revascularization options.77,78 For treatment of proximal vertebral artery stenosis, reimplantation of the vertebral artery on the common carotid is used most commonly (Fig. 6). The vertebral artery is exposed through supraclavicular incision, which frequently requires division of the sternocleidomastoid, sternohyoid, and sternothyroid muscles. The common carotid artery is followed proximally to either the left subclavian artery or the right brachiocephalic artery, after which the vertebral artery can be identified. For the left vertebral artery, the thoracic duct is ligated and divided. The proximal end of the vertebral artery is ligated while the distal end is shaped for anastomosis. An arteriotomy is made on the common carotid artery and the anastomosis is performed using 6–0 or 7–0 Prolene sutures (Ethicon Johnson and Johnson, Somerville, NJ).
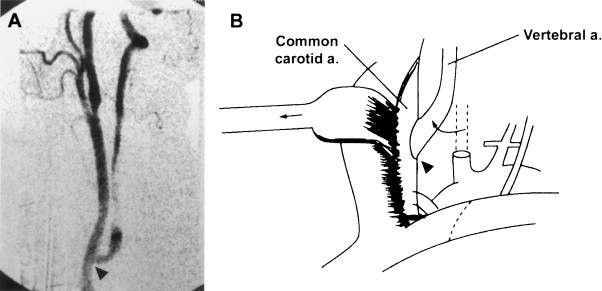
Figure 6.
Reimplantation (transposition) of the vertebral artery into the common carotid artery is depicted on (A, arrowhead) postoperative angiography and (B) schematically. From Stoodley et al,78 reprinted with permission.
Vertebral artery stenosis distal to the origin, which is not amenable to direct endarterectomy, can be treated using a carotid-to-vertebral vein or synthetic graft bypass (Fig. 1). The common carotid artery is exposed through an incision along the anterior border of the sternocleidomastoid muscle with the patient in supine position, the head rotated to the contralateral side. The vertebral artery is exposed through an incision over C1. While the carotid is cross-clamped, an end-to-side anastomosis is performed using 6–0 Prolene. The graft is passed deep to the sternocleidomastoid muscle and an end-to-side anastomosis is made between the graft and the vertebral artery using 7–0 to 8–0 Prolene. A lateral position with rotated head or prone position is used for more distal cervical vertebral artery stenosis, identifying the vertebral artery at C1.
Focal vertebral artery stenosis can occasionally be treated with endarterectomy. The incision is located in the supraclavicular region and is parallel to and above the clavicle. After division of the sternocleidomastoid muscle and identification of the subclavian vein and thoracic duct on the left side, the scalene muscle is divided while avoiding damage to the phrenic nerve. Following back the subclavian artery, the thyrocervical trunk is identified first, distal to the vertebral artery. After careful dissection of the sympathic chain and stellate ganglion, the vertebral artery is circumferentially dissected. For proximal control the subclavian artery should also be dissected. After the subclavian artery is cross-clamped and the thyrocervical trunk, vertebral artery, and internal thoracic artery are clipped with temporary aneurysm clips, a curvilinear incision is made in the subclavian artery near the origin of the vertebral artery. Removal of the plaque using a dissector is facilitated by a second incision in the vertebral artery 1 cm distal to its origin. Intimal flaps must be tacked down. Temporary release of the vertebral artery clip will allow verification of back flow. The vertebral artery is closed with 7–0 Prolene sutures and saphenous vein or Hemashield (Boston Scientific Corporation, Natick, MA) for patching. The proximal subclavian clamp is removed first followed by the distal clamp, the internal thoracic artery clip, thyrocervical trunk clip, and the vertebral artery clip. After reapproximation of the scalene and sternocleiomastoid muscles, the wound is closed in layers. Postoperative measures include blood pressure control and long-term aspirin. Care should be taken to prevent compression of the graft caused by wound dressing. Complications include damage to the phrenic nerve, the thoracic duct, internal jugular vein, subclavian artery, thyrocervical trunk, and internal thoracic artery.
Intracranial Reconstructions
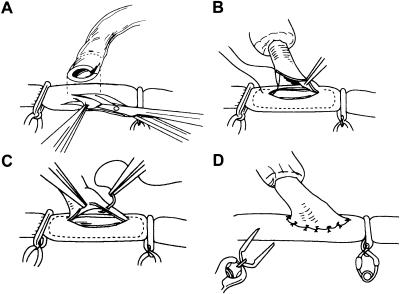
Doppler ultrasonography is often used to identify donor vessels. Microinstruments and microscope are used routinely for all intracranial and some extracranial revascularization procedures. Selection of donor and recipient vessels is based on size, location, and atherosclerosis. Diluted papaverine is used to prevent vasospasm. The recipient vessel is exposed over a 6- to 10-mm segment. After placement of a high-visibility background, a temporary clip is placed on the donor vessel. The donor vessel is ligated and divided at length. To verify good flow, the temporary clip on the donor vessels is temporarily opened. After flushing with heparinized saline the distal tip is stripped of adventitia and cut obliquely. A proximal and distal microvascular clip (Accurate Surgical and Scientific Instruments Corporations, Westbury, NY) or temporary aneurysm clips are placed and an elliptic or teardrop-shaped arteriotomy is made (Fig. 7A) in the superior wall of the vessel. The recipient vessel is irrigated with heparanized saline. The anastomosis is performed using a neuromicroscope with 10–0 or 9–0 interrupted or running monofilament sutures. First, corner stitches are placed (Fig. 7B), followed by the far wall and the near wall (Fig. 7C and 7D)78a. With each interrupted stitch the intimal layer should be included but narrowing should be avoided. Temporary clips are removed and the need for additional stitches assessed. Slight leakage of blood can be treated with Gelfoam (Pharmacia and Upjohn, Kalamazoo MI) or Surgicell (Johnson and Johnson, New Brunswick, NJ). Doppler ultrasonography can be used to test patency while quantitative and directional Doppler ultrasound (Charbel Micro-flowprobe: Transonic Systems, Ithaca, NY) can be useful in the assessment of flow through donor and recipient vessels before and after the bypass. The dura is closed loosely around the graft to avoid compression or kinking. The bone flap is also adjusted to avoid compression on the graft.
Figure 7.
After placement of vascular clips on both sides of the anastosmosis, the (A) elliptic arteriotomy is performed in the recipient vessel using microscissors. The donor vessel is cut in a fish-mouth manner to facilitate anastomosis. (B) The two end sutures are placed first, followed by (C) interrupted sutures along the back and front wall of the anastomosis. (D) The vascular clips are removed on completion of the anastomosis. From Chang and Steinberg,78a reprinted with permission.
Hemodynamic control is of great importance in postoperative management. Postoperative hypertension can cause excessive bleeding at the anastomosis site or intraparenchymal hemorrhage from hyperperfusion and should thus be avoided. On the other hand, hypotension could result in graft occlusion and ischemia, necessitating emergent angiography and reoperation. Antiplatelet therapy (aspirin, 325 mg) is routinely used on a long-term basis. CSF leakage from loose dural closure is also a potential postoperative problem.
Interposition Grafts
If the donor artery is too short a venous or arterial graft can be used. The blood flow demand limits use of radial artery grafts to moderate flows of 40 to 70 mL/min while venous graft flows can go up to 60 to 80 mL/min.79 In arterial grafts, in contrast to venous grafts, flow can be compromised by vasospasm. The obvious drawback of interposition grafts is the need to create two anastomosis sites.
The saphenous vein is most commonly used as a venous graft. It is located proximal to the medial malleolus and dissection is continued proximally. Small side branches should be carefully ligated with 4–0 or 5–0 silk or Prolene. Once the appropriate length is achieved the vein is usually left in continuity until just before the anastomosis to minimize the risk of injuring the vessel. Prolene 6–0 is used to mark alignment of the vessel. Harvesting of the venous graft is preceded by proximal and distal ligation with 4–0 silk. The venous graft is irrigated with and stored in heparinized saline (10 U heparin per mL saline).
In short-vein interposition grafts between the proximal superficial temporal artery (STA) or OA and PCA or SCA branches, the proximal anastomosis is performed first. Optimizing length of the graft will prevent kinking. The arteriotomy is made in the donor vessel and 10–0 or 9–0 monofilament sutures are used to anchor the corner, followed by the back and front walls. The distal anastomosis proceeds similarly to the direct arterial graft. In long-vein grafts to SCA or PCA branches, the distal anastomosis is performed first. The vein is then tunneled using a 20-French catheter. The proximal anastomosis is performed end-to-side using a 6–0 or 7–0 monofilament suture. The need for additional stitches, Gelfoam, or Surgicel is assessed after removal of the temporary clips.
The radial artery can be used as an interposition graft when the hand is adequately perfused during compression of the radial artery (Allen test). A linear lateral ventral incision is made in the wrist between the flexor carpi radialis tendons and brachioradialis muscle with extension distally depending on the length needed. The proximal and distal anastomoses are usually performed using 7–0 and 8–0 monofilament sutures, respectively.
Reimplantation (Transposition) of the Vertebral Artery
This procedure is also used for treatment of proximal vertebral artery stenosis. Vertebral artery reimplantation is depicted schematically and on angiogram in Figure 6. The supraclavicular incision is extended along the superior border of the sternocleidomastoid muscle. The origin of the vertebral artery is identified following the subclavian artery retrograde. As for carotid endarterectomy, the common carotid artery is freed circumferentially avoiding the vagus and phrenic nerves. If collateral circulation is insufficient, a shunt should be prepared. A temporary aneurysm clip is placed on the distal vertebral artery and the proximal vertebral artery is occluded permanently using Weck clips. Proximally on the carotid artery a clamp or tourniquet is used, depending on the necessity of a shunt. To accommodate an end-to-side anastomosis using a 7–0 monofilament suture, a 4- to 5-mm arteriotomy is made in the carotid artery. Saphenous vein or Hemashield grafts can be used to correct for insufficient length. Subintimal flaps have to be tacked down carefully. Backflow from the distal common carotid can minimize air emboli. Heparinized saline irrigation is used before placement of final sutures. Wound closure includes reconstruction of previously divided scalene and sternocleidomastoid muscles. Doppler ultrasonography can confirm patency while quantitative directional Doppler ultrasound and angiography give more detailed estimates of flow.
The transverse foramen can be removed with additional osteophyte if necessary to relieve stenosis of the vertebral artery in the second segement, extending from the transverse foramen of C6 to the anterior rim of C1. The skin incision follows the anterior border of the sternocleidomastoid muscle. The trachea and esophagus are retracted medially and the common carotid laterally to expose the anterior cervical spine. Injury must be avoided to the jugular vein, and vagus, hypoglossal, and accessory nerves. Fluoroscopy is used in the lateral plane to confirm the correct level. The ipsilateral longus coli muscle is dissected off the anterior spine. Care is taken to avoid injury to the sympathetic chain to prevent Horner's syndrome. Curets and a drill are used to unroof the neuroforamen and decompress the vertebral artery. To enable removal of medial osteophytes, the artery is gently retracted laterally. If a focal area of stenosis is the result of atherosclerosis an endarterectomy can be performed. Alternatively, the vertebral artery can be reimplanted into the ipsilateral common carotid either directly or using a venous interposition graft. If an external carotid artery (ECA) branch is available a direct anastomosis with the cervical vertebral artery may be possible.
CONSIDERING EXTRACRANIAL AND INTRACRANIAL TREATMENT OPTIONS
The location of the stenosis or occlusion, the symptoms of the patient, and the experience of the surgeon will determine whether revascularization will be extra- or intracranial. Revascularization should generally be focused on the source of the hemodynamic insufficiency, with endarterectomy or vertebral artery reimplantation for focal stenosis as examples. Intracranial procedures carry a small risk of complications such as intracranial hematoma, hygromas, meningitis, and brain injury due to retraction.
Temporary occlusion of intracranial arteries in both intra- and extracranial procedures may cause ischemia or stroke in the territory of the occluded vessel.
INTRACRANIAL SURGICAL OPTIONS
STA to PCA
Revascularization of the distal PCA territory is controversial because of the excellent native collateral flow to the occipital lobe and the small size of recipient arteries. Occlusion of the P2 segment or PCA trapping for peripheral PCA aneurysm resulted in acquired visual field deficit in a small number of patients.80 Development of more severe deficits after deliberate PCA occlusion has led to the use of a revascularization procedure combined with Hunterian ligation.22 The patient is positioned supine with head turned to the contralateral side fixed in a three-point Mayfield headrest. Doppler ultrasonography is used to mark the course of the STA. The skin incision is made directly superficial to the STA or OA, after which the artery is carefully dissected out while leaving a generous layer of adventitia surrounding the artery to avoid damage. The length of the graft is determined by the planned anastomosis site and by whether a short venous interposition graft is used. A small temporal craniotomy is performed flush with the middle fossa floor. The dura mater is opened and the gentle subtemporal retraction is used to identify the recipient PCA. Dissection of the arachnoid results in exposure of the perimesencephalic PCA over 10- to 15-mm lengths to attain direct anastomosis. Using a more peripheral cortical PCA branch will reduce the required length of the donor vessel.
STA to SCA
For this procedure, positioning is similar to that of the STA-PCA bypass. After gentle and limited retraction of the ipsilateral temporal lobe and division of the tentorium, a 10- to 15-mm portion of the perimesencephalic SCA is exposed. The SCA can often be reached more easily than the PCA because of increased mobility. A direct STA-SCA anastomosis is performed as described previously. In a 1990 series of 49 patients with STA-SCA bypasses, a high (98%) patency rate was reported. Resolution of ischemic symptoms occurred in 70% while 8% had recurrent symptoms despite patent grafts.14
External Carotid (EC) to PCA and SCA Bypass Using Venous Interposition Graft
If an STA-PCA/SCA bypass is expected to provide insufficient flow, an external carotid (EC)-PCA/SCA bypass using a venous interposition graft can be used. An example of an EC-PCA bypass is depicted in Figure 8. To allow adequate exposure of the EC, the patient is positioned supine with head turned to the contralateral side and slightly extended. A standard linear carotid incision is used to expose the carotid bifurcation. A subtemporal craniectomy similar to that for STA-PCA bypasses is made and the PCA is exposed over a 10- to 15-mm segment. A long saphenous vein graft is harvested. First the proximal anastomosis to the ECA is performed. The arteriotomy on the PCA should be adjusted for the larger venous graft size. Care should be taken to prevent vein thrombosis or graft occlusion. Drawbacks of this surgery are similar to the ECA-PCA with venous interposition including longer operative time (two-anastomosis) and lower patency rates. An example of an EC-PCA is depicted in Figure 8.
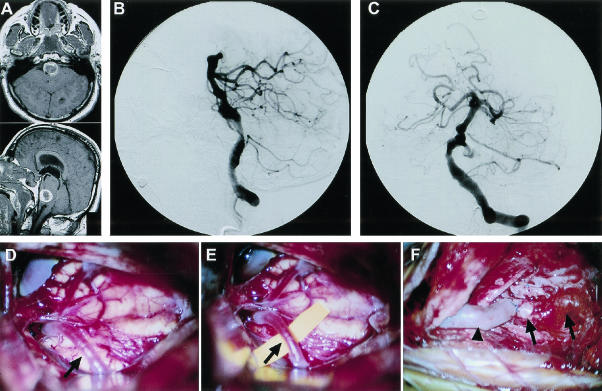
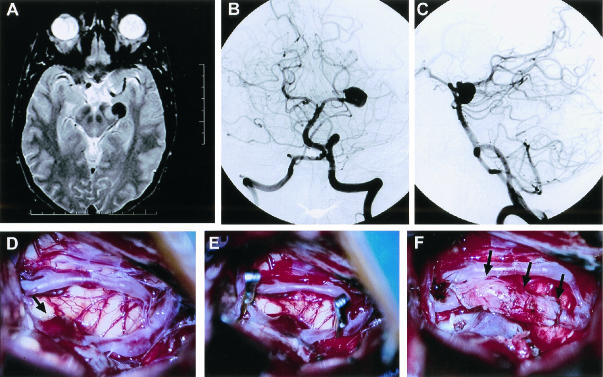
Figure 8.
This 61-year-old man presented with a subarachnoid hemorrhage in poor condition (Hunt and Hess grade 4). MR scan and vertebrobasilar cerebral angiogram revealed a large left vertebrobasilar junction aneurysm (A–C). Trial balloon occlusion of the left vertebral artery was not tolerated. Treatment included a right-sided EC-PCA saphenous vein bypass and deliberate left vertebral artery occlusion using GDC coils. Intraoperative photographs (D-F) depict the PCA (arrows) and the EC-PCA (arrowhead) vein bypass. Unfortunately, the patient did not recover from his initial subarachnoid hemorrhage and died several months later. MR, magnetic resonance; EC, external carotid; PCA, posterior cerebral artery; GDC, Guglielmi detachable coils.
OA to PICA or AICA
The OA can be used as an alternative donor vessel (Fig. 5). The patient is positioned in one of the standard suboccipital approach positions (sitting, lateral oblique, semi-sitting, supine with shoulder roll, prone). A U-shaped or curved, hockey stick-shaped incision is made and the OA is dissected from the undersurface of the skin flap. After a suboccipital craniotomy and C1 laminectomy are performed, the dura mater is opened linearly and PICA identified on the vermal surface. The anastomosis is performed using 9–0 or 10–0 monofilament suture. To avoid compression on the graft and the inherent risks of CSF leakage and pseudomeningoceles, the dura mater cannot be closed tightly. For the OA-AICA anastomosis, the AICA is identified coming off the vertebral artery after a small occipital craniotomy, and the OA is dissected out as previously described. The anastomosis is performed using a 9–0 or 10–0 monofilament suture.
In a 46-patient series of OA-PICA bypasses, excellent results were obtained in 83% of cases with no morbidity or mortality.80a,80b These results were recently confirmed, with 83% of patients improving and 48% achieving normal function after revascularization.14 In this study, patency rate was 73% for the OA-PICA bypass, and 53% of patients had complete relief of symptoms. For OA-AICA bypass, excellent results have also been reported12,13 including angiographic patency in 94% and complete symptom relief in 70%.14 Data on long-term outcome have revealed more failures with recurrence of symptoms.81
PICA to PICA
This bypass procedure is mostly used in the treatment of fusiform aneurysms or stenosis of PICA that are not amenable to endovascular treatment (angioplasty). Both PICAs are identified through a midline suboccipital exposure with C1 laminectomy. A side-to-side anastomosis is performed between the two arteries distal to the stenosis. On completion of the bypass the proximally located fusiform PICA aneurysm can be treated with trapping.
Reanastomosis (Using an Interposition Graft)
In the posterior circulation aneurysms can sometimes be treated with excision followed by anastomosis of the proximal and distal ends. An interposition venous or arterial graft can be used if necessary to allow for reconstruction without tension. An example of a PCA-PCA bypass using an autologous saphenous vein graft is depicted in Figure 9.
Figure 9.
This 32-year-old man first presented with a 6-week history of throbbing headaches. (A) An MR scan revealed a large aneurysm involving the left PCA. Vertebrobasilar angiography confirmed the presence of a 1.5-cm fusiform left P2 aneurysm (B and C). Surgical therapy included (F, three arrows) a PCA-PCA bypass using an autologous saphenous vein graft and (D, arrow) resection of the aneurysm after (E) placement of temporary clips. The patient experienced mild transient right-sided weakness and third nerve paresis, but made a complete recovery in 1 month and is doing well, working full time at a 61-month follow-up.
Intracranial Vertebral Endarterectomy
Atherosclerosis in the posterior circulation is not a rare phenomenon. In contrast to carotid disease, however, stenoses are often more generalized. Beneficial effects have been documented82,83 in smaller series. Exposure of the vertebral artery should extend beyond the plaque. During temporary occlusion cerebroprotective measures such as hypothermia, barbiturates, and induced hypertension help reduce risk of ischemic damage.
Indirect Revascularization
Indirect revascularization procedures have been devised for the treatment of moyamoya disease.77 Encephalomyosynangiosis (EMS), encephaloduroarteriosynangiosis (EDAS), and omentum transpositions are most commonly used for revascularization of the anterior circulation and rarely for PCA territory. In EDAS an intact arterial pedicle is placed directly on the brain. Whether thick arachnoid adhesions covering the brain should be opened remains controversial. In weeks to months small vascular branches will penetrate and supply the underlying cerebral cortex. The OA or STA can be used for this type of indirect revascularization in the posterior circulation. This procedure can be used when there is no adequately sized recipient vessel within reach and is technically easier. The delay in revascularization by the indirect graft seems to be an obvious disadvantage. However, there may be some advantage to the more gradual reversal of flow direction, such as avoiding opposition of residual anterograde and new retrograde flow, and hyperperfusion. Neovascularization may not occur in adults treated with indirect grafts.
In EMS, vascularized muscle is placed on the cerebral cortex to provide a new blood supply. Since muscle is scarce in the occipital region this technique in rarely used.
Omental transposition is a technique in which omentum is either tunneled subcutaneously on a pedicle to the cranium or is dissected and used as a free graft. The omentum is placed on the brain surface. As with EDAS and EMS the benefit of this indirect technique is delayed by weeks to months.
CLINICAL RESULTS OF EXTRACRANIAL TECHNIQUES
Clinical series on vertebral artery reimplantation have reported good results. In the series of 32 patients of Anson and Spetzler,11 preoperative symptoms resolved in 84% of cases. Diaz and colleagues,17 in their 1984 series of 107 patients, revealed improvement of symptoms in 94%. More series have confirmed excellent results for this procedure.11,15,16,18,19 Carotid-to-C1 segment vertebral artery bypass resulted in relief of ischemic symptoms in 63 to 71% with surgical morbidity of 4 to 7% and mortality of 1 to 2%.16,19 Edwards and Mulherin18 reported good results in 32 patients with minimal morbidity and mortality. Midcervical vertebral artery decompressions have yielded good results in several small series.84,85 Midvertebral endarterectomy followed by reimplantation or transposition with or without venous interposition graft resulted in relief of symptoms in all patients.80a
CLINICAL RESULTS OF INTRACRANIAL TECHNIQUES
In the group of patients stable without medication, 75% improved with direct STA-PCA and SCA bypasses while 59% of the group requiring anticoagulation therapy improved.86 Patency was confirmed in 48 of 49 patients in a follow-up study. Review of a series of 86 bypasses by Hopkins and Bundy81 revealed a 79% patency rate with 14% mortality. In this study 20% of patients had a complication that was classified as serious, including hydrocephalus, meningitis, stroke, pneumonia, cardiac dysrhythmias, hematomas, midbrain contusions, and death. A mortality rate of 23% was reported by El-Fiki and associates80b in a series of 13 STA-SCA bypasses. ECA-PCA and -SCA bypass series include the large 132-patient series from Sundt et al87 which reported good results in 68% of patients with 15% mortality. In a later series from the same institution, Regli and colleagues88 demonstrated overall patency rates of 86% at 1 year to 82% at 5 years and 73% at 13 years. This resulted in a yearly failure rate of 1 to 1.5%. The OA-AICA bypass is less frequently performed than the other revascularization procedures. Relief of vertebrobasilar ischemic symptoms was obtained in 83% of patients in a series of 18 patients by Ausman and coworkers13 in 1981. There was one death and three recurrences of symptoms in that study. Patency overall was 94%. Success rate for OA-PICA was 7 of 9 (78%) initially86 and 53% at follow-up.14 Hopkins and Bundy's series81 of posterior circulation bypasses revealed a patency rate of 91% for OA-PICA bypasses with 3% mortality and complications in 22%. In Sundt and Piepgras's study89 on 39 patients, improvement of symptoms was obtained in 46% and relief in 38%. Roski and colleagues90 had successful bypasses in all 14 of their patients with a 100% patency rate. In a series of 76 OA-PICA bypasses Hopkins and Bundy81 noted 91% patency and 4% mortality with 10% of patients having serious complications.
COMPLICATIONS
Potential complications include restenosis, graft occlusion, wound bed hematomas, and emboli. Angiography can usually help identify kinking or the location of stenosis. Post-treatment hypertension may cause parenchymal hemorrhage or cerebral edema and leakage at the anastomosis resulting in sub- or epidural hematoma. Loose dural closure can cause CSF leaking, pseudomeningocele, and subdural hygroma. Scalp ischemia caused by STA or OA diversion is extremely unusual. General postoperative complications include epilepsy, wound, urinary or respiratory tract infection, deep venous thrombosis, and pulmonary embolism.
ACKNOWLEDGMENTS
The authors thank Ms. Beth Hoyte for preparation of the illustrations and David Schaal, Ph.D., for his editorial comments.
REFERENCES
- Yasargil M G, Krayenbuhl H A, Jacobson J H. Microneurosurgical arterial reconstruction. Surgery. 1970;67:221–233. [PubMed] [Google Scholar]
- Chater N. Neurosurgical extracranial-intracranial bypass for stroke: with 400 cases. Neurol Res. 1983;5:1–9. doi: 10.1080/01616412.1983.11739637. [DOI] [PubMed] [Google Scholar]
- Collice M, Arean O, Eermino F, Riva M. In: Spetzler RF, Caster LP, Selman WR, Martin N, editor. Cerebral Revascularization for Stroke. New York, NY: Thieme-Stratton; 1985. Surgical and long term results in 100 consecutive patients with ICA occlusion. p. 445.
- Eguchi T, Mayanagi Y, Hanamura T, et al. Treatment of bilateral spontaneous carotid-cavernous fistula by Hamby's method combined with an extracranial-intracranial bypass procedure. Neurosurgery. 1982;11:706–711. doi: 10.1227/00006123-198211000-00018. [DOI] [PubMed] [Google Scholar]
- Gagliardi R, Benvenuti L, Onesti S. In: Spetzler RF, Caster LP, Selman WR, Martin N, editor. Cerebral Revascularization for Stroke. New York, NY: Thieme-Stratton; 1985. Seven years experience with extracranial-intracranial arterial bypass for cerebral ischemia. p. 413.
- Gratzl O, Roun J. In: Spetzler RF, Caster LP, Selman WR, Martin N, editor. Cerebral Revascularization for Stroke. New York, NY: Thieme-Stratton; 1985. Quality grading of bypass surgery. Operative combined risks less than 4 percentm. p. 467.
- Reale F, Benericettti E, Benvenuti L, et al. Extra–intracranial arterial bypass in typical carotid reversible ischaemic deficits: long-term follow-up in 100 patients. Neurol Res. 1984;6:113–114. doi: 10.1080/01616412.1984.11739673. [DOI] [PubMed] [Google Scholar]
- Reichman O H. Complications of cerebral revascularization. Clin Neurosurg. 1976;23:318–335. doi: 10.1093/neurosurgery/23.cn_suppl_1.318. [DOI] [PubMed] [Google Scholar]
- Sundt T M, Jr, Whisnant J R, Fode N C, Piepgras D G, Houser O W. Results, complications, and follow-up of 415 bypass operations for occlusive disease of the carotid system. Mayo Clin Proc. 1985;60:230–240. doi: 10.1016/s0025-6196(12)60315-2. [DOI] [PubMed] [Google Scholar]
- Yasargil M G, Yonekawa Y. Results of microsurgical extra–intracranial arterial bypass in the treatment of cerebral ischemia. Neurosurgery. 1977;1:22–24. doi: 10.1227/00006123-197707000-00005. [DOI] [PubMed] [Google Scholar]
- Anson J A, Spetzler R F. In: Carter LP, Spetzler RF, Hamilton MG, editor. Neurovascular Surgery. New York, NY: McGraw-Hill Inc; 1995. Surgery for vertebrobasilar insufficiency: extracranial. pp. 383–404.
- Ausman J I, Diaz F G, de los Reyes R A, et al. Posterior circulation revascularization. Superficial temporal artery to superior cerebellar artery anastomosis. J Neurosurg. 1982;56:766–776. doi: 10.3171/jns.1982.56.6.0766. [DOI] [PubMed] [Google Scholar]
- Ausman J I, Pak H, Patel S, Boulos R. Superficial temporal to proximal superior cerebellar artery anastomosis for basilar artery stenosis. Neurosurgery. 1981;9:56–60. doi: 10.1227/00006123-198107000-00009. [DOI] [PubMed] [Google Scholar]
- Ausman J I, Diaz F G, Vacca D F, Sadasivan B. Superficial temporal and occipital artery bypass pedicles to superior, anterior inferior, and posterior inferior cerebellar arteries for vertebrobasilar insufficiency. J Neurosurg. 1990;72:554–558. doi: 10.3171/jns.1990.72.4.0554. [DOI] [PubMed] [Google Scholar]
- Berguer R, Feldman A J. Surgical reconstruction of the vertebral artery. Surgery. 1983;93:670–675. [PubMed] [Google Scholar]
- Branchereau A, Magnan P E. Results of vertebral artery reconstruction. J Cardiovasc Surg (Torino) 1990;31:320–326. [PubMed] [Google Scholar]
- Diaz F G, Ausman J I, de los Reyes R A, et al. Surgical reconstruction of the proximal vertebral artery. J Neurosurg. 1984;61:874–881. doi: 10.3171/jns.1984.61.5.0874. [DOI] [PubMed] [Google Scholar]
- Edwards W H, Mulherin J L. The surgical approach to significant stenosis of the vertebral and subclavian arteries. Surgery. 1980;87:20–28. [PubMed] [Google Scholar]
- Habozit B. Vertebral artery reconstruction: results in 106 patients. Ann Vasc Surg. 1991;5:61–65. doi: 10.1007/BF02021780. [DOI] [PubMed] [Google Scholar]
- Sundt T M, Jr, Piepgras D G. Occipital to posterior inferior cerebellar artery bypass surgery. J Neurosurg. 1978;48:916–928. doi: 10.3171/jns.1978.48.6.0916. [DOI] [PubMed] [Google Scholar]
- Sundt T M, Jr, Piepgras D G, Fode N C. In: Meyer FB, editor. Sundt's Occlusive Cerebrovascular Disease. Philadelphia, PA: WB Saunders Co; Philadelphia:PA; 1994. Techniques, results and complications of occipital and temporal artery bypass pedicles to branches of the vertebral and basilar arteries. pp. 456–465.
- Vishteh A G, Smith K A, McDougall C G, Spetzler R G. Distal posterior cerebral artery revascularization in multimodality management of complex peripheral posterior cerebral artery aneurysms: technical case report. Neurosurgery. 1998;43:166–170. doi: 10.1097/00006123-199807000-00114. [DOI] [PubMed] [Google Scholar]
- Drake C G, Peerless S J, Ferguson G G. Hunterian proximal arterial occlusion for giant aneurysms of the carotid circulation. J Neurosurg. 1994;81:656–665. doi: 10.3171/jns.1994.81.5.0656. [DOI] [PubMed] [Google Scholar]
- de Oliveira E, Tedeschi H, Rhoton A, Peace D A. In: Carter LP, Spetzler RF, Hamilton MG, editor. Neurovascular Surgery. New York, NY: McGraw-Hill Inc; 1995. Microsurgical anatomy of the posterior circulation: vertebral and basilar arteries. pp. 25–34.
- Lister J R, Rhoton A L. Microsurgical anatomy of the posterior inferior cerebellar artery. Neurosurgery. 1982;10:170–199. [PubMed] [Google Scholar]
- Ciceri E F, Kluezmik R P, Grossman R G, Rose G E, Mawad M E. Aneurysms of the posterior cerebral artery: classification and endovascular treatment. AJNR Am J Neuroradiol. 2001;22:27–34. [PMC free article] [PubMed] [Google Scholar]
- Schomer D F, Marks M P, Steinberg G K, et al. The anatomy of the posterior communicating artery as a risk factor for ischemic cerebral infarction. N Engl J Med. 1994;330:1565–1570. doi: 10.1056/NEJM199406023302204. [DOI] [PubMed] [Google Scholar]
- Steinberg G K, Drake C G, Peerless S J. Deliberate basilar or vertebral artery occlusion in the treatment of intracranial aneurysms: immediate results and long-term outcome in 201 patients. J Neurosurg. 1993;79:161–173. doi: 10.3171/jns.1993.79.2.0161. [DOI] [PubMed] [Google Scholar]
- Donnelly D F, Jiang C, Haddad G G. Comparative responses of brain stem and hippocampal neurons to O2 deprivation: in vitro intracellular studies. Am J Physiol. 1992;262:L549–L554. doi: 10.1152/ajplung.1992.262.5.L549. [DOI] [PubMed] [Google Scholar]
- Cartlidge N EF, Whisnant J P, Evelback L R. Carotid and vertebral basilar transient cerebral ischemic attacks. Mayo Clin Proc. 1977;52:117–120. [PubMed] [Google Scholar]
- Stoodley M A, Thompson R C, Mitchell R S, Marks M P, Steinberg G K. Neurosurgical and neuroendovascular management of Takayasu's arteritis. Neurosurgery. 2000;46:841–852. doi: 10.1097/00006123-200004000-00014. [DOI] [PubMed] [Google Scholar]
- Golby A J, Marks M P, Thompson R C, Steinberg G K. Direct and combined revascularization in pediatric moyamoya disease. Neurosurgery. 1999;45:50–60. doi: 10.1097/00006123-199907000-00013. [DOI] [PubMed] [Google Scholar]
- Kawaguchi S, Okuno S, Sakaki T. Effect of direct arterial bypass on the prevention of future stroke in patients with the hemorrhagic variety of moyamoya disease. J Neurosurg. 2000;93:397–401. doi: 10.3171/jns.2000.93.3.0397. [DOI] [PubMed] [Google Scholar]
- Carlson D H, Thomson D. Spontaneous thrombosis of a giant cerebral aneurysm in five days. Report of a case. Neurology. 1976;26:334–336. doi: 10.1212/wnl.26.4.334. [DOI] [PubMed] [Google Scholar]
- Scott R M, Ballantine H T., Jr Spontaneous thrombosis in a giant middle cerebral artery aneurysm. Case report. J Neurosurg. 1972;37:361–363. doi: 10.3171/jns.1972.37.3.0361. [DOI] [PubMed] [Google Scholar]
- Bull J. Massive aneurysms at the base of the brain. Brain. 1969;92:535–570. doi: 10.1093/brain/92.3.535. [DOI] [PubMed] [Google Scholar]
- Duvoisin R C, Yahr M D. Posterior fossa aneurysms. Neurology. 1965;15:231–241. doi: 10.1212/wnl.15.3.231. [DOI] [PubMed] [Google Scholar]
- Michael W F. Posterior fossa aneurysms simulating tumours. J Neurol Neurosurg Psychiatry. 1974;37:218–223. doi: 10.1136/jnnp.37.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley T P, Barr H W. Giant intracranial aneurysms: diagnosis, course, and management. Clin Neurosurg. 1969;16:73–94. doi: 10.1093/neurosurgery/16.cn_suppl_1.73. [DOI] [PubMed] [Google Scholar]
- Ojemann R G, Ogilvy C S, Crowell R M, Heros R C. Surgical Management of Cerebrovascular Disease. 3rd ed. Baltimore: William and Wilkins; 1995.
- Drake C G. Ligation of the vertebral (unilateral or bilateral) or basilar artery in the treatment of large intracranial aneurysms. J Neurosurg. 1975;43:255–274. doi: 10.3171/jns.1975.43.3.0255. [DOI] [PubMed] [Google Scholar]
- Eckard D A, O'Boynick P L, McPherson C M, et al. Coil occlusion of the parent artery for treatment of symptomatic peripheral intracranial aneurysms. AJNR Am J Neuroradiol. 2000;21:137–142. [PMC free article] [PubMed] [Google Scholar]
- Graves V B, Perl J, II, Strother C M, Wallace R C, Kesava P P, Masaryk T J. Endovascular occlusion of the carotid or vertebral artery with temporary proximal flow arrest and microcoils: clinical results. AJNR Am J Neuroradiol. 1997;18:1201–1206. [PMC free article] [PubMed] [Google Scholar]
- Aymard A, Gobin Y P, Hodes J E, et al. Endovascular occlusion of vertebral arteries in the treatment of unclippable vertebrobasilar aneurysms. J Neurosurg. 1991;74:393–398. doi: 10.3171/jns.1991.74.3.0393. [DOI] [PubMed] [Google Scholar]
- Hopkins L N, Bundy J L, Castellani D. Extracranial-intracranial arterial bypass and basilar artery ligation in the treatment of giant basilar artery aneurysms. Neurosurgery. 1983;13:189–194. doi: 10.1227/00006123-198308000-00016. [DOI] [PubMed] [Google Scholar]
- Rozario R A, Stein B M. Ligation of the basilar artery as the definitive treatment for a giant aneurysm of the basilar artery apex: case report. Neurosurgery. 1980;6:87–91. doi: 10.1227/00006123-198001000-00013. [DOI] [PubMed] [Google Scholar]
- Shintani A, Zervas N T. Consequence of ligation of the vertebral artery. J Neurosurg. 1972;36:447–450. doi: 10.3171/jns.1972.36.4.0447. [DOI] [PubMed] [Google Scholar]
- Spetzler R F, Carter L P. Revascularization and aneurysm surgery: current status. Neurosurgery. 1985;16:111–116. [PubMed] [Google Scholar]
- Yamada K, Hayakawa T, Ushio Y, et al. Therapeutic occlusion of the vertebral artery for unclippable vertebral aneurysm: relationship between site of occlusion and clinical outcome. Neurosurgery. 1984;15:834–838. [PubMed] [Google Scholar]
- Chang S D, Lopez J R, Steinberg G K. The usefulness of electrophysiologic monitoring during resection of central nervous system vascular malformations. J Stroke Cerebrovas Dis. 1999;8:411–422. doi: 10.1016/s1052-3057(99)80049-4. [DOI] [PubMed] [Google Scholar]
- Grosso S, Mostardini R, Venturi C, et al. Recurrent torticollis caused by dissecting vertebral artery aneurysm in a pediatric patient: results of endovascular treatment by use of coil embolization: case report. Neurosurgery. 2002;50:204–207. discussion 207–208. doi: 10.1097/00006123-200201000-00031. [DOI] [PubMed] [Google Scholar]
- Fox A J, Vineula F, Pelz D M, et al. Use of detachable balloons for proximal artery occlusion in the treatment of unclippable cerebral aneurysms. J Neurosurg. 1987;66:40–46. doi: 10.3171/jns.1987.66.1.0040. [DOI] [PubMed] [Google Scholar]
- Halsey J H, Jr, Morawetz R B, Blauenstein U W. The hemodynamic effect of STA-MCA bypass. Stroke. 1982;13:163–167. doi: 10.1161/01.str.13.2.163. [DOI] [PubMed] [Google Scholar]
- Holzschuh M, Brawenski A, Ullrich W, Meixensgerger J. Cerebral blood flow and cerebrovascular reserve 5 years after EC-IC bypass. Neurosurg Rev. 1991;14:275–278. doi: 10.1007/BF00383261. [DOI] [PubMed] [Google Scholar]
- Schmiedek P, Piepgras A, Leinsinger G, Kirsch C M, Einhupl K. Improvement of cerebrovascular reserve capacity by EC-IC arterial bypass surgery in patients with ICA occlusion and hemodynamic cerebral ischemia. J Neurosurg. 1994;81:236–244. doi: 10.3171/jns.1994.81.2.0236. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Kashigawa S, Nakano S, et al. The effect of EC-IC bypass surgery on resting cerebral blood flow and cerebrovascular reserve capacity studied with stable Xe-CT and acetazolamide test. Neuroradiology. 1991;33:217–222. doi: 10.1007/BF00588221. [DOI] [PubMed] [Google Scholar]
- Klijn C JM, Kappelle L J, Tulleken C A, Gijn J van. Symptomatic carotid artery occlusion: a reappraisal of hemodynamic factors. Stroke. 1997;28:2084–2093. doi: 10.1161/01.str.28.10.2084. [DOI] [PubMed] [Google Scholar]
- Higashida R T, Tsai F Y, Halback V V. Transluminal angioplasty for atherosclerotic disease of the vertebral and basilar arteries. J Neurosurg. 1993;78:192–198. doi: 10.3171/jns.1993.78.2.0192. [DOI] [PubMed] [Google Scholar]
- [No authors listed] Prognosis of patients with symptomatic vertebral or basilar artery stenosis. The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Study Group. Stroke. 1998;29:1389–1392. doi: 10.1161/01.str.29.7.1389. [DOI] [PubMed] [Google Scholar]
- Marks M P, Marcellus M, Norbash A M, Steinberg G K, Tong D, Albers G W. Outcome of angioplasty for atherosclerotic intracranial stenosis. Stroke. 1999;30:1065–1069. doi: 10.1161/01.str.30.5.1065. [DOI] [PubMed] [Google Scholar]
- Levy E I, Horowitz M B, Koebbe C K, et al. Transluminal stent-assisted angiplasty of the intracranial vertebrobasilar system for medically refractory, posterior circulation ischemia: early results. Neurosurgery. 2001;48:1215–1221. discussion 1221–1223. doi: 10.1097/00006123-200106000-00002. [DOI] [PubMed] [Google Scholar]
- Kellogg J X, Nesbit G M, Clark W M, Barnwell S L. The role of angioplasty in the treatment of cerebrovascular disease. Neurosurgery. 1998;43:549–555. discussion 555–556. doi: 10.1097/00006123-199809000-00077. [DOI] [PubMed] [Google Scholar]
- Mori T, Fukuoka M, Kazita K, Mori K. Follow-up study after intracranial percutaneous transluminal cerebral balloon angioplasty. AJNR Am J Neuroradiol. 1998;19:1525–1533. [PMC free article] [PubMed] [Google Scholar]
- Fischman D L, Leon M B, Baim D S, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994;331:496–501. doi: 10.1056/NEJM199408253310802. [DOI] [PubMed] [Google Scholar]
- Ko Y G, Park S, Kim J Y, et al. Percutaneous interventional treatment of extracranial vertebral artery stenosis with coronary stents. Yonsei Med J. 2004;45:629–634. doi: 10.3349/ymj.2004.45.4.629. [DOI] [PubMed] [Google Scholar]
- Levy E I, Hanel R A, Bendak B R, et al. Staged stent-assisted angioplasty for symptomatic intracranial vertebrobasilar artery stenosis. J Neurosurg. 2002;97:1294–1301. doi: 10.3171/jns.2002.97.6.1294. [DOI] [PubMed] [Google Scholar]
- Lopez J R, Chang S D, Steinberg G K. The utility of electrophysiologic monitoring during microsurgery of cerebral aneurysms. J Neurol Neurosurg Psychiatry. 1999;66:189–196. doi: 10.1136/jnnp.66.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S D, Steinberg G K. In: Bogousslavsky J, Ginsberg M, editor. Cerebrovascular Disease. London, England: Blackwell Scientific Publications; 1998. Other treatment for stroke prevention. pp. 1945–1963.
- Liu A Y, Lopez J R, Do H M, Steinberg G K, Cockroft K, Marks M P. Neurophysiological monitoring in the endovascular therapy of aneurysms. AJNR Am J Neuroradiol. 2003;24:1520–1527. [PMC free article] [PubMed] [Google Scholar]
- Steinberg G K, Grant G, Yoon E J. In: Andrews RJ, editor. Intraoperative Neuroprotection. Baltimore, MD: Williams & Wilkins; 1996. Deliberate hypothermia. pp. 65–84.
- Ogilvy C S, Carter B S, Kaplan S, Rich C, Crowell R M. Temporary vessel occlusion for aneurysm surgery: risk factors for stroke in patients protected by induced hypothermia and hypertension and intravenous mannitol administration. J Neurosurg. 1996;84:785–791. doi: 10.3171/jns.1996.84.5.0785. [DOI] [PubMed] [Google Scholar]
- Maier CM, Steinberg GK, editor. Hypothermia and Cerebral Ischemia: Mechanisms and Clinical Application. Totowa, NJ: Humana Press; 2003.
- Steinberg G K, Ogilvy C S, Shuer L M, et al. Comparison of endovascular and surface cooling during unruptured cerebral aneurysm repair. Neurosurgery. 2004;55:307–314. discussion 314–315. doi: 10.1227/01.neu.0000129683.99430.8c. [DOI] [PubMed] [Google Scholar]
- Lenhardt R, Marker E, Goll V, et al. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology. 1997;87:1318–1323. doi: 10.1097/00000542-199712000-00009. [DOI] [PubMed] [Google Scholar]
- Schmied H, Kurz A, Sessler D I, Kozek S, Reiter A. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet. 1996;347:289–292. doi: 10.1016/s0140-6736(96)90466-3. [DOI] [PubMed] [Google Scholar]
- Kurz A, Sessler D I, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334:1209–1215. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- Chang S D, Steinberg G K. In: Tindall GT, editor. Contemporary Neurosurgery. Baltimore, MD: Lippicott, Williams & Wilkins; 2000. Surgical management of moyamoya disease.
- Stoodley M A, Mitchell R S, Steinberg G K. Extracranial brachiocephalic reconstruction and grafts. Techniques in Neurosurgery. 2000;6:152–163. [Google Scholar]
- Chang S D, Steinberg G K. Superficial temporal artery to middle cerebral artery anastomosis. Techniques in Neurosurgery. 2000;6:86–100. [Google Scholar]
- Sekhar L N, Bucur S D, Bank W O, Wright D C. Venous and arterial bypass grafts for difficult tumors, aneurysms, and occlusive vascular lesions: evolution of operative management and improved results. Neurosurgery. 1999;44:1207–1224. doi: 10.1097/00006123-199906000-00028. [DOI] [PubMed] [Google Scholar]
- Drake C G. In: Drake PSJ, Hernesniemi CC, JA, editor. Surgery of Vertebrobasilar Aneurysms: London Ontario Experience on 1767 Patients. New York, NY: Thieme-Stratton; 1985. Giant posterior cerebral artery aneurysms: 66 patients. pp. 548–554.
- Diaz F G, et al. Surgical correction of lesions affecting the second portion of the vertebral artery. Neurosurgery. 1986;19:93–100. doi: 10.1227/00006123-198607000-00014. [DOI] [PubMed] [Google Scholar]
- El-Fiki M, Chater N L, Weinstein P R. In: Spetzler RF, editor. Cerebral Revascularization for Stroke. New York, NY: Thieme Stratton; 1985. Extracranial-intracranial arterial bypass for vertebrobasilar insufficiency. pp. 483–489.
- Hopkins L N, Bundy J L. Complications of intracranial bypass for vertebrobasilar insufficiency. J Neurosurg. 1989;70:207–211. doi: 10.3171/jns.1989.70.2.0207. [DOI] [PubMed] [Google Scholar]
- Allen G S, Cohen R J, Preziosi T J. Microsurgical endarterectomy of the intracranial vertebral artery for vertebrobasilar transient ischemic attacks. Neurosurgery. 1981;8:56–59. doi: 10.1227/00006123-198101000-00011. [DOI] [PubMed] [Google Scholar]
- Ausman J I, Diaz F G, Sadasian B. Intracranial endarterectomy. Neurosurgery. 1990;26:465–471. doi: 10.1097/00006123-199003000-00014. [DOI] [PubMed] [Google Scholar]
- Hardin C A, Williamson W P, Steegmann A T. Vertebral artery insufficiency produced by cervical osteoarthritic spurs. Neurology. 1960;10:855–858. doi: 10.1212/wnl.10.9.855. [DOI] [PubMed] [Google Scholar]
- Nagashima C. Surgical treatment of vertebral artery insufficiency caused by cervical spondylosis. J Neurosurg. 1970;32:512–521. doi: 10.3171/jns.1970.32.5.0512. [DOI] [PubMed] [Google Scholar]
- Ausman J I, Diaz F G, Dujovny M. Posterior circulation revascularization. Clin Neurosurg. 1986;33:331–343. [PubMed] [Google Scholar]
- Sundt T M, et al. Interposition saphenous vein grafts for advanced occlusive disease and large aneurysms in the posterior circulation. J Neurosurg. 1982;56:205–215. doi: 10.3171/jns.1982.56.2.0205. [DOI] [PubMed] [Google Scholar]
- Regli L, Piepgras D G, Hansen K K. Late patency of long saphenous vein bypass grafts to the anterior and posterior cerebral circulation. J Neurosurg. 1995;83:806–811. doi: 10.3171/jns.1995.83.5.0806. [DOI] [PubMed] [Google Scholar]
- Sundt T M, Piepgras D G. In: Wilkins RH, Rengachary SS, editor. Neurosurgery. New York, NY: McGraw-Hill; 1985. Extracranial to intracranial bypass grafting: posterior circulation. pp. 1281–1292.
- Roski R A, Spetzler R F, Hopkins L N. Occipital artery to posterior inferior cerebellar artery bypass for vertebrobasilar ischemia. Neurosurgery. 1982;10:44–49. [PubMed] [Google Scholar]