Abstract
1. Melanin-concentrating hormone (MCH)-containing neurons within the lateral hypothalamus affect a broad spectrum of physiological and behavioral processes. The aim of this study was to examine a differentiation of rat MCH neurons dependent on their functionally related projections to the intermediolateral column (IML) of the spinal cord, the midbrain periaqueductal gray (PAG), and the brainstem locus coeruleus (LC).
2. A combination of retrograde fluorescent neuronal tracing and immunocytochemical identification revealed MCH neurons that project to IML, PAG, and LC. Subsequent to isolation of these single neurons by laser microdissection, multi-transcriptional profiling by means of real-time RT-PCR demonstrated different expression patterns between sympathetically projecting MCH neurons and those targeting the PAG or LC.
3. These results provide evidence for the existence of projection-dependent MCH neuronal subpopulations with functional differentiation in the context of controlling energy homeostasis, autonomic functioning, and arousal.
KEY WORDS: gene expression, hypothalamus, food intake, wakefulness, rat
INTRODUCTION
Lateral hypothalamic melanin-concentrating hormone (MCH) neurons have widespread projections throughout the central nervous system (Bittencourt et al., 1992) and are implicated in the regulation of energy homeostasis (Qu et al., 1996; Shimada et al., 1998). The great variety of MCH projections supports the involvement of MCH in a broad spectrum of appetite-associated physiological and behavioral processes, which addresses the question whether MCH cells represent a uniform neuronal population or employ differentiated signaling or output to target systems depending on functionally related projections. Some studies have indicated that the MCH neuronal population is not homogeneous and consists of subpopulations characterized by specific peptide or receptor expression (Griffond et al., 1997; Broberger, 1999; Vrang et al., 1999; Toumaniantz et al., 2000; Elias et al., 2001; Brischoux et al., 2002, Cvetkovic et al., 2003), however a differentiation of expression patterns with connection to functionally related projections is still lacking. The primary aim of this study is to examine whether there is a differentiation of the intrinsic molecular composition dependent on the anatomical divergence of MCH projections toward neurons of the intermediolateral column (IML) of the spinal cord, the mesencephalic periaqueductal gray (PAG), and the brainstem locus coeruleus (LC), respectively, being targets for sympathetic regulation of energy homeostasis, control of accompanying autonomic functions, and wakefulness (Behbehani, 1995; Horvath et al., 1999; Van den Pol, 1999). For that purpose, retrograde fluorescent neuronal tracing was made in rats combined with immunocytochemical staining to identify MCH neurons that project to the IML, PAG, or LC. By an approach of laser microdissection and pressure catapulting (LMPC) these projection-defined MCH neurons were isolated separately, and subsequently analyzed for their transcriptional profiles based on real-time RT-PCR, as described and validated recently (Harthoorn et al., 2005).
MATERIALS AND METHODS
Experiments were conducted with male Wistar rats (250–300 g, Harlan, Zeist, The Netherlands) under the approval of the Animal Care Committee of the Royal Netherlands Academy of Arts and Sciences. In total, 29 rats were used in this study. The animals were housed on a 12-h light, 12-h dark cycle. Water and standard food pellets were usually available ad libitum. The retrograde fluorescent neuronal tracer Cholera Toxin subunit B (CTB) Alexa Fluor 555 was injected either unilaterally in the IML of the spinal cord (T4 segment) (n=10), bilaterally in the dorsomedial and dorsolateral parts of the PAG (bregma −6.5 mm) (n=9), or bilaterally in the LC (bregma −10.1 mm) (n=10). Seven days after tracing, animals were sacrificed with 50 mg sodium pentobarbital i.p., followed by transcardial perfusion with RNase-free (diethylpyrocarbonate (DEPC)-treated) phosphate-buffered saline (PBS) (pH 7.4), followed by a solution of 4% paraformaldehyde in PBS.
Tissue preparations were performed as described previously (Harthoorn et al., 2005). Coronal cryostat sections (18 μm) were light-protected and treated free-floating with DEPC-treated solutions. From the thoracic spinal cord, PAG, and LC, 40 μm sections were examined under fluorescence microscopy to verify injection placement. Incubations with rabbit anti-MCH, biotinylated goat anti-rabbit IgG, ABC complex (Vector Laboratories), and cytochemical staining with diaminobezidine (DAB) were as reported previously (Harthoorn et al., 2005). Sections were mounted on Superfrost®Plus glasses.
DAB-stained MCH neurons were analyzed for fluorescence under a PALM® Microbeam System (PALM Microlaser Technologies, Germany) with the appropriate fluorescence filter set (Carl Zeiss). Per brain the percentage of double-labeled MCH cell profiles was determined in three sections. Samples contained about 50 projection-defined MCH neurons per brain and were collected selectively by means of this PALM® Microbeam System via LMPC into PALM® AdhesiveCaps (PALM Microlaser Technologies, Germany) filled with 5 μL lysis buffer (4 M guanidine thiocyanate, 25 mM sodium citrate, 0.5% Sarcosyl, pH 7.8). All samples were further processed for subsequent real-time RT-PCR with a GeneAmp 5700 sequence detector and SYBR® Green PCR master mix (Applied Biosystems) as previously reported (Harthoorn et al., 2005). PCR primer pairs were designed using Primer Express 2.0 (Applied Biosystems), giving amplicons between 90 and 300 basepairs. Primers pairs were MCH, CCGCAGAAAGATCGGTTGTT, TGGTCCTTTCAGAGCGAGGTA (M62641); cocaine- and amphetamine-regulated transcript (CART), TGGATGATGCGTCCCATGA, CGGAATGCGTTTACTCTTGAGC (U10071); neuropeptide-5 receptor (Y5R), GCCGAAGCATAAGCTGTGGAT, TTTTCTGGAACGGCTAGGTGC (U66274); melanocortin-4 receptor (MC4R), TGATGGCGAGGCTTCACATT, TGAGACATGAAGCACACGCAG (U67863); leptin receptor (isoform b) (OB-Rb), CAGAGCACCCAGGGAACCT, CTGTTTTCACGTTGCTGACCA (U60151); glucocorticoid receptor (GR), TGCTCTGCTTTGCTCCTGATC, TGTCAGTTGGTAAAACCGTTGC (M14053); GABA-A receptor α1 subunit (GABAARα1), GCAAAAGCGTGGTTCCAGAA, TTAGCAATAGTGGCCAAGCCG (L08490); vesicular glutamate transporter-1 (VGLUT1), GGTGCAATGACCAAGCACAAG, TTCACTTTCGTCACTGCCAGC (U07609); glutamic acid decarboxylase (GAD)-65, GGAAGCCTCAGCACACAAATG, ACCATGCGGAAGAAGTTGACC (M72422); GAD-67, ATGGTGAGCCTGAGCACACAA, TGAGGCTGGTAACCAACCATG (M76177). As previously reported at the validation of this approach (Harthoorn et al., 2005), primer pair concentrations were optimized at 150 nM, and non-reverse transcribed samples (n=3) and lysis buffer blanks (n=4) were included as negative controls. The outcomes of real-time RT-PCR results were expressed as threshold cycle (C t), which was the number of PCR rounds needed to pass a fluorescence intensity set at 0.500. The results were validated based on the quality of dissociation curves of individual real-time PCR runs, according to previously reported criteria (Harthoorn et al., 2005).
Expression analysis involved a first screening of positive real-time RT-PCR signals of MCH neuronal samples. Relative expression of individual transcripts was represented as C t values ± SD. For expression levels between the IML-, PAG-, and LC-projecting MCH neuronal populations statistical significance was established using Student’s t-test. Differences were considered statistically significant when P < 0.05.
RESULTS
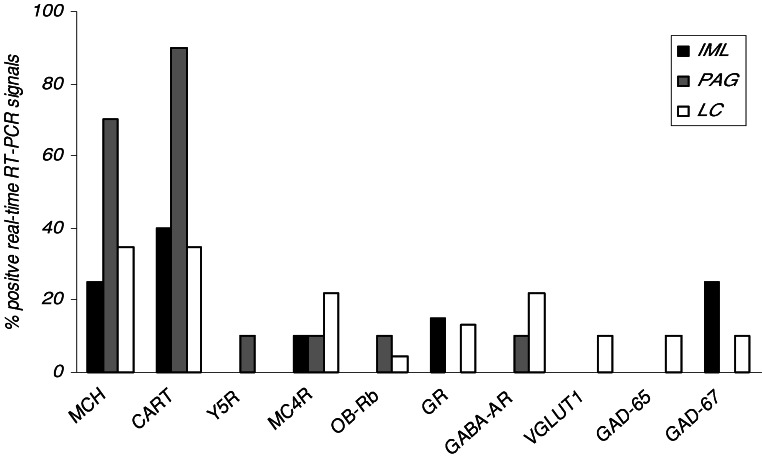
Verification of injection placements of fluorescent tracer showed that CTB injections largely covered the IML and LC, and partially covered the dorsomedial and dorsolateral parts of the PAG. After retrograde tracer injections into IML, PAG, and LC, a mean of ∼22%, ∼12%, and ∼18% of DAB-stained MCH neurons were found to be labeled with fluorescence, respectively, and were at randomly found throughout the entire MCH population. These projection-defined subpopulations were dissected out of the sections by LMPC as shown in Fig. 1A–C. The immunocytochemical phenotype of the three distinctly projection-related MCH populations was cohered to specific expression of MCH mRNA as illustrated in Fig. 2. Among the three projection-dependent subpopulations, the PAG-projecting MCH samples showed most positive MCH mRNA signals. Fig. 2 also shows the same differential expression pattern for CART, having most positive signals among PAG-projecting MCH samples compared to the IML- and LC-related MCH samples. Furthermore, Table I shows that positive PAG-projecting MCH samples have lower C t values, indicating higher relative expression of MCH mRNA compared to the IML-projecting MCH population. From the total screening, as further graphed in Fig. 2, there was a low but different signaling for mRNAs of Y5R, MC4R, OB-Rb, GR, GABAARα1, VGLUT1, GAD-65, and GAD-67, which suggests a low but differentiated expression of those transcripts among the IML-, PAG-, or LC-projecting MCH subpopulations. At the level of relative expression, Table I shows a significant difference in GR between IML- and LC-projecting MCH populations, the latter having higher C t values, which indicates a lower expression of this transcript.
Fig. 1.
Three images showing the successive stages of LMPC of CTB Alexa Fluor 555 traced and DAB-stained MCH neurons in an 18 μm thick immunocytochemical section. (A) Typical example of MCH neurons that were first measured for staining intensity. (B) Fluorescent image of the same section that revealed some MCH neurons with IML projection (arrowheads). (C) After uncovering the immunocytochemical section, the images were compatibly transferred to a PALM® Microbeam system where the double-labeled neurons were selectively harvested by contact-free laser pressure catapulting.
Fig. 2.
Multi-transcriptional profiling of projection-defined MCH neurons showing the percentage of samples containing positive real-time RT-PCR signaling for mRNAs of MCH, CART, Y5R, MC4R, OB-Rb, GR, GABAARα1, VGLUT1, GAD-65, and GAD-67.
Table I.
Relative Expression of IML-, PAG-, and LC-Projecting MCH Neurons as C t Values ± SD from Real-Time RT-PCR
| Transcript | IML-projecting MCH neurons | PAG-projecting MCH neurons | LC-projecting MCH neurons |
|---|---|---|---|
| MCH | 34.25 ± 1.97 | 32.71 ± 1.06a | 32.86 ± 1.27 |
| CART | 32.34 ± 1.84 | 31.48 ± 1.77 | 31.39 ± 1.98 |
| Y5R | 35.60 | ||
| MC4R | 36.25 ± 1.36 | 37.28 | 40.02 ± 3.57 |
| OB-Rb | 41.69 | 40.12 | |
| GR | 37.68 ± 1.22b | 39.78 ± 0.56 | |
| GABAARα1 | 36.96 | 35.34 ± 1.38 | |
| VGLUT1 | 40.52 | ||
| GAD-65 | 35.00 | ||
| GAD-67 | 37.74 ± 1.08 | 39.04 |
a p < 0.05; PAG versus IML (Student’s t-test).
b p < 0.05; IML versus LC (Student’s t-test).
DISCUSSION
This study demonstrates a novel strategy of molecular featuring of individual neurons within the rat hypothalamus dependent on their functionally related projections, involving a combination of retrograde fluorescent neuronal tracing, immunocytochemical phenotyping, and LMPC followed by real-time RT-PCR. Herein, a fundamental projection-related subdivision of the MCH neuronal population was found that couples differentiated functional in- and output of those neurons toward target neurons of the spinal IML, the midbrain PAG, and the brainstem LC.
In addition to what has been proposed about the role of MCH in linking energy balance, reward, and arousal, dependent on their functionally related projections (Bittencourt et al., 1992; Elias and Bittencourt, 1997; Horvath et al., 1999; Griffond and Baker, 2002), the coherence of sympathetic innervation of organs, the control of accompanying autonomic functions, and the maintenance of wakefulness is not intimately dictated by a uniform transmission of a homogeneous hypothalamic system of MCH neurons. This study shows that MCH neurons certainly render a central link between regulation of energy homeostasis, autonomic functions, and the arousal state, but are specialized by comprising a differentiated output by both native MCH and the concomitant co-transmitter CART.
Furthermore, MCH neurons may monitor to the same extend indicators like first-order signaling from other central sources and peripheral hormones. The present data of multi-transcriptional profiling of these projection-defined MCH neurons, however, demonstrate a heterogeneous MCH cell population that possesses differential putative responsiveness to specific input both from other central regions by GABA, neuropeptide Y, melanocortins, and by peripheral leptin and glucocorticoids. This might well result in a differentially intrinsic control of sympathetic activity by the IML versus autonomic regulation by PAG, and wakefulness by LC. Further studies on a differential regulation of projection-dependent hypothalamic systems would be of great importance.
REFERENCES
- Behbehani, M. M. (1995). Functional characteristics of the midbrain periaqueductal gray. Prog. Neurobiol.46:575–605. [DOI] [PubMed] [Google Scholar]
- Bittencourt, J. C., Presse, F., Arias, C., Peto, C., Vaughan, J., Nahon, J. L., Vale, W., and Sawchenko, P. E. (1992). The melanin-concentrating hormone system of the rat brain: An immuno- and hybridization histochemical characterization. J. Comp. Neurol.319:218–245. [DOI] [PubMed] [Google Scholar]
- Brischoux, F., Cvetkovic, V., Griffond, B., Fellmann, D., and Risold, P. Y. (2002). Time of genesis determines projection and neurokinin-3 expression patterns of diencephalic neurons containing melanin-concentrating hormone. Eur. J. Neurosci.16:1672–1680. [DOI] [PubMed] [Google Scholar]
- Broberger, C. (1999). Hypothalamic cocaine- and amphetamine-regulated transcript (CART) neurons: Histochemical relationship to thyrotropin-releasing hormone, melanin-concentrating hormone, orexin/hypocretin and neuropeptide Y. Brain Res.848:101–113. [DOI] [PubMed] [Google Scholar]
- Cvetkovic, V., Poncet, F., Fellmann, D., Griffond, B., and Risold, P. Y. (2003). Diencephalic neurons producing melanin-concentrating hormone are influenced by local and multiple extra-hypothalamic tachykininergic projections through the neurokinin 3 receptor. Neuroscience119:1113–1145. [DOI] [PubMed] [Google Scholar]
- Elias, C. F., and Bittencourt, J. C. (1997). Study of the origins of melanin-concentrating hormone and neuropeptide EI immunoreactive projections to the periaqueductal gray matter. Brain Res.755:255–271. [DOI] [PubMed] [Google Scholar]
- Elias, C. F., Lee, C. E., Kelly, J. F., Ahima, R. S., Kuhar, M., Saper, C. B., and Elmquist, J. K. (2001). Characterization of CART neurons in the rat and human hypothalamus. J. Comp. Neurol.432:1–19. [DOI] [PubMed] [Google Scholar]
- Griffond, B., and Baker, B. I. (2002). Cell and molecular cell biology of melanin-concentrating hormone. Int. Rev. Cytol.213:233–277. [DOI] [PubMed] [Google Scholar]
- Griffond, B., Ciofi, P., Bayer, L., Jacquemard, C., and Fellmann, D. (1997). Immunocytochemical detection of the neurokinin B receptor (NK3) on melanin-concentrating hormone (MCH) neurons in rat brain. J. Chem. Neuroanat.12:183–189. [DOI] [PubMed] [Google Scholar]
- Harthoorn, L. F., Sañé, A., Nethe, M., and Van Heerikhuize, J. J. (2005). Multi-transcriptional profiling of melanin-concentrating hormone and orexin-containing neurons. Cell. Mol. Neurobiol.25:1209–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath, T. L., Peyron, C., Diano, S., Ivanov, A., Aston-Jones, G., Kilduff, T. S., and Van Den Pol, A. N. (1999). Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J. Comp. Neurol.415:145–159. [PubMed] [Google Scholar]
- Qu, D., Ludwig, D. S., Gammeltoft, S., Piper, M., Pelleymounter, M. A., Cullen, M. J., Mathes, W. F., Przypek, R., Kanarek, R., and Maratos-Flier, E. (1996). A role for melanin concentrating hormone in the central regulation of feeding behaviour. Nature380:243–247. [DOI] [PubMed] [Google Scholar]
- Shimada, M., Tritos, N. A., Lowell, B. B., Flier, J. S., and Maratos-Flier, E. (1998). Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature396:670–674. [DOI] [PubMed] [Google Scholar]
- Toumaniantz, G., Ferreira, P. C., Allaeys, I., Bittencourt, J. C., and Nahon, J. L. (2000). Differential neuronal expression and projections of melanin-concentrating hormone (MCH) and MCH-gene-overprinted-polypeptide MGOP) in the rat brain. Eur. J. Neurosci.12:4367–4380. [DOI] [PubMed] [Google Scholar]
- Van Den Pol, A. N. (1999) Hypothalamic hypocretin (orexin): Robust innervation of the spinal cord. J. Neurosci.19:3171–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrang, N., Larsen, P. J., Clausen, J. T., and Kristensen, P. (1999). Neurochemical characterization of hypothalamic cocaine-amphetamine-regulated transcript neurons. J. Neurosci.19:RC5. [DOI] [PMC free article] [PubMed] [Google Scholar]