Abstract
The FpvA protein of Pseudomonas aeruginosa strain PAO1 mediates uptake of a siderophore, ferripyoverdine. It is also a component of a signal transduction pathway that controls production of an exotoxin, a protease, pyoverdine, and FpvA itself. The purpose of the research described here was to dissect these different functions of FpvA. Signaling involves an N-terminal domain of FpvA, and it was shown that this domain is probably located in the periplasm, as expected. Short peptides were inserted at 36 sites within FpvA by linker insertion mutagenesis. The effects of these mutations on the presence of FpvA in the outer membrane, on FpvA-mediated uptake of ferripyoverdine, and on pyoverdine synthesis and gene expression were determined. Five of the mutations resulted in the absence of FpvA from the outer membrane of the bacteria. All of the remaining mutations eliminated either the transport or signaling function of FpvA and most affected both functions. Three mutations prevented transport of ferripyoverdine but had no effect on the signal transduction pathway showing that transport of ferripyoverdine is not required for the trans-membrane signaling process. Conversely, eight mutations affected pyoverdine-mediated signaling but had no effect on transport of ferripyoverdine. These data show that insertions throughout FpvA resulted in loss of function and that signaling and transport are separate and discrete functions of FpvA.
Many microorganisms secrete iron-chelating organic compounds (siderophores) to obtain ferric (Fe3+) ions from the environment. For Gram negative bacteria, the ferrisiderophore complexes formed following iron chelation are taken up through receptor proteins located in the outer membrane in a process involving the energy-transducing protein TonB (35, 38). The receptor proteins have a high degree of specificity, transporting only the cognate siderophore or closely related compounds. The processes involved in ferrisiderophore uptake are best characterized in Escherichia coli and structures have been determined for the receptor proteins from this species for ferric enterobactin (FepA) (7), ferrichrome (FhuA) (14, 28), and ferric citrate (FecA) (13, 49). Each protein consists of a 22-stranded β-barrel that forms a pore in the membrane. The pore is blocked by a plug, or cork, structure located within the barrel. Extracellular loops of protein connecting the β strands provide a surface for recognition of ferrisiderophore and the plug also contributes to substrate binding. Crystallization of FhuA in the presence of ferrichrome (28) and of FecA in the presence of citrate and ferric citrate (13, 49) gave insights into the interactions of ferrisiderophore receptor proteins with their substrates.
Ferrisiderophore receptor proteins have been identified in a wide range of other Gram negative bacterial genera (3, 6, 8, 11, 39, 47). All such proteins that have been characterized to date have features in common with the E. coli receptor proteins, being of comparable size (70 to 90 kDa) and having sequence similarities. This suggests that all of these proteins have broadly similar structures and mechanisms for internalization of ferrisiderophores. The presence of TonB homologues in many species also suggests that the processes of transport will be comparable, with the differences lying in the details of substrate recognition and internalization.
The structures of receptor proteins from species other than E. coli had not been reported until very recently. However, the structure of the ferrisiderophore receptor protein FpvA from Pseudomonas aeruginosa strain PAO1 has now been determined (10). This protein is responsible for the uptake of the siderophore ferripyoverdine by this strain (37). The structure and sequence of FpvA show that it is similar to other TonB-dependent ferrisiderophore receptors. Uptake is a multistep process involving binding of apopyoverdine to FpvA, exchange of apopyoverdine for ferripyoverdine, and internalization of ferripyoverdine (44). Production of FpvA in P. aeruginosa PAO1 is induced by the presence of the corresponding pyoverdine (16). Production of FecA in E. coli (20) and a ferrisiderophore receptor PupB from Pseudomonas putida (25) are also induced by the presence of the cognate siderophore.
In each case, induction involves a transmembrane-signaling system that is induced by binding of the (ferri-)siderophore to the receptor protein. A signal is then transmitted by the receptor protein to a regulatory protein (FpvR, FecR, or PupR) that spans the cytoplasmic membrane and this leads to increased activity of an alternative sigma factor protein (FpvI, FecI, or PupI) that directs expression of genes required for ferrisiderophore transport (4; reviewed in references 5 and 46). The FpvA signaling pathway also controls the activity of a second sigma factor, PvdS, that directs production of pyoverdine and two secreted proteins, exotoxin A and PrpL protease (26). Synthesis of the alternative sigma factors is regulated by the iron-responsive Fur repressor protein so that production of transport proteins is not induced in iron-replete conditions, even if the ferrisiderophore is present. Signal transduction involves the N-terminal regions (signaling domains) of the FecA, PupB, and FpvA receptor proteins (12, 22, 25, 45), and this part of FecA has been shown to be located in the periplasm (22).
The purpose of the research described here was to determine whether the signaling domain of FpvA is located in the periplasm and to identify parts of FpvA that are required for transport of ferripyoverdine and for transmembrane signaling. The approach used was to introduce mutations at random sites within FpvA and then determine the effects of these mutations on the presence of the protein in the membrane, on transport of ferripyoverdine, and on signal transduction.
MATERIALS AND METHODS
Growth of bacteria.
Bacteria were grown at 37°C using L broth and L agar (Gibco BRL) for E. coli and King's B broth and agar (23) for the growth of P. aeruginosa, unless otherwise stated. Antibiotics were added as required at the following concentrations for work with E. coli, with the concentrations used for P. aeruginosa shown in parentheses: ampicillin, 50 μg/ml; chloramphenicol, 30 μg/ml (200 μg/ml); gentamicin, 4 μg/ml (20 μg/ml); and kanamycin, 50 μg/ml.
Linker insertion mutagenesis of fpvA.
The fpvA gene from P. aeruginosa PAO1 was amplified from pRV2 (37) by PCR using Expand DNA polymerase (Roche Molecular Biochemicals) with primers fpvA1 (5′-GAGCTCGAAGAGCAATCACCCAT-3′) and fpvA2 (5′-AAGCTTGGCGTTCTTTTTCGCA-3′) that contain synthetic SacI and HindIII restriction sites (shown in bold). The PCR product was treated with SacI and HindIII restriction enzymes and cloned into plasmid pUCP22 (48) using standard methods (42). In the resulting plasmid (pUCP22::fpvA) the fpvA gene is expressed from the E. coli lac promoter.
The cloned DNA was sequenced and the sequence was identical to the wild-type fpvA sequence. pUCP22::fpvA DNA was linearized using restriction enzymes that cut only once in the insert (ClaI, KpnI, and SalI) or by partial digestion with limiting amounts of more frequently cutting enzymes (AluI, EcoRV, Sau3AI, and TacI) in the presence of ethidium bromide (2) and linearized plasmid DNA was purified from agarose gels using the Qiaex II Gel Extraction Kit (Qiagen). The linearized DNA was ligated with kanamycin resistance cassettes that had been purified from agarose gels following treatment of plasmids pUC4KISS (2) and pNRE1 (29) with appropriate restriction enzymes. The ligated DNA was transformed into E. coli MC1061 (9), and bacteria resistant to ampicillin, gentamicin and kanamycin were selected.
The approximate locations of the kanamycin cassette insertions in the resulting plasmids were determined by restriction analysis. The precise locations of insertions in fpvA were determined by sequencing of plasmid DNA using primers Km1 (AGATTTTGAGACACAACG) and Km2 (TTACGCTGACTTGACGGG) that correspond to outward-facing kanamycin cassette sequences in conjunction with an Applied Biosystems Automated sequencer (Centre for Gene Research, University of Otago). The kanamycin cassettes were then excised from the plasmids using a suitable flanking restriction enzyme and the plasmids treated with DNA ligase and transformed into E. coli MC1061 with selection for Apr Gmr Kms bacteria. This procedure resulted in the insertion of short DNA segments (12 or 24 oligonucleotides) at 36 different sites in fpvA (Table 1). The DNA spanning each insertion site was sequenced to confirm that the expected mutational events had occurred. Plasmids containing wild-type and mutant DNA, as well as a vector-only control, were transformed into the P. aeruginosa fpvA mutant strain K690 fpvA::Tc (32) which has a Tetr interposon inserted at an internal ScaI site (K. Poole, personal. communication).
TABLE 1.
Insertion mutations in fpvA
| Mutationa | Restriction siteb | Insertionc
|
||
|---|---|---|---|---|
| Start | Amino acids inserted | End | ||
| A-12 | HaeIII-35 | M-13 | GPAGP | L-11 |
| E4 | TaqI 10 | V3 | ARSG | E4 |
| R21 | HaeIII 59 | G20 | TCRS | R21 |
| 125 | EcoRV 72 | D24 | DLEV | I25 |
| L46 | AluI 135 | K45 | DLQV | L46 |
| L55 | AluI 162 | E54 | DLQV | L55 |
| T90 | Sau3AI 267 | 189 | RRPAGRRI | T90 |
| N172 | Sau3AI 513 | 1171 | RRPAGRRI | N172 |
| N224 | Sau3AI 669 | I223 | RRPAGRRI | N224 |
| K228 | Sau3AI 680 | R227 | RPAGRRIR | K228 |
| H237 | HaeIII 707 | G236 | TCRS | H237 |
| P257 | HaeIII 767 | G256 | TCRS | P257 |
| E294 | TaqI 880 | L293 | ARSG | E294 |
| L297 | Sau3AI 889 | D296 | PSTCRSTD | L297 |
| G318 | Sau3AI 950 | S317 | VDLQVDGS | G318 |
| E360 | TaqI 1078 | L359 | ARSG | E360 |
| H375 | Sau3AI 1123 | D374 | PSTCRSTD | H375 |
| N378 | Sau3AI 1131 | 1377 | RRPAGRRI | N378 |
| V401 | Sau3AI 1200 | I400 | RRPAGRRI | V401 |
| I416 | EcoRV 1245 | D415 | DLEV | I416 |
| L431 | AluI 1290 | E430 | DLQV | L431 |
| S446 | AluI 1337 | K445 | RTCR | S446 |
| P475 | KpnI 1422 | T474 | ACGT | P475 |
| D480 | ClaI 1438 | I479 | ARSG | D480 |
| D510 | TaqI 1528 | V509 | ARSG | D510 |
| Y511 | SalI 1530 | D510 | LQVD | Y511 |
| L564 | AluI 1689 | K563 | DLQV | L564 |
| E566 | TaqI 1696 | L565 | ARSG | E566 |
| Q571 | HaeIII 1709 | G570 | TCRS | Q571 |
| G576 | Sau3AI 1725 | I575 | RRPAGRRI | G576 |
| E594 | TaqI 1780 | F593 | ARSG | E594 |
| I653 | Sau3AI 1956 | I652 | RRPAGRRI | I653 |
| L685 | AluI 2052 | K684 | DLQV | L685 |
| A724 | HaeIII 2170 | M723 | GPAGP | R725 |
| D743 | TaqI 2225 | F742 | ARSG | D743 |
| R762 | Sau3AI 2282 | P761 | STCRSTDP | R762 |
Alleles are named as the first amino acid following the inserted peptide or (for A-12 and A724) the amino acid that is lost as a consequence of the mutation.
Restriction site and location of the insertion in fpvA. Numbering is relative to the first nucleotide corresponding to the mature FpvA protein.
The amino acid preceding the inserted sequence, the sequence of the inserted peptide, and the amino acid following the inserted sequence are shown. Numbering is relative to the first amino acid residue in mature FpvA protein (37).
Western blotting.
Outer membranes were prepared from cells of P. aeruginosa and FpvA was analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and Western blotting using a polyclonal anti-FpvA serum as described previously (4). Antibodies against the C terminus of FpvA were obtained by conjugating a peptide (GDPRNLMFSTRWDF, corresponding to residues 802 to 815 of the mature protein) with an equal amount (5 mg) of keyhole limpet hemocyanin (Roche) by overnight incubation in the presence of 0.1% glutaraldehyde (19). Following dialysis against phosphate-buffered saline, one tenth of the resulting mixture was mixed with an equal amount of Freund's complete adjuvant and used to immunize a New Zealand White rabbit. Nine booster immunizations were carried out at seven-day intervals after mixing the remainder of the conjugated peptide with an equal volume of Freund's incomplete adjuvant. Three days after the final immunization the animal was bled and serum was recovered from clotted blood. The serum was used in Western blotting in conjunction with goat anti-rabbit immunoglobulin G (whole molecule) horseradish peroxidase conjugate and 3-amino-9-ethylcarbazole (Sigma) as described (19).
To analyze the FpvA protein in whole cells of P. aeruginosa, bacteria were grown in Kings B medium overnight. The optical density at 600 nm (OD600) was measured and approximately 1.5 × 109 cells were removed from each culture and collected by centrifugation. The bacterial pellet was resuspended in 10 μl of double-distllled H2O, 2 μl of 6× SDS-PAGE loading dye was added and the samples were boiled for 5 min. Samples were then electrophoresed on a 10% SDS-PAGE gel for 1 h at 180 V. Protein was transferred to nitrocellulose membrane by Western blotting and FpvA was detected using the polyclonal anti-FpvA serum.
Treatment of FpvA with proteinase K.
Overnight cultures (approximately 1.5 × 109 cells) were centrifuged and the pellets were washed in 1 ml of Tris buffer (0.2 M Tris-HCl, 150 mM NaCl, pH 8.0) and then suspended in 1 ml Tris-CaCl2 buffer (0.2 M Tris-HCl, 150 mM NaCl, 10 mM CaCl2, pH 8.0). Proteinase K (20 μl; 5 mg ml−1) was added and the samples were incubated for 30 min at 37°C. The reaction was stopped by the addition of 10 μl of 0.2 M phenylmethylsulfonyl fluoride and the cells were spun for 10 min at 13,200 rpm. The cell pellet was then washed with 1 ml Tris buffer supplemented with 10 mM EDTA and 2 mM phenylmethylsulfonyl fluoride.
For digestion of outer membrane preparations, samples were boiled in 0.5 M NaOH and the amount of protein was then assayed (DC protein assay, Bio-Rad). Purified outer membranes containing 50 to 60 μg of protein were suspended in 1 ml of Tris-CaCl2 buffer, 20 μl of proteinase K was added and the mixture was incubated for 30 min at 37°C. The reaction was stopped by addition of 10 μl of 0.2 M phenylmethylsulfonyl fluoride and the samples were centrifuged for 1 h at 21,000 rpm in a Beckman J2-MC ultracentrifuge. The outer membrane pellet was then washed with 1 ml Tris buffer supplemented with 10 mM EDTA and 2 mM phenylmethylsulfonyl fluoride. The outer membrane and whole-cell samples were resuspended in 30 μl of double-distilled H2O and placed at −20°C for 1 to 2 h. SDS-PAGE loading dye (5 μl) was added and the samples were boiled for 5 min and SDS-PAGE was carried out.
The sequence of amino acids at the N terminus of a truncated form of FpvA was determined by preparing outer membrane samples, carrying out SDS-PAGE, and transferring protein to a polyvinylidene difluoride membrane (Bio-Rad) with protein being detected by light staining with Ponceau S (41). The position of FpvA was marked, the sample was destained by washing in acetic acid (1%), the band was excised and the N-terminal sequence was determined by the Protein Microchemistry Facility, Department of Biochemistry, University of Otago.
Measurement of ferripyoverdine transport.
Pyoverdine was purified from P. aeruginosa strain PAO using the method described (31). Ferripyoverdine uptake assays were performed using a modification of the method described (36). Bacteria were grown in 5 ml of succinate medium (33) to an OD600 of 1.0. The cells were collected by centrifugation (7700 g, 4°C, 5 min), washed twice in 5 ml of ice-cold succinate medium and resuspended in the same medium to an OD600 of 0.67. Portions of cells (150 μl) were placed in microfuge tubes (1.5 ml) and incubated at 37°C for 15 min. Uptake was initiated by the addition of 55 μl of a solution comprising 50 μl of succinate medium, 4 μl (16 μg) of pyoverdine and 1 μl (24 pmol) of 55Fe (Amersham Life Sciences; 1.2 mCi/μmol). Tubes were removed at intervals and the cells collected by centrifugation (30 seconds). The supernatants were immediately aspirated off and the pellets were resuspended in 40 μl of 20% (vol/vol) Triton X-100 and transferred to a scintillation vial. Scintillant (3.75 ml optiPhase Hisafe 2 [Wallac]) was added to the vials and the radioactivity was measured using an LKB Wallac RackBeta liquid scintillation counter; 1 pmol of 55Fe gave 2,000 cpm.
Measurement of pyoverdine and FpvA-dependent signaling.
Strains of P. aeruginosa were grown until the OD600 was between 0.8 and 1.2 and the amount of pyoverdine was measured spectrophotometrically as described previously (30). The amounts of pyoverdine were calculated using the molar extinction coefficient 1.4 × 104 M−1.cm−1 at 405 nm (1) and were normalized for OD600. Pyoverdine-mediated signaling was measured using P. aeruginosa K690 fpvA(pMP190::pvdE) (26) that had been transformed with pUCP22-derived plasmids carrying the fpvA derivatives to be tested and that contains the promoter of the pvdE gene upstream of the lacZ reporter gene (40). The amounts of β-galactosidase made by the bacteria were measured as described previously (26) and reflect the ability of the FpvA protein to initiate the signaling process.
RESULTS
Signaling domain of FpvA is likely to lie in the periplasm.
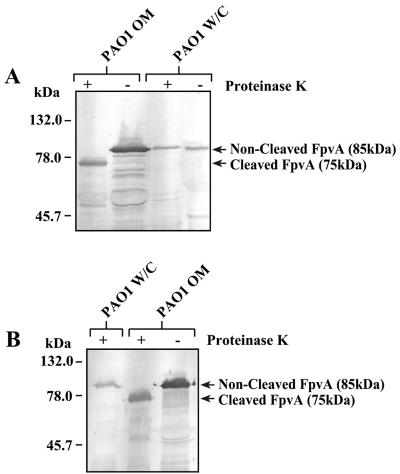
FpvA consists of a β-barrel with a plug domain in the outer membrane and an N-terminal signaling domain that is predicted to interact with FpvR in the periplasm (4, 10, 15, 26, 45). To test whether this part of FpvA is located in the periplasm, purified outer membranes and whole cells of P. aeruginosa were treated with proteinase K. FpvA in whole cells was unaffected by proteinase K treatment, whereas treatment of outer membranes resulted in FpvA's being shortened to about 75 kDa (Fig. 1A). Serum raised against a 12-residue peptide corresponding to the C terminus of FpvA also detected the truncated form of FpvA obtained following treatment of outer membranes with proteinase K, indicating that this treatment had removed the N terminus of FpvA (Fig. 1B). These data are consistent with at least part of the 12-kDa N-terminal portion of FpvA being exposed at the periplasmic face of the outer membrane, as expected. Consistent with this result, the N-terminal region of FpvA was degraded in the absence of proteinase inhibitors during the purification of FpvA, suggesting that this part of FpvA is prone to proteolysis (43), most likely because it is not within the outer membrane.
FIG. 1.
Subcellular localization of the N-terminal region of FpvA. Intact cells and outer membranes of wild-type P. aeruginosa were incubated with (+) or without (−) proteinase K for 1 h. Proteins were then separated by SDS-PAGE and FpvA was detected by Western blotting using a polyclonal anti-FpvA antibody (A) or an antibody specific for the C terminus of FpvA (B). The positions of molecular size markers are indicated. OM, outer membranes; W/C, whole cells.
Mutagenesis of fpvA and incorporation of mutant proteins into the outer membrane.
Linker insertion mutagenesis was performed on pUCP22::fpvA as described in Materials and Methods. This method allowed the incorporation of 12- or 24-bp DNA segments into fpvA, resulting in four- or eight-amino-acid insertions at 36 different sites within the FpvA protein. The mutations were scattered throughout the fpvA gene (Fig. 2) and are listed in Table 1.
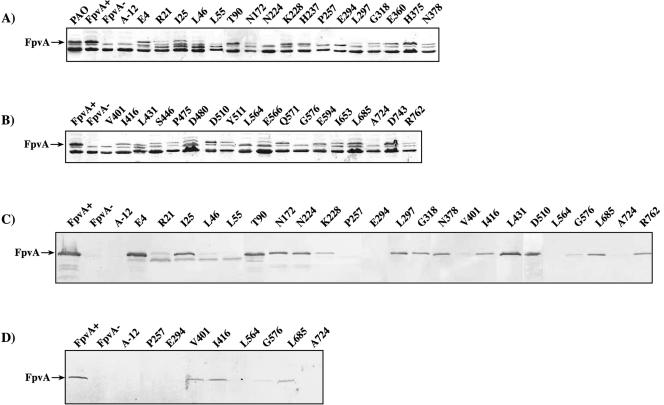
FIG. 2.
Locations of mutations in FpvA. The predicted boundaries of domains of FpvA were identified by aligning the FpvA sequence with those of FepA, FhuA, and FecA. The positions of mutations obtained in this study that allowed detection of FpvA by Coomassie staining (▾), reduced amounts of FpvA so that it could only be detected by Western blotting (dotted triangle) or prevented incorporation (▿) (Fig. 3 and Table 1) are shown. Mutations identified previously (21) as permitting (•) or preventing (○) incorporation of FpvA into the outer membrane are also shown. Numbering is relative to the first amino acid residue of mature FpvA (37).
Plasmids carrying the mutated genes were transformed into strain K690, an fpvA mutant of P. aeruginosa, and the presence of FpvA in the outer membranes of the bacteria was assessed following purification of outer membranes and SDS-PAGE gel electrophoresis. Mutant proteins that were not clearly detected by Coomassie staining were also analyzed by Western blotting. Twenty-seven of the mutant proteins were detected by Coomassie staining (Fig. 3A and B; Table 2), indicating that the mutations did not prevent incorporation of the protein into the outer membrane. Four mutations (L55, N224, V401, and G576) resulted in proteins that could only be detected by Western blotting (Fig. 3C), with the L55 protein being smaller than the wild-type protein. The remaining five mutations (A-12, P257, E294, L564, and A724) resulted in no FpvA protein being detectable in the outer membrane fraction. These strains were examined for the presence of the mutant protein in the cells by Western blotting (Fig. 3D). The five mutant proteins that could not be detected in outer membranes also could not be detected in cells, indicating that they were not synthesized or were rapidly degraded.
FIG. 3.
Production and membrane incorporation of mutant FpvA proteins. A and B. Detection of FpvA by Coomassie staining. Outer membranes were prepared from P. aeruginosa containing wild-type fpvA or the mutant alleles shown and analyzed by SDS-PAGE followed by Coomassie staining. The position of FpvA is indicated. C. Detection of FpvA in outer membranes by Western blotting. Outer membrane samples were analyzed by Western blotting using a polyclonal antibody against FpvA. D. Detection of FpvA in whole cells. Protein was prepared from whole cells of P. aeruginosa and analyzed by Western blotting.
TABLE 2.
Effects of mutations on transport of ferripyoverdine, pyoverdine synthesis, and FpvA-mediated signaling
| Strain or mutationa | Pyoverdine-dependent iron transportb (mean fmol/ml ± SD) | Pyoverdine synthesisc (mean μmol/ml ± SD) | Signalingd (mean U ± SD) |
|---|---|---|---|
| PAO | 22.52 ± 1.74 | 103.4 ± 12.1 | 527.3 ± 49.0 |
| FpvA+ | 20.81 ± 2.30 | 93.3 ± 2.3 | 482.2 ± 35.0 |
| FpvA− | 3.27 ± 0.34 | 22.3 ± 7.3 | 177.7 ± 28.4 |
| E4 | 20.72 ± 1.97 | 35.7 ± 12.9 | 113.7 ± 31.4 |
| R21 | 21.33 ± 2.21 | 23.7 ± 6.4 | 78.4 ± 23.8 |
| I25 | 19.64 ± 4.49 | 22.3 ± 6.5 | 115.5 ± 45.0 |
| L46 | 19.56 ± 4.14 | 13.3 ± 4.6 | 160.0 ± 35.6 |
| L55 | 23.19 ± 1.01 | 6.7 ± 3.9 | 111.3 ± 21.1 |
| T90 | 7.05 ± 0.73 | 24.3 ± 3.9 | 382.5 ± 30.6 |
| N172 | 3.66 ± 0.40 | 32.7 ± 22.2 | NDe |
| N224 | 4.48 ± 0.61 | 11.8 ± 0.6 | 148.2 ± 16.9 |
| K228 | 3.90 ± 0.29 | 12.3 ± 4.3 | ND |
| H237 | 13.67 ± 1.57 | 31.4 ± 9.8 | ND |
| L297 | 17.69 ± 1.15 | 22.4 ± 7.8 | 185.2 ± 66.2 |
| G318 | 3.62 ± 0.20 | 14.9 ± 10.7 | 130.1 ± 39.0 |
| E360 | 8.56 ± 0.24 | 17.7 ± 5.2 | ND |
| H375 | 3.54 ± 0.21 | 17.7 ± 8.5 | 151.1 ± 35.4 |
| N378 | 3.83 ± 0.33 | 14.9 ± 8.9 | ND |
| V401 | 6.47 ± 0.51 | 39.9 ± 21.2 | ND |
| I416 | 3.75 ± 0.24 | 27.2 ± 12.9 | ND |
| L431 | 12.76 ± 1.34 | 31.5 ± 18.7 | ND |
| S446 | 4.24 ± 0.19 | 26.7 ± 3.9 | 109.6 ± 51.7 |
| P475 | 17.03 ± 2.94 | 23.7 ± 7.2 | 101.8 ± 28.8 |
| D480 | 5.97 ± 0.34 | 25.5 ± 4.9 | ND |
| D510 | 3.43 ± 0.28 | 80.5 ± 10.1 | 637.3 ± 37.1 |
| Y511 | 4.25 ± 0.65 | 68.4 ± 10.1 | 611.9 ± 76.0 |
| E566 | 6.96 ± 0.64 | 26.5 ± 6.2 | ND |
| Q571 | 4.00 ± 0.29 | 19.6 ± 9.3 | ND |
| G576 | 3.56 ± 0.22 | 31.9 ± 7.0 | ND |
| E594 | 5.01 ± 1.71 | 78.2 ± 20.8 | 542.5 ± 90.3 |
| I653 | 9.75 ± 0.95 | 43.3 ± 11.9 | ND |
| L685 | 17.80 ± 3.28 | 21.2 ± 3.8 | 241.2 ± 61.4 |
| D743 | 7.32 ± 1.30 | 23.1 ± 8.6 | ND |
| R762 | 3.56 ± 0.31 | 20.3 ± 8.1 | 241.7 ± 65.1 |
Mutations A-12, P257, E294, L564, and A724 resulted in FpvA being undetectable in the outer membrane (Fig 3A and B) and resulted in pyoverdine-dependent iron transport at levels indistinguishable from that of the FpvA mutant (data not shown); pyoverdine production and signaling were not measured for these mutants.
In pmol of 55Fe taken up in 30 minutes per ml of bacteria. All values are the means of at least three experiments with standard deviations shown.
In μmol of pyoverdine per ml of culture. All values are the means of at least three experiments with standard deviations shown.
In units of β-galactosidase expressed from a pvdE::lacZ fusion construct. All values are the means of at least three experiments with standard deviations shown.
ND, not determined.
Mutations in the signaling domain of FpvA (R21, I25, L46, and L55) resulted in the presence of truncated FpvA as well as full-size FpvA in the outer membrane (Fig. 3C). This suggested that these mutations affected the structure of the signaling domain so that it was more susceptible to proteolysis. The sequence at the N terminus of the truncated FpvA resulting from the insertion at L46 was determined in order to locate the site of the truncation in this mutant. The sequence was found to be EAXDS, where the identity of the third amino acid could not be determined. This sequence corresponds to residues 76 to 80 of mature FpvA and is consistent with the size (78 kDa) of the truncated FpvA arising from the L46 mutation.
Effects of mutations on ferripyoverdine transport.
P. aeruginosa K690 containing the mutated fpvA genes was tested for the ability to transport ferripyoverdine using a time course assay (36). The relative amounts of iron acquired by different mutants are shown in Table 2. As expected, mutations that resulted in the absence of detectable FpvA in the outer membrane also reduced uptake of ferripyoverdine to levels that were not significantly different from those of the FpvA− mutant. The residual ferripyoverdine transport observed with these strains may be due to a second ferripyoverdine transporter, FpvB (17).
Fourteen of the remaining 31 mutations resulted in uptake of ferripyoverdine at rates comparable to those of the FpvA mutant and a further nine resulted in levels of uptake above those of the FpvA mutant but below those seen with wild-type FpvA. Only three of the mutations in the plug or barrel regions of FpvA (L297, P475, and L685) did not have a major effect on ferripyoverdine transport. None of the five mutations in the signaling domain (E4 to L55) affected ferripyoverdine transport.
Effects of the mutations on production of pyoverdine and expression of pyoverdine synthesis genes.
FpvA is part of a signaling system that controls production of pyoverdine by P. aeruginosa, and consistent with this, deletion of fpvA or its signaling domain results in reduced expression of pyoverdine synthesis genes and reduced production of pyoverdine (26, 45). The amounts of pyoverdine made by P. aeruginosa carrying the mutant fpvA alleles were determined. All five of the mutations in the signaling domain (E4 to L55) resulted in reduced pyoverdine production. Most of the remaining mutations also resulted in reduced pyoverdine synthesis. This included mutations (L297, P475, and L685) that had only a small effect on transport of ferripyoverdine (Table 2). Strikingly, mutations D510, Y511, and E594 that abolished the transport activity of FpvA did not reduce pyoverdine synthesis.
The pyoverdine signaling system involves posttranslational control of the activity of the alternative sigma factor PvdS by the pyoverdine-FpvA-FpvR signaling pathway (26). It was likely that the effects of mutations in fpvA on pyoverdine synthesis were due to changes in the expression of pyoverdine synthesis genes that are controlled by this pathway. To test this, the effects of selected mutations in fpvA on expression of a representative pyoverdine synthesis gene (pvdE) was measured using a pvdE::lacZ fusion. The amount of expression of lacZ from this construct was a measure of the ability of FpvA to initiate the signaling process. As expected, expression of pvdE::lacZ correlated well with the amount of pyoverdine that was made by P. aeruginosa containing different alleles of fpvA, with the T90 mutation being the only clear exception (Table 2).
DISCUSSION
FpvA has two known functions, transport of ferripyoverdine and initiation of the signaling pathway that controls production of exotoxin A, PrpL protease, pyoverdine, and FpvA itself. The purpose of the research described here was to carry out a mutational study of FpvA in order to identify parts of the protein that contribute to these functions. The approach used was to engineer 4- or 8-amino-acid insertions at 36 sites in FpvA and then determine the effects of the mutations on the properties of the protein. The results showed that most of the mutated parts of FpvA are required for both transport of ferripyoverdine and signaling, although some parts are required for only one of these functions. Previous researchers inserted a larger peptide (18 residues) into a smaller number of sites (6) in FpvA (21), and their results complement those presented here.
Twenty-seven of the 36 mutations resulted in forms of FpvA that were present in the outer membrane, indicating that the overall protein topology was retained. Five of the 36 mutations resulted in the apparent absence of detectable FpvA from the outer membrane of the bacteria, and a further four mutations resulted in reduced amounts of FpvA that could only be detected by Western blotting. Mutant proteins may be absent from the outer membrane because the insertions occur at sites in the proteins that are essential for membrane incorporation, because the mutations cause reduced expression of the mutated gene or because of degradation of the altered protein.
Combining data for the 23 mutations in this study and the five mutations in the previous study (21) that are in the plug/barrel regions of FpvA and did not detectably affect the amount of protein in the outer membrane, 22 of 28 mutations resulted in reduced ability or an inability to transport ferripyoverdine. These mutations may affect any of the processes in pyoverdine-mediated iron uptake involving FpvA—binding of apopyoverdine, exchange of apopyoverdine with ferripyoverdine, and TonB-mediated internalization of ferripyoverdine with consequent recycling of the apopyoverdine (reviewed in reference 44).
The high proportion of mutations that affect transport contrasts with studies of FhuA in which a high proportion of mutations created by insertion of 4- to 22-residue peptides did not significantly affect the ability of the protein to act as a receptor for ferrichrome, albomycin, bacteriophages, or bacteriocins (24, 34). Similarly, 18 of 26 dipeptide insertions in the TonB-dependent BtuB receptor did not affect its ability to transport cobalamin (27). The lower proportion of silent mutations in FpvA compared with FhuA and BtuB may reflect differences in the inserted peptides or the assays that were used to investigate the effects of mutations on protein function. Alternatively it may be that the differences reflect differences in the mechanisms of substrate binding and internalization by the different proteins. It remains to be determined which of the mutations generated here affect binding of apopyoverdine and ferripyoverdine by FpvA and which affect internalization of ferripyoverdine once binding has taken place.
The N-terminal signaling domain of FpvA is predicted to lie in the periplasm and to interact with FpvR as part of the pyoverdine signaling system (4, 26, 45). Experiments with proteinase K (Fig. 1) were consistent with this portion of FpvA being located in the periplasm. Mutations within the signaling domain affected the amount of pyoverdine produced by the bacteria while having no effect on transport of ferripyoverdine (Table 2) (45). Collectively these data show that the role of the signaling domain of FpvA is purely regulatory and it is most likely to be located in the periplasm. This conclusion is the same as that obtained for FecA, the only other protein that has been studied in a comparable way (22).
Mutations L46 and L55, which are in the signaling domain, resulted in lower levels of pyoverdine synthesis and pvd gene expression than observed for the fpvA mutant strain. The same was true, to various extents, for mutations N224 and K228 that are predicted to lie in the plug domain and mutations G318, E360, H375, and N378 that are predicted to lie in the β-barrel domain. This indicates that these mutations result in a form of FpvA that suppresses the low-level pyoverdine synthesis obtained with the fpvA mutant. Understanding the molecular basis of this suppression will require an understanding of the molecular events involved in signal transmission to FpvR in the signaling pathway.
Twenty-three of the 26 mutations that were examined in the plug and barrel domains of FpvA affected pyoverdine synthesis and, for those examined, expression from the pvdE::lacZ reporter construct. It is not known whether signaling is initiated by the binding of apopoverdine or ferripyoverdine to FpvA or in a process involving the TonB-dependent transport of ferripyoverdine into the periplasm. Mutations at three sites (D510, Y511, and G594) that prevented transport of ferripyoverdine did not result in reduced signaling and indeed, bacteria carrying these alleles secreted more pyoverdine and had higher activity in the signaling assay than bacteria carrying wild-type fpvA (Table 2). The phenotypes of these mutants show that net transport of ferripyoverdine is not required for the transmembrane signaling process so that the molecular events that are involved in transmission of a signal from FpvA to FpvR can take place without internalization of ferripyoverdine into the periplasm.
Mutants of FecA have also been isolated in which transport and signaling are uncoupled (18), including mutants in which signaling occurs in the absence of inducer (ferric citrate). The mutant alleles D510, Y511, and G594 were tested for the ability to induce pvd gene expression in a derivative of the K690 fpvA mutant that is also unable to synthesis pyoverdine. All three alleles, like wild-type fpvA, caused expression of the pvdE::lacZ construct only when pyoverdine was present (data not shown) so that they did not cause constitutive expression of pyoverdine synthesis genes. Conversely, three mutations (L297, P475, and L685) that are predicted to lie in the barrel region of FpvA reduced pyoverdine synthesis and signaling activity to amounts similar to those of the fpvA mutant while having only slight effects on iron transport. These data show that uptake of iron and signaling are distinct, though clearly closely linked, processes.
The structure of the beta-barrel and cork domains of FpvA was reported at 3.6 Å resolution while this paper was under revision (10) and this provides a structural basis for analyzing the effects of the mutations reported here. Of the mutations that resulted in the absence of FpvA from the outer membrane, E294 and A722 lie within membrane-spanning beta strands and insertions following these residues may prevent incorporation of the mutant proteins into the outer membrane. Mutation P257 is at the end of a beta strand on the periplasmic face of the membrane and mutation L564 within a surface loop, so that these mutations are less likely to affect incorporation of FpvA into the membrane and may instead affect expression of fpvA or result in degradation of the protein.
The three mutations that abolished ferripyoverdine transport while enabling signaling (D510, Y511, and E594) all lie quite close to each other near the extracellular surface of FpvA, consistent with the similarities in their phenotypes, although mutation Q571 that abolishes signaling also lies within this region. Two of the three mutations in the beta-barrel that affected signaling but had only minimal effects on transport of ferripyoverdine (L297 and L685) are at the periplasmic face of FpvA and this part of the protein may be involved in interactions with the signaling domain (which was not present in the structure) or with the anti-sigma-factor FpvR; by contrast, P475 is at a surface-exposed loop. The availability of a structure for FpvA, coupled to the information gained from this study, will provide an excellent platform for exploring structure-function relationships in this protein.
Acknowledgments
This research was supported by funding from the New Zealand Health Research Council, the Marsden Fund administered by the Royal Society of New Zealand, and the University of Otago.
We are very grateful to K. Poole and F. Barany for providing plasmids and strains, to Peter Lambert and Anthony Smith for their assistance in preparing the anti-FpvA antibody, and to two anonymous referees for their comments on an earlier version of the manuscript.
REFERENCES
- 1.Abdallah, M. A. 1991. Pyoverdines and pseudobactins, p. 139-153. In G. Winkelmann (ed.), CRC handbook of microbial iron chelates. CRC Press, Boca Raton, Florida.
- 2.Barany, F. 1988. Procedures for linker insertion mutagenesis and use of new kanamycin resistance cassettes. DNA Protein Eng. Techniques 1:29-44. [Google Scholar]
- 3.Baumler, A. J., and K. Hantke. 1992. Ferrioxamine uptake in Yersinia enterocolitica: characterization of the receptor protein FoxA. Mol. Microbiol. 6:1309-1321. [DOI] [PubMed] [Google Scholar]
- 4.Beare, P. A., R. J. For, L. W. Martin, and I. L. Lamont. 2003. Siderophore-mediated cell signalling in Pseudomonas aeruginosa: divergent pathways regulate virulence factor production and siderophore receptor synthesis. Mol. Microbiol. 47:195-207. [DOI] [PubMed] [Google Scholar]
- 5.Braun, V., and M. Braun. 2002. Iron transport and signaling in Escherichia coli. FEBS Lett. 529:78-85. [DOI] [PubMed] [Google Scholar]
- 6.Brickman, T. J., and S. K. Armstrong. 1999. Essential role of the iron-regulated outer membrane receptor FauA in alcaligin siderophore-mediated iron uptake in Bordetella species. J. Bacteriol. 181:5958-5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchanan, S. K., B. S. Smith, L. Venkatramani, D. Xia, L. Esser, M. Palnitkar, R. Chakraborty, D. van der Helm, and J. Deisenhofer. 1999. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat. Struct. Biol. 6:56-63. [DOI] [PubMed] [Google Scholar]
- 8.Butterton, J. R., J. A. Stoebner, S. M. Payne, and S. B. Calderwood. 1992. Cloning, sequencing, and transcriptional regulation of viuA, the gene encoding the ferric vibriobactin receptor of Vibrio cholerae. J. Bacteriol. 174:3729-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casabadan, M. J., and S. N. Cohen. 1980. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli cells. Gene 6:23-28. [DOI] [PubMed] [Google Scholar]
- 10.Cobessi, D., C. Herve, N. Folschweiller, I. J. Schalk, M. A. Abdallah, and F. Pattus. 2005. The crystal structure of the pyoverdine outer membrane receptor FpvA from Pseudomonas aeruginosa at 3.6Å resolution. J. Mol. Biol. 347:121-134. [DOI] [PubMed] [Google Scholar]
- 11.Cornelis, P., and S. Matthijs. 2002. Diversity of siderophore-mediated iron uptake systems in fluorescent pseudomonads: not only pyoverdines. Environ. Microbiol. 4:787-798. [DOI] [PubMed] [Google Scholar]
- 12.Enz, S., S. Mahren, U. H. Stroeher, and V. Braun. 2000. Surface signaling in ferric citrate transport gene induction: interaction of the FecA, FecR, and FecI regulatory proteins. J. Bacteriol. 182:637-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson, A. D., R. Chakraborty, B. S. Smith, L. Esser, D. van der Helm, and J. Deisenhofer. 2002. Structural basis of gating by the outer membrane transporter FecA. Science 295:1715-1719. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson, A. D., E. Hofmann, J. W. Coulton, K. Diederichs, and W. Welte. 1998. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science 282:2215-2220. [DOI] [PubMed] [Google Scholar]
- 15.Folschweiller, N., I. J. Schalk, H. Celia, B. Kieffer, M. A. Abdallah, and F. Pattus. 2000. The pyoverdin receptor FpvA, a TonB-dependent receptor involved in iron uptake by Pseudomonas aeruginosa. Mol. Membr. Biol. 17:123-133. [DOI] [PubMed] [Google Scholar]
- 16.Gensberg, K., K. Hughes, and A. W. Smith. 1992. Siderophore-specific induction of iron uptake in Pseudomonas aeruginosa. J. Gen. Microbiol. 138:2381-2387. [DOI] [PubMed] [Google Scholar]
- 17.Ghysels, B., B. T. M. Dieu, S. A. Beatson, J.-P. Pirnay, U. A. Ochsner, M. L. Vasil, and P. Cornelis. 2004. FpvB, an alternative type I ferripyoverdine receptor of Pseudomonas aeruginosa. Microbiology 150:1671-1680. [DOI] [PubMed] [Google Scholar]
- 18.Härle, C., I. Kim, A. Angerer, and V. Braun. 1995. Signal transfer through three compartments: transcription initiation of the Escherichia coli ferric citrate transport system from the cell surface. EMBO J. 14:1430-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 20.Hove, B. V., H. Staudenmaier, and V. Braun. 1990. Novel two-component transmembrane transcription control: regulation of iron dicitrate transport in Escherichia coli K-12. J. Bacteriol. 172:6749-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilburn, L., K. Poole, J. M. Meyer, and S. Neshat. 1998. Insertion mutagenesis of the ferric pyoverdine receptor FpvA of Pseudomonas aeruginosa: identification of permissive sites and a region important for ligand binding. J. Bacteriol. 180:6753-6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, I. S., A. Stiefel, S. Plantör, A. A., and V. Braun. 1997. Transcription induction of the ferric citrate transport genes via the N-terminus of the FecA outer membrane protein, the Ton system and the electrochemical potential of the cytoplasmic membrane. Mol. Microbiol. 23:333-344. [DOI] [PubMed] [Google Scholar]
- 23.King, E. O., M. K. Ward, and D. E. Raney. 1954. Two simple media for the demonstration of pyocyanin and fluorescein. J. Lab. Med. 44:301-307. [PubMed] [Google Scholar]
- 24.Koebnik, R., and V. Braun. 1993. Insertion derivatives containing segments of up to 16 amino acids identify surface- and periplasm-exposed regions of the FhuA outer membrane receptor of Escherichia coli K-12. J. Bacteriol. 175:826-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koster, M., W. V. Klompenburg, W. Bitter, J. Leong, and P. Weisbeek. 1994. Role for the outer membrane ferric siderophore receptor PupB in signal transduction across the bacterial cell envelope. EMBO J. 13:2805-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamont, I. L., P. A. Beare, U. Ochsner, A. I. Vasil, and M. L. Vasil. 2002. Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proc, Natl. Acad. Sci. USA 99:7072-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lathrop, J. T., B. Y. Wei, G. A. Touchie, and R. J. Kadner. 1995. Sequences of the Escherichia coli BtuB protein essential for its insertion and function in the outer membrane. J. Bacteriol. 177:6810-6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Locher, K. P., B. Rees, R. Koebnik, A. Mitschler, L. Moulinier, J. P. Rosenbusch, and D. Moras. 1998. Transmembrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell 95:771-778. [DOI] [PubMed] [Google Scholar]
- 29.Markie, D., D. F. Hill, and R. Poulter. 1986. The construction of a modified drug resistance cassette. Proceedings of the Otago Medical School 64:69-70. [Google Scholar]
- 30.McMorran, B. J., H. M. C. S. Kumara, K. Sullivan, and I. L. Lamont. 2001. Involvement of a transformylase enzyme in siderophore synthesis in Pseudomonas aeruginosa. Microbiology 147:1517-1524. [DOI] [PubMed] [Google Scholar]
- 31.Meyer, J., A. Stintzi, D. D. Vos, P. Cornelis, R. Tappe, K. Taraz, and H. Budzikiewicz. 1997. Use of siderophores to type pseudomonads: the three Pseudomonas aeruginosa pyoverdine systems. Microbiology 143:35-43. [DOI] [PubMed] [Google Scholar]
- 32.Meyer, J.-M., A. Stintzi, and K. Poole. 1999. The ferripyoverdine receptor FpvA of Pseudomonas aeruginosa PAO1 recognizes the ferripyoverdines of P. aeruginosa PAO1 and P. fluorescens ATCC 13525. FEMS Microbiol. Lett. 170:145-150. [DOI] [PubMed] [Google Scholar]
- 33.Meyer, J. M., and M. A. Abdallah. 1978. The fluorescent pigment of Pseudomonas fluorescens: biosynthesis, purification and physio-chemical properties. J. Gen. Microbiol. 107:319-328. [Google Scholar]
- 34.Moeck, G. S., B. S. Bazzaz, M. F. Gras, T. S. Ravi, M. J. Ratcliffe, and J. W. Coulton. 1994. Genetic insertion and exposure of a reporter epitope in the ferrichrome-iron receptor of Escherichia coli K-12. J. Bacteriol. 176:4250-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moeck, G. S., and J. W. Coulton. 1998. TonB-dependent iron acquistion: mechanisms of siderophore-mediated active transport. Mol. Microbiol. 28:675-681. [DOI] [PubMed] [Google Scholar]
- 36.Poole, K., S. Neshat, and D. Heinrichs. 1991. Pyoverdine-mediated iron transport in Pseudomonas aeruginosa: involvement of a high-molecular-mass outer membrane protein. FEMS Microbiol. Lett. 62:1-5. [PubMed] [Google Scholar]
- 37.Poole, K., S. Neshat, K. Krebes, and D. Heinrichs. 1993. Cloning and nucleotide analysis of the ferripyoverdine receptor gene fpvA of Pseudomonas aeruginosa. J. Bacteriol. 175:4597-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Postle, K., and R. J. Kadner. 2003. Touch and go: tying TonB to transport. Mol. Microbiol. 49:869-882. [DOI] [PubMed] [Google Scholar]
- 39.Rakin, A., E. Saken, D. Harmsen, and J. Heesemann. 1994. The pesticin receptor of Yersinia enterocolitica: a novel virulence factor with dual function. Mol. Microbiol. 13:253-263. [DOI] [PubMed] [Google Scholar]
- 40.Rombel, I. T., B. J. McMorran, and I. L. Lamont. 1995. Identification of a DNA sequence motif required for expression of iron-regulated genes in pseudomonads. Mol. Gen. Genet. 246:519-528. [DOI] [PubMed] [Google Scholar]
- 41.Salinovich, O., and R. C. Montelaro. 1986. Reversible staining and peptide mapping of proteins transferred to nitrocellulose after separation by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Anal. Biochem. 156:341-347. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook, J., D. W. Russell, and N. Irwin. 2000. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 43.Schalk, I. J., P. Kyslik, D. Prome, A. van Dorsselaer, K. Poole, M. A. Abdallah, and F. Pattus. 1999. Copurification of the FpvA ferric pyoverdin receptor of Pseudomonas aeruginosa with its iron-free ligand: implications for siderophore-mediated iron transport. Biochemistry 38:9357-9365. [DOI] [PubMed] [Google Scholar]
- 44.Schalk, I. J., W. W. Yue, and S. K. Buchanan. 2004. Recognition of iron-free siderophores by TonB-dependent iron transporters. Mol. Microbiol. 54:14-22. [DOI] [PubMed] [Google Scholar]
- 45.Shen, J., A. Meldrum, and K. Poole. 2002. FpvA receptor involvement in pyoverdine biosynthesis in Pseudomonas aeruginosa. J. Bacteriol. 184:3268-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Visca, P., L. Leoni, M. J. Wilson, and I. L. Lamont. 2002. Iron transport and regulation, cell signalling and genomics: lessons from Escherichia coli and Pseudomonas. Mol. Microbiol. 45:1177-1190. [DOI] [PubMed] [Google Scholar]
- 47.Webster, A. C., and C. M. Litwin. 2000. Cloning and characterization of vuuA, a gene encoding the Vibrio vulnificus ferric vulnibactin receptor. Infect Immun. 68:526-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.West, S. E., H. P. Schweizer, C. Dall, A. K. Sample, and L. J. Runyen-Janecky. 1994. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for replication in P. aeruginosa. Gene 148:81-86. [DOI] [PubMed] [Google Scholar]
- 49.Yue, W. W., S. Grizot, and S. K. Buchanan. 2003. Structural evidence for iron-free citrate and ferric citrate binding to the TonB-dependent outer membrane transporter FecA. J. Mol. Biol. 332:353-368. [DOI] [PubMed] [Google Scholar]