Abstract
1. The aim of the present study was to examine the distribution of unmyelinated, small-diameter myelinated neuronal nitric oxide synthase immunoreactive (nNOS-IR) axons and large-diameter myelinated neuronal nitric oxide synthase and parvalbumin-immunoreactive (PV-IR) axons in the dorsal funiculus (DF) of sacral (S1–S3) and lumbar (L1–L7) segments of the dog.
2. nNOS and PV immunohistochemical methods were used to demonstrate the presence of nNOS-IR and PV-IR in the large-diameter myelinated, presumed to be proprioceptive, axons in the DF along the lumbosacral segments.
3. Fiber size and density of nNOS-IR and PV-IR axons were used to compartmentalize the DF into five compartments (CI–CV). The first compartment (CI) localized in the lateralmost part of the DF, containing both unmyelinated and small-diameter myelinated nNOS-IR axons, is homologous with the dorsolateral fasciculus, or Lissauer tract. The second compartment (CII) having similar fiber organization as CI is situated more medially in sacral segments. Rostrally, in lower lumbar segments, CII moves more medially, and at upper lumbar level, CII reaches the dorsomedial angle of the DF and fuses with axons of CIV. CIII is the largest in the DF and the only one containing large-diameter myelinated nNOS-IR and PV-IR axons. The largest nNOS-IR and PV-IR axons of CIII (8.0–9.2 μm in diameter), presumed to be stem Ia proprioceptive afferents, are located in the deep portion of the DF close to the dorsal and dorsomedial border of the dorsal horn. The CIV compartment varies in shape, appearing first as a small triangular area in S3 and S2 segments, homologous with the Philippe–Gombault triangle. Beginning at S1 level, CIV acquires a more elongated shape and is seen throughout the lumbar segments as a narrow band of fibers extending just below the dorsal median septum in approximately upper two-thirds of the DF. The CV is located in the basal part of the DF. In general, CV is poor in nNOS-IR fibers; among them solitary PV-IR fibers are seen.
4. The analysis of the control material and the degeneration of the large- and medium-caliber nNOS-IR fibers after unilateral L7 and S1 dorsal rhizotomy confirmed that large-caliber nNOS-IR and and PV-IR axons, presumed to be proprioceptive Ia axons, and their ascending and descending collaterals are present in large number in the DF of the lumbosacral intumescence. However, in the DF of the upper lumbar segments, the decrease in the number of nNOS-IR and PV-IR fibers is quite evident.
KEY WORDS: primary afferents, dorsal funiculus, neuronal nitric oxide synthase, parvalbumin, immunoreactivity
INTRODUCTION
The white matter of the mammalian DF was traditionally thought to consist predominantly of large myelinated axons, the majority of which are primary afferents originating from large dorsal root ganglion (DRG) neurons (Willis and Coggeshall, 1991). Large myelinated axons leave the corresponding DRG at its proximal end and proceed centripetally within the dorsal root, becoming intermingled with thin, mostly unmyelinated axons until they reach the posterior spinal cord rootlet junction at the level of the pial ring situated 1–2 mm outside the entry of rootlets into the dorso-lateral sulcus, where a rearrangement of large myelinated and thin unmyelinated fibers takes place (Sindou et al., 1974). The fibers become grouped according to their size and function, and here, the large myelinated fibers occupy the central and dorsomedial portion in the root. The segregation of the large myelinated and the small mainly unmyelinated fibers is completed upon entering the white matter of the spinal cord. Classical and tract-tracing studies have established that the collaterals of the small-caliber fibers linked to the transmission of high-threshold tissue-damaging stimuli triggering nociceptive sensations (Besson and Chaouch, 1987; Willis and Westlund, 1997; Pearson, 1952; White and Sweet, 1969) enter the spinal cord and give off ascending and descending collaterals into the Lissauer tract before penetrating the dorsal horn through its dorsal surface and terminating preferentially in the marginal layer (lamina I) and the substantia gelatinosa (laminae II–III). The main bulk of large myelinated axons contributing to the DF comes from the MB of the dorsal root, especially at the level of the lumbosacral and cervical enlargement (Ranson, 1914). Large myelinated axons of the MB are responsible for transmitting the information required for discriminative touch, vibratory sensibility, position sense, and proprioception, the latter being conveyed by PV-IR fibers. PV is known as a member of the family of low molecular weight, calcium-binding proteins which have been shown to be preferentially expressed within large-diameter primary afferents (Celio, 1990; Zhang et al., 1990).
The use of impregnation technique and electron microscopy has revealed that the DF is rich, apart from the large-and medium-sized myelinated axons, also in unmyelinated primary afferent fibers (Chung and Coggeshall, 1985; Chung et al., 1987). Recent studies provide evidence that a substantial population of thin fibers located in the medial portion of the DF are long, ascending, unmyelinated primary afferent axons transmitting signals of visceral pain (Patterson et al., 1990, 1992; Hirshberg et al., 1996; Willis et al., 1999).
In the search for the presence of nNOS-IR somata and the origin of punctate nNOS-IR in the spinal cord gray matter of various segments in rabbit and dog, a noticeable number of nNOS-IR and/or NADPH diaphorase (NADPHd)-stained small- and large-caliber fibers has been consistently found in the white matter all along the rostro-caudal axis of the spinal cord (Maršala et al., 1997, 1998, 1999, 2002, 2003, 2004, 2005, 2006). Moreover, recent studies provide evidence that large-diameter nNOS-IR axons occur in the DF of lumbosacral segments in the dog, and medium- and large-sized somata located in the DRGs may form the putative afferent limb of the monosynaptic Ia-motoneuron pathway (Maršala et al., 2005, 2006; Lukáčová et al., 2006).
The aims of the present study were threefold: (1) to test the hypothesis that nNOS-IR dorsal root afferents in the lumbosacral segments segregate according to fibre size, thus forming a small-caliber nNOS-IR LB and a massive large-caliber nNOS-IR MB in S2-L4 segments, (2) to confirm the hypothesis that large-caliber nNOS-IR and PV-IR axons enter the DF all along the lumbosacral intumescence (S2-L4 segments), and 3) finally, to show that dorsal rhizotomy can induce anterograde degeneration coupled with nNOS-IR depletion in the MB of the dorsal root and in the DF.
MATERIAL AND METHODS
Animal Model, Surgical Procedures, Tissue Sampling, Sectioning, and Staining of Sections
Adult mongrel dogs (n = 8) of both sexes weighing 8–13 kg were used in these experiments. Experimental protocols were approved by the Institute of Neurobiology Animal Care Committee on animal welfare according to the rules of the Slovak government. The dogs were divided into two groups. In the first group of control animals (n = 4), the occurrence and compartmental distribution of nNOS-IR and PV-IR fibers in the DF of S3-L1 segments were studied. From all segments, coronal, horizontal, and parasagittal sections were prepared, allowing precise localization of nNOS-IR and PV-IR axons and their collaterals in the DF. In the second group (n = 4), unilateral dorsal rhizotomy of L7 and S1 dorsal roots midway between DRG and the DREZ-one was performed. After 6 days of survival, the S3-L1 segments, S3-L1 DRGs, and corresponding dorsal roots were dissected and processed for immunolabeling.
nNOS-IR and PV-IR in S3-L1 Segments
Fiber nNOS-IR and PV-IR were studied in the DF of LS segments in the first group of animals (n = 4), anesthetized with a mixture of ketamine and xylazine (100 and 15 mg/kg bw, i.m.) and artificially ventilated in a respirator with oxygen and nitrous oxide (Anemat N8 Chirana, ČSSR). Afterwards, the animals were perfused intracardially with heparinized saline and subsequently with freshly-prepared 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS, ph 7.4). The lumbosacral spinal cord was removed in toto and postfixed in the same fixative for an additional 12 h. The following day, the S3-L1 segments were dissected and cryoprotected in PBS containing graded sucrose (15–30%). After postfixation, the lumbosacral spinal cord was divided into S3-L1 segments, and each segment was further divided into three small blocks representing the upper, middle, and lower segmental levels (Maršala et al., 1999). Because a spinal cord segment is, by definition, that part of the cord which gives rise to those root fibers that unite to form a pair of spinal nerves (Brodal, 1969), the caudal border of each segment was defined as its most caudal dorsal rootlet (McKenna and Nadelhaft, 1986). Sixty transverse, 20 horizontal, and 20 parasagittal sections, all 36 μm thick, were cut from each segment. Pairs of sections were then processed for nNOS-IR (Bredt et al., 1990) and PV-IR.
Free-floating tissue sections were pretreated with 0.3% H2O2 in PBS for 30 min, then washed and blocked with 10% normal horse serum. For processing nNOS-IR, sections were incubated in monoclonal antinitric oxide synthase-brain antiserum (bNOS, Sigma, Product Number, N2280), 1:1000 dilution with 0.3% Triton X-100 in PBS overnight at 4°C with gentle agitation. This antibody specifically recognizes nNOS derived from rat brain (nNOS, 150–160 kDa) and several breakdown products of lower molecular weight. It does not react with NOS derived from macrophages (mNOS) and endothelial cells (eNOS). To produce this antibody, a recombinant neuronal fragment (amino acids 1–181) from rat brain was used as chromogen (Dinerman et al., 1994). After several washes in PBS the sections were incubated with a biotinylated anti-mouse secondary antibody (1:200, Vector Laboratories) for 2 h. The next day, the sections were rinsed with PBS and mixed with avidin-biotin complex (ABC) solution for 1 h (1:50, Vector Labs) at room temperature. Finally, the sections were developed in diaminobenzidine-H2O2 (DAB) as the chromogen, and on a few selected sections, the nNOS staining was intensified by 1 min incubation in nickel chloride enhancing solution. The sections were washed in distilled water, mounted on Colorfrost/Plus microscope slides (Fisher Scientific, USA), air-dried, and dehydrated with graded alcohol (50–100%) followed by Xylene and coverslipped with Permount. On a few sections the primary antibody nNOS was omitted from the staining procedures, so that no nNOS-IR fibers were detected.
The pattern of PV-IR was examined on alternate sections cut from S3-L1 segments. The spinal cord sections were incubated for 1 h in 3% normal horse serum (Vector) and then incubated overnight at 4°C in mouse anti-parvalbumin monoclonal antibody (Chemicon International), used at a dilution of 1:1000. Negative control sections, on which the primary antibody was omitted, were also prepared. After thorough washing in phosphate-buffered saline with 0.1% Triton-X-100 (Sigma), the sections were incubated for 2 h at room temperature in biotinylated anti-mouse secondary antibody (dilution 1:100, Vector Labs) and then incubated for 1 h in avidin-biotin complex (Vector). Finally, they were processed using diaminobenzidine (DAB). After three rinses in 0.1 M phosphate buffer, free-floating sections were dehydrated in a graded series of ethanols, cleared in Xylene and coverslipped with Permount.
L7 and S1 Dorsal Rhizotomy-Induced Depletion of nNOS-IR in the DF of Sacral and Lower Lumbar Segments
In the second group (n = 4), L7 and S1 dorsal roots were approached and divided unilaterally as follows. The animals were anesthetized with a mixture of ketamine and xylazine (100 and 15 mg/kg bw im), and artificially ventilated in a respirator with oxygen and nitrous oxide (Anemat N8 Chirana, ČSSR). L7 and S1 dorsal roots were approached through lumbar laminectomy of the sixth and seventh laminas, thus gaining access to the lower lumbar segments and cauda equina (Maršala et al., 1995; Orendáčová et al., 2000, 2001). The L7 and S1 dorsal roots were divided 2–4 mm proximally to the corresponding DRGs. Extreme care was taken to avoid damage to the radicular and spinal blood vessels. Following recovery from the anesthetic, each dog was transferred from the operating room to a holding cage for observation. Six days later the animals were deeply anesthetized (as above) and intracardially perfused with heparinized saline, and subsequently with freshly-prepared 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS; pH 7.4). Lumbosacral intumescence (L4-S2 segments) and L4-S2 DRGs were removed, postfixed, and processed for nNOS-IR in the same way as for the first group.
Quantitative Assessment of the Square Area of the DF in LS Segments, Density of nNOS-IR Fibers, and Percentage of the Compartmental Distribution of CI–CV in the Lumbosacral DF
To determine the cross-sectional area of the DF in LS segments, digital images of five transverse sections taken from the DF of S3-L1 segments of all control animals were captured using a light microscope (Olympus BX51), a digital camera (Olympus DP50), and Olympus DP-soft (version 3.0). Final cross-sectional area, expressed in square centimeters of each unilateral DF as given in Figs. 2 and 6, was determined by outlining the border of the DF laterally against the dorsal horn and medially along the dorsal median septum using the UTHSCSA Image Tool 3. The results were statistically evaluated using ANOVA as well as the Tukey–Kramer test, and are expressed as means±SEM.
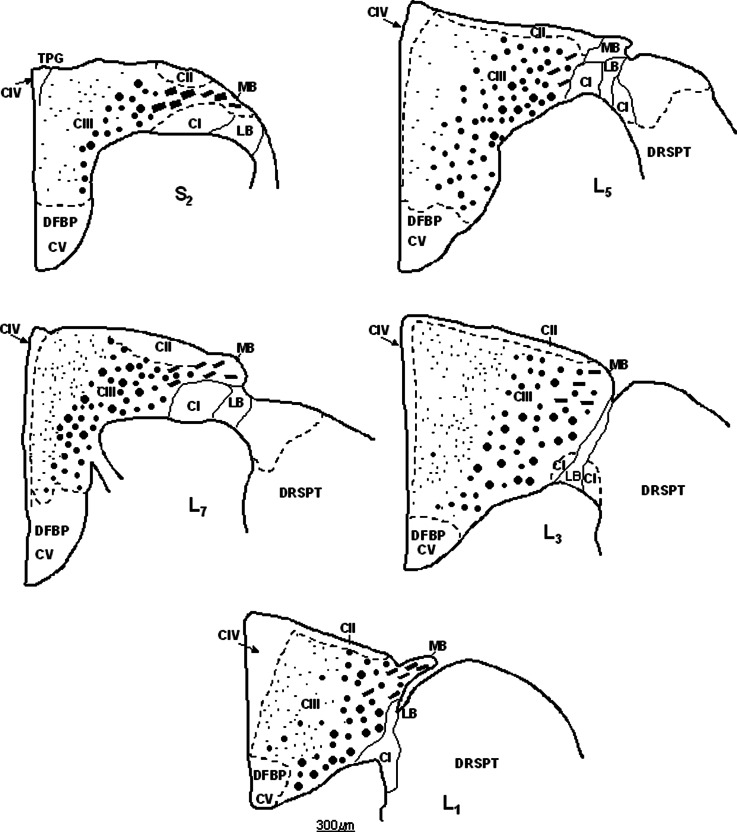
Fig. 2.
Schematic depiction of the distribution and compartmentalization of nNOS-IR axons in the DF of S3-S1 segments. The demarcation between CI–CV is shown by the dashed line. The distribution of nNOS-IR axons is given by black dots of different sizes in the white matter of DF in S3-S1 segments. Note that large-caliber nNOS-IR fibers in CIII tend to be distributed dorsally and dorsomedially to the dorsal horn. The cross-sectional square area of the DF in S3-S1 segments is given. Longitudinally and obliquely cut large-caliber nNOS-IR axons in the MB are depicted by the thick dashed line. DFBP, basal part of the DF.
Fig. 6.
Schematic depiction of the distribution and compartmentalization of nNOS-IR axons in the DF of L7-L1 segment. The border between CI–CV is shown by the dashed line. The distribution of nNOS-IR axons is given by dots of different sizes in the white matter of DF in L7-L1 segments. Note that large-caliber nNOS-IR axons, presumed to be Ia afferents in CIII, tend to be distributed dorsally and dorsomedially to the DH. Cross-sectional square area of the DF sq.a. is given for each segment (top right). DFBP, basal part of the DF corresponding to CV; DRSPT, dorsal reticulospinal tract containing large-caliber nNOS-IR axons; LB fibers in the DREZ-one; MB, longitudinally and obliquely cut large-caliber nNOS-IR axons are depicted by thick dashed line.
The distribution and density of nNOS-IR fibers was evaluated using five transverse sections from all lumbosacral segments of three animals. Altogether, 150 sections processed for nNOS-IR were used for quantitative analysis. After identification of CI–CV, high-power pictures (100X/1.30 OIL) were captured from randomly selected areas of the compartment under study. Then the microphotographs prepared from fivefold enlarged primary pictures and a micrometer grid (MEOPTA, ČSSR) were used to assess the number of unmyelinated and small-diameter myelinated nNOS-IR axons in 1 mm2 areas of CI, CII, CIV, and CV and, similarly, the number of large-diameter myelinated nNOS-IR axons were counted in 1 mm2 areas of CIII in all LS segments. Percentage distribution of small- and large-diameter nNOS-IR fibers in CI–CV was calculated by substracting the square area value of CI–CV from the total square area value of the DF, as shown in Figs. 2 and 6.
RESULTS
The Appearance and General Characteristics of nNOS-IR Fibers in the DF of the LS Segments
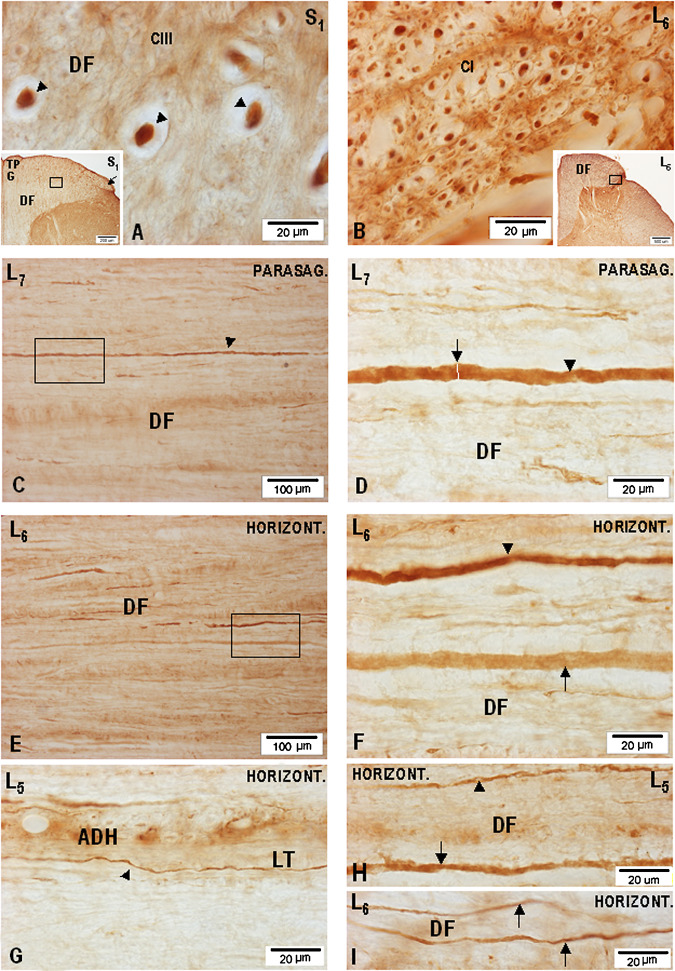
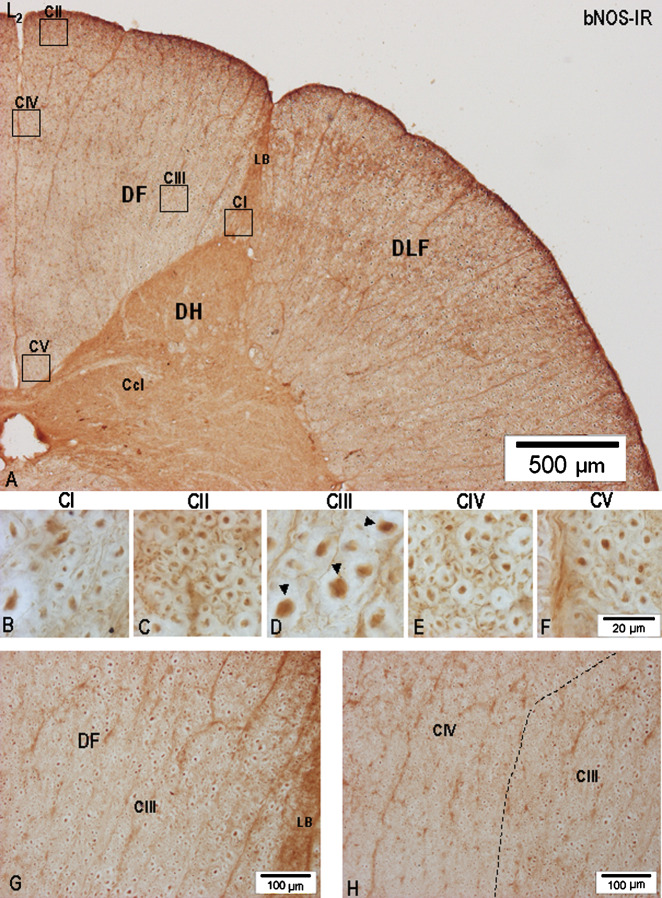
To identify nNOS-IR axons, combined transverse, parasagittal, and horizontal sections (all 36 μm thick) were used, allowing the course of nNOS-IR fibers to be pursued for several hundreds of μm (Fig. 1A–I). It should be noted that all quantitative data concerning the size of nNOS-IR fibers were collected from the central processes of primary sensory cells travelling in the MB and LB bundle of the dorsal roots in S3-L1 segments and in the corresponding S3-L1 DF. Light microscopic examination of the S3-L1 DF revealed large numbers of unmyelinated nNOS-IR axons ranging from 0.4 to 1.0 μm in diameter (Fig. 1G), small-caliber myelinated nNOS-IR axons ranging from 1.1 to 2.2 μm in diameter (Fig. 1B, H, I), and large-caliber myelinated bNOS-IR axons and their collaterals ranging from 2.3 to 8.0–9.2 μm in diameter (Fig. 1A, C–F). Occasionally, even larger (>9.2 μm in diameter) nNOS-IR axons, presumed to be stem Ia proprioceptive afferents, could be identified in the DF, mostly in S2-L7 segments. However, an interesting finding was noticed in connection with the largest nNOS-IR axons in the lumbosacral DF, mainly in the extent of S2-L6 segments, having direct relation with the MB of S2-L6 dorsal roots. It was repeatedly seen that a massive MB at S2-L6 level, usually divided into two or three fascicles, penetrates the DF not only through the dorsomedial sulcus, but quite often takes a more medial course, entering more medially into the dorsomedial sulcus, passing through the dorsal portion of the DF and proceeding in the medial direction until it reaches the dorsomedial quadrant of the DF. A noticeable number of large-caliber myelinated nNOS-IR axons could therefore be detected in the dorsomedial portion of the DF, many of them being only 25–35 μm below the dorsal surface of the DF.
Fig. 1.
Microphotographs showing the appearance of nNOS-IR fibers in the DF of the lumbosacral segments on transverse (A–B), parasagittal (C–F), and horizontal (G–I) sections processed for nNOS-IR. (A) Group of large-caliber nNOS-IR fibers (arrowheads) presumed to be Ia proprioceptive afferents taken from the central part of the DF of S1 segment (S1). Inset: low-power microphotograph of a transverse section through S1 segment showing the DF and the location of the TPG and MB of the dorsal root entering the DREZ-one (arrow); a high-power microphotograph of the boxed area is seen in (A) and (B). (C) Low-power microphotograph of a parasagittal section (parasag.) through the DF of L7 segment depicting a large-caliber nNOS-IR axon (arrowhead) presumed to be Ia fiber cut parallel to its sectioning in the coronal plane; nNOS-IR axon size measured at the level of the white bar (arrow) was 5.64–7.55 μm (n = 8). (E) Low-power microphotograph taken from a horizontal (horizont.) section cut through the middle portion of the DF in L6 segment. Two large-caliber axons are seen in the boxed area. (F) High-power microphotograph (boxed area in E) depicting two differently nNOS-IR axons, one (arrowhead) demonstrating intensely nNOS-IR axoplasm, while in the other (arrow) the axoplasm is only lightly nNOS-IR. (G) Small-caliber unmyelinated nNOS-IR fiber (0.6–0.8 μm in diameter-arrowhead) generating varicosities varying in shape from spherical to elliptical, located in the Lissauer tract close to the apex of the dorsal horn (ADH) on a horizontal section cut through the DF of L5 segment. (H) Horizontal section through the dorsal portion of the DF; small-(arrowhead) and large-(arrow) caliber nNOS-IR fibers are seen. (I) Small-caliber nNOS-IR fibers (arrows) are seen on a horizontal sectionthrough the dorsomedial portion of the DF.
Moreover, apparent incongruity in the segmental distribution of the largest nNOS-IR axons, 8.0–9.2 μm in diameter, was noticed in the DF along the lumbosacral intumescence. However, the largest nNOS-IR axons were consistently found in the DF of S2, S1, and L7 segments.
Compartmentalization and Packing Density of nNOS-IR Fibers in the DF of S3-S1 Segments
The normal S3-S1 DF contains a noticeable number of large-caliber myelinated nNOS-IR axons which, before entering the DF, can be seen in the MB of the dorsal root and in the DREZ-one. Unmyelinated and small-caliber myelinated nNOS-IR fibers form an LB located more ventrolaterally, which, upon entering the spinal cord, approaches the dorsolateral portion of the dorsal horn and form a dense plexus of nNOS-IR fibers in the superficial laminae of the dorsal horn. The massive nNOS-IR MB of S3-S1 dorsal roots passes through the DREZ-one and enters the dorsolateral portion of the DF. Fiber analysis shows that the DF is relatively small at lower sacral level but expands greatly in the upper sacral and then in the lower lumbar segments. Proceeding rostrally, more and more ascending nNOS-IR fibers of the dorsal roots and nNOS immunonegative axons join the DF laterally and, the caudal fibers are gradually displaced medially. For this reason, the description of the nNOS-IR axons in the DF is given in ascending order beginning with S3 segment.
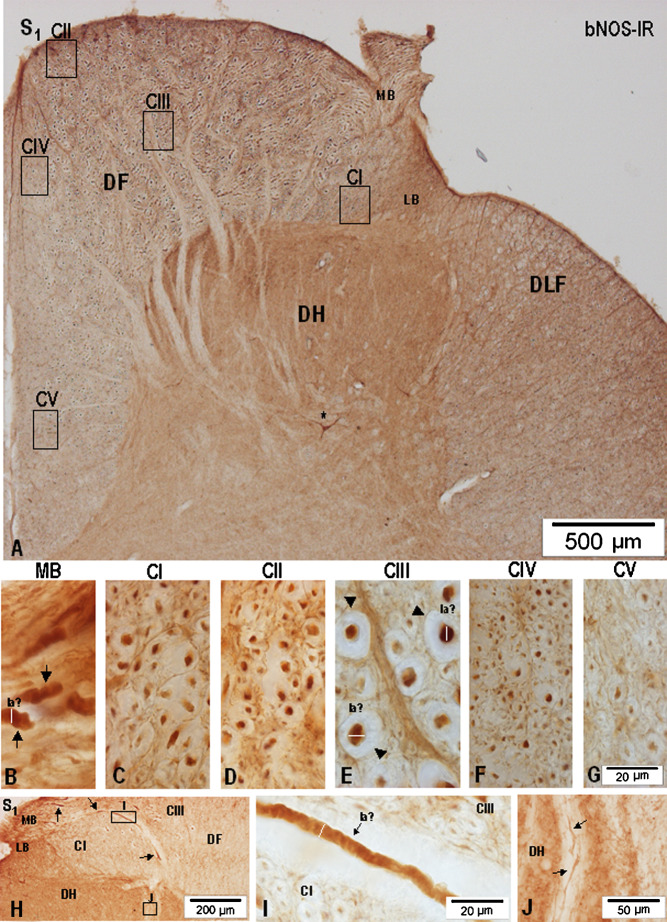
Considering the size, density, and distribution pattern of myelinated and unmyelinated nNOS-IR axons, CI–CV nNOS-IR fiber compartments could be detected in the S3-S1 segments (Fig. 2). CI, located just above the dorsolateral and dorsal border of the dorsal horn in S3 segment, is reduced in size in the upper half of S1 segment and covers the lateral half of the dorsal horn. Although small-caliber myelinated nNOS-IR axons and transversally cut unmyelinated nNOS-IR axons are discernible in the medial half of CI, a more ventrolaterally located CI portion contains a higher number of obliquely and longitudinally cut nNOS-IR axons, forming in fact the spinal trajectory of the LB just before branching into ascending and descending collaterals (Figs. 2, 3A–C, 4A–B, 5A–C). Although the light-microscopic appearance of nNOS-IR axons in CII appears to be almost the same in all sacral segments (Figs. 3D, 4C, 5D), passing rostrally, the extent and location of CII changes, and, while at S3 level, CII is seen only as a narrow band reaching 250–350 μm mediolaterally and abutting on large-caliber nNOS-IR axons of the MB, in S2 segment level, CII moves more medially (Figs. 2, 4A), then, in S1 segment, CII approaches the TPG and finally fuses with the CIV (Figs. 2, 5A). Large-caliber nNOS-IR fibers, some cut parallel with the long axis seen in the MB of all sacral roots, are clearly distinguishable in the DREZ-one of S3-S1 segments (Figs. 2, 3A, B, 4A, 5A, B, H, I). Upon entering the DF, large-caliber nNOS-IR fibers penetrate more deeply; those seen in the DF of S3 and S2 segments can be found not only medially along the dorsal horn but some of them, though limited in number, can almost reach the superficial portion of the DF (Figs. 2, 3C, 4C, 5E).
Fig. 3.
Microphotographs showing the appearance and distribution of nNOS-IR axons in the DF of S3 segment (A–G). (A) Low-power microphotograph showing the DF, the location of the MB of the dorsal root, and the LB of the dorsal root. The extent of samples representing the five fiber compartments is given by the boxed areas (CI–CV) in the DF. (B) Microphotograph depicting the DREZ-one of the same section; arrows point to large-caliber nNOS-IR axons in the MB, some cut parallel to their sectioning in the coronal plane; the dotted line separates CI, CII, and CIII in the dorsolateral part of the DF. (C–G) High-power microphotographs showing axonal nNOS-IR in CI–CV; large-caliber nNOS-IR axons in CIII (asterisks) were the largest nNOS-IR fibers identified in the white columns of the lumbosacral spinal cord.
Fig. 4.
(A) Low-power microphotograph depicting the dorsal half of a transverse section cut through S2 segment including the DH and DF. (B–F) Samples taken from the boxed areas (CI–CV) were used to demonstrate the nNOS-IR fiber patterns in DF. Note the occurrence of large-caliber nNOS-IR axons (arrowheads) in CIII (D).
Fig. 5.
(A) Low-power microphotograph depicting the distribution of nNOS-IR axons in the horizontal and vertical limbs of the DF in the S1 segment; samples from the boxed areas (CI–CV) were used to demonstrate the fibre pattern and density of nNOS-IR axons. (B) High-power microphotograph showing a transverse section through the MB demonstrating large-caliber nNOS-IR axons (arrows) presumed to be proprioceptive afferents (Ia); the diameter of the large-caliber nNOS-IR axons (white bar) was measured close to the DREZ-one. (C–G) nNOS-IR fibre patterns in CI–CV in the DF of S1 segment; arrowheads and white bars in CIII point to large-caliber nNOS-IR axons, presumed to be proprioceptive afferents (Ia). Scale bar in (B–G equals 20 μm. (H) Low-power microphotograph depicting the DREZ-one in S1 segments; large-caliber nNOS-IR fibers (arrows) of the MB are seen to proceed in medial direction just above the DH; some are cut parallel to their sectioning in the coronal plane (boxed area); a sample from the small boxed area in the superficial dorsal horn was used; CI, CIII of the DF; LB of the dorsal root. (I) High-power microphotograph depicting a large-caliber nNOS-IR axon (arrow, Ia) taken from the boxed area (I) drawn in (H); CI, CIII in DF. (J) Penetration of small-caliber nNOS-IR fibers (arrows) through the dorsomedial portion of the dorsal horn (for DH see boxed area in H).
Generally, CIII is characterized by the occurrence of a broad nNOS-IR fiber range of 1.1–9.2 μm in diameter and distributed throughout a large portion of the DF. The largest nNOS-IR fibers ranging from 8.0–9.2 μm in diameter are seen to concentrate in close vicinity of the dorsomedial and medial border of the dorsal horn (Figs. 2, 5H, I). Considerably tapered collaterals, ranging usually between 1.2–2.5 μm, are seen to proceed deeply through the dorsal horn (Fig. 5J). CIV is located in the dorsomedial part of DF localized along the upper and middle part of the dorsal median septum including the TPG, containing small-caliber myelinated and unmyelinated nNOS-IR fibers (Figs. 2, 3F, 4E, 5F). CV located in the basal part of the DF in S3-S1 segments is poor in nNOS-IR fibers (Figs. 2, 3G, 4F, 5G).
Compartmentalization and Differences in nNOS-IR Fiber Spectra in the DF of L7-L1 Segments
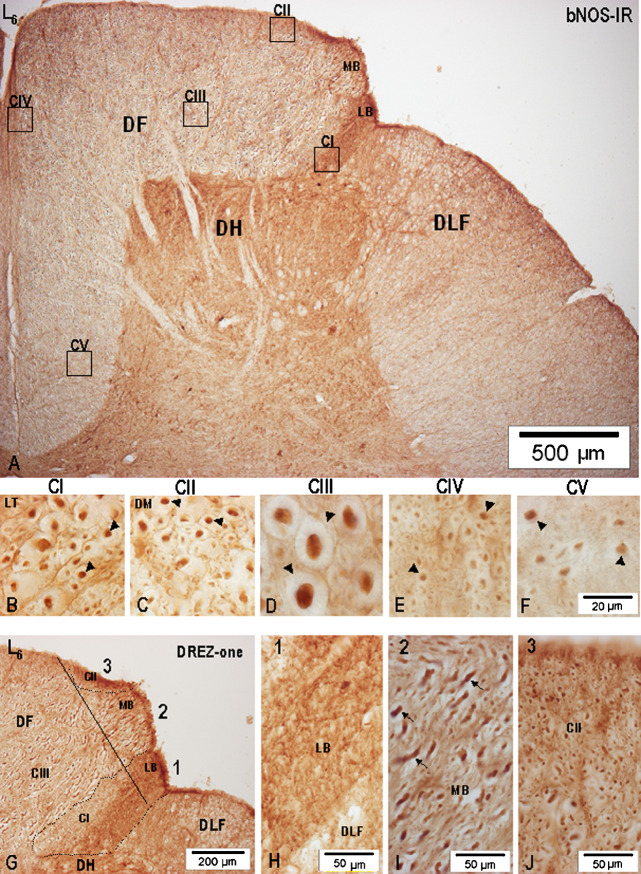
Noticeable differences were found in the configuration and compartmentalization of nNOS-IR fibers in the DF of lower lumbar (L7-L4) segments forming a part of the lumbosacral intumescence and upper lumbar (L3-L1) segments (Fig. 6). A prominent increase in the number and extent of the dorsal root afferents was found, and among them both large- and small-caliber nNOS-IR fibers in L7-L4 dorsal roots were clearly discernible before the rootlet’s spinal cord junction, thus allowing for identification of the MB and LB of the L7-L4 dorsal roots (Figs. 7A, 7G–J, 8A and G, 9A). While an intensely nNOS-IR LB in L7-L4 segments after passing through the DREZ-one proceeded ventromedially and entered the dorsolateral portion of the dorsal horn, large-caliber nNOS-IR fibers of the MB entered the DREZ-one either as a single massive bundle or, more often, divided into two or three smaller fascicles and penetrated the DF from its dorsolateral border, thus dividing the small-caliber nNOS-IR axons into a ventrolateral portion, i.e., CI (homologous with the Lissauer tract) and a dorsomedial portion, CII (Fig. 7G). Distinct segregation of large- and small-caliber nNOS-IR fibers, just before entering the spinal cord, points to the fact that a substantial portion of the DREZ-one, at least in L7-L4 segments, contains a high number of large-caliber nNOS-IR fibers, some among them ranging 8.0–9.2 μm in diameter.
Fig. 7.
(A) Low-power microphotograph demonstrating the distribution of nNOS-IR axons in the vertical and horizontal limbs of the DF of L6 segment. Specimens taken from the boxed areas were used to demonstrate the fiber pattern in CI–CV. (B–F) High-power microphotographs demonstrating the nNOS-IR fiber patterns in compartments CI–CV. Arrows point to identified nNOS-IR axons of various diameters. (G) Arrangement of nNOS-IR fibers in the DF, DREZ-one (full line) in L6 segments, divided arbitrarily into 1–3 sections; dotted line demarcates the location of CI and CIII; 1, ventrolateral sector of the DREZ-one containing the LB; 2, middle sector represented by large-caliber nNOS-IR axons of the MB; 3, dorsomedial sector corresponding to CII. Note the difference between nNOS-IR of the LB and CI. (H) Prominent nNOS-IR of the LB-1. (I) Obliquely cut large-caliber nNOS-IR axons (arrows) of the MB-2 in the DREZ-one. (J) Large accumulation of transversally cut nNOS-IR axons occurring in the dorsomedial part of the DREZ-one (CII-3).
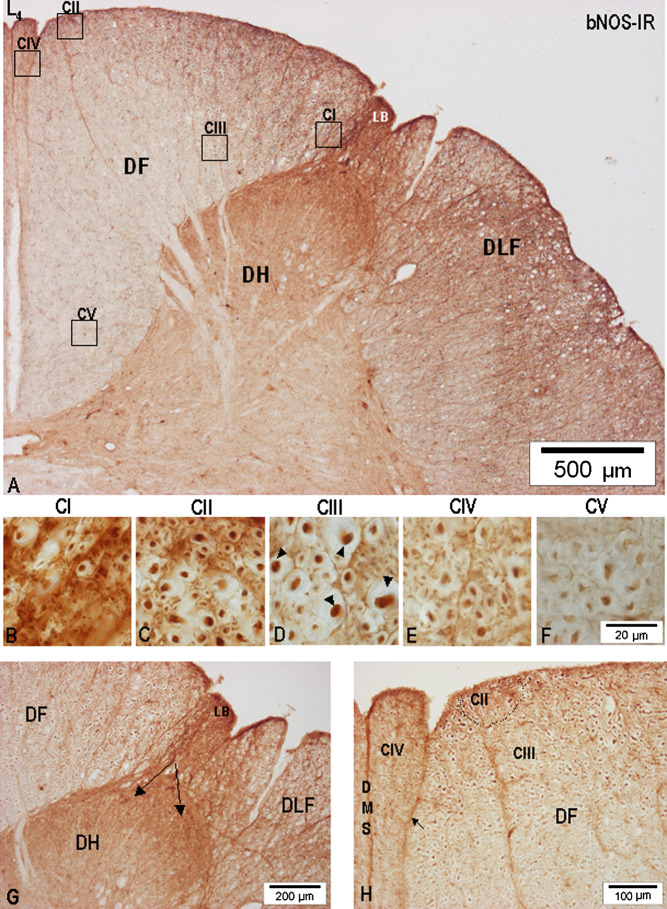
Fig. 8.
(A) Low-power microphotograph of the dorsal half of L4 segment cut through the uppermost portion of the lumbar intumescence just above the entry of the L4 dorsal root. Samples from the boxed areas in the DF were used to depict the nNOS-IR fiber patterns in CI–CV. (B–F) High-power microphotographs of nNOS-IR in CI–CV; arrowheads point to identified nNOS-IR axons. (G) High nNOS-IR of the LB is seen to proceed (arrows) into the dorsal and lateral superficial parts (asterisks) of the dorsal horn (DH); DLF, dorsolateral funiculus. H, large-caliber nNOS-IR (CIII) and small-caliber nNOS-IR CIV located along the dorsal median sulcus and septum (DMS) is (at least in some sections) divided by a septum (arrow) similar to but not identical with the dorsal intermediate septum seen at midthoracic level.
Fig. 9.
(A) Low-power microphotograph depicting the wedge-shaped DF in L2 segment greatly reduced in size in comparison with middle and lower lumbar segments; samples taken from the boxed areas (CI–CV) were used to demonstrate nNOS-IR fibre patterns. CcL, Clarke’s column. (B–F) High-power microphotographs of nNOS-IR fiber patterns in CI–CV; note that large-caliber nNOS-IR axons (arrowheads) could be identified in CIII. (G) Characteristic appearance of nNOS-IR axons in the lateral part of CIII in the DF close to the LB. (H) Dashed line bordering CIII and CIV; large-caliber nNOS-IR axons could not be seen in CIV.
While the ventrolateral portion, i.e., CI of small-caliber nNOS-IR fibers remain closely related to the dorsolateral part of the dorsal horn throughout all lumbar segments, detailed analysis reveals that only the ventrally oriented part of CI abutting on the dorsal horn contains transversally cut nNOS-IR fibers (Figs. 7B, 8B, 9B), and those detected just below the dorsolateral sulcus, appearing as an intensely nNOS-IR area, are in fact longitudinally and obliquely cut small-caliber nNOS-IR fibers of the LB (Figs. 7G, 8G). Although CI is fairly well identified in all lumbar segments, its extent in L3-L1 segments, i.e., above the lumbosacral intumescence, is considerably reduced and, where it closely adjoins the apical portion of the dorsal horn, CI consists of loosely dispersed fibers, some demonstrating quite low nNOS-IR (Figs. 6, 7B, 8B, 9B). CII or dorsomedial portion of the small-caliber nNOS-IR fibers located along or between the incoming large-caliber nNOS-IR axons of the MB, is usually seen as a superficial 150–250 μm broad fiber sheath in L7-L4 segments (Figs. 6, 7A, C, 8A, C, H); more rostrally, in L3-L1 segments, CII gets narrower and after reaching the dorsomedial angle of the DF, it is seen close to the dorsal median septum (Figs. 6, 9A, C). Here, CII passes into CIV extending along the dorsal median septum.
In contrast to the small-caliber nNOS-IR fibers of the LB, large-caliber nNOS-IR axons pass through the DREZ-one and then proceed medially to spread through the major part of the horizontal and vertical limbs of the DF, thus forming the large CIII in all lumbar segments (Figs. 6, 7A, D, G, 8 A, D, H, 9A, D). However, a more detailed analysis shows that a differential distribution of nNOS-IR fibers exists, regarding their caliber range and considering the dorsoventral and mediolateral extent of CIII. Although CIII contains nNOS-IR fibers of varying diameter ranging from 1.1 to 8.0–9.2 μm, the packing density in comparison with CI, CII, and CIV is not only considerably lower but, more importantly, the largest transversally cut nNOS-IR axons ranging between 8.0–9.2 μm in diameter are almost solely seen in the deep portion of the DF and are located around the dorsomedial border of the dorsal horn (Fig. 9G, H). Marked differences in the distribution of large-caliber primary nNOS-IR afferents and heterogeneity of primary afferent fibers in CIII could be identified mainly throughout L7-L4 segments.
A significant number of small-caliber myelinated nNOS-IR and unmyelinated nNOS-IR axons was found in CIV, appearing as a 150–220 μm broad band located in the upper two-thirds of the medialmost part of the DF (Figs. 6, 7E, 8E, 9E), whereas CV was found in the ventral third of the vertical limb of DF in L7-L1 segments (Fig. 6). In the L7-L4 segments CV was characterized by loosely dispersed, some intensely immunolabeled nNOS axons, 1.5–3.5 μm in diameter (Figs. 7F and 8F), whereas those found in CV in L3-L1 segments displayed an apparently lower nNOS-IR (Fig. 9F). Remarkable differences were found in the distribution of nNOS-IR fibers in CI, CII, and CIII, expressed in percentages relative to the DF cross-sectional square area (sq.a.) in S3-L1 segments. While in S3, S2, and S1 segments, the CI, homologous with the Lissauer tract, represents 22.86%, 13.45%, and 9.65%, respectively, of the DF sq.a., i.e., close to the value seen in L7 (10.95%) and L6 (8.44%) segments, the percentage of CI sq.a. detected in the middle (L5, L4) and upper (L3-L1) segments was slightly decreased (Table I). CIII is characterized by great heterogeneity of nNOS-IR fibers ranging from 1.1 to 9.2 μm in diameter, and is clearly the largest in the DF of S3-L1 segments. In proportion to the total DF sq.a. it ranges from 58.40% in S3 segment to 78.84% in L1 segment, while the largest percentage distribution in CII was found in S1 segment. Noticeable differences were detected in the density of nNOS-IR fibers across CI–CV (Table II). The highest density of nNOS-IR fibers was detected in CIV located along the dorsal median septum and, although thin unmyelinated nNOS-IR fibers could invariably be found in CI–CV, there was a concentration of these fibers in the dorsal and middle portions of CIV. Although a decrease in the density of nNOS-IR fibers in CI–CV was noted in the DF of L3-L1 segments, an abrupt decline of such fibers could be identified in CI and CIII related to L3-L1 segments.
Table I.
Percentage Distribution of nNOS-IR Axons in Compartments (CI–CV) of the Dorsal Funiculus in S3-L1 Segments*
| Compartments | |||||
|---|---|---|---|---|---|
| (Percentage (%) distribution) | |||||
| Segments | CI | CII | CIII | CIV | CV |
| S3 | 22.86 | 5.32 | 58.40 | 6.15 | 7.27 |
| S2 | 13.45 | 4.87 | 69.20 | 1.87 | 10.61 |
| S1 | 9.65 | 7.43 | 62.22 | 10.22 | 10.48 |
| L7 | 10.95 | 5.16 | 63.34 | 4.95 | 15.60 |
| L6 | 8.44 | 5.60 | 71.26 | 3.40 | 11.30 |
| L5 | 6.84 | 5.35 | 72.90 | 4.88 | 10.03 |
| L4 | 6.78 | 3.87 | 68.16 | 6.61 | 14.48 |
| L3 | 5.62 | 4.03 | 79.98 | 4.70 | 5.67 |
| L2 | 5.52 | 4.70 | 77.73 | 5.34 | 6.71 |
| L1 | 6.40 | 3.94 | 78.84 | 3.79 | 7.03 |
*Total square area of each dorsal funiculus represents 100%. The description of the quantitative assessment of the square area of the DF in LS segments is given in the Material and Methods section.
Table II.
Number of nNOS-IR Axons in the Dorsal Column of S3-L1 Segments*
| Compartments | |||||
|---|---|---|---|---|---|
| Segments | CI | CII | CIII | CIV | CV |
| S3 | 51.4 ± 1.77 | 89.2 ± 2.05 | 20.6 ± 1.28 | 78.0 ± 2.38 | 35.8 ± 2.78 |
| S2 | 75.6 ± 2.42 | 65.8 ± 1.85 | 23.6 ± 1.43 | 104.4 ± 4.63 | 19.2 ± 1.39 |
| S1 | 72.4 ± 1.86 | 71.4 ± 2.08 | 31.0 ± 1.78 | 110.4 ± 3.29 | 23.0 ± 2.12 |
| L7 | 64.2 ± 2.08 | 63.0 ± 1.81 | 24.2 ± 1.65 | 89.4 ± 1.50 | 30.2 ± 2.43 |
| L6 | 55.4 ± 1.91 | 52.0 ± 1.81 | 24.0 ± 1.51 | 65.0 ± 1.84 | 23.0 ± 1.73 |
| L5 | 35.8 ± 1.93 | 68.0 ± 2.07 | 24.6 ± 1.56 | 77.0 ± 2.64 | 26.2 ± 2.08 |
| L4 | 45.8 ± 1.85 | 74.4 ± 1.93 | 45.8 ± 1.98 | 54.6 ± 2.13 | 30.2 ± 1.15 |
| L3 | 18.0 ± 1.22 | 50.0 ± 1.22 | 19.8 ± 0.86 | 33.0 ± 1.52 | 24.0 ± 1.30 |
| L2 | 16.4 ± 1.53 | 42.8 ± 1.59 | 14.2 ± 1.28 | 24.0 ± 1.58 | 18.8 ± 1.15 |
| L1 | 13.0 ± 0.89 | 52.6 ± 1.60 | 12.6 ± 0.87 | 23.8 ± 1.71 | 15.4 ± 1.21 |
*The description of the quantitative assessment and density in CI–CV of the DF in LS segments is given in the Material and Methods section.
L7-S1 Dorsal Rhizotomy-Induced Depletion of nNOS-IR in the DF of Sacral and Lower Lumbar Segments
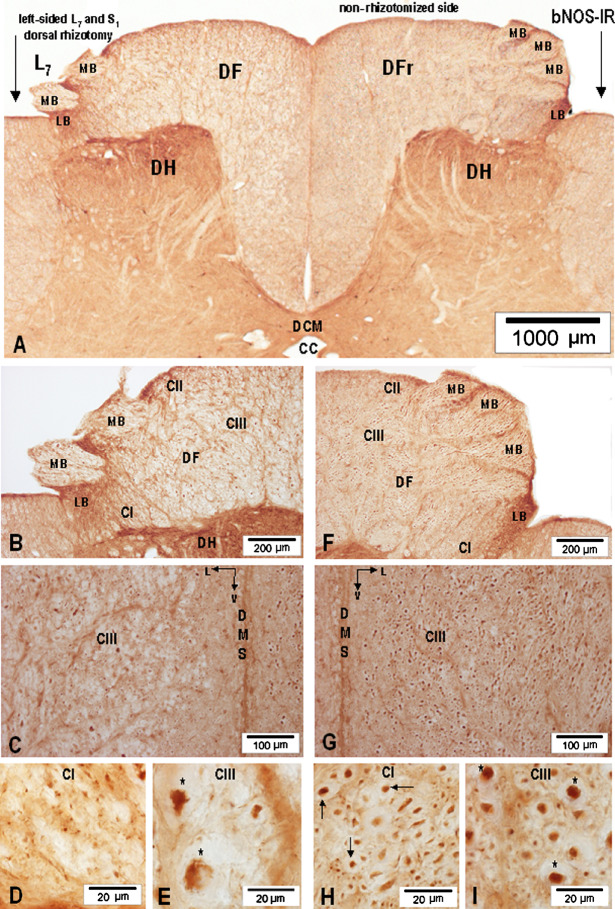
Signs of L7-S1 dorsal rhizotomy-induced depletion of axonal nNOS-IR and anterograde degeneration were revealed in the L7 and S1 MB of the dorsal root, DREZ-one and DF at the level of L7 and S1 segments on the rhizotomized side (Fig. 10A(left half)–B), while nNOS-IR axons identified on the homotopic non-rhizotomized side retained their axonal nNOS-IR (Figs. 10A(right half), F–G). More detailed analysis of sections cut from deafferented segments and processed for nNOS-IR demonstrated that the DF on the rhizotomized side acquired a spongy appearance and, whereas the basal part of the DF along the dorsal median septum containing CV and CIV retained an almost unaltered appearance, the major portion of the DF (i.e., CI–CIII) was beset by numerous lacunae of various sizes and shapes accompanied by fragments of degenerating and nNOS-IR-depleted axons (Fig. 10B, C). Comparison of homotopic loci of the DF on the rhizotomized side, i.e., CI and CIII (Fig. 10D, E), with the contralateral, non-rhizotomized side (Fig. 10H, I) revealed that the texture of CI and CIII was visibly altered; nNOS-IR axonal profiles and axolemma within CI and CIII lost their smooth circular outlines, tended to become wrinkled and distorted, and as a rule demonstrated a variously expressed decrease in the intensity of axonal nNOS-IR. Characteristic signs of anterograde degeneration and nNOS-IR depletion were seen more clearly on transverse sections cut through the large-caliber nNOS-IR fibers, presumed to be stem Ia proprioceptive afferents.
Fig. 10.
(A) Low-power microphotograph showing depletion of axonal nNOS-IR and degeneration of nNOS-IR axons in the MB of the dorsal root (MB-2X) and DF of L7 segment after unilateral left-sided L7 and S1 dorsal rhizotomy. The right (MB-3X) and right dorsal funiculus (DFr) on the non-rhizotomized side remained unchanged; cc, central canal; DCM, dorsal commissure. (B–E) Depletion of axonal nNOS-IR in the medial bundle (MB-2X), DF, CI–CIII, and degeneration of small-caliber nNOS-IR axons in CI and large-caliber nNOS-IR axons (asterisks) in CIII; v, ventrally. (F, G) Normally-appearing nNOS-IR fibers in the medial bundle (MB-3X). (H) High-power microphotograph depicting normally-appearing nNOS-IR axons (arrows) in CI. (I) High-power microphotograph showing normally-appearing large-caliber nNOS-IR axons (asterisks) in CIII.
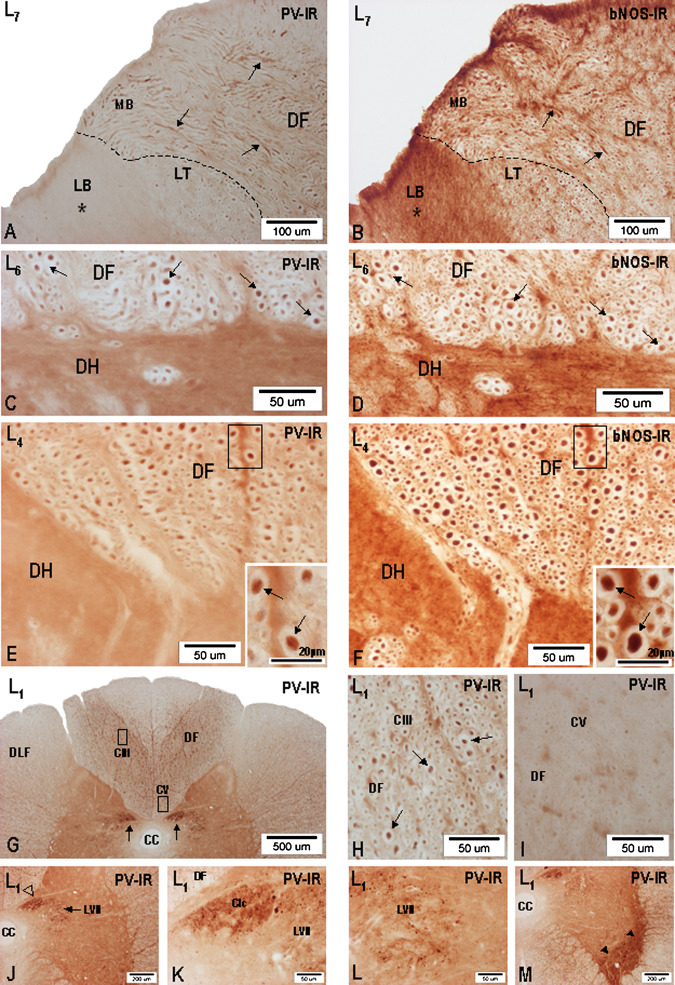
The Distribution and Co-Localization of PV-IR and nNOS-IR Fibres in the DF of S3-L1 Segments
Large-diameter PV-IR fibers were followed on transverse and longitudinal sections cut through S3-L1 segments. First, PV-IR fibers could be seen in the MB of all S3-L1 dorsal roots. However, the occurrence of large-diameter PV-IR axons was most prominent in the S2-L4 dorsal roots related to the lumbosacral intumescence (Fig. 11A). In all specimens studied, the main bulk of large-diameter PV-IR fibers, some 8.0–9.2 μm thick, was identified in the S1, L7, and L6 dorsal roots. After entering the spinal cord through the dorsolateral sulcus, PV-IR fibers were seen to pass through the DREZ-one and then proceed medially into the dorsolateral portion of the DF, thus taking the course corresponding to the path followed by large-diameter primary afferent fibers. A rather complex appearance of PV-IR axons was found in the DREZ-one, consisting of many large-diameter PV-IR profiles oriented not only perpendicularly to the level of the section, but often seen running obliquely or transversally. The largest PV-IR axons were repeatedly found in the deep portion of the DF along the dorsal and dorsomedial borders of the dorsal horn. The distribution of PV-IR fibers in the DF of S3-L1 segments closely mimic the position of large-caliber nNOS-IR fibers described above, and encompassing CIII (Fig. 12). The majority of PV-IR fibers is therefore found concentrated in the deep central part of the DF and, occasionally, a small number of such large-caliber PV-IR axons could be identified close to the surface of the DF in L7 and L6 segments. Detailed analysis discloses a rather stepwise transition in the density of large- and medium-sized PV-IR fibers passing from the central portion into the ventral third of the DF, which almost completely lacks PV-IR fibers, compared with a distinct demarcation of the dorsomedial portion of the DF containing many PV-IR axons against a narrow vertically oriented band of the white matter located along the dorsal median sulcus, apparently lacking PV-IR and characterized as CIV in the preceding description.
Fig. 11.
Microphotographs showing the distribution of PV-IR (A, C, E, G, H, J, L, M) and nNOS-IR (B, D, F) fibers in the DF of L7, L6, L4, and L1 segments and terminal arborizations of PV-IR fibers in Clarke’s column, lamina VII, and ventral horn. (A) Low-power microphotograph showing the occurrence of large-diameter PV-IR fibers (PV-IR axons) of the MB, entering the DF of L7 segment. No signs of PV-IR can be seen in the entering LB (asterisk). (B) Occurrence of large-diameter nNOS-IR fibers (arrows) in the MB entering the DF of L7 segment. High nNOS-IR is seen in the entering LB. (C–D) Pair of transverse sections (PV-IR: nNOS-IR) cut through the L6 segment and depicting the deep portion of the DF close to the DH. Co-localisation of PV-IR and nNOS-IR in large-diameter axons (arrows). (E–F) Pair of transverse sections (PV-IR: nNOS-IR) cut through the L4 segment and depicting the deep portion of the DF close to the dorsomedial border of the DH. Insets in the lower right corner in (E) and (F) present high-power microphotographs taken from the small framed area demonstrating co-localisation of PV-IR and nNOS-IR in large-diameter axons (arrows). (G) Low-power microphotograph showing the distribution of PV-IR in the DF and terminal arborizations of PV-IR fibers in Clarke’s column (arrows) in L1 segment. (H) Occurrence of PV-IR axons (arrows) in CIII (framed area in G) of the DF in L1 segment. (I) PV-IR fibers are almost completely lacking in CV (framed area in G) of the DF. (J) PV-IR in Clarke’s column (open arrowhead) and in the medial part of lamina VII (arrow) in L1 segment. (K) Dense PV-IR in the neuropil of Clarke’s column (Clc) in L1 segment; LVII, lamina VII. (L) PV-IR in the neuropil of lamina VII (LVII) of L1 segment. (M) Dense PV-IR in the neuropil of the ventrolateralpart of the ventral horn (arrowheads) in L1 segment; cc, central canal.
Fig. 12.
Schematic depiction of the distribution of PV-IR axons in CIII of S2, L7, L5, L3, and L1 segments. The distribution of PV-IR axons is given by black dots of different sizes in the white matter of CIII. Obliquely and/or longitudinally cut PV-IR axons entering the dorsolateral part of the DF are depicted as short rods. CI, CII, and CIV were free of PV-IR fibers.
While in L1-L3 segments, i.e., above the lumbosacral intumescence, the distribution of PV-IR axons in the DF, when viewed bilaterally, appeared as two divergently oriented fiber columns rich in PV-IR and extending along the inner border of the dorsal horn, no signs of PV-IR fibers could be seen in the dorsomedial portion of the DF, triangular in outline (Fig. 11G–I). To compare the distribution of PV-IR axons in relation to that of nNOS-IR ones throughout the DF of the lumbosacral segments, we used immunohistochemistry on pairs of adjacent sections alternately processed for PV-IR and nNOS-IR (Fig. 11B). Although specific fiber immunolabeling for PV-IR and nNOS-IR was found profusely throughout the DF of S3-L1 segments, distinct immunolabeling for both compounds was quite apparent in large-caliber fibers, presumed to be Ia axons in the deep portion of the DF close to the dorsal and along the medial border of the dorsal horn (Fig. 11C–F).
While individually running small-caliber PV-IR fibers were seen in the deep laminae of the dorsal horn, across the lateral part of the intermediate zone (lamina VII) of all segments studied, a dense accumulation of PV-IR axonal arborizations was found in the neuropil of the medial half of lamina VII, the ventral and ventrolateral parts of the ventral horn, especially throughout the extent of the lumbosacral intumescence (S2-L4 segments) and, notably, in the neuropil of Clarke’s column in L4-L1 segments (Fig. 11J–M). Among the densely packed PV-IR axonal fragments, spherical or ovoid bouton-like structures 5.0–7.0 μm in diameter were found. Sporadically, PV-IR elements similar to giant boutons were noted.
DISCUSSION
The present study is concerned with the occurrence and compartmentalization of primary afferent nNOS-IR and PV-IR fibers in the DF of the lumbosacral spinal cord in the dog. An interesting finding is that dorsal root nNOS-IR and PV-IR afferents in all lumbosacral segments, shortly before entering the DREZ-one, segregate in such a way that large-diameter nNOS-IR and PV-IR axons enter the MB of the dorsal root, while small-diameter nNOS-IR axons but not PV-IR participate in the formation of the LB which upon entering the DREZ-one tend to proceed in the ventrolateral direction and approach the dorsolateral border of the dorsal horn. Thus, our study based on nNOS-IR and PV-IR immunohistochemistry documents that the MB of the dog, previously well described in man and monkey (Sindou et al., 1974; Snyder, 1977) but absent in cat (Snyder, 1977) and hardly detectable in rat (Willis and Coggeshall, 1991), is a large anatomical entity containing nNOS-IR and PV-IR fibers, the latter being a highly reliable, although not exclusive, marker for fast-conducting Ia proprioceptive muscle afferents originating from muscle spindles (Clowry et al., 2000, 2005; Dekkers et al., 2002). Once the small-diameter LB and large-diameter MB enters the DREZ-one, the labeling of nNOS-IR is quite apparent in the LB, while PV-IR is strictly limited to the large-diameter axons of the MB. In contrast, the LB is lacking in any PV-IR fibers (see Fig. 11A, B).
The presence of large-diameter nNOS-IR and PV-IR fibers identified in the DREZ-one of lumbosacral segments may be of clinical significance, since guided neurosurgical interventions using microsurgery and intraoperative neural stimulation (i.e., drezotomy) targeted at surgical management of spasticity, a technique mainly indicated in patients suffering from localized spasticity without useful mobility (Lazorthes et al., 2002), potentially affects not only small-diameter fibers transporting nNOS, substance P (Molander et al., 1987) and calcitonin gene-related peptide (Patterson et al., 1990, 1992), but also the large-diameter nNOS-IR and PV-IR axons, thus adversely influencing the anterograde transport of nNOS, PV confirmed in the present study and glutamate verified in the afferent limb of the Ia-motoneuron pathway (Maxwell et al., 1990).
Immunohistochemistry of nNOS alone and in combination with PV revealed distinct differences in the distribution and density of axons divisible into CI–CV in all lumbosacral segments. Moreover, the co-localization of nNOS-IR and PV-IR suggests that the role of nNOS in the processing of the proprioceptive, exteroceptive, nociceptive, and visceroceptive afferentation needs to be reconsidered. Small unmyelinated and myelinated nNOS-IR fibers and large-diameter myelinated nNOS-IR and PV-IR fibers were found unevenly distributed across the DF of lumbosacral segments. With regard to the fiber spectra and packing density, the DF was divided into CI–CV traceable throughout the S3-L1 segments. When the extent, location, and fiber spectra of CI and CII were compared with the distribution of small-diameter fibers in the DREZ-one, it was apparent that CI was homologous with the Lissauer (dorsolateral) tract (Chung and Coggeshall, 1985, 1987; Chung et al., 1985, 1987) or, more exactly, that CI represents the nNOS-IR component of this pathway, while CII located dorsomedially to the MB closely resembles the dorsomedial tract described in the developing human spinal cord (Altman and Bayer, 2001). Examination of CI and CII, both characterized by an almost homotypic fiber pattern and location in the dorsolateral part of the DF in sacral and lumbar segments, showed that CI achieves its largest extent in the sacral segments. However, passing rostrally, CI is steadily decreasing. A noticeable decrease in the density of nNOS-IR fibers in CI was found in L3-L1 segments, thus confirming previous findings that small-diameter primary nociceptive afferents are thought to travel over relatively few segments as they enter the cord, and assemble in the Lissauer tract and dorsolateral funiculus (Willis and Coggeshall, 1991). Rostrally passing nNOS-IR fibers in CII move more medially, covering the dorsal portion of the DF as a narrow layer of small-diameter nNOS-IR fibers (see Figs. 2 and 3). At mid-lumbar level, nNOS-IR fibers in CII approach the dorsomedial edge of the DF and merge with nNOS-IR fibers of the Philippe–Gombault triangle and CIV located close to the dorsal median septum (see Fig. 2). The finding of a quantitatively unspecified number of small-diameter unmyelinated and myelinated nNOS-IR fibers in CII, originating from the sacral dorsal roots, brings evidence that a previously described long, ascending unmyelinated primary visceral pain-transmitting pathway in the DF (Patterson et al., 1990, 1992; Hirshberg et al., 1996; Willis et al., 1999) contains nNOS-IR axons. The occurrence of a considerable number of small-diameter nNOS-IR and/or NADPHd-stained somata detected in S3-S1 DRGs in the dog strongly supports this view (Lukáčová et al., 2006).
Not only do the fibers of CIII take up the largest area of the DF in the lumbosacral segments, but also display great heterogeneity in fiber size—they are evidently nNOS-IR and PV-IR. Co-localization of nNOS-IR and PV-IR is seen throughout CIII. The heterogeneous appearance of myelinated nNOS-IR and PV-IR fibers varying in size seen throughout CIII bears some resemblance to the description of the funicular trajectory of proprioceptive afferents stained with horse-radish peroxidase in the lumbosacral spinal cord of the cat (Ishizuka et al., 1979) and the functional organization of afferents in the DF (Uddenberg, 1968). As shown on transverse sections cut from S3-L1 segments, the large-diameter nNOS-IR and PV-IR axons passing through the DREZ-one proceed in a ventromedial direction, and many of them are seen in the DF close to the dorsal and dorsomedial border of the dorsal horn. Considering their size and general appearance, one can reasonably assume that they represent the stem Ia axons before their bifurcation into an ascending branch and a descending branch (see Fig. 1A, D). Co-localization of nNOS-IR and PV-IR in axons of the MB of the dorsal root and in the DF implies that both compounds, i.e., nNOS and PV, may play a role in the processing of the proprioceptive and, perhaps exteroceptive afferentation carried by the large-diameter myelinated axons of the MB (Knyihár-Csillik et al., 1999; Altman and Bayer, 2001). Recent developmental and tract-tracing studies strongly support the view that, of the four different classes of sensory fibers that reach the spinal cord (Sherrington, 1906), the large-diameter proprioceptive fibers are strongly PV-IR. The finding that nNOS-IR was found co-localized with PV-IR in the large-diameter, fast-conducting primary proprioceptive fibers providing afferent input to motoneurons suggests that the pattern of motor activity may be, at least at monosynaptic Ia-motoneuron level, under control of PV and nNOS (Dekkers et al., 2002). The potential functional significance of bNOS-IR and PV-IR co-localization may be anticipated on the basis of known facts, that fast-conducting PV-IR proprioceptive Ia afferents coiling around intrafusal muscle fibers (Celio, 1990; Zhang et al., 1990) form the basis of the monosynaptic stretch reflex circuit (Brown and Fyffe, 1981; Kudo and Yamada, 1987).
The occurrence of large-diameter nNOS-IR and PV-IR axons in the dog DF is consistent with recent findings characterizing nNOS-IR and/or NADPHd-stained somata in L1-L7 and S1-S3 DRGs of the dog. nNOS-IR was present in large number of small (1000 μm2) and medium-sized (1000–2000 μm2) neurons, and in a limited number of large-diameter (2000 μm2) neurons (Lukáčová et al., 2006). Both staining procedures mentioned above proved to be highly effective in visualizing intraganglionic fibers of various diameters. In general, the largest nNOS-IR fibers revealed at the peripheral end of lumbar and sacral DRGs were larger, while those running centrally were slightly reduced in size, ranging between 6.49–9.15 μm in diameter. Large- and small-diameter nNOS-IR central processes of L1-S3 DRGs neurons segregated shortly before entering the spinal cord, the former making a massive MB of the dorsal root, while the small-diameter nNOS-IR fibers represented the LB penetrated Lissauer tract (Lukáčová et al., 2006).
Distinct differences in the distribution of unmyelinated and small-diameter myelinated nNOS-IR axons versus large-diameter myelinated nNOS-IR and PV-IR fibers in the DF were found all along the lumbosacral spinal cord. The total square area taken up by large-diameter nNOS-IR and PV-IR fibers in the DF of S3-S1 segments, mainly in the extent of the lumbosacral intumescence, clearly exceeds that taken up by aggregated small-diameter nNOS-IR fibers. However, a noticeable decrease in the DF square area noted in the upper lumbar segments together with a marked decline in large-diameter nNOS-IR and PV-IR fibers in the DF, seen in the transition of the upper portion of the lumbosacral intumescence (L4) into the upper lumbar segments (L3-L1), suggests that a substantial portion of nNOS-IR and PV-IR fibers leaves the DF and, as would be expected, terminate as proprioceptive Ia afferents in laminae VI, VII, and IX of LS segments and in Clarke’s column of L4 and higher lumbar segments (Brown and Fyffe, 1978; Fyffe, 1979; Brown, 1981). We can only speculate as to whether our nNOS-IR and PV-IR technique visualized both groups of large-diameter fibers, i.e., proprioceptive and exteroceptive, entering the DF, or whether only stem Ia proprioceptive axons, their ascending, descending, and main collaterals as characterized after horse-radish peroxidase injection (Ishizuka et al., 1979) were immunolabeled.
In search of further evidence explaining the existence of the remarkable number of large-diameter nNOS-IR fibers in CIII of the DF in LS segments, one-sided L7 and S1 dorsal rhizotomy was performed. Six days after this rhizotomy, clear signs of anterograde degeneration accompanied by nNOS-IR depletion were readily established in CI–CIII containing the previously-described axons of various thicknesses, but then a strikingly homotypic type of Wallerian degeneration coupled with nNOS-IR-depletion emerged, affecting nNOS-IR axons and their collaterals in CIII, which are known for their great heterogeneity (see Fig. 10A, B versus F, C versus G, and E versus L).
The present nNOS-IR study supports our previous findings based on radioassay analysis documenting that a measurable amount of catalytic nNOS activity in the DF of the dog exists (Lukáčová et al., 2002), and it presents evidence that large-diameter nNOS-IR axons of extrinsic, i.e., DRGs neurons contribute to nNOS-IR in the gray matter of the lumbosacral intumescence. Dorsal rhizotomy-induced depletion of nNOS-IR detected in the DF of L7 and S1 segments, along with the reduction of nNOS-IR in axon terminals of nNOS-IR proprioceptive afferents issuing in the muscle spindles of the triceps surae and quadriceps femoris muscle strongly supports this view (Maršala et al., 2005, 2006).
The functional significance of the present finding that large-diameter nNOS-IR and PV-IR axons and their collaterals in CIII occur throughout the lumbosacral intumescence (S2-L4 segments), may consist in their ability to release NO upon stimulation. This assumption is based on the results obtained using the luminol chemiluminiscence method allowing for visualization of NO formation (Wiklund et al., 1997, 1999). Notwithstanding that our present study is aimed mainly at a detailed description of the distribution pattern in CI–CV in the DF of lumbosacral segments, it may be linked with a recent study dealing with the monosynaptic Ia-motoneuron pathway in the dog (Maršala et al., 2005), which described the nitrergic afferent limb of the stretch reflex circuit, and disclosed proprioceptive nNOS-IR terminal arbors and Ia boutons in terminal regions detected previously by tract-tracing methods in lamina VI and VII (Maxwell and Bannatyne, 1983; Fyffe and Light, 1984), Clarke’s column (Walmsley et al., 1985; Nicol and Walmsley, 1991), and lamina IX (Ishizuka et al., 1979; Hongo et al., 1987; Pierce and Mendell, 1993). Analyzing the proprioceptive afferent pathway from the gastrocnemius-soleus muscles after L7 and S1 dorsal rhizotomy, nNOS-IR Ia boutons could be identified in all gray matter layers known to receive proprioceptive afferents from the gastrocnemius-soleus muscle.
The compartmental distribution of unmyelinated and small-diameter myelinated nNOS-IR axons in S3-L1 segments of the dog DF (see Figs. 2, 6 and 12) clearly differs from that given for funicular distribution of unmyelinated axons in the DF of the rat (Chung et al., 1987; see Fig. 5), where most of such fibers appear to be concentrated in the dorsolateral part of the DF close to the Lissauer tract. Further differences were found in the compartmental distribution of large-diameter nNOS-IR and PV-IR axons, occurring in CIII and taking up a large area of the DF, but lacking in the medial, basal, and dorsolateral portions of the DF, whereas large myelinated primary afferent fibers in the rat were found to be homogenously distributed throughout the DF, with the exception of an oval area located in the ventromedial part of the DF known to contain axons of the corticospinal tract (Chung et al., 1987; see Fig. 4).
In conclusion, the results of the immunohistochemical analysis of the lumbosacral DF suggest that large-caliber nNOS-IR and PV-IR axons, their ascending and descending collaterals present in large number in the DF of the lumbosacral intumescence are primary proprioceptive afferents participating in the formation of the monosynaptic Ia-motoneuron pathway.
Acknowledgments
The authors thank Mr. D. Krokavec, Ms. M. Špontáková, Mrs. M. Syneková, and Mrs. A.M. Košová for their excellent technical assistance. The experimental work was supported by the VEGA Grants No. 2/3217/23 and 2/5134/25 from the SAS, APVT Grant No. 51-013002, and NIH grants NS 32794 and NS 40386 to M.M.
Abbreviations used:
- CI–CV
fibre compartments in DF
- DF
dorsal funiculus
- DRG
dorsal root ganglion
- DREZ-one
dorsal root entry zone
- eNOS
endothelial nitric oxide synthase
- LB
lateral bundle of the dorsal root
- LS
lumbosacral segments
- MB
medial bundle of the dorsal root
- mNOS
macrophage nitric oxide synthase
- NADPHd
nicotinamide adenine dinucleotide phosphate diaphorase
- nNOS-IR
neuronal nitric oxide synthase immunoreactivity
- PV-IR
parvalbumin immunoreactivity
- TPG
the triangle of Philippe–Gombault.
REFERENCES
- Altman, J., and Bayer, S. A. (2001). Development of the Human Spinal Cord. An Interpretation Based on Experimental Studies in Animals. Oxford University Press, Oxford. [Google Scholar]
- Besson, J. M., and Chaouch, A. (1987). Peripheral and spinal mechanisms of nociception. Physiol. Rev.67:67–186. [DOI] [PubMed] [Google Scholar]
- Bredt, D. S., Hwang, P. M., and Snyder, S. H. (1990). Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature347:768–770. [DOI] [PubMed] [Google Scholar]
- Brodal, A. (1969). Neurological Anatomy. Oxford University Press, New York. [Google Scholar]
- Brown, A. G., and Fyffe, R. E. (1978). The morphology of group Ia afferent fiber collaterals in the spinal cord of the cat. J. Physiol.274:111–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, A. G., and Fyffe, R. E. (1981). Direct observations on the contacts made between Ia afferent fibres and alpha-motoneurones in the cat’s lumbosacral spinal cord. J. Physiol.313:121–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, A. G. (1981). Organization in the Spinal Cord: The Anatomy and Physiology of Identified Neurones. Springer-Verlag, Berlin. [Google Scholar]
- Celio, M. R. (1990). Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience35:375–475. [DOI] [PubMed] [Google Scholar]
- Chung, K., and Coggeshall, R. E. (1985). Unmyelinated primary afferent fibers in dorsal funiculi of cat sacral spinal cord. J. Comp. Neurol.238:365–369. [DOI] [PubMed] [Google Scholar]
- Chung, K. S., and Coggeshall, R. E. (1987). Postnatal development of the rat dorsal funiculus. J. Neurosci.7:972–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, K., Langford, L. A., and Coggeshall, R. E. (1987). Primary afferent and propriospinal fibers in the rat dorsal and dorsolateral funiculi. J. Comp. Neurol.263:68–75. [DOI] [PubMed] [Google Scholar]
- Chung, K., Sharma, J., and Coggeshall, R. E. (1985). Numbers of myelinated and unmyelinated axons in the dorsal, lateral, and ventral funiculi of the white matter of the S2 segment of cat spinal cord. J. Comp. Neurol.234:117–121. [DOI] [PubMed] [Google Scholar]
- Clowry, G. J., Arnott, G. A., Clement-Jones, M., Fallah, Z., Gould, S., and Wright, C. (2000). Changing pattern of expression of parvalbumin immunoreactivity during human fetal spinal cord development. J. Comp. Neurol.423:727–735. [PubMed] [Google Scholar]
- Clowry, G. J., Moss, J. A., and Clough, R. L. (2005). An immunohistochemical study of the development of sensorimotor components of the early fetal human spinal cord. J. Anat.207:313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers, J., Greensmith, L., and Navarrete, R. (2002). Changes in the expression of parvalbumin immunoreactivity in the lumbar spinal cord of the rat following neonatal nerve injury. Dev. Neurosci.24:283–293. [DOI] [PubMed] [Google Scholar]
- Dinerman, J. L., Dawson, T. M., Schell, M. J., Snowman, A., and Snyder, S. H. (1994). Endothelial nitric oxide synthase localized to hippocampal pyramidal cells: Implications for synaptic plasticity. Proc. Natl. Acad. Sci. USA91:4214–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe, R. E. W. (1979). The morphology of Group II muscle afferent fiber collaterals. J. Physiol.296:39–40. [PubMed] [Google Scholar]
- Fyffe, R. E. W., and Light, A. R. (1984). The ultrastructure of group Ia afferent fiber synapses in the lumbosacral spinal cord of the cat. Brain Res.300:201–209. [DOI] [PubMed] [Google Scholar]
- Hirshberg, R. M., Al-Chaer, E. D., Lawand, N. B., Westlund, K. N., and Willis, W. D. (1996). Is there pathway in the posterior funiculus that signals visceral pain? Pain67:291–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo, T., Kudo, N., Sasaki, S., Yamashita, M., Yoshida, K., Ishizuka, N., and Mannen, H. (1987). Trajectory of group Ia and Ib fibers from the hind-limb muscles at the L3 and L4 segments of the spinal cord of the cat. J. Comp. Neurol.262:159–194. [DOI] [PubMed] [Google Scholar]
- Ishizuka, N., Mannen, H., Hongo, T., and Sasaki, S. (1979). Trajectory of group Ia afferent fibers stained with horseradish peroxidase in the lumbosacral spinal cord of the cat: Three dimensional reconstructions from serial sections. J. Comp. Neurol.186:189–212. [DOI] [PubMed] [Google Scholar]
- Knyihár-Csillik, E., Rakic, P., and Csillik, B. (1999). Illusive transience of parvalbumin expression during embryonic development of the primate spinal cord. Int. J. Dev. Neurosci.17:79–97. [DOI] [PubMed] [Google Scholar]
- Kudo, N., and Yamada, T. (1987). Morphological and physiological studies of development of the monosynaptic reflex pathway in the rat lumbar spinal cord. J. Physiol.389:441–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazorthes, Y., Sol, J. C., Sallerin, B., and Verdié, J. C. (2002). The surgical management of spasticity. Europ. J. Neurol.9:35–41. [DOI] [PubMed] [Google Scholar]
- Lukáčová, N., Čížková, D., Maršala, M., Lukáč, I., and Maršala, J. (2002). The regional distribution of nitric oxide synthase activity in the spinal cord of the dog. Brain Res. Bull.58:173–178. [DOI] [PubMed] [Google Scholar]
- Lukáčová, N., Kolesár, D., Maršala, M., and Maršala, J. (2006). Immunohistochemical, histochemical and radioassay analysis of nitric oxide synthase immunoreactivity in the lumbar and sacral dorsal root ganglia of the dog. Cell. Mol. Neurobiol.26:17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maršala, J., Maršala, M., Vanický, I., and Taira, Y. (1999). Localization of NADPHd-exhibiting neurons in the spinal cord of the rabbit. J. Comp. Neurol.406:263–284. [DOI] [PubMed] [Google Scholar]
- Maršala, J., Lukáčová, N., Čížková, D., Kafka, J., Katsube, N., Kuchárová, K., and Maršala, M. (2002). The case for the bulbospinal respiratory nitric oxide synthase-immunoreactive pathway in the dog. Exp. Neurol.177:115–132. [DOI] [PubMed] [Google Scholar]
- Maršala, J., Kluchová, D., and Maršala, M. (1997). Spinal cord gray matter layers rich in NADPH diaphorase-positive neurons are refractory to ischemia-reperfusion-induced injury: a histochemical and silver impregnation study in rabbit. Exp. Neurol.145:165–179. [DOI] [PubMed] [Google Scholar]
- Maršala, J., Lukáčová, N., Čížková, D., Lukáč, I., Kuchárová, K., and Maršala, M. (2004). Premotor nitric oxide synthase immunoreactive pathway connecting lumbar segments with the ventral motor nucleus of the cervical enlargement in the dog. J. Chem. Neuroanat.27:43–54. [DOI] [PubMed] [Google Scholar]
- Maršala, J., Lukáčová, N., Kolesár, D., Kuchárová, K., and Maršala, M. (2006). Nitrergic proprioceptive afferents originating from quadriceps femoris muscle are related to monosynaptic Ia-motoneuron stretch reflex circuit in the dog. Cell. Mol. Neurobiol. 26:1385–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maršala, J., Lukáčová, N., Šulla, I., Wohlfahrt, P., and Maršala, M. (2005). The evidence for nitric oxide synthase immunopositivity in the monosynaptic Ia-motoneuron pathway of the dog. Exp. Neurol.195:161–178. [DOI] [PubMed] [Google Scholar]
- Maršala, J., Maršala, M., Lukáčová, N., Ishikawa, T., and Čížková, D. (2003). Localization and distribution patterns of nicotinamide adenine dinucleotide phosphate diaphorase exhibiting axons in the white matter of the spinal cord of the rabbit. Cell. Mol. Neurobiol.23:57–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maršala, J., Šulla, I., Jalč, P., and Orendáčová, J. (1995). Multiple protracted cauda equina constrictions cause deep derangement in the lumbosacral spinal cord circuitry in the dog. Neurosci. Lett.193:97–100. [DOI] [PubMed] [Google Scholar]
- Maršala, J., Vanický, I., Maršala, M., Jalč, P., Orendáčová, J., and Taira, Y. (1998). Reduced nicotinamide adenine dinucleotide phosphate diaphorase in the spinal cord of dogs. Neuroscience85:847– 862. [DOI] [PubMed] [Google Scholar]
- Maxwell, D. J., and Bannatyne, B. A. (1983). Ultrastructure of muscle spindle afferent terminations in lamina VI of the cat spinal cord. Brain Res.288:297–301. [DOI] [PubMed] [Google Scholar]
- Maxwell, D. J., Christie, W. M., Ottersen, O. P., and Storm-Mathisen, J. (1990). Terminals of group Ia primary afferent fibers in Clarkes column are enriched with L-glutamate-like immunoreactivity. Brain Res.510:346–350. [DOI] [PubMed] [Google Scholar]
- McKenna, K. E., and Nadelhaft, I. (1986). The organization of the pudendal nerve in the male and female rat. J. Comp. Neurol.22:248:532–549. [DOI] [PubMed] [Google Scholar]
- Molander, C., Ygge, J., and Dalsgaard, C. J. (1987). Substance P-, somatostatin- and calcitonin gene-related peptide-like immunoreactivity and fluoride resistant acid phosphatase-activity in relation to retrogradely labeled cutaneous, muscular and visceral primary sensory neurons in the rat. Neurosci. Lett.74:37–42. [DOI] [PubMed] [Google Scholar]
- Nicol, M. J., and Walmsley, B. (1991). A serial section electron microscope study of an identified Ia afferent collateral in the cat spinal cord. J. Comp. Neurol.314:257–277. [DOI] [PubMed] [Google Scholar]
- Orendáčová, J., Čížková, D., Kafka, J., Lukáčová, N., Maršala, M., Sulla, I., Maršala, J., and Katsube, N. (2001). Cauda equina syndrome. Prog. Neurobiol.64:613–637. [DOI] [PubMed] [Google Scholar]
- Orendáčová, J., Maršala, M., Sulla, I., Kafka, J., Jalč, P., Čížková, D., Taira, Y., and Maršala, J. (2000). Incipient cauda equina syndrome as a model of somatovisceral pain in dogs: Spinal cord structures involved as revealed by the expression of c-fos and NADPH diaphorase activity. Neuroscience95:543–557. [DOI] [PubMed] [Google Scholar]
- Patterson, J. T., Coggeshall, R. E., Lee, W. T., and Chung, K. (1990). Long ascending unmyelinated primary afferent axons in the rat dorsal column: Immunohistochemical localizations. Neurosci. Lett.108:6–10. [DOI] [PubMed] [Google Scholar]
- Patterson, J. T., Chung, K., and Coggeshall, R. E. (1992). Further evidence for the existence of long ascending unmyelinated primary afferent fibers within the dorsal funiculus: effects of capsaicin. Pain49:117–120. [DOI] [PubMed] [Google Scholar]
- Pearson, A. A. (1952). Role of gelatinous substance of spinal cord in conduction of pain. Arch. Neurol. Psych.68:515–529. [DOI] [PubMed] [Google Scholar]
- Pierce, J. P., and Mendell, L. M. (1993). Quantitative ultrastructure of Ia boutons in the ventral horn: Scaling and positional relationships. J. Neurosci.13:4748–4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson, S. W. (1914). An experimental study of Lissauer's tract and the dorsal roots. J. Comp. Neurol.24:531–545. [Google Scholar]
- Sherrington, C. S. (1906). The Integrative Action of the Nervous System. Yale University Press, New Haven. [Google Scholar]
- Sindou, M., Quoex, C., and Baleydier, C. (1974). Fiber organization at the posterior spinal cord-rootlet junction in man. J. Comp. Neurol.153:15–26. [DOI] [PubMed] [Google Scholar]
- Snyder, R. (1977). The organization of the dorsal root entry zone in cats and monkeys. J. Comp. Neurol.174:47–70. [DOI] [PubMed] [Google Scholar]
- Uddenberg, N. (1968). Functional organization of long, second-order afferents in the dorsal funiculus. Exp. Brain Res.4:377–382. [DOI] [PubMed] [Google Scholar]
- Walmsley, B., Wieniawa-Narkiewicz, E., and Nicol, M. J. (1985). The ultrastructural basis for synaptic transmission between primary muscle afferents and neurons in Clarke’s column of the cat. J. Neurosci.5:2095–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, J. C., and Sweet, W. H. (1969). Pain and Neurosurgeon. Thomas, Springfield, IL. [Google Scholar]
- Wiklund, N. P., Cellek, S., Leone, A. M., Iversen, H. H., Gustafsson, L. E., Brundin, L., Furst, V. W., Flock, A., and Moncada, S. (1997). Visualisation of nitric oxide released by nerve stimulation. J. Neurosci. Res.47:224–232. [DOI] [PubMed] [Google Scholar]
- Wiklund, N. P., Iversen, H. H., Leone, A. M., Cellek, S., Brundin, L., Gustafsson, L. E., and Moncada, S. (1999). Visualization of nitric oxide formation in cell cultures and living tissue. Acta Physiol. Scand.167:161–166. [DOI] [PubMed] [Google Scholar]
- Willis, W. D., Al-Chaer, E. D., Quast, M. J., and Westlund, K. N. (1999). A visceral pain pathway in the dorsal column of the spinal cord. Proc. Natl. Acad. Sci. USA96:7675–7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis, W. D., and Coggeshall, R. E. (1991). Sensory Mechanisms of the Spinal Cord, Plenum Press, New York. [Google Scholar]
- Willis, W. D., and Westlund, K. N. (1997). Neuroanatomy of the pain system and of the pathways that modulate pain. J. Clinic. Neurophysiol.14:2–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. H., Morita, Y., Hironaka, T., Emson, P. C., and Tohyama, M. (1990). Ontological study of calbindin-D28k-like and parvalbumin-like immunoreactivities in rat spinal cord and dorsal root ganglia. J. Comp. Neurol.302:715–728. [DOI] [PubMed] [Google Scholar]