Abstract
(1) Axons contain numerous mRNAs and a local protein synthetic system that can be regulated independently of the cell body. (2) In this study, cultured primary sympathetic neurons were employed, to assess the effect of local protein synthesis blockade on axon viability and mitochondrial function. (3) Inhibition of local protein synthesis reduced newly synthesized axonal proteins by 65% and resulted in axon retraction after 6 h. Acute inhibition of local protein synthesis also resulted in a significant decrease in the membrane potential of axonal mitochondria. Likewise, blockade of local protein transport into the mitochondria by transfection of the axons with Hsp90 C-terminal domain decreased the mitochondrial membrane potential by 65%. Moreover, inhibition of the local protein synthetic system also reduced the ability of mitochondria to restore axonal levels of ATP after KCl-induced depolarization. (4) Taken together, these results indicate that the local protein synthetic system plays an important role in mitochondrial function and the maintenance of the axon.
Keywords: Superior cervical ganglia, Axon, Local protein synthesis, Mitochondrial membrane potential, ATP levels
Introduction
Axons and nerve terminals are unique subcellular structures of the neuron that play a critical role in the development and maintenance of neural connectivity. One of the central tenets in neuroscience has been that protein synthesis occurs exclusively in the somatodendritic domain of neurons, and that anterograde transport provides the axons and nerve terminals with molecules required for growth, compartmental specificity, and plasticity of the axon and presynaptic nerve terminal.
Although the majority of neuronal mRNAs are indeed transcribed and translated in the neuronal cell soma, a subset of these gene transcripts are selectively transported to the distal structural/functional domains of the neuron to include the axon and presynaptic nerve terminal (for review, see Giuditta et al. 2002; Piper and Holt 2004). It has also become well established that proteins synthesized from these mRNAs play a key role in the development of the neuron and the function of the axon and nerve terminal, including navigation of the axonal growth cone (Campbell and Holt 2001; Ming et al. 2002; Zhang and Poo 2002), synthesis of membrane receptors employed as axon guidance molecules (Brittis et al. 2002), axon transport (Li et al. 2004), and synapse formation (Schacher and Wu 2002). In invertebrate model systems, the synthesis of proteins in the presynaptic nerve terminal also plays an important part in activity-dependent synaptic plasticity, such as long-term facilitation (Martin et al. 1997; Casadio et al. 1999; Beaumont et al. 2001; Liu et al. 2003; Si et al. 2003). In this regard, the axon might share much in common with dendrites, where local protein synthesis is involved in growth, synaptic plasticity, and long-term potentiation (for review, see Schuman et al. 2006).
In previous studies, we reported that several nuclear-encoded mitochondrial mRNAs were present in the squid giant axon and the presynaptic terminals of photoreceptor neurons (Gioio et al. 2001, 2004). Approximately 25% of the total protein synthesized locally in the nerve terminal was destined for the mitochondria. The synthesis of these proteins was sensitive to cycloheximide, but was resistant to inhibition by chloramphenicol and hence was derived from cytosolic polyribosomes. Based upon these findings, we hypothesized that the local protein synthetic system plays a critical role in the maintenance of the local mitochondrial population and ultimately, the function of the axon and presynaptic nerve terminal.
Mitochondria produce ATP, buffer cytosolic calcium, and sequester apoptotic factors, and thus play a critical role in neuronal function. These organelles are transported to the distal axon and nerve terminal from the neuronal cell soma by fast anterograde transport (see Hollenbeck and Saxton 2005). The organelle is comprised of approximately 1,000–2,000 different proteins, of which <1–2% are encoded by the mitochondrial genome (Anderson et al. 1981; Attardi and Schatz 1988; Truscott et al. 2003). For example, 10 of the 13 subunits of Complex IV, proteins involved in the electron transport chain, derive from the nuclear genome (Heales et al. 2006). However, the half-life of these organelles in the axon and whether there is a turnover of nuclear-encoded mitochondrial proteins or biogenesis of mitochondria in the distal structural domains of the neuron is unknown. In this study, we have investigated the effects of local protein synthesis on the function of mitochondria and axon viability, using primary rat sympathetic neurons cultured in Campenot chambers.
Methods
Neuronal cell cultures
Compartmented cultures were constructed essentially following the protocol of Campenot and Martin (2001). Nunclon-treated, polystyrene plastic dishes (35 mm) with airvent were coated with 1% Vitrogen (Cohesion, Palo Alto, CA) in sterile tissue culture grade ultra pure water. The collagen-coated dishes were scratched using a pin-rake (Tyler Research Corp., Edmonton, Alberta, Canada) to form parallel tracks. Sympathetic superior cervical ganglia (SCG) were obtained from 3 days-old Harlan Sprague–Dawley rats and enzymatically and mechanically dissociated, essentially following the protocol by Johnson (2001). About twenty ganglia were incubated in 0.125% Trypsin (Invitrogen Life Technologies, Carlsbad, CA) for approximately 30 min at 37°C with slow rotation at 25 rpm. Afterwards, the SCG ganglia were rinsed twice in Neurobasal™ Medium with the additives 2% B-27 supplement and 0.5 mM l-Glutamine (Invitrogen Life Technologies, Carlsbad, CA), and 25 μM Glutamic Acid (Sigma-Aldrich, St. Louis, MO). The ganglia were pipetted 20 times with a fire polished sterile cotton-plugged Pasteur pipette and supernatant transferred to a centrifuge tube. This pipetting procedure was repeated twice and the combined supernatants, containing the dissociated neurons, were centrifuged for 5 min at 1,100 rpm (Beckham Allegra 6R Benchtop Centrifuge and GH-3.8A Rotor, Beckman Coulter, Inc., Fullerton, CA). Thereafter, the supernatant was discarded and the pellet containing the cells resuspended in culture medium consisting of Neurobasal™ Medium with additives and 20 U/ml Penicillin/20 μg/ml Streptomycin (Hyclone, Logan, UT) and 50 ng/ml Mouse Nerve Growth Factor 2.5S (Alomone Labs Ltd., Jerusalem, Israel). The neurons were plated in the center compartment at a cell density of about 6,000 neurons per plate. Cytosin-β-d-arabinofuranoside (AraC, 10 μM; Sigma-Aldrich, St. Louis, MO) or 50 nM 5-Fluoro-2′-deoxyuridine (FuDR; Sigma-Aldrich, St. Louis, MO) were added to the culture medium to inhibit the growth of non-neuronal cells 1 day after plating and remained in the media for six additional days. The culture medium was changed every 3–4 days. The complete culture media, including NGF, was present in both the central and side compartments throughout the culture period and all experimental procedures save the metabolic labeling experiments in which methionine-free culture media (Invitrogen Life Technologies, Carlsbad, CA) was employed (see below). A Nikon Eclipse TE300 microscope was used to obtain phase-contrast images of the neuronal cell cultures.
mRNA abundance
Quantitative RT-PCR analyses were performed on total RNA isolated from SCG axons and somas using Trizol Reagent (Invitrogen Life Technologies, Carlsbad, CA), following the manufacturer’s protocol. Axons and somas were harvested and lyzed in Trizol Reagent. First-strand cDNA was obtained using random nucleotide primers and the SuperScript First-strand Synthesis Kit (Invitrogen Life Technologies, Carlsbad, CA). Amplification was effected using a battery of rat gene-specific primers obtained for several mRNAs previously identified in squid axons (Gioio et al. 2001). The primers used in the analyses were as follows:
β Tubulin: (forward) TGAGGCCTCCTCTCAGAAGT (reverse) TGCAGGCAGTCACAATTCTC; cytochrome c oxidase subunit IV (COX IV): (forward) ACTACCCCTTGCCTGATG, (reverse) ACTCATTGGTGCCCTTGTTC; cytochrome c oxidase subunit Va (COX Va): (forward) GTGGTCGCCGTCATGCTC, (reverse) CGGGTCAAACTCATTTCCT; ATP synthase, H+ transporting, mitochondrial F1 complex, alpha subunit, isoform 1 (H+-ATP synthase): (forward) AGGAACGTTCAAGCTGAGGA, (reverse) ACTACACGGCCCAACAGTTC; DNA Polymerase γ (DNA Pol γ): (forward) TCAGGAAGATCCTGGACCAC, (reverse) CTCAGCCCTGAACTCTCCAC.
In situ Hybridization
COX IV cDNA (Genbank accession’s number X15029, nucleotides 10–544) was reverse transcribed from rat brain poly (A+) RNA (Clontech, Mountain View, CA), PCR-amplified, and subcloned into the transcription vector pSC-A (Stratagene, La Jolla, CA), which contains a T3 and T7 promotor adjacent to the inserted PCR product. Dioxigenin-labeled sense and antisense riboprobes were synthesized using the T3/T7 MAXIscript in vitro transcription kit (Ambion, Austin, TX) and purified by ammonium acetate precipitation according to manufacturer’s instructions. The concentration of the probes was estimated in a dot blot with a Dioxigenin-labeled control RNA (Roche Applied Sciences, Indianapolis, IN) following manufacturer’s instructions. In situ hybridization was performed essentially following the protocol of Moccia et al. (2003), with the following modifications: The cells were fixed in 4% paraformaldehyde containing 4% sucrose for 1 h and subsequently permeabilized in PBS containing 0.1% Triton-X100 for 10 min at room temperature. Pre-hybridization and hybridization were carried out at 50°C in a mix consisting of 50% formamide, 5 × SSC, 40 μg/ml salmon sperm DNA, 2% blocking reagent, 250 μg/ml yeast tRNA. The riboprobe’s concentration in the hybridization mix was 1 ng/μl. Images were captured using a Nikon Eclipse TE300 microscope equipped with a Nikon digital Sight DS-L1 camera (Nikon, Melville, NY).
[35S]Methionine metabolic labeling
Labeling medium was prepared by adding 250 μCi/ml l-[35S]-Methionine (PerkinElmer, Boston, MA) to culture medium lacking methionine. The axons in both side compartments were pre-incubated with the protein-synthesis inhibitors emetine, cycloheximide, or chloramphenicol in regular culture medium at 37°C for 30 min. Thereafter, the medium in the side compartments was changed to labeling medium with or without inhibitors and axons incubated for an additional 4 h. Axons were harvested in 10 mM Tris-Cl (pH 7.4), 100 mM NaCl, and 1 mM EDTA (pH 8) and axons from three culture dishes combined. Axons incubated with l-[35S]-Methionine, but no inhibitors were used as controls. The combined volume from three dishes was centrifuged at 14,000 rpm for 15 min at 4°C in an Eppendorff microcentrifuge and acid precipitable, alkaline resistant radioactivity measured on Whatman GFC filter discs (Giuditta et al. 1991).
Staining of axonal mitochondria
To evaluate changes in mitochondria membrane potential, axons were stained with the mitochondria specific dyes 5,5′,6,6′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) (Cossarizza et al. 1993) or 3,6-bis(dimethylamino)-9-[2-(ethoxycarbonyl)phenyl]-Xanthylium perchlorate (TMRE; Molecular Probes/Invitrogen Detection Technologies, Eugene, OR) (Ehrenberg et al. 1988). Axons stained with JC-1 were washed twice with warm (37°C) Dulbecco’s Modified Eagle Medium (DMEM) and 10 mM N-[2-Hydroxyethyl]piperazine-N’-[2-ethanesulfonic acid] (HEPES; Sigma-Aldrich, St. Louis, MO), pre-incubated in DMEM and 10 mM HEPES for 1 h at 37°C, and incubated with 15.4 μM JC-1 in warm DMEM and 10 mM HEPES for 20 min at 37°C. Afterwards, the axons were washed twice with warm DMEM and 10 mM HEPES and kept in culture medium. To stain axons with TMRE, the axons were incubated with 1 μM TMRE in Hank’s Balanced Salt Solution (HBSS; Invitrogen Life Technologies, Carlsbad, CA) for 10 min at 37°C, and subsequently washed twice with HBSS and kept in HBSS. A Nikon Eclipse TE300 with Nikon Super High Pressure Mercury Lamp power supply was used to excite the stained mitochondria (green fluorescence with excitation at 485 nm and emission at 525 nm and red fluorescence with excitation at 535 nm and emission at 590 nm for JC-1 and excitation at 549 nm and emission at 574 nm for TMRE). Photomicrographs of the stained axons were collected and saved as RBG images. The intensity of fluorescence acquired from clusters of mitochondria present in single axons or thin nerve fiber bundles and background signals were analyzed using ImageJ 1.32j software (NIH; http://rsb.info.nih.gov/ij/). For RBG images, each pixel was converted to grayscale from 0 (black) to 255 (white) and average gray value within each selection was calculated as the sum of each pixel in the selection divided by the number of pixels. In this study, we were interested in the changes of mitochondrial membrane potential compared to controls, and hence true Δψm values were not calculated. Instead, for each mitochondria cluster, the corresponding background signal present in the axoplasm immediately adjacent to the mitochondria was subtracted from the measured mitochondrial fluorescent values and the average value for each side compartment was compared to the respective control. Additionally, the ratio of each mitochondria cluster and corresponding axoplasmic background signal was determined. In normal mitochondria, JC-1 forms mono-aggregates that emit red fluorescence relative to the degree of membrane polarization. The absence of such mono-aggregates will result in JC-1 emitting green fluorescence. The calculated ratio between the red and green fluorescence is proportional to the mitochondrial membrane potential. Thus in experiments using JC-1 as the fluorescent dye, the average calculated JC-1 red/green ratio for each culture dish was compared to the average ratio of the respective control.
Mitochondrial protein import blockade
The plasmid pPROEXHTa-C90 (provided by Dr. Jason Young (Young et al. 2003)) encoding amino acid 566 to the stop codon of human HSP90α, was transformed into DH5α competent E. coli (Invitrogen Life Technologies, Carlsbad, CA). The bacteria containing the expressed His tagged C90 peptide were lysed with 8 M urea, 0.1 M NaH2PO4, and 0.01 M Tris-HCl (pH 8.0) and the fusion protein subsequently purified on a Ni-NTA Agarose column (Qiagen Inc., Valencia, CA) per manufacturer’s instructions. The purified C-90 peptide was labeled using Alexa Fluor 488 protein labeling Kit (Molecular Probes/Invitrogen Detection Technologies, Eugene, OR) following manufacturer’s protocols. The C90 molecules were labeled with fluorescent Alexa Fluor 488 (Alexa) with an efficiency of about 80%. SCG axons were lipofected with C90 (500 ng/side compartment) using Bioporter (Gene Therapy Systems, San Diego, CA), following protocol suggested by the manufacturer, for 4 h and were subsequently stained with TMRE (1 μM for 10 min at 37°C).
ATP measurement
The ATPlite 1step luminescence ATP detection assay system (PerkinElmer, Boston, MA) was used to measure total ATP in SCG distal axons, following the manufacturer’s protocol. SCG axons were harvested using Neurobasal Medium without phenol red (Invitrogen Life Technologies, Carlsbad, CA) and the axons from one side compartment added per well in a white, 96-well Optiplate (PerkinElmer, Boston, MA). ATP levels were determined by measuring the luciferase activity using a Wallace 3 Microplate reader (PerkinElmer, Boston, MA) following the protocol provided by the manufacturer.
Results
Campenot compartmented cultures
The Campenot compartmented culture system has been shown to provide an excellent model to study axons of sympathetic neurons (Eng et al. 1999). To evaluate the effects of the inhibition of local protein synthesis on axonal viability and mitochondrial function, we used a three-compartmented culture system assembled following the protocol of Campenot and Martin (2001) (Fig. 1A). The use of three-compartmented chambers allowed us to plate primary rat sympathetic SCG neurons in the center compartment and grow the axons into the two side compartments, providing pure axonal populations in the side compartments (Fig. 1B). One advantage of using two side compartments is that it facilitates the inhibition of local protein synthesis in the axons of one compartment, while the axons in the opposite side-compartment serve as controls. The SCG neurons were obtained from 3-days old rat pups essentially following the protocol of Johnson (2001) and both the cell bodies and proximal axons located in the center compartment and distal axons present in the side-compartments cultured in serum-free culture medium containing NGF (50 ng/ml) for about 14 days prior to use. As shown in Fig. 1C, somas, confined to the center compartment, began to form clusters of neurons after 1 day in culture and neuronal clusters were clearly visible after about 5 days. The axons were able to penetrate the barrier between the center and the side compartments and continue to grow into the side compartments at the rate of 0.4–0.6 mm per day, reaching the end of the side compartments after about 10 days (Fig. 1C). The anti-mitotic agents AraC or FuDR were used to inhibit the growth of non-neuronal cells in the center compartment. The side compartments were also treated with antimitotic agents to expose the distal axons to the same culture conditions as the cell soma and proximal axons. The side compartments used in these experiments contained no non-neuronal cells, as judged by phase contrast microscopy, as well as ethiduim bromide and acridine orange staining.
Fig. 1.
Campenot compartmented cultures. (A) Photo image of a collagen coated 35-mm tissue culture dish with a Teflon chamber attached to the bottom of the dish. The tracks in the collagen covering the area of the lower part of the chamber were made using a pin rake. (B) Schematic drawing of a single track in the bottom of the culture dish showing the location of cell bodies and proximal axons in the center compartment and the growing distal axons in the side compartments. (C) Representative phase contrast photomicrographs of cell bodies in center compartments and axons in side compartments on days 2, 5, and 14 after plating. Images were collected using a Nikon Eclipse TE300 microscope equipped with a KODAK MDS 290 digital camera and saved with Adobe Photoshop 6.0. Bar = 50 μm
SCG axons contain a heterogeneous population of mRNA
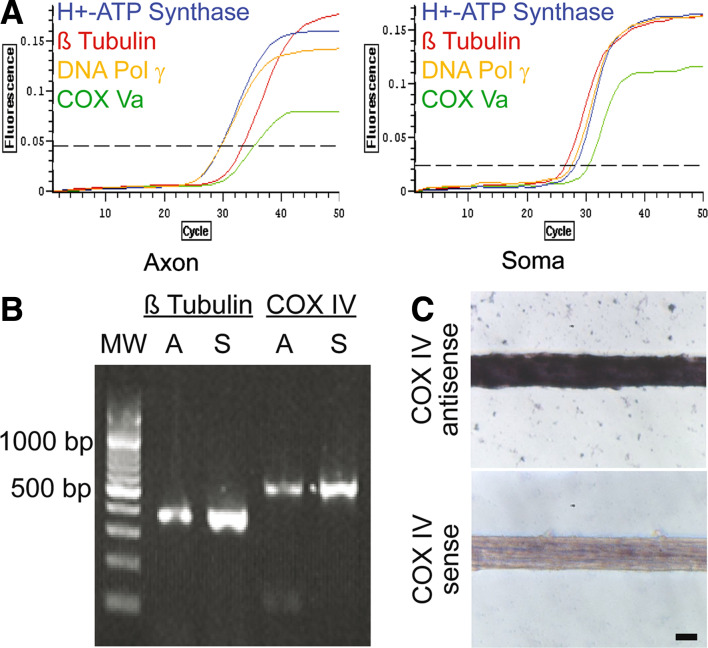
It has been shown in previous studies that axons in invertebrate systems, such as squid (Perrone Capano et al. 1987; Kaplan et al. 1992; Gioio et al. 2004) and Aplysia (Martin et al. 2000), and in chick sympathetic neurons (Olink-Coux and Hollenbeck 1996; Lee and Hollenbeck 2003) contain a heterogeneous population of mRNAs. To evaluate the generality of these findings, we used RT-PCR to identify the mRNAs in rat SCG axons. Consistent with previous findings, we found that SCG axons contain a heterogeneous population of mRNAs, including several mRNAs for nuclear-encoded mitochondrial proteins involved in the electron transport chain, such as H+-ATP synthase (ATP synthase, H+ transporting, mitochondrial F1 complex, alpha subunit, isoform 1; Fig. 2A) and cytochrome C oxidase subunit IV (COX IV; Fig. 2B), and Va (COX Va; Fig. 2A). The presence of COX IV in axons was confirmed using in situ hybridization (Fig. 2C). Additionally, mRNAs coding for the molecular chaperones Hsp70 and Hsp90 (data not shown), proteins facilitating the import of pre-proteins into the mitochondria, as well as nuclear-encoded proteins essential for the transcription (DNA Polymerase γ; Fig. 2A), and translation (mitochondrial ribosomal proteins; data not shown) of mitochondrial-encoded proteins were present in the axons. The identification of mRNAs for cytoskeletal proteins (β actin and β tubulin; Fig. 2A, B) is consistent with the protein metabolic labeling studies by Campenot and colleagues (Eng et al. 1999), as well as the findings obtained from protein synthesis studies conducted on cultured chick sympathetic neurons (Lee and Hollenbeck 2003). Using quantitative RT-PCR, we were able to measure the relative abundance of different mRNAs present in the distal axons, as well as the proximal axons and cell somas located in the central compartment. In somas and proximal axons, β tubulin mRNA was more abundant than the mRNAs for the nuclear-encoded mitochondrial proteins H+-ATP synthase and DNA Polymerase γ, as judged by the number of PCR cycles required to amplify the mRNAs (Fig. 2A). However, in the distal axons we noted a shift in relative abundance of these mRNAs, with the mRNAs for nuclear-encoded mitochondrial proteins being more abundant compared to β tubulin mRNA. For example, the ratio of the number of PCR cycles required to amplify ATP synthase relative to that of β tubulin in the distal axonal compartment was 0.89, whereas the ratio was 1.05 in the proximal axons and cell somas, a 15.3% decrease in relative ratios. Similar results were obtained for DNA polymerase γ mRNA. The alterations in the relative abundance of these mRNAs suggest that there is a differential transport of nuclear-encoded mitochondrial mRNAs into the axon or alternatively there are differences in mRNA turnover in the distal axon compartment.
Fig. 2.
SCG axons contain a heterogeneous population of mRNA. (A and B) RT-PCR analyses performed on total RNA isolated from SCG axons and somas. Note the shift in abundance of β tubulin mRNA relative to H+-ATP synthase and DNA Pol γ mRNAs between axon and soma. Axon, A; Soma, S. (C) COX IV subunit is present in SCG axons, as demonstrated with in situ hybridization. Representative phase contrast photomicrographs of SCG axons after fixation and hybridization with antisense (COX IV antisense) and sense (Control; COX IV sense) riboprobes for the cytochrome c oxidase subunit IV mRNA. Bar = 10 μm
Protein synthesis inhibitors reduce SCG axon viability and translation activity
To test the hypothesis that the local protein synthetic system plays a key role in the maintenance of the axon, we investigated the effects of protein synthesis inhibitors on axonal viability, as judged by the distal axons ability to adhere to the substratum and thrive in the side compartment. In this study, axons in one side-compartment were exposed to cycloheximide, chloramphenicol, or emetine, and the viability of the axons was compared to control axons located in the opposite non-treated side compartment. Throughout the course of this experiment, all compartments of the culture dish contained complete media with NGF. After inhibition of local protein synthesis with either emetine, which inhibits both cytosolic and mitochondrial protein synthesis, for about 6 h, axons started to detach from the substrata and began to retract and after 15 h only the proximal parts of the distal axons were visible in the side compartments (for example, see Fig. 3A, B). Similar effects on the distal axons were seen using cycloheximide, an agent that inhibits cytosolic protein synthesis (data not shown). After 24 h of exposure to either emetine or cycloheximide, the axons had completely retracted in the antibiotic-treated side-compartment (Fig. 3C). Neither the control axons in the contralateral side compartment, nor the cell bodies in the center compartment showed morphological changes during this time. In contrast to these results, chloramphenicol, an inhibitor of mitochondrial protein synthesis, did not affect axonal maintenance to any great degree after 24 h of exposure (Fig. 3C). It is also noteworthy, that the acute effects of these agents on axon maintenance were reversible, as judged by the unaffected growth of axons 24 h after 3 h of exposure to the inhibitors (data not shown).
Fig. 3.
Protein synthesis inhibitors decrease SCG axon maintenance and translation activity. (A) The attachment of the distal axons in the side compartment was decreased by inhibition of the local protein synthetic system by emetine (100 μM) after 6 h. (B) Inhibition of local protein synthesis by emetine (100 μM) for 15 h resulted in retraction of distal axons toward the center compartment. Representative phase contrast photomicrographs of 14 days-old axons exposed to emetine for 6 h or 15 h and untreated control distal axons in contralateral side compartments. Culture media containing NGF was present in all compartments throughout the duration of the experiment. Arrows indicate regions of axonal detachment and retraction. (C) Axonal maintenance was decreased by inhibition of the local protein synthetic system by emetine, cycloheximide, or chloramphenicol. Representative phase contrast photomicrographs of 14 days-old axons before (0 h) and after 24 h of exposure to emetine (100 μM), cycloheximide (3.5 μM), or chloramphenicol (1 mM). Bars = 50 μm. (D) Axons were pretreated with the protein synthesis inhibitors emetine (10 μM), cycloheximide (350 nM), or chloramphenicol (1 mM) for 30 min and were subsequently incubated for 4 h in methionine-free media containing inhibitors and [35S]Methionine (250 μCi/ml). Axons exposed to culture media containing reagent vehicle lacking inhibitors served as controls. All compartments contained culture media with NGF throughout the experiment. Acid precipitable, alkaline resistant radioactivity was measured on Whatman GFC filter discs by liquid scintillation spectometry as described in methods. Data shown are the mean ± s.e.m. with *P < 0.05 and **P < 0.01 using one sample t-test (two-tailed, α = 0.05, n = 4)
To confirm that protein synthesis occurs in SCG axons, we exposed axons in both side compartments of the culture dish to complete media containing the antibiotics cycloheximide, chloramphenicol, or emetine for 30 min prior to the addition of methionine-free media containing the appropriate antibiotic and [35S]Methionine (250 μCi/ml). Distal axons exposed to the culture media containing reagent vehicle lacking inhibitors served as controls. After 4 h of incubation, the amount of newly synthesized [35S]Methionine-labeled total protein was assessed in the distal axons. Cycloheximide significantly decreased the amount of newly synthesized protein in the distal axons (Fig. 3D). In contrast, chloramphenicol had only modest effects on axonal protein synthesis, a finding that suggests that mitochondria contribute approximately 20–25% of the total protein synthetic activity in the axon. Not unexpectedly, emetine markedly reduced local protein synthesis (Fig. 3D).
Blockade of mitochondrial protein import decreases axonal mitochondrial membrane potential
Many of the functions of mitochondria require proteins synthesized from the nuclear genome and these proteins have to be imported from the cytosol (for an overview of import of pre-proteins into the mitochondria, see Truscott et al. 2003; Bauer and Hofmann 2006). The import of pre-proteins utilizes the molecular chaperones Hsp70 and Hsp90, and the translocase of the outer membrane (TOM) complex. The Tom70 receptor is located on the cytosolic side of the TOM complex, and it has been shown that disruption of the interaction between Hsp90 and the Tom70 receptor inhibits the import of proteins into the mitochondria (Young et al. 2003).
One means of inhibiting Tom70/Hsp90 function is to expose the Tom70 receptor to C90, the carboxyl terminal portion of Hsp90 (Young et al. 1998). C90 molecules will bind to the Tom70 receptor when introduced into the axon and block full-length, functional Hsp90 molecules from binding to the receptor. The C90 molecules were prepared according to the protocol of Young et al. (1998), and thereafter labeled with fluorescent Alexa Fluor 488 to facilitate the detection of mitochondria with C90 molecules bound to their Tom70 receptors.
In these experiments, the Alexa-labeled C90 fragment was lipofected into the distal axons in one side compartment, whereas axons in the contralateral compartment were treated with reagent vehicle and served as controls. Alterations in mitochondrial membrane potential were employed to evaluate the effects of decreased protein import into the organelle using the mitochondrial specific dye TMRE (Ehrenberg et al. 1988), whose fluorescence is proportional to the mitochondrial membrane potential. In this experiment, the intensity of fluorescence was converted to a grayscale (0–255 maximum; see Methods). Membrane potential was first compared by correcting the mitochondrial fluorescence values for the fluorescence background present in the axoplasm immediately adjacent to the organelle. Additionally, a ratio analysis was conducted in which mitochondrial fluorescence in experimental and control axons was expressed relative to the adjacent axoplasm fluorescence.
After 4 h of exposure to C90, the membrane potential of Alexa-labeled mitochondria was reduced to 35.9 ± 2.6% (mean ± s.e.m.) of control values (n = 3; P = 0.002). The relative ratio of Alexa-labeled mitochondrial fluorescence was 89.5 ± 0.23% (mean ± s.e.m.) of control mitochondria (P = 0.0005). During the 4 h of exposure, we observed no Alexa-labeled mitochondria in the proximal axons or cell somas in the central compartment. Taken together, these findings indicate that mitochondria in the distal axon compartment require the import of local nuclear-encoded proteins to maintain their membrane potential.
Inhibition of local protein synthesis decreases axonal mitochondrial membrane potential
The findings that SCG axons contain mRNAs coding for nuclear-encoded mitochondrial proteins, that local protein synthesis occurs in the axons, and that axonal mitochondria require import of nuclear-encoded mitochondrial proteins, raised the possibility that nuclear-encoded mitochondrial proteins required by the mitochondria might be locally synthesized. To investigate this possibility, we inhibited local protein synthesis in the SCG axons and monitored mitochondrial membrane potential. In these experiments, axons in the left side-compartments were exposed to emetine, cycloheximide, or chloramphenicol for 3 h. The axons in the right side compartments served as controls. Changes in mitochondrial membrane potential were monitored by measuring changes in fluorescent intensity using JC-1 (Cossarizza et al. 1993) or TMRE (Fig. 4). Acute inhibition of local protein synthesis for 3 h by emetine, cycloheximide, or chloramphenicol significantly decreased the mitochondrial membrane potential (Fig. 4A, B). The relative ratio of JC-1 labeled mitochondrial fluorescence for emetine, cycloheximide, or chloramphenicol treated axons compared to controls were 86.2 ± 5.3% (P = 0.05), 79.2 ± 3.4% (P = 0.002), and 92.2 ± 2.8% (P = 0.04), respectively. Similar decreases in mitochondrial membrane potential were observed in distal axons exposed to emetine, cycloheximide, or chloramphenicol as judged by staining with TMRE (Fig. 4B). Corresponding TMRE ratio values for emetine, cycloheximide, or chloramphenicol treated axons compared to controls were 85.7 ± 2.2% (P = 0.02), 89.3 ± 3.1% (P = 0.04), and 90.4 ± 1.7% (P = 0.03), respectively. These results indicate that the maintenance of axonal mitochondrial membrane potential is dependent, at least in part, on locally synthesized proteins.
Fig. 4.
Inhibition of local protein synthesis decreases axonal mitochondrial membrane potential and generation of ATP. Axons in one side compartment were exposed to the protein synthesis inhibitors emetine (10 μM), cycloheximide (350 nM), or chloramphenicol (1 mM) for 3 h and mitochondrial membrane potential assessed by subsequent treatment with JC-1 at 15.4 μM for 20 min at 37°C (A) or TMRE at 1 μM for 10 min at 37°C (B). Values for mitochondrial membrane potentials, as determined with each dye, were obtained as described in Methods. Data shown are mean ± s.e.m. with *P < 0.05, **P < 0.01, and ***P < 0.01 using one sample t-test (two-tailed, α = 0.05, n = 3–6). (C) Protein synthesis inhibitors impede the recovery of depolarization-induced decreases of axonal ATP levels. Depolarization was induced by exposing untreated SCG axons and axons exposed to emetine or cycloheximide for 3 h followed by a 5 min exposure to 50 mM KCl. Axonal ATP levels were measured using ATPlite 1step kit and a microplate reader. Data shown are mean ± s.e.m. with *P < 0.05 and **P < 0.01 using one sample t-test (two-tailed, α = 0.05, n = 8–9)
Acute inhibition of local protein synthesis impedes the recovery of depolarization-induced decreases in axonal ATP levels
To evaluate the hypothesis that inhibition of local synthesis will have a deleterious effect on the ability of axonal mitochondria to generate ATP, levels of ATP in distal axons were determined after acute exposure to protein synthesis inhibitors. In these experiments, axons in one side compartment were exposed to emetine or cycloheximide for 3 h and non-treated axons located in the contralateral side compartment served as positive controls. After 3 h of exposure, axonal ATP levels were measured by chemiluminescence. Treatment of axons with emetine decreased basal ATP levels in the axons to 80.5 ± 7.4% of controls (P = 0.030), whereas, cycloheximide had no effect on basal ATP levels (99.4 ± 8.5% of controls; P = 0.9). To evaluate the possibility that mitochondria in axons with inhibited local protein synthesis may have more difficulty in regenerating ATP levels after stress, we exposed axons to emetine or cycloheximide for 3 h and subsequently induced a stress-like condition by depolarizing the axons with 50 mM KCl for 5 min. Control axons in the contralateral side compartments were not exposed to inhibitors, but underwent KCl-induced depolarization. This depolarization lead to a marked decrease in ATP levels in emetine- or cycloheximide-treated axons compared to controls (Fig. 4C). Taken together, these results suggest that inhibition of local protein synthesis has significant effects on the ability of mitochondria to maintain ATP levels in distal axons under stressful conditions.
Discussion
In this communication, we have shown that axons of primary sympathetic neurons contain mRNAs and that inhibition of the local protein synthesis has marked effects on axon viability and mitochondrial function. The Campenot culture system used in this study provided a valuable tool to investigate biological functions of local protein synthesis in pure axonal populations. One disadvantage of this system, however, was the requirement of plastic tissue culture dishes, and hence SCG axons could not be visualized using confocal microscopy. Culture dishes suitable for confocal microscopy could not be coated with collagen nor scratched to form the necessary tracks required for SCG axons to grow into the side compartments. Hence, a Nikon Eclipse TE300 microscope with Nikon Super High Pressure Mercury Lamp power supply was used to visualize axons for bright field and fluorescent studies. This equipment provided reasonably high quality images of mitochondria labeled with the mitochondria membrane specific dyes JC-1 and TMRE.
The presence of a heterogeneous axonal mRNA population and the ability of the axon to incorporate [35S]Methionine into proteins in a cycloheximide-sensitive manner confirmed the hypothesis that local protein synthesis occurs in mammalian SCG axons. The presence of several nuclear-encoded mitochondrial mRNAs raised the possibility that local protein synthesis played a key role in mitochondrial function and provided the opportunity to initiate study of the functions of the local synthetic system.
The use of the antibiotics cycloheximide or chloramphenicol to inhibit protein synthesis enabled us to distinguish between the effects derived from decreased synthesis of nuclear-encoded proteins by cycloheximide and mitochondrial-encoded proteins by chloramphenicol. These antibiotics, or similar drugs, have been widely used to distinguish between nuclear-encoded and mitochondrial-encoded protein synthesis (Eng et al. 1999; Gioio et al. 2001; Hanz et al. 2003). In addition to these drugs, the effects of emetine on both nuclear and mitochondrial-encoded protein synthesis were studied to confirm effects derived from these agents. The reduction in newly synthesized axonal proteins seen after exposure to cycloheximide and emetine established that the locally present mRNAs can serve as templates for the synthesis of proteins in the axon. Similar findings were previously reported by Campenot and colleagues (Eng et al. 1999).
The detachment of axons from the substrate of culture dish and their subsequent retraction after inhibition of local protein synthesis indicate that the viability of the distal axon requires locally synthesized proteins, at least in this primary cell culture system. In view of the rate of slow axonal transport of proteins (0.1–3 mm/day), relatively little newly synthesized (Methionine-labeled) proteins from the cell bodies should theoretically reach the distal axons in the side compartments during the acute incubation times (3–4.5 h) used in this study, and hence the effects observed could be attributed in large measure to inhibition of the local protein synthetic system. It bears mention, however, that these findings differ from those of Eng et al. (1999) who reported that the inhibition of local protein synthesis did not negatively impact axon viability. Reason(s) for the discrepancy in these findings is unclear, but could derive from differences in cell culture conditions to include differences in NGF concentrations and substratum, as well as the use of serum-free culture medium in this study.
The finding of several mRNAs in the axon coding for nuclear-encoded mitochondrial proteins and the molecular chaperones Hsp70 and Hsp90 suggests that axonal mitochondria may be dependant on proteins synthesized locally in the axoplasm. It has previously been shown that inhibiting local protein synthesis in isolated nerve terminals of squid photoreceptor neurons decreased the presence of newly synthesized proteins in the nerve terminal mitochondrial population (Gioio et al. 2001). In this study, determination of changes in mitochondrial membrane potential, as judged by two mitochondrial specific cationic dyes, was used to evaluate the biological effects of decreased import of proteins into axonal mitochondria. Blockade of the import of axonal proteins into the mitochondria greatly diminished the mitochondrial membrane potential, indicating that the mitochondria require replenishment of nuclear-encoded proteins to function properly in the distal axon.
Although the 3-h used to inhibit protein synthesis in this study represents a relatively short time period, it was sufficient to obtain effects on the membrane potential and function of axonal mitochondria. This observation was particularly striking in view of the low concentration of protein synthesis inhibitors employed in the study and the subsequent amount of protein synthetic activity that remained in the axon. At the concentrations employed in these experiments, our best estimate is that cycloheximide (350 nM) reduced cytosolic protein synthetic activity by approximately 70–80%. This estimate is based upon the probability that 20–25% of the total residual translation activity observed in the presence of cycloheximide derives from the mitochondrial protein synthesis (Fig. 3D). In our initial experiments, high levels of inhibitors were used, but as shown in Fig. 3C, these dosages had debilitating effects on the distal axons. Hence, inhibitor concentrations in the culture media were reduced 10-fold to minimize drug side-effects and enhance the viability of the distal axons. Under these conditions, we did not observe changes in distal axon morphology or maintenance, as judged by phase contrast microscopy. Interestingly, the pronounced effects observed on the mitochondria under these experimental conditions suggest that a significant portion of mitochondrial protein is being rapidly turned over in the axon, and raises the possibility that one of the major functions of the local protein synthetic system is to replace these highly labile constituents of the organelle.
Acute inhibition of local protein synthesis had only modest effects on basal levels of axonal ATP. There may be several reasons for the lack of response. For example, not all mitochondria were affected by protein synthesis inhibition and those mitochondria could potentially increase their ATP production as a compensatory response. Another explanation may be that there are high basal levels of ATP in the normal axon and the relatively short time period used to inhibit the local protein synthetic system was not sufficient to lower the total amount of ATP in the axons. In addition, basal levels of axonal ATP could be maintained by anaerobic metabolism, as there are significant amounts of glucose in the culture medium. Nonetheless, after massive depolarization of the axon with KCl, the inhibition of local synthesis resulted in marked decrements in the recovery of axonal ATP levels, suggesting that mitochondrial function is dependent on local protein synthesis.
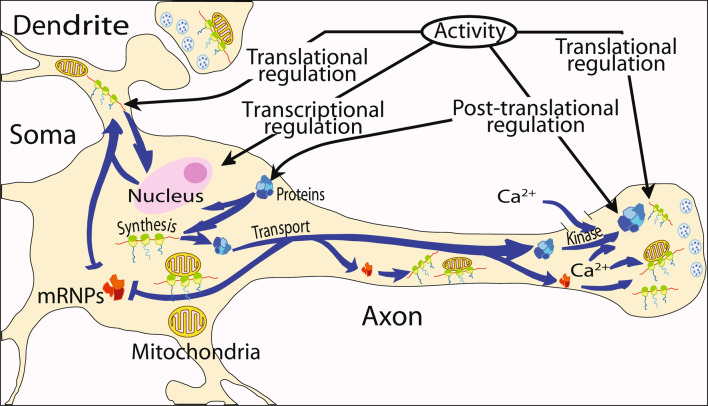
Taken together, these results support the hypothesis that local protein synthesis in distal regions of the axons is vital for mitochondrial function and the viability of the axon. In this regard, we suggest a modified model of the maintenance of mitochondria in the axon and the nerve terminal (Fig. 5). In this model, mRNA can be translocated to specific cellular destinations, including dendrites, axons, and nerve terminals, as non-translated, and relatively stable ribonucleoprotein particles (mRNP) (Li et al. 1999) whose translation can be regulated in response to neuronal activity. These mRNPs can serve as templates for the synthesis of mitochondrial pre-proteins in the axoplasm. It has been shown by Liu and Wong-Riley (1994) that mitochondria in rat brain distal dendrites and axon nerve terminals contain COX IV precursor protein. Based upon this finding, they concluded that nuclear-encoded mitochondrial precursor proteins were transported within the mitochondria to the distal axon and were later converted to mature proteins locally in the axons. In the light of new findings, we believe that these precursor proteins can also be synthesized locally in the distal axons and transported into the organelle at the site of function. In this regard, it has been demonstrated, using light and electron microscopy, that ribosome clusters in the squid giant axon are often surrounded by mitochondria (Bleher and Martin 2001) and that the close localization of mRNA to the vicinity of mitochondria in yeast cells is essential for respiratory function (Margeot et al. 2005). Taken together, these reports suggest that proteins may be synthesized near, or even on the surface of the axonal mitochondria and that local translation may play an essential role in the biosynthesis, maintenance, and function of the axonal mitochondria.
Fig. 5.
Model of neuronal protein synthesis. Protein synthesis occurs in multiple compartments within neurons to include the dendrite, axon, and nerve terminal. Key features of the model include the rapid and selective transport of stable messenger ribonucleoprotein complexes (mRNPs) to the neuronal periphery and the local regulation of mRNA translation in response to neuronal activity. The model also shows that synthesis of proteins occurs in the vicinity or on the surface of mitochondria in the distal parts of the neuron. The activity in the neuron can be modulated by transcriptional regulation in the soma and translational and post-translational regulation in soma, dendrites, axons, and nerve terminals
Acknowdgement
We thank Dr. J. Young (McGill Faculty of Medicine, Montreal, Quebec, Canada) for providing the C90 plasmid.
References
- Anderson S, Bankier AT, Barrell BG, de Bruijn MHL, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJH, Staden R, Young IG (1981) Sequence and organization of the human mitochondrial genome. Nature 290:457–465 [DOI] [PubMed] [Google Scholar]
- Attardi G, Schatz G (1988) Biogenesis of mitochondria. Annu Rev Cell Biol 4:289–331 [DOI] [PubMed] [Google Scholar]
- Bauer MF, Hofmann S (2006) Import of mitochondrial proteins. In: Schapira AHV (ed) Mitochondrial function and dysfunction. Academic Press, San Diego, CA, pp 57–90 [Google Scholar]
- Beaumont V, Zhong N, Fletcher R, Froemke RC, Zucker RS (2001) Phosphorylation and local presynaptic protein synthesis in calcium- and calcineurin-dependent induction of crayfish long-term facilitation. Neuron 32:489–501 [DOI] [PubMed] [Google Scholar]
- Bleher R, Martin R (2001) Ribosomes in the squid giant axon. Neuroscience 107:527–534 [DOI] [PubMed] [Google Scholar]
- Brittis PA, Lu Q, Flanagan JG (2002) Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell 110:223–235 [DOI] [PubMed] [Google Scholar]
- Campbell DS, Holt CE (2001) Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron 32:1013–1026 [DOI] [PubMed] [Google Scholar]
- Campenot RB, Martin G (2001) Construction and use of compartmented cultures for studies of cell biology in neurons. In: Federoff S, Richadrson A (ed) Protocols for neural cell culture. Humana Press, Inc., Totowa, NJ, pp 49–57 [Google Scholar]
- Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER (1999) A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell 99:221–237 [DOI] [PubMed] [Google Scholar]
- Cossarizza A, Baccaranicontri M, Kalashnikova G, Franceschi C (1993) A new method for the cytofluorometric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1). Biochem Biophys Res Commun 197:40–45 [DOI] [PubMed] [Google Scholar]
- Ehrenberg B, Montana V, Wei MD, Wuskell JP, Loew LM (1988) Membrane potential can be determined in individual cells from the nernstian distribution of cationic dyes. Biophys J 53:785–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng H, Lund K, Campenot RB (1999) Synthesis of beta-tubulin, actin, and other proteins in axons of sympathetic neurons in compartmented cultures. J Neurosci 19:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioio AE, Eyman M, Zhang H, Lavina ZS, Giuditta A, Kaplan BB (2001) Local synthesis of nuclear-encoded mitochondrial proteins in the presynaptic nerve terminal. J Neurosci Res 64:447–453 [DOI] [PubMed] [Google Scholar]
- Gioio AE, Lavina ZS, Jurkovicova D, Zhang H, Eyman M, Giuditta A, Kaplan BB (2004) Nerve terminals of squid photoreceptor neurons contain a heterogeneous population of mRNAs and translate a transfected reporter mRNA. Eur J Neurosci 20:865–872 [DOI] [PubMed] [Google Scholar]
- Giuditta A, Kaplan BB, van Minnen J, Alvarez J, Koenig E (2002) Axonal and presynaptic protein synthesis: new insights into the biology of the neuron. Trends Neurosci 25:400–404 [DOI] [PubMed] [Google Scholar]
- Giuditta A, Menichini E, Perrone Capano C, Langella M, Martin R, Castigli E, Kaplan BB (1991) Active polysomes in the axoplasm of the squid giant axon. J Neurosci Res 28:18–28 [DOI] [PubMed] [Google Scholar]
- Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, Fainzilber M (2003) Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron 40:1095–1104 [DOI] [PubMed] [Google Scholar]
- Heales SJR, Gegg ME, Clark JB (2006) Oxidative phosporylation: structure, function, and intermediary metabolism. In: Schapira AHV (ed) Mitochondria function and dysfuntion. Academic Press, San Diego, CA, pp 25–56 [Google Scholar]
- Hollenbeck PJ, Saxton WM (2005) The axonal transport of mitochondria. J Cell Sci 118:5411–5419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MI (2001) Primary cultures of sympathetic ganglia. In: Federoff S, Richadrson A (eds) Protocols for neural cell culture. Humana Press, Inc., Totowa, NJ, pp 71–94 [Google Scholar]
- Kaplan BB, Gioio AE, Perrone Capano C, Crispino M, Giuditta A (1992) β-Actin and β-Tubulin are components of a heterogeneous mRNA population present in the squid giant axon. Mol Cell Neurosci 3:133–144 [DOI] [PubMed] [Google Scholar]
- Lee SK, Hollenbeck PJ (2003) Organization and translation of mRNA in sympathetic axons. J Cell Sci 116:4467–4478 [DOI] [PubMed] [Google Scholar]
- Li C, Sasaki Y, Takei K, Yamamoto H, Shouji M, Sugiyama Y, Kawakami T, Nakamura F, Yagi T, Ohshima T, Goshima Y (2004) Correlation between Semaphorin3A-induced facilitation of axonal transport and local activation of a translation initiation factor eukaryotic translation initiation factor 4E. J Neurosci 24:6161–6170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J-Y, Volknandt W, Dahlstrom A, Herrmann C, Blasi J, Das B, Zimmermann H (1999) Axonal transport of ribonucleoprotein particles (Vaults). Neuroscience 91:1055–1065 [DOI] [PubMed] [Google Scholar]
- Liu K, Hu J-Y, Wang D, Schacher S (2003) Protein synthesis at synapse versus cell body: enhanced but transient expression of long-term facilitation at isolated synapses. J Neurobiol 56:275–286 [DOI] [PubMed] [Google Scholar]
- Liu S, Wong-Riley M (1994) Nuclear-encoded mitochondrial precursor protein: intramitochondrial delivery to dendrites and axon terminals of neurons and regulation by neuronal activity. J Neurosci 14:5338–5351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeot A, Garcia M, Wang W, Tetaud E, di Rago JP, Jacq C (2005) Why are many mRNAs translated to the vicinity of mitochondria: a role in protein complex assembly? Gene 354:64–71 [DOI] [PubMed] [Google Scholar]
- Martin KC, Barad M, Kandel ER (2000) Local protein synthesis and its role in synapse-specific plasticity. Curr Opin Neurobiol 10:587–592 [DOI] [PubMed] [Google Scholar]
- Martin KC, Casadio A, Zhu H, E Y, Rose JC, Chen M, Bailey CH, Kandel ER (1997) Synapse-specific, long-term facilitation of Aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell 91:927–938 [DOI] [PubMed] [Google Scholar]
- Ming GL, Wong ST, Henley J, Yuan XB, Song HJ, Spitzer NC, Poo M (2002) Adaptation in the chemotactic guidance of nerve growth cones. Nature 417:411–418 [DOI] [PubMed] [Google Scholar]
- Moccia R, Chen D, Lyles V, Kapuya E, E Y, Kalachikov S, Spahn CMT, Frank J, Kandel ER, Barad M, Martin KC (2003) An unbiased cDNA library prepared from isolated Aplysia sensory neuron processes is enriched for cytoskeletal and translational mRNAs. J Neurosci 23:9409–9417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olink-Coux M, Hollenbeck PJ (1996) Localization and active transport of mRNA in axons of sympathetic neurons in culture. J Neurosci 16:1346–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone Capano C, Giuditta A, Castigli E, Kaplan BB (1987) Occurrence and sequence complexity of polyadenylated RNA in squid axoplasm. J Neurochem 49:698–704 [DOI] [PubMed] [Google Scholar]
- Piper M, Holt C (2004) RNA translations in axons. Annu Rev Cell Dev Biol 20:505–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacher S, Wu F (2002) Synapse formation in the absence of cell bodies requires protein synthesis. J Neurosci 22:1831–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman EM, Dynes JL, Steward O (2006) Synaptic regulation of translation of dendritic mRNAs. J Neurosci 26:7143–7146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si K, Giustetto M, Etkin A, Hsu R, Janisiewicz AM, Miniaci MC, Kim JH, Zhu H, Kandel ER (2003) A neuronal isoform of CPEB regulates local protein synthesis and stabilizes synapse-specific long-term facilitation in Aplysia. Cell 115:893–904 [DOI] [PubMed] [Google Scholar]
- Truscott KN, Brandner K, Pfanner N (2003) Mechanisms of protein import into mitochondria. Curr Biol 13:R326–R337 [DOI] [PubMed] [Google Scholar]
- Young JC, Hoogenraad NJ, Hartl FU (2003) Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell 112:41–50 [DOI] [PubMed] [Google Scholar]
- Young JC, Obermann WM, Hartl FU (1998) Specific binding of tetratricopeptide repeat proteins to the C-terminal 12-kDa domain of Hsp90. J Biol Chem 273:18007–18010 [DOI] [PubMed] [Google Scholar]
- Zhang X, Poo M (2002) Localized synaptic potentiation by BDNF requires local protein synthesis in the developing axon. Neuron 36:675–688 [DOI] [PubMed] [Google Scholar]