Abstract
Transposon mutagenesis of Bordetella pertussis was used to discover mutations in the cytochrome c biogenesis pathway called system II. Using a tetramethyl-p-phenylenediamine cytochrome c oxidase screen, 27 oxidase-negative mutants were isolated and characterized. Nine mutants were still able to synthesize c-type cytochromes and possessed insertions in the genes for cytochrome c oxidase subunits (ctaC, -D, and -E), heme a biosynthesis (ctaB), assembly of cytochrome c oxidase (sco2), or ferrochelatase (hemZ). Eighteen mutants were unable to synthesize all c-type cytochromes. Seven of these had transposons in dipZ (dsbD), encoding the transmembrane thioreduction protein, and all seven mutants were corrected for cytochrome c assembly by exogenous dithiothreitol, which was consistent with the cytochrome c cysteinyl residues of the CXXCH motif requiring periplasmic reduction. The remaining 11 insertions were located in the ccsBA operon, suggesting that with the appropriate thiol-reducing environment, the CcsB and CcsA proteins comprise the entire system II biosynthetic pathway. Antiserum to CcsB was used to show that CcsB is absent in ccsA mutants, providing evidence for a stable CcsA-CcsB complex. No mutations were found in the genes necessary for disulfide bond formation (dsbA or dsbB). To examine whether the periplasmic disulfide bond pathway is required for cytochrome c biogenesis in B. pertussis, a targeted knockout was made in dsbB. The DsbB− mutant makes holocytochromes c like the wild type does and secretes and assembles the active periplasmic alkaline phosphatase. A dipZ mutant is not corrected by a dsbB mutation. Alternative mechanisms to oxidize disulfides in B. pertussis are analyzed and discussed.
Three pathways for the synthesis of c-type cytochromes, called systems I, II, and III, have been described previously (26, 41, 59). Proteins in these pathways bring the vinyl side chains of heme to the thiol groups of apocytochrome c (in a CXXCH motif), whereby spontaneous ligations to form two thioether bonds occur. The system III pathway, present in yeast species, invertebrates, and vertebrates, is comprised of a single enzyme called cytochrome c heme lyase, which is present in the mitochondrial intermembrane space. Systems I and II are more complicated, although studies of system II have lagged behind those of system I, and the complete catalog of components for system II has not yet been definitively determined by genetic analysis.
For studies of system I, proteobacteria such as Rhodobacter capsulatus (4, 5, 12, 25, 27), Paracoccus denitrificans (38-40), and Bradyrhizobium japonicum (44, 47, 48) have undergone extensive mutagenesis analysis and screening to discover genes involved in the system I pathway. Nine proteins are proven to be required for system I, two of which (DipZ/DsbD and HelX/CcmG) are thioreduction proteins for the reduction of the cytochrome c CXXCH signature motif; seven additional proteins are putatively involved in delivering heme to the periplasm (HelABCD/CcmABCD), as a periplasmic heme chaperone (CycJ/CcmE), and in the final ligation step (Ccl1/CcmF and Ccl2/CcmH). (See Discussion for a review of disulfide oxidizing requirements).
In contrast, no organism that possesses a system II pathway has been as thoroughly screened after random transposon mutagenesis to discover new genes involved in the pathway. Some studies of the thiol reduction components have been reported for the gram-positive bacterium Bacillus subtilis (15, 50, 51), but for unknown reasons, insertions or null mutations in the ccsB and ccsA genes (called resBC), known to be part of system II, are lethal (29, 57). For the unicellular alga Chlamydomonas reinhardtii, in which the ccsB (ccs1) and ccsA genes were originally uncovered (22, 63), it has been suggested previously that components in addition to thioreduction proteins are necessary (22). This prediction is based on mutants whose defects map to other uncharacterized loci. Other genetic analyses of organisms with system II pathways (for examples, see reference 60) have been reviewed recently, as has the rationale for the use of the proteobacterium Bordetella pertussis to determine if additional components of the system II pathway exist (28). To date, four genes in B. pertussis have been shown to be necessary for system II (3). Constructed by directed mutagenesis, these include the genes for CcsB, CcsA, and two proteins called DipZ and CcsX, which are involved in the general thioreduction of periplasmic proteins.
We previously reported that a tetramethyl-p-phenylenediamine (TMPD) oxidase screen was able to distinguish B. pertussis wild-type colonies (oxidase positive) from strains that are deficient in the assembly of c-type cytochromes (oxidase negative), although these mutants grow like wild-type strains (3). This screen is identical to that frequently used to uncover the system I genes in those organisms for which biogenesis mutations are not lethal. In the present study, we used the oxidase screen with transposon-based random mutagenesis of B. pertussis to search for genes involved in system II. Because the mechanism(s) needed for oxidation of the CXXCH disulfide did not emerge in this screen, we investigated the typical disulfide bond (dsb) pathway. The generation of a targeted dsbB mutant and alkaline phosphatase screens in the dsbB background were carried out to uncover redundancy in the B. pertussis periplasmic oxidation pathway. From these results, we hypothesize that low-molecular-weight compounds (e.g., oxygen and cystine) supplement the normal dsb oxidation pathway in B. pertussis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The B. pertussis strains used in this work (Table 1) were grown as previously described (3). Briefly, BC39 and its derivatives were grown at 37°C either on blood agar plates for 2 to 4 days or in supplemented Stainer-Scholte medium for 24 to 36 h (53). The antibiotic concentrations used for B. pertussis were as follows: 50 μg ml−1 nalidixic acid, 100 μg ml−1 streptomycin, 1 μg ml−1 tetracycline, 10 μg ml−1 kanamycin, and 10 μg ml−1 gentamicin. Ampicillin was used for Escherichia coli at a concentration of 100 μg ml−1.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| Strains | ||
| B. pertussis | ||
| BC39 | Wild type; TOHAMA III background; Nalr Smr | 9, 10 |
| RGK343 | BC39 dsbB::pRGK340; Nalr Smr Kanr | This study |
| Tn5 mutants | See Table 2 | This study |
| E. coli | ||
| S17-1 | λ pir conjugation mobilizing strain | Laboratory collection |
| TB1 | General cloning vector | Laboratory collection |
| CC118 | Pho− strain | 30 |
| Tuner (DE3) | T7 expression host | Novagen |
| Plasmids | ||
| pET2-Blue | T7 expression vector | Novagen |
| pTGN | Mini-Tn5gfp-km transposon; Kanr | 58 |
| pss2141 | Suicide vector for homologous recombination; Gmr Sms | 56 |
| pUC119 | Cloning vector; Ampr | Laboratory collection |
| pUC4-KIXX | Aminoglycoside phosphotransferase Kanr cassette | Pharmacia |
| pRGK309 | CcsB:PhoA expression vector; Tetr | 3 |
| pRGK338 | puc119-DsbB; 2,469-bp PCR product including dsbB ORF as HinDIII fragment in puc119; Ampr | This study |
| pRGK339 | dsbB::Kanr; pRGK338 with Kanr cassette from pUC4-KIXX replacing dsbB ORF; Ampr Kanr | This study |
| pRGK340 | dsbB::Kanr HinDIII fragment from pRGK339 in pss2141; Gmr Kanr Sms | This study |
| pRGK341 | ccsB gene cloned into pET2-Blue with C-terminal 6 × His tag; Ampr | This study |
| pRGK342 | CcsA replacing PhoA from pRGK309; Tetr | This study |
ORF, open reading frame.
B. pertussis transposon mutagenesis and analysis.
The mini-Tn5gfp-km transposon carried on pTGN gave good results for B. pertussis mutagenesis in our study. The wild-type strain BC39 of B. pertussis was used as the recipient. The donor strain, E. coli S17-1 (λ pir), was mixed with B. pertussis and allowed to conjugate on blood agar plates overnight. These pools were resuspended and plated on blood agar containing antibiotics. Since the B. pertussis BC39 chromosome carries nalidixic acid resistance, plating exconjugants on this drug in addition to kanamycin ensures selection for only B. pertussis with a chromosomally integrated transposon. Either kanamycin or gentamicin resistance genes, both of which are located on the transposon, were used for selection, with no significant difference in yield. A total of 240 independent conjugations were carried out to yield the 16,000 colonies that were screened. (The colonies from single conjugations expressed green fluorescent protein [GFP] at various levels. This observation and the fact that no two oxidase mutants that were sequenced gave identical inserts indicate that very few siblings were screened.) Colonies were tested for defects in cytochrome c biogenesis by the TMPD oxidase assay. Colonies were transferred by toothpick to antibiotic blood agar plates with a nitrocellulose overlay, incubated for 3 days, and then soaked in a 0.1% TMPD solution and compared to wild-type (blue) and mutant (colorless) controls for phenotype, as described previously. After restreaking the strains and performing a second oxidase test, we studied the strains further. For cytochrome c profiles, cells were grown in 1.5 ml minimal medium, harvested, and extracted with B-PER reagent (Pierce) for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and heme stains. Twenty-seven mutants are characterized further in the present study. (Three additional transposon mutants that produced the oxidase-negative/heme stain-negative phenotype were not complemented by the respective wild-type gene, in which Tn5 was located, to an oxidase-positive phenotype. However, these were complemented by a ccsBA plasmid [pRGK342]. Furthermore, we could not recapitulate the oxidase-negative defect by directly inactivating the respective gene in BC39. We conclude that a small percentage of transposon mutants actually have either point mutations in unlinked loci [here, ccsBA] or additional IS50 transpositions, as has been noted previously for B. pertussis.) For sequencing the regions of insertions, we used genomic DNA that was purified by the QIAGEN bacterial genomic DNA kit. Cycle sequencing was accomplished using this DNA as the template and pTGN as the control with BigDye terminators. Initially, sequencing with three different oligonucleotides that were complementary to the Tn5 ends in pTGN was attempted. However, for unknown reasons, only one of these primers gave consistent results that could be unambiguously read using the genomic DNA as the template. The sequence of that oligonucleotide is 5′-CGTCAATTCGAGGGCCGCACTT-3′ (TGNUP3), the last four bases of which are part of the Tn5 inverted repeat. We estimate that the automated sequence output allowed us to determine the sequence accurately within eight nucleotides.
Construction of RGK343 (dsbB knockout).
DsbB and adjoining DNA was amplified from Tohama III genomic DNA with oligonucleotides containing BamHI restriction sites. The oligonucleotides used were 5′-CGCTGGATCCCGTGTTCAAGGAGCTGGAAG-3′ (forward dsbB) and 5′-AACGGATCCAGTCCTGGCGCCTGTCGG-3′ (reverse dsbB). The amplified genes were cloned into pUC119 as a BamHI fragment to form pRGK338 (pUC119dsbB). To construct pUC-derived plasmids with dsbB replaced with a Kanr cassette, the pUC and upstream and downstream portions of dsbB were outwardly amplified with the following oligonucleotides containing MluI sites: 5′-CCGCTCGAGGTTGCATGATCTGCCGCATGAC-3′ (5′ outward dsbB) and 5′-GATCTCGAGCCTGGCGCTGTTCGTGATCGTGCT-3′ (3′ outward dsbB). The resulting PCR products are linearized plasmids containing dsbB with the center of the open reading frame deleted. The Kanr cassette from pUC4-KIXX (Pharmacia) was inserted into the XhoI sites of pRGK338 to yield pRGK339 (pUC119dsbB-Kan). The BamHI fragment from pRGK339 containing the Kanr cassette was then subcloned into the BamHI site of the Gmr Sms suicide plasmid vector pSS2141 to yield pRGK340 (pSS2141dsbB-Kan). pRGK340 was conjugated into BC39 and grown on blood agar containing kanamycin and streptomycin (the latter was used to select against the vector and for double recombinants with the Kanr cassette in the chromosome).
Alkaline phosphatase assay.
Alkaline phosphatase activity was detected visually in B. pertussis colonies that were grown on nitrocellulose disks overlaying blood agar plates and then transferred to LB plates containing the chromogenic indicator 5-bromo-4-chloro-3-indolylphosphate (XP; Sigma Chemical). Alkaline phosphatase activities were measured in cells grown in liquid cultures by a method adapted from that described by Brickman and Beckwith (7). Briefly, a 5-ml B. pertussis culture was grown to mid-log phase. A 20-μl aliquot of each culture was diluted in 0.04% Sigma 104 to a final volume of 200 μl and incubated at 37°C until the optical density at 450 nm (OD450) of the solution was between 0.1 and 0.4 (20 min to 3 h). Reactions were stopped by the addition of 20 μl of monobasic potassium phosphate. Protein concentrations in each culture were determined using a modified bicinchoninic acid (BCA) assay (Pierce). Relative activities were calculated using the formula (OD450 × 103)/protein concentration (mg)/time (min).
Production of antibodies to CcsB.
For overexpression of the periplasmic domain of the CcsB protein in E. coli, the coding sequence of this region was amplified by PCR using Tohama III genomic DNA as the template and the oligonucleotides 5′-CAGCCCATGGCCATCAACGGCGAAATG-3′ and 5′-CGGGGATCCTGGAAAACGCTGCCCTGG-3′. The left primer engineers an NcoI site, and the right primer engineers a BamHI site. The resulting amplified product was digested with NcoI and BamHI and cloned into the plasmid pET2-Blue (Novagen) to create the plasmid pRGK341 (pET2ccsB-His). The CcsB periplasmic protein containing a C-terminal six-His tag was overexpressed from pRGK341 in the Tuner strain of E. coli. Cells were grown to mid-exponential phase (A600 = 0.6) in LB containing 50 μg/ml carbenicillin at 37°C and induced with IPTG (isopropyl-β-d-thiogalactopyranoside; 1 mM) for 3 h. Cells were harvested, washed in 20 mM Tris (pH 8), and resuspended in lysis buffer (20 mM Tris [pH 8], lysozyme [1 mg/ml], Triton X-100 [1%]). After incubation on ice for 20 min, the bacteria were lysed by sonication (twice for 3 min each; Branson model 200 sonicator) using a microtip at a power setting of 40% and a duty cycle of 50%. Debris was removed by centrifugation (10,000 × g, 15 min, 4°C), and the six-His-tagged proteins were purified from the supernatant fluids by chromatography over a nickel affinity resin according to the recommendations of the manufacturer (Novagen). The eluted protein was concentrated with Centricon-30 columns (Millipore) and dialyzed against storage buffer (20 mM Tris [pH 8], 200 mM NaCl) to remove the imidazole. The purity of the preparations, assessed by SDS-PAGE and staining with Coomassie brilliant blue, was greater than 95%. Protein concentrations were determined using a BCA assay (Pierce). Antiserum was generated in New Zealand White rabbits at a commercial facility (Cocalico Biologicals, Inc.). The antibodies were purified from the serum by ammonium sulfate precipitation and used at a 1:700 dilution for Western blotting.
Membrane fractionation.
B. pertussis strain BC39 and derivatives were grown at 37°C in Stainer-Scholte medium containing the appropriate antibiotics for 24 to 36 h to an A600 of 1.3 to 1.5. Cells were treated as follows. Cell cultures were harvested by centrifugation at 15,000 × g for 15 min at 4°C. Cells were resuspended at 1/100 the original culture volume in 10 mM Tris (pH 8) and sonicated twice for 3 min each at 4°C (Branson model 200 sonicator with a microtip at a power setting of 40% and a duty cycle of 50%). Unbroken cells were removed by centrifugation at 10,000 × g for 15 min at 4°C and discarded. Soluble and membrane fractions were isolated by ultracentrifugation of the crude sonicate fraction at 100,000 × g for 2 h. The supernatant contained soluble periplasmic and cytoplasmic proteins. The pelleted membranes were washed once and resuspended in 10 mM Tris, pH 8. Solubilized membrane extracts were prepared by the addition of Triton X-100 to a final concentration of 1%, incubation on ice for 1 h, and centrifugation for 15 min at 15,000 × g. Insoluble proteins were resuspended in 10 mM Tris, pH 8.
Construction of pRGK342 (pBA).
The coding sequence of B. pertussis ccsA was amplified from the Tohama III genomic DNA template with the oligonucleotides 5′-CGTAAAGATCTTTGAATGTCTACCACCACCAGC-3′ and 5′-AAGGGGTACCAAAGACAATGCCCCGCATCTG-3′. The left primer corresponds to the N terminus of ccsA and engineers a BglII site followed by a stop codon, and the right primer corresponds to the C terminus of ccsA and engineers a KpnI site. The amplified product was digested with BglII and KpnI and ligated to the vector pRGK309 (3), which was digested with BamHI and KpnI. pRGK309 contains the coding sequences of cycC and ccsB with an in-frame C-terminal translational fusion to alkaline phosphatase, which is replaced by CcsA in the resulting plasmid, pRGK342 (pBA). pRGK342 was verified by restriction digestion and DNA sequencing.
B-PER extraction reagent.
B-PER protein extracts from B. pertussis and E. coli were prepared as directed by the supplier (Pierce). B-PER uses a proprietary nonionic detergent in 20 mM Tris, pH 7.5, to extract proteins; thus, the resulting B-PER extract contains soluble, periplasmic, and some membrane proteins. Briefly, 1.5- to 5-ml cultures were pelleted at 13,000 rpm in a microcentrifuge and frozen at −80°C for a minimum of 15 min to aid in cell disruption (longer freezing [overnight] resulted in enhanced membrane protein extraction in our experiment). Cell pellets were thawed on ice and resuspended in 1/25 to 1/10 of the original culture volume in B-PER extraction reagent (determined empirically). The resuspension was vortexed for 1 min and centrifuged at 13,000 rpm for 10 min to separate insoluble debris. The supernatant was saved, and the protein concentration was determined by BCA assay using bovine serum albumin as a standard.
Chemiluminescent heme staining.
Heme staining was performed as described previously (17). Heme staining of E. coli extracts was performed using SuperSignal Femto chemiluminescent substrate (Pierce), and the results were exposed using an LAS-1000plus luminescent image analyzer charge-coupled-device camera system (Fujifilm). B. pertussis heme staining was performed using SuperSignal Pico chemiluminescent substrate (Pierce), and the results were revealed by exposure to X-ray film (Kodak X-OMAT AR).
Western blotting.
For analysis of the CcsB protein, extracts were first separated by 12% or 15% SDS-PAGE and electroblotted onto Hybond-C nitrocellulose filters (Amersham). Western analyses were performed using the SuperSignal West Pico ECL detection system (Pierce). Protein A peroxidase (Sigma) was used as the secondary label (1:1,300 dilution). Blots probed with anti-CcsB were exposed to X-ray film (Kodak X-OMAT AR).
RESULTS AND DISCUSSION
B. pertussis Tn5 mutants are deficient in cytochrome c oxidase activity.
A TMPD oxidase screen, sometimes called the NADI or “oxidase test,” is based on the ability of TMPD to donate electrons specifically to cytochrome c, which reduces cytochrome c oxidase, resulting in a blue oxidized TMPD. Because B. pertussis has many soluble c-type cytochromes, including four different diheme cytochromes c4 (3), it is likely that multiple, redundant cytochromes c feed into the single predicted cytochrome c oxidase (and indeed this is consistent with the genetic results reported below.) Thus, with this colony screen, it is expected that Tn5 insertions in the genes for cytochrome c biogenesis (system II) and for cytochrome c oxidase subunits and assembly would be discovered. We screened approximately 16,000 colonies of B. pertussis that were resistant to kanamycin or gentamicin, due to the insertion of Tn5-GFP from pTGN. Twenty-seven independent TMPD oxidase-negative mutants were characterized further (Table 2). The site of insertion for each mutant was determined by genomic sequencing using an oligonucleotide specific for the transposon end. This analysis was possible due to the availability of the complete sequence of the B. pertussis genome (43). The genes in which Tn5-GFP was inserted and the locations of the insertions within the proteins encoded by the gene are shown in Table 2. To evaluate whether the mutants were deficient in the ability to assemble all c-type cytochromes, we performed heme staining of B-PER fractions from cells of each strain (Fig. 1A). The heme present in c-type cytochromes is retained upon SDS-PAGE of the polypeptides, and this is detected by heme staining.
TABLE 2.
B. pertussis Tn5 mutants with defects in TMPD oxidase
| Straina | Gene with Tn5 insertion | Location of Tn5 in geneb | TMPD oxidase activityc | % of cytochrome c producedde | Synthesis of cytochromes corrected by DTTef |
|---|---|---|---|---|---|
| CB08 | ctaC (CoxII) | G217→ | — | NR | NR |
| CB01 | ctaD (CoxI) | G247→ | — | NR | NR |
| CB27 | ctaD | V95← | — | NR | NR |
| CB134 | ctaD | G87→ | — | NR | NR |
| CB116 | ctaE (CoxIII) | A270← | — | NR | NR |
| CB28 | ctaB | N195→ | — | NR | NR |
| CB114 | ctaB | I147→ | — | NR | NR |
| CB106* | sco2 | V67→ | — | NR | NR |
| CB15 | hemZ (hemH) | Upstream | — | <10 | No |
| CB05 | dipZ (dsbD) | E99← | +/− | <50 | Yes |
| CB07 | dipZ | L387→ | +/− | <50 | Yes |
| CB10 | dipZ | P137← | — | <1 | Yes |
| CB26 | dipZ | A273→ | — | <1 | Yes |
| CB82* | dipZ | G205← | — | <1 | Yes |
| CB128 | dipZ | L290→ | — | <1 | Yes |
| CB115 | dipZ | R82← | — | <1 | Yes |
| CB84* | cycC | P117← | — | <10 | No |
| CB75 | ccsA | V132→ | — | <1 | No |
| CB83* | ccsA | G43← | — | <1 | No |
| CB121 | ccsA | D348→ | — | <1 | No |
| CB127 | ccsA | D30← | — | <1 | No |
| CB133 | ccsB | Y58→ | — | <1 | No |
| CB110* | ccsB | S55→ | — | <1 | No |
| CB111* | ccsB | V550← | — | <1 | No |
| CB129 | ccsB | N501→ | — | <1 | No |
| CB130 | ccsB | G429→ | — | <1 | No |
| CB123 | ccsB | Y79← | — | <1 | No |
An asterisk indicates a strain with a Tn5 insertion in a dsbB background.
The amino acid residue is indicated, followed by an arrow which refers to the direction of the GFP (in the mini-Tn5) with respect to the orientation of the gene.
Activity is noted as detected in single colonies and restreaked from each strain. —, not detectable above background; +/−, less than that of the wild type but above background.
Profiles of c-type cytochromes are as shown in Fig. 1 and described in the text. The values are relative to that produced by the WT.
NR, not relevant, since these mutants have wild-type levels of c-type cytochromes.
DTT was added to the growing cultures at various concentrations, and then extracts were assayed for c-type cytochromes, as depicted in Fig. 1.
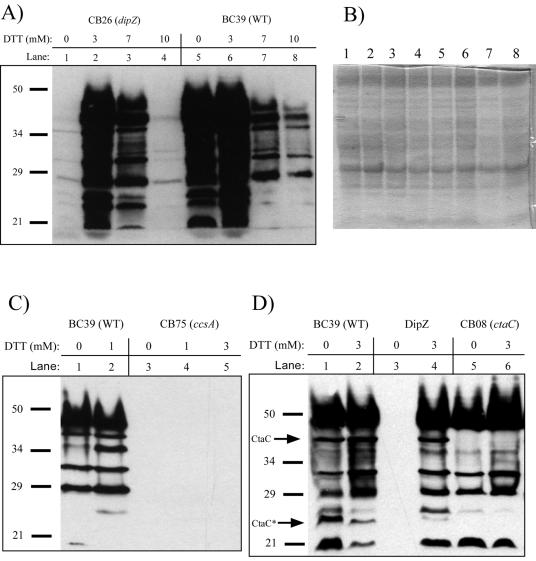
FIG. 1.
(A, C, and D) Cytochrome c profiles of representatives from three classes of B. pertussis Tn5 mutants deficient in cytochrome c oxidase activity. Heme staining was carried out on B-PER extracts from the indicated strains. Equivalent amounts of protein (approximately 20 μg) were loaded in each lane, shown stained in panel B, which represents a duplication of the strains and conditions shown in panel A. The concentration of DTT used for cells grown overnight in cultures is designated above each lane number (A, C, and D). The location of full-length CtaC as well as a heme-containing truncated CtaC (CtaC*: see text) is indicated (D).
Seven mutants had insertions in the dipZ gene, encoding a membrane-thioreducing protein that transfers reducing equivalents from inside to outside the cell (2, 11, 34, 35, 45) (see reference 24 for a review). Figure 1A shows the heme stains (cytochrome c profiles) of the BC39 wild-type strain and one of the dipZ mutants called CB26. We previously reported that wild-type B. pertussis has at least eight different c-type cytochromes in these B-PER fractions (3). Less than 1% of wild-type c-type cytochromes are detectable in the CB26 cells grown in basal medium to stationary phase (Fig. 1A, lane 1), whereas BC39 has a significant level of many different c-type cytochromes (Fig. 1A, lane 5). Equal amounts of protein, as shown in Fig. 1B, were loaded in each lane. All B. pertussis dipZ Tn5 mutants, including CB26, were corrected for cytochrome c assembly by exogenous dithiothreitol (DTT) (Fig. 1A, lanes 2 and 3 [results with CB26]; Table 2), which is consistent with the function of DipZ in the reduction of the periplasmic CXXCH motif in cytochromes c. Cell growth and cytochrome c assembly correction was maximal at 3 mM DTT, whereas higher concentrations resulted in reduced levels of correction and cell growth.
Eleven Tn5 insertions were located in the cycC-ccsB-ccsA operon. Since the single insertion in cycC, which encodes a cytochrome c4, still produced low levels of all c-type cytochromes (Table 2), it is likely that this insertion is partially polar on the ccsB gene and does not represent a new biosynthetic gene. The 10 remaining mutants, 4 with insertions in ccsB and 6 with insertions in ccsA, produce <1% of the wild-type levels of c-type cytochromes (Fig. 1C, lane 3). The synthesis of cytochromes in these mutants is not corrected by DTT (Fig. 1C, lanes 4 and 5 [example of results with CB75]).
Seven mutants possessed Tn5 insertions in genes encoding the cytochrome c oxidase subunits (ctaD, ctaC, and ctaE) or the biosynthesis of the a-type heme associated with the oxidase (ctaB). These genes are located at a single locus in B. pertussis, as described previously using the genomic sequence (28). B-PER extracts of cells from the ctaB and ctaD mutants showed wild-type levels and profiles of c-type cytochromes (not shown). Interestingly, the ctaC mutant CB08 had wild-type levels of c-type cytochromes but was completely lacking two c-type cytochromes that migrated at approximately 40 kDa and 24 kDa on SDS-PAGE (Fig. 1D, compare lanes 5 and 6 to lanes 1, 2, and 4). The ctaC-encoded subunit of the B. pertussis cytochrome c oxidase has a CXXCH motif and is a predicted 42-kDa c-type cytochrome, explaining this deficiency in CB08. Bengtsson et al. (6) showed that CtaC in B. subtilis is truncated in the N-terminal end, resulting in a 28-kDa heme-containing fragment in addition to the 40-kDa full-length protein. We suggest that a similar truncation occurred in B. pertussis, resulting in a second heme staining band around 24 kDa that is absent in CB08. One can also surmise from this result that the cytochrome c oxidase in B. pertussis is a caa3 type, similar to that in B. subtilis (6, 61). B. pertussis has homologues of the CcoNOP subunits of the cbb3 oxidase as determined previously by BLAST analysis (36). However, B. pertussis ccoN is a pseudogene, due to a frameshift mutation after codon 220; this fact has been reconfirmed by the Sanger Sequencing Center (43). Based on the ccoN frameshift and our ability to isolate oxidase-negative colonies in transposon insertion mutants of the caa3 oxidase, we speculate that the cbb3 oxidase is not functional in B. pertussis.
An insertion in one mutant was located in a gene that we designated sco2, which is related to the Sco1 protein in eukaryotes (19, 23, 42, 52) and B. subtilis known to be required for assembly of the cytochrome c oxidase. A study of B. subtilis has suggested that the Sco1 homolog, called YpmQ, is involved in CuA insertion into the oxidase (32). As in our study, the B. subtilis ypmQ mutant was TMPD oxidase negative. Interestingly, the B. pertussis genome has two genes related to sco, one directly downstream of the aforementioned cta genes, called sco1 previously (28), and the sco2 discovered here. Clearly the Sco2 protein is necessary for activity (assembly) of the cytochrome c oxidase in B. pertussis. It is possible that a Sco1/Sco2 heteromer is functioning in B. pertussis, since there is precedence for such a model with yeast (31). We have not studied this aspect further.
The insertion of one mutant was located in the DNA directly upstream of the hemZ (hemH) ATG start codon. This gene encodes the B. pertussis ferrochelatase, which inserts reduced iron into protoporphyrin IX (62). Indeed, this mutant secretes large amounts of a fluorescent compound which could be protoporphyrin IX, as other hemZ mutants have previously been reported to secrete (18, 37). We speculate that as a result of this insertion, the transcription of hemZ is directed by a weak promoter within Tn5, reducing the levels of heme produced and thus having a pleiotropic effect on cytochrome production. Consistent with this hypothesis, CB15 produces less than 10% of the c-type cytochromes, and it grows more slowly than any of the other mutants which have wild-type growth properties under the conditions tested.
In conclusion, for most genes described here, we isolated multiple strains with insertions and in different regions of the genes. Given this result and the genome size (4.1 megabase pairs) and number of colonies analyzed (16,000 colonies), we estimate approximately 95% saturation with the screen. We suggest that it is likely that no new genes for the system II pathway exist. Other than the thioreduction system, CcsB and CcsA are necessary and may be sufficient for the assembly process. One question that emerges is why no genes involved in disulfide bond formation were isolated, which we address next.
The dsb pathway is not essential for cytochrome c biogenesis in B. pertussis.
It is surprising that the screen did not yield strains with insertions in genes encoding the dsb oxidizing pathway. The dsb oxidizing pathway, consisting of DsbA and DsbB, is responsible for forming disulfide bonds in the periplasm of gram-negative bacteria (8, 46). In E. coli, DsbA was shown to be required for formate-dependent nitrate reduction due to a defect in cytochrome c biosynthesis (33). Additionally, the synthesis of endogenous holocytochromes c and heterologously produced Paracoccus denitrificans holocytochrome c550 in E. coli was shown to be lost in dsbA and dsbB mutants (49). B. pertussis possesses a single DsbA and DsbB homolog (by BLAST analysis with dsbA exhibiting 24% identity and dsbB with 28% identity to E. coli homologs) (see also reference 54). To investigate if B. pertussis DsbB is required for holocytochrome synthesis and disulfide bond production, a directed knockout strain was generated via the insertion of a kanamycin resistance cassette by homologous recombination. The dsbB insertion was confirmed by PCR analysis, and the dsbB mutant was analyzed for cytochrome c assembly. The DsbB− strain (RGK343) produces wild-type levels of holocytochromes c (Fig. 2, lanes 1 and 6), which suggests that an alternative mechanism to oxidize the CXXCH motif exists in B. pertussis.
FIG. 2.
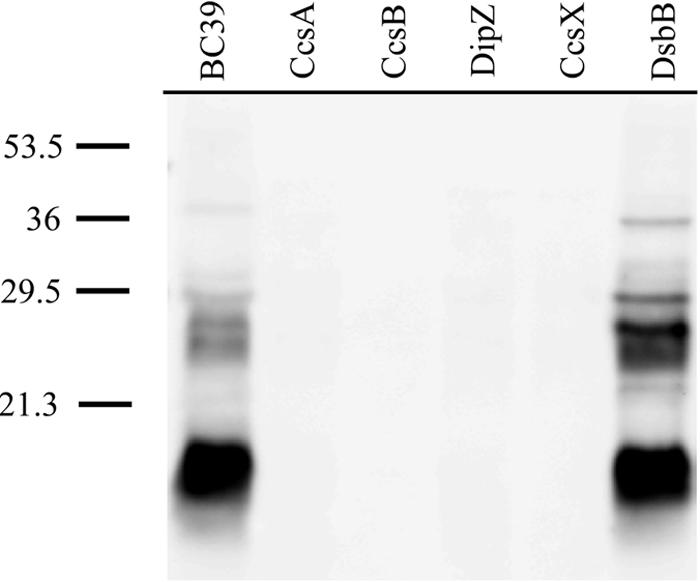
Heme stain blot of indicated B. pertussis B-PER protein fractions. Equivalent amounts of protein (19 μg) were loaded in each lane. Negative controls CcsA, CcsB, DipZ, and CcsX were defective for cytochrome c synthesis. This heme stain is representative of a soluble cytochrome c profile (see reference 4 for soluble versus membrane cytochrome c profiles) since the cell pellet was frozen for less than 15 min. Molecular size markers (in kilodaltons) are noted on the left.
To assay the status of another disulfide-bonded protein, alkaline phosphatase, in the periplasm of dsbB, we transformed both BC39 and RGK343 with a plasmid possessing the B. pertussis cycC (cytochrome c4) fused with alkaline phosphatase (pCycC:PhoA). Alkaline phosphatase is a periplasmic homodimer that contains two intramolecular disulfide bridges that are required for activity (1). A disrupted periplasmic dsb pathway in E. coli results in inactive alkaline phosphatase and a Pho− phenotype (45). When pCycC:PhoA was expressed in RGK343, alkaline phosphatase activity was reduced only approximately twofold (Table 3) compared to that of the wild type. Pho activity was well above background levels, indicating that there is another, potentially redundant system capable of oxidizing alkaline phosphatase.
TABLE 3.
Alkaline phosphatase activities of bacteria with CycC:Pho gene fusion reporter
| B. pertussis strain | Plasmid | Alkaline phosphatase activitya |
|---|---|---|
| BC39 | None | 3.9 ± 0.8 |
| pCycC:Pho | 761.6 ± 33.8 | |
| RGK343 | None | 3.4 ± 2.9 |
| pCycC:Pho | 330.7 ± 10.1 |
Activity is given in OD units min−1 mg−1 protein. Assays were performed three times. B. pertussis strains were grown in Stainer-Scholte media to mid-log phase at 37°C. Cultures containing pCycC:PhoA were grown in the presence of tetracycline.
We have attempted to discover a second oxidizing system in B. pertussis by using a genetic approach. Starting with RGK343 harboring pCycC:PhoA, we searched for Tn5 mutants that are Pho−. Colonies were screened on plates containing the chromogenic indicator XP (Sigma Chemical). Colonies were initially grown on nitrocellulose disks overlaying blood agar plates, at which time they were removed and shifted to LB plates containing XP. Active PhoA catalyzes a conversion of XP resulting in blue colonies. Based on this screen, it is expected that transposon insertions in genes affecting periplasmic disulfide bonding, or the plasmid-borne phoA gene itself, would be identified by their white color. Of approximately 12,000 kanamycin-resistant colonies screened, 60 colonies were Pho−.
To establish whether insertions were located in phoA or a secondary dsb pathway, plasmid DNA was isolated from the Pho− colonies and subsequently transformed into the CC118 (Pho−) strain of E. coli. Transformants were plated on LB agar containing tetracycline (pCycC:PhoA selection). Of 30 Pho− B. pertussis mutants checked in this manner, all 30 had insertions in the plasmid-borne phoA as determined by their resistance to kanamycin and Pho− phenotypes. As a control, plasmids isolated from Pho+ B. pertussis strains yielded Pho+, kanamycin-sensitive CC118 strains. These results suggest that under the growth conditions used, a second redundant periplasmic oxidation protein(s) does not exist (or is essential for growth). Potentially, a small molecule such as cystine or oxygen may be sufficient for disulfide bond formation and apocytochrome c oxidation in B. pertussis. Since B. pertussis is an obligate aerobe, testing for direct oxygen oxidation is technically not feasible. Cystine is a required growth factor for B. pertussis; thus, completely removing it from growth media is not possible (53, 54). However, changing the levels of cystine did not perturb cytochrome c synthesis (not shown). The dsbB mutant is also no more sensitive to DTT, as determined by doubling time or cytochrome c biosynthesis.
Our results are in agreement with previous studies on pertussis toxin production and secretion in B. pertussis dsbA and dsbB mutants (55). DsbA and DsbB are not required for generating an intermolecular disulfide between the pertussis toxin secretion proteins PtlF and PtlI (55). However, formation of the 11 intramolecular disulfide bonds that are required for pertussis toxin assembly is defective in dsbA or dsbB mutants. This supports the hypothesis that an additional, non-protein-based process may be responsible for oxidizing a subset of periplasmic proteins in B. pertussis, possibly those proteins with a single or few disulfide bonds.
Similarly, DsbA and DsbB are not directly required for cytochrome c biosynthesis in R. capsulatus (13) or B. subtilis (16). However, experiments with R. capsulatus and B. subtilis show that a dsbB mutation suppresses the TMPD− phenotype of a ccdA mutant. CcdA is functionally and structurally related to DipZ/DsbD. It has been proposed previously (13) that after the secretion of apocytochrome into the periplasm, the cysteine residues of the heme-binding CXXCH motif are oxidized by DsbB/DsbA. In a ccdA mutant, the oxidized heme-binding cysteines cannot be rereduced; thus, heme ligation is blocked. In the B. subtilis and R. capsulatus ccdA mutants, a compensatory dsbA or dsbB knockout restores thiol redox homeostasis with respect to cytochromes c. This allows reduced apocytochrome secreted into the periplasm to remain in a reduced state that is competent for heme ligation. Thus, R. capsulatus and B. subtilis may not strictly require the heme-binding cysteine residues of apocytochrome c to be oxidized and subsequently rereduced prior to heme ligation. This situation does not exist in E. coli, possibly because E. coli requires immediate apocytochrome oxidation to escape rapid proteolysis (13). We have investigated whether a dsbB defect will compensate for a dipZ defect in B. pertussis. With RGK343, we found that an insertion in mutant strain CB82 is located in dipZ and is TMPD negative. Therefore, a B. pertussis dipZ dsbB double mutant is unable to synthesize cytochromes c and is corrected by exogenous DTT. This mutant is also PhoA+ when pCycC:PhoA is present. This demonstrates that cytochrome c biogenesis is blocked in the dsbB dipZ double mutant, as it is in the dipZ mutant alone (e.g., CB82 compared to CB10). In contrast to R. capsulatus and B. subtilis, a dsbB mutation does not suppress the dipZ mutation in B. pertussis, presumably due to complete (non-DsbB-catalyzed) oxidation of the heme-binding cysteines of the apocytochrome. These results are also consistent with the hypothesis that in B. pertussis, oxygen or another small oxidizing molecule is responsible for oxidizing disulfide bonds in the periplasm in the absence of DsbB/DsbA.
Evidence that CcsB and CcsA form a complex in B. pertussis.
The results of random insertion mutagenesis and oxidase screening suggest that with the proper periplasmic reducing environment and heme availability, all that is necessary for cytochrome c assembly are the CcsA and CcsB proteins. We decided to further investigate CcsB and CcsA from B. pertussis and their localizations, syntheses, and quaternary structures. CcsB (3) and CcsA (20) are predicted membrane proteins with four and six transmembrane regions, respectively. The domain topology was demonstrated using lacZ and phoA methods. In C. reinhardtii, CcsB (Ccs1) appears to be present but at reduced levels in strains with a ccsA mutation or any of three other ccs mutations (14). In another study by Hamel and colleagues, CcsB appears to migrate in a complex on native gels, but this putative complex is not present in a ccsA mutant (21). Hamel et al. concluded that the large size of this complex, greater than 200 kDa, suggests that other components besides CcsA and CcsB are present, a result that is consistent with the C. reinhardtii genetic analysis cited above.
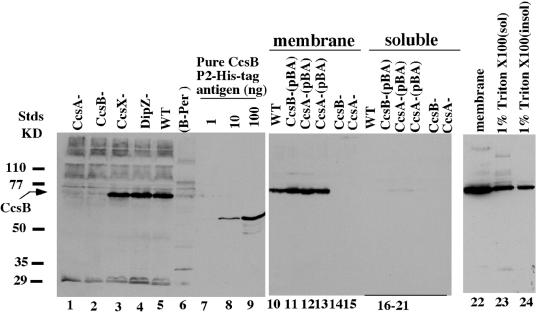
To begin to study the locations and potential complex formations of CcsB and CcsA from proteobacteria, we generated antisera to the 435-residue periplasmic domain (P2) of the B. pertussis CcsB protein (3). This was used to probe various B. pertussis strains and soluble and membrane fractions (Fig. 3). The antisera reacted with the isolated 50-kDa P2 polypeptide used as an antigen (Fig. 3, lanes 8 and 9). These sera also reacted well on Western blots to a polypeptide of approximately 72 kDa in B. pertussis crude extracts from the wild type (WT) (Fig. 3, lane 5) that is absent in a ccsB mutant (lane 2). The predicted molecular size of CcsB was 76 kDa. CcsB was also absent in a ccsA mutant (lane 1) but present in dipZ (and ccsX) mutants. A lack of CcsB in a ccsA mutant cannot be due to polarity, as ccsA is downstream of ccsB and only ccsA is necessary to complement this mutation. We conclude that the CcsB protein is unstable and degraded in the ccsA mutant. This suggests that the bacterial CcsB and CcsA proteins form a stable complex. We isolated membrane and soluble fractions from the WT and the ccsB and ccsA mutants, as well as various ccsB or ccsA mutants with a complementing plasmid. These fractions were probed for CcsB. The CcsB protein is a membrane protein (Fig. 3, lanes 10 to 13) that can be solubilized by 1% Triton X-100 (lane 23). The membrane fractions of strains without a functional ccsB or ccsA gene did not possess the CcsB polypeptide (Fig. 3, lanes 14 and 15). These results support the contention that a CcsBA complex provides both the heme export and cytochrome c-heme ligation functions of system II.
FIG. 3.
Western blot analysis using CcsB antisera of the indicated B. pertussis protein fractions and strains. CcsB antisera generated against the 50-kDa periplasmic domain (P2) of the B. pertussis CcsB protein reacted with the hexahistidine-tagged P2 antigen (lanes 7 to 9). Extracts in lanes 1 to 5 were from crude sonicated cells of the indicated B. pertussis strains. A B-PER extract from the WT strain BC39 is shown in lane 6. Membrane and soluble fractions, as described in the text, are shown in lanes 10 to 24.
Acknowledgments
We thank Duncan Maskell and Julian Parkhill at the Sanger Institute for making the B. pertussis genomic sequence publicly available before publication. We thank Brian San Francisco for culturing and testing the B. pertussis strains.
This work was supported by NIH grant GM47909 to R.G.K.
REFERENCES
- 1.Akiyama, Y., and K. Ito. 1993. Folding and assembly of bacterial alkaline phosphatase in vitro and in vivo. J. Biol. Chem. 268:8146-8150. [PubMed] [Google Scholar]
- 2.Beck, R., H. Crooke, M. Jarsch, J. Cole, and H. Burtscher. 1994. Mutation in dipZ leads to reduced production of active human placental alkaline phosphatase in Escherichia coli. FEMS Microbiol. Lett. 124:209-214. [DOI] [PubMed] [Google Scholar]
- 3.Beckett, C. S., J. A. Loughman, K. A. Karberg, G. M. Donato, W. E. Goldman, and R. G. Kranz. 2000. Four genes are required for the system II cytochrome c biogenesis pathway in Bordetella pertussis, a unique bacterial model. Mol. Microbiol. 38:465-481. [DOI] [PubMed] [Google Scholar]
- 4.Beckman, D. L., and R. G. Kranz. 1993. Cytochromes c biogenesis in a photosynthetic bacterium requires a periplasmic thioredoxin-like protein. Proc. Natl. Acad. Sci. USA 90:2179-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckman, D. L., D. R. Trawick, and R. G. Kranz. 1992. Bacterial cytochromes c biogenesis. Genes Dev. 6:268-283. [DOI] [PubMed] [Google Scholar]
- 6.Bengtsson, J., H. Tjalsma, C. Rivolta, and L. Hederstedt. 1999. Subunit II of Bacillus subtilis cytochrome c oxidase is a lipoprotein. J. Bacteriol. 181:685-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brickman, E., and J. Beckwith. 1975. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and phi80 transducing phages. J. Mol. Biol. 96:307-316. [DOI] [PubMed] [Google Scholar]
- 8.Collet, J. F., and J. C. Bardwell. 2002. Oxidative protein folding in bacteria. Mol. Microbiol. 44:1-8. [DOI] [PubMed] [Google Scholar]
- 9.Cookson, B. T., H.-L. Cho, L. A. Herwaldt, and W. E. Goldman. 1989. Biological activities and chemical composition of purified tracheal cytotoxin of Bordetella pertussis. Infect. Immun. 57:2223-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cookson, B. T., A. N. Tyler, and W. E. Goldman. 1989. Primary structure of the peptidoglycan-derived tracheal cytotoxin of Bordetella pertussis. Biochemistry 28:1744-1749. [DOI] [PubMed] [Google Scholar]
- 11.Crooke, H., and J. Cole. 1995. The biogenesis of c-type cytochromes in Escherichia coli requires a membrane-bound protein, DipZ, with a protein disulphide isomerase-like domain. Mol. Microbiol. 15:1139-1150. [DOI] [PubMed] [Google Scholar]
- 12.Deshmukh, M., G. Brasseur, and F. Daldal. 2000. Novel Rhodobacter capsulatus genes required for the biogenesis of various c-type cytochromes. Mol. Microbiol. 35:123-138. [DOI] [PubMed] [Google Scholar]
- 13.Deshmukh, M., S. Turkarslan, D. Astor, M. Valkova-Valchanova, and F. Daldal. 2003. The dithiol:disulfide oxidoreductases DsbA and DsbB of Rhodobacter capsulatus are not directly involved in cytochrome c biogenesis, but their inactivation restores the cytochrome c biogenesis defect of CcdA-null mutants. J. Bacteriol. 185:3361-3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dreyfuss, B. W., P. P. Hamel, S. S. Nakamoto, and S. Merchant. 2003. Functional analysis of a divergent system II protein, Ccs1, involved in c-type cytochrome biogenesis. J. Biol. Chem. 278:2604-2613. [DOI] [PubMed] [Google Scholar]
- 15.Erlendsson, L. S., R. M. Acheson, L. Hederstedt, and N. E. Le Brun. 2003. Bacillus subtilis ResA is a thiol-disulfide oxidoreductase involved in cytochrome c synthesis. J. Biol. Chem. 278:17852-17858. [DOI] [PubMed] [Google Scholar]
- 16.Erlendsson, L. S., and L. Hederstedt. 2002. Mutations in the thiol-disulfide oxidoreductases BdbC and BdbD can suppress cytochrome c deficiency of CcdA-defective Bacillus subtilis cells. J. Bacteriol. 184:1423-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feissner, R., Y. Xiang, and R. G. Kranz. 2003. Chemiluminescent-based methods to detect subpicomole levels of c-type cytochromes. Anal. Biochem. 315:90-94. [DOI] [PubMed] [Google Scholar]
- 18.Frustaci, J. M., and M. R. O'Brian. 1993. The Escherichia coli visA gene encodes ferrochelatase, the final enzyme of the heme biosynthetic pathway. J. Bacteriol. 175:2154-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glerum, D. M., A. Shtanko, and A. Tzagoloff. 1996. SCO1 and SCO2 act as high copy suppressors of a mitochondrial copper recruitment defect in Saccharomyces cerevisiae. J. Biol. Chem. 271:20531-20535. [DOI] [PubMed] [Google Scholar]
- 20.Goldman, B. S., D. L. Beck, E. M. Monika, and R. G. Kranz. 1998. Transmembrane heme delivery systems. Proc. Natl. Acad. Sci. USA 95:5003-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamel, P. P., B. W. Dreyfuss, Z. Xie, S. T. Gabilly, and S. Merchant. 2003. Essential histidine and tryptophan residues in CcsA, a system II polytopic cytochrome c biogenesis protein. J. Biol. Chem. 278:2593-2603. [DOI] [PubMed] [Google Scholar]
- 22.Inoue, K., B. W. Dreyfuss, K. L. Kindle, D. B. Stern, S. Merchant, and O. A. Sodeinde. 1997. Ccs1, a nuclear gene required for the posttranslational assembly of chloroplast c-type cytochromes. J. Biol. Chem. 272:31747-31754. [DOI] [PubMed] [Google Scholar]
- 23.Jaksch, M., I. Ogilvie, J. Yao, G. Kortenhaus, H. G. Bresser, K. D. Gerbitz, and E. A. Shoubridge. 2000. Mutations in SCO2 are associated with a distinct form of hypertrophic cardiomyopathy and cytochrome c oxidase deficiency. Hum. Mol. Genet. 9:795-801. [DOI] [PubMed] [Google Scholar]
- 24.Kadokura, H., F. Katzen, and J. Beckwith. 2003. Protein disulfide bond formation in prokaryotes. Annu. Rev. Biochem. 72:111-135. [DOI] [PubMed] [Google Scholar]
- 25.Koch, H.-G., O. Hwang, and F. Daldal. 1998. Isolation and characterization of Rhodobacter capsulatus mutants affected in cytochrome cbb3 oxidase activity. J. Bacteriol. 180:969-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kranz, R., R. Lill, B. Goldman, G. Bonnard, and S. Merchant. 1998. Molecular mechanisms of cytochrome c biogenesis: three distinct systems. Mol. Microbiol. 29:383-396. [DOI] [PubMed] [Google Scholar]
- 27.Kranz, R. G. 1989. Isolation of mutants and genes involved in cytochromes c biosynthesis in Rhodobacter capsulatus. J. Bacteriol. 171:456-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kranz, R. G., C. S. Beckett, and B. S. Goldman. 2002. Genomic analyses of bacterial respiratory and cytochrome c assembly systems: Bordetella as a model for the system II cytochrome c biogenesis pathway. Res. Microbiol. 153:1-6. [DOI] [PubMed] [Google Scholar]
- 29.Le Brun, N. E., J. Bengtsson, and L. Hederstedt. 2000. Genes required for cytochrome c synthesis in Bacillus subtilis. Mol. Microbiol. 36:638-650. [DOI] [PubMed] [Google Scholar]
- 30.Lee, E., and C. Manoil. 1994. Mutations eliminating the protein export function of a membrane-spanning sequence. J. Biol. Chem. 269:28822-28828. [PubMed] [Google Scholar]
- 31.Lode, A., M. Kuschel, C. Paret, and G. Rodel. 2000. Mitochondrial copper metabolism in yeast: interaction between Sco1p and Cox2p. FEBS Lett. 485:19-24. [DOI] [PubMed] [Google Scholar]
- 32.Mattatall, N. R., J. Jazairi, and B. C. Hill. 2000. Characterization of YpmQ, an accessory protein required for the expression of cytochrome c oxidase in Bacillus subtilis. J. Biol. Chem. 275:28802-28809. [DOI] [PubMed] [Google Scholar]
- 33.Metheringham, R., L. Griffiths, H. Crooke, S. Forsythe, and J. Cole. 1995. An essential role for DsbA in cytochrome c synthesis and formate-dependent nitrite reduction by Escherichia coli K-12. Arch. Microbiol. 164:301-307. [DOI] [PubMed] [Google Scholar]
- 34.Metheringham, R., K. L. Tyson, H. Crooke, D. Missiakas, S. Raina, and J. A. Cole. 1996. Effects of mutations in genes for proteins involved in disulphide bond formation in the periplasm on the activities of anaerobically induced electron transfer chains in Escherichia coli K12. Mol. Gen. Genet. 253:95-102. [DOI] [PubMed] [Google Scholar]
- 35.Missiakas, D., F. Schwager, and S. Raina. 1995. Identification and characterization of a new disulfide isomerase-like protein (DsbD) in Escherichia coli. EMBO J. 14:3415-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myllykallio, H., and U. Liebl. 2000. Dual role for cytochrome cbb3 oxidase in clinically relevant proteobacteria? Trends Microbiol. 8:542-543. [DOI] [PubMed] [Google Scholar]
- 37.Nakahigashi, K., K. Nishimura, K. Miyamoto, and H. Inokuchi. 1991. Photosensitivity of a protoporphyrin-accumulating, light-sensitive mutant (visA) of Escherichia coli K-12. Proc. Natl. Acad. Sci. USA 88:10520-10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Page, M. D., and S. J. Ferguson. 1993. Mutants of Methylobacterium extorquens and Paracoccus denitrificans deficient in c-type cytochrome biogenesis synthesise the methylamine-dehydrogenase polypeptides but cannot assemble the tryptophan-tryptophylquinone group. Eur. J. Biochem. 218:711-717. [DOI] [PubMed] [Google Scholar]
- 39.Page, M. D., and S. J. Ferguson. 1999. Mutational analysis of the Paracoccus denitrificans c-type cytochrome biosynthetic genes ccmABCDG: disruption of ccmC has distinct effects suggesting a role for CcmC independent of CcmAB. Microbiology 145:3047-3057. [DOI] [PubMed] [Google Scholar]
- 40.Page, M. D., and S. J. Ferguson. 1997. Paracoccus denitrificans CcmG is a periplasmic protein-disulphide oxidoreductase required for c- and aa3-type cytochrome biogenesis; evidence for a reductase role in vivo. Mol. Microbiol. 24:977-990. [DOI] [PubMed] [Google Scholar]
- 41.Page, M. D., Y. Sambongi, and S. J. Ferguson. 1998. Contrasting routes of c-type cytochrome assembly in mitochondria, chloroplasts and bacteria. Trends Biochem. Sci. 23:103-108. [DOI] [PubMed] [Google Scholar]
- 42.Papadopoulou, L. C., C. M. Sue, M. M. Davidson, K. Tanji, I. Nishino, J. E. Sadlock, S. Krishna, W. Walker, J. Selby, D. M. Glerum, R. V. Coster, G. Lyon, E. Scalais, R. Lebel, P. Kaplan, S. Shanske, D. C. De Vivo, E. Bonilla, M. Hirano, S. DiMauro, and E. A. Schon. 1999. Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat. Genet. 23:333-337. [DOI] [PubMed] [Google Scholar]
- 43.Parkhill, J., M. Sebaihia, A. Preston, L. D. Murphy, N. Thomson, D. E. Harris, M. T. G. Holden, C. M. Churcher, S. D. Bentley, K. L. Mungall, A. M. Cerdeño-Tárraga, L. Temple, K. James, B. Harris, M. A. Quail, M. Achtman, R. Atkin, S. Baker, D. Basham, N. Bason, I. Cherevach, T. Chillingworth, M. Collins, A. Cronin, P. Davis, J. Doggett, T. Feltwell, A. Goble, N. Hamlin, H. Hauser, S. Holroyd, K. Jagels, S. Leather, S. Moule, H. Norberczak, S. O'Neil, D. Ormond, C. Price, E. Rabbinowitsch, S. Rutter, M. Sanders, D. Saunders, K. Seeger, S. Sharp, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, L. Unwin, S. Whitehead, B. G. Barrell, and D. J. Maskell. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 35:32-40. [DOI] [PubMed] [Google Scholar]
- 44.Ramseier, T. M., H. V. Winteler, and H. Hennecke. 1991. Discovery and sequence analysis of bacterial genes involved in the biogenesis of c-type cytochromes. J. Biol. Chem. 266:7793-7803. [PubMed] [Google Scholar]
- 45.Rietsch, A., D. Belin, N. Martin, and J. Beckwith. 1996. An in vivo pathway for disulfide bond isomerization in Escherichia coli. Proc. Natl. Acad. Sci. USA 93:13048-13053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ritz, D., and J. Beckwith. 2001. Roles of thiol-redox pathways in bacteria. Annu. Rev. Microbiol. 55:21-48. [DOI] [PubMed] [Google Scholar]
- 47.Ritz, D., M. Bott, and H. Hennecke. 1993. Formation of several bacterial c-type cytochromes requires a novel membrane-anchored protein that faces the periplasm. Mol. Microbiol. 9:729-740. [DOI] [PubMed] [Google Scholar]
- 48.Ritz, D., L. Thony-Meyer, and H. Hennecke. 1995. The cycHJKL gene cluster plays an essential role in the biogenesis of c-type cytochromes in Bradyrhizobium japonicum. Mol. Gen. Genet. 247:27-38. [DOI] [PubMed] [Google Scholar]
- 49.Sambongi, Y., and S. J. Ferguson. 1996. Mutants of Escherichia coli lacking disulphide oxidoreductases DsbA and DsbB cannot synthesise an exogenous monohaem c-type cytochrome except in the presence of disulphide compounds. FEBS Lett. 398:265-268. [DOI] [PubMed] [Google Scholar]
- 50.Schiött, T., M. Throne-Holst, and L. Hederstedt. 1997. Bacillus subtilis CcdA-defective mutants are blocked in a late step of cytochrome c biogenesis. J. Bacteriol. 179:4523-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schiött, T., C. von Wachenfeldt, and L. Hederstedt. 1997. Identification and characterization of the ccdA gene, required for cytochrome c synthesis in Bacillus subtilis. J. Bacteriol. 179:1962-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schulze, M., and G. Rodel. 1988. SCO1, a yeast nuclear gene essential for accumulation of mitochondrial cytochrome c oxidase subunit II. Mol. Gen. Genet. 211:492-498. [DOI] [PubMed] [Google Scholar]
- 53.Stainer, D. W., and M. J. Scholte. 1970. A simple chemically defined medium for the production of phase I Bordetella pertussis. J. Gen. Microbiol. 63:211-220. [DOI] [PubMed] [Google Scholar]
- 54.Stenson, T. H., A. K. Patton, and A. A. Weiss. 2003. Reduced glutathione is required for pertussis toxin secretion by Bordetella pertussis. Infect. Immun. 71:1316-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stenson, T. H., and A. A. Weiss. 2002. DsbA and DsbC are required for secretion of pertussis toxin by Bordetella pertussis. Infect. Immun. 70:2297-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stibitz, S. 1994. Use of conditionally counterselectable suicide vectors for allelic exchange. Methods Enzymol. 235:458-465. [DOI] [PubMed] [Google Scholar]
- 57.Sun, G., E. Sharkova, R. Chesnut, S. Birkey, M. F. Duggan, A. Sorokin, P. Pujic, S. D. Ehrlich, and F. M. Hulett. 1996. Regulators of aerobic and anaerobic respiration in Bacillus subtilis. J. Bacteriol. 178:1374-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang, X., B. F. Lu, and S. Q. Pan. 1999. A bifunctional transposon mini-Tn5gfp-km which can be used to select for promoter fusions and report gene expression levels in Agrobacterium tumefaciens. FEMS Microbiol. Lett. 179:37-42. [DOI] [PubMed] [Google Scholar]
- 59.Thöny-Meyer, L. 1997. Biogenesis of respiratory cytochromes in bacteria. Microbiol. Mol. Biol. Rev. 61:337-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tichy, M., and W. Vermaas. 1999. Accumulation of pre-apocytochrome f in a Synechocystis sp. PCC 6803 mutant impaired in cytochrome c maturation. J. Biol. Chem. 274:32396-32401. [DOI] [PubMed] [Google Scholar]
- 61.van der Oost, J., C. von Wachenfeld, L. Hederstedt, and M. Saraste. 1991. Bacillus subtilis cytochrome oxidase mutants: biochemical analysis and genetic evidence for two aa3-type oxidases. Mol. Microbiol. 5:2063-2072. [DOI] [PubMed] [Google Scholar]
- 62.Woodard, S. I., and H. A. Dailey. 1995. Regulation of heme biosynthesis in Escherichia coli. Arch. Biochem. Biophys. 316:110-115. [DOI] [PubMed] [Google Scholar]
- 63.Xie, Z., and S. Merchant. 1996. The plastid-encoded ccsA gene is required for heme attachment to chloroplast c-type cytochromes. J. Biol. Chem. 271:4632-4639. [DOI] [PubMed] [Google Scholar]