Abstract
The MS2 coat protein binds specifically to an RNA hairpin formed within the viral genome. By soaking different RNA fragments into crystals of MS2 coat protein capsids it is possible to determine the X-ray structure of the RNA–protein complexes formed. Here we present the structure to 2.85 Å resolution of a complex between a chemically modified RNA hairpin variant and the MS2 coat protein. This RNA variant has a substitution at the –5 base position, which has been shown previously to be pyrimidine-specific and is a uracil in the wild-type RNA. The modified RNA hairpin contains a pyridin-4-one base (4one) at this position that lacks the exocyclic 2-oxygen eliminating the possibility of forming a hydrogen bond to asparagine A87 in the protein. The 4one complex structure shows an unprecedented major conformational change in the loop region of the RNA, whereas there is almost no change in the conformation of the protein.
INTRODUCTION
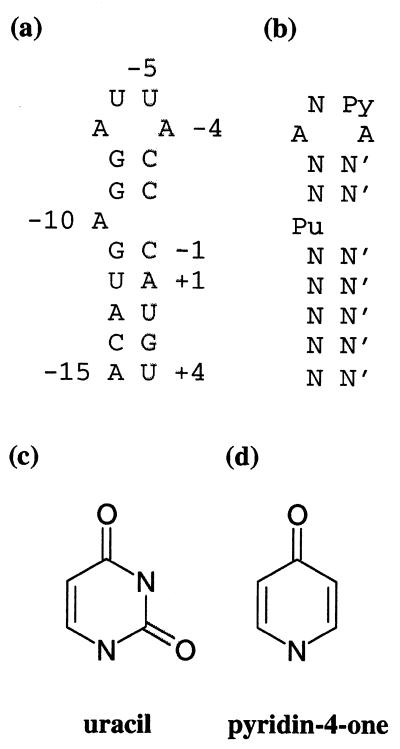
The binding of the Escherichia coli phage MS2 coat protein to a hairpin in the viral genome has been widely used as a model system for studying sequence specific protein–RNA interactions (1–4). A dimer of the MS2 coat protein binds specifically to a 19 nt long hairpin (the operator) in the single-stranded viral RNA genome, located at the 5′-end of the viral replicase gene and encompassing both the start codon and the Shine–Dalgarno sequence. Protein binding to this site on the RNA molecule results in repression of the replicase translation. The complex also appears to function as an initiation site for viral assembly and controls the encapsidation of the cognate RNA in vivo (2). The RNA hairpin comprises a 7 bp stem closed by a 4 nt loop and is interrupted by one unpaired residue between base pairs five and six at the 5′-end of the stem (Fig. 1a).
Figure 1.
(a) The secondary structure of the 19 nt wt RNA hairpin. Numbering is relative to the first nucleotide in the replicase start codon (+1). (b) The consensus RNA hairpin as deduced from operator sequence variant binding experiments (1,2). N, any nucleotide; N′, its complementary nucleotide; Pu, purine; Py, pyrimidine. (c) The uracil base present at position –5 in the wt hairpin. (d) The pyridin-4-one derivative present at position –5 in the 4one RNA hairpin variant.
X-ray crystallography has been used to determine the structure of complexes where variants of the RNA hairpin are soaked into crystals of protein capsids formed by MS2 coat protein recombinantly expressed in E.coli (5). The T = 3 icosahedral viral capsid of MS2 consists of 180 chemically identical copies of the coat protein, folded as three slightly different conformers denoted A, B and C, arranged as 90 dimers of two types, A/B and C/C (6). Crystal structures of RNA hairpin–MS2 complexes have been determined for the wild-type (wt) hairpin (7), the cytosine –5 variant hairpin, which binds with higher affinity than the wt (5,7), two hairpins with longer or shorter stems than the normal RNA hairpin (8) and three different RNA aptamers selected to bind to the MS2 coat protein despite their different secondary structures (9,10). The structures have also been determined for complexes of the hairpin with MS2 capsids having mutations in the coat protein (11). These structures, however, all show mainly similar modes of binding of the RNA hairpin to a dimer of the MS2 coat protein. The stronger binding of the C-5 RNA was shown to be due to an additional intramolecular hydrogen bond compared to the wt operator (7).
Binding assays (1,2) have shown that in the context of the wt operator, four positions in the RNA hairpin sequence are important for strong binding; –4 and –7 should be adenines, –5 should be a pyrimidine and –10 should be a purine (Fig. 1b). There is a structural basis for this preference (7). The adenine bases at positions –4 and –10 are bound in corresponding pockets in the two subunits of the dimer. The adenine at position –7 is stacked between bases –8 and –5. The uracil at position –5 is, in turn, stacked on a tyrosine side chain (Tyr A85). The presence of an asparagine side chain (Asn A87) forming a hydrogen bond with the –5 base appears to be the main determinant of the pyrimidine specificity at this position. The hydrogen bond is formed between the amino group of the asparagine and the exocyclic 2-oxygen of either Ura –5 in the wt complex or Cyt –5 in the C-5 variant complex (5,7). Data from mutation studies of asparagine 87 do not lead to a clear picture of the importance of the hydrogen bond between Asn87 and the –5 base for complex formation. An Asn87Ser MS2 mutant (12) was shown to bind to the wt hairpin (U-5) with ~20-fold lower affinity than the wt protein, suggesting that the hydrogen bond is important for the affinity. The Asn87Ala mutant (13), in contrast, showed an increased affinity for purines at position –5 by a factor of 3–5 and almost no change in the pyrimidine affinity when compared to the wt protein.
The RNA binding to MS2 capsids offers a unique opportunity to study the structural details of protein–RNA interactions. In this work, we have used X-ray crystallography to study a complex where an RNA hairpin modified at position –5 has been soaked into MS2 coat protein crystals. In this hairpin, the wt uracil (Fig. 1a and c) has been replaced by a pyridin-4-one derivative (Fig. 1d). This modified base is missing the 2-oxygen, which forms the hydrogen bond to the asparagine side chain mentioned above, allowing the importance of this hydrogen bond to be investigated structurally.
MATERIALS AND METHODS
The pyridin-4-one derivative RNA (4one) was produced by solid-phase synthesis and purified to homogeneity by HPLC (14). The pyridin-4-one phosphoramidite was synthesised as described (15). The variant RNA was characterised by electrospray mass spectrometry using negative ionisation at a concentration of 10 pmol/µl in aqueous methanol with 1% (v/v) triethylamine. This confirmed the predicted molecular weight of the RNA. The MS2 coat protein was overexpressed in E.coli (16) and purified as capsids, free of native RNA, as described previously (17). Crystals of the MS2 coat protein capsid were grown in space group R32 with cell dimensions a = b = 288 Å and c = 654 Å (17). The maximal size of the crystals used was 1.5 × 1.0 × 0.4 mm3. The crystals were soaked with RNA at a final concentration of 2 mg/ml for 1 week before data collection (5). The X-ray data for the 4one complex were collected using a Mar345 image plate detector at Max lab II, station 711, Lund, Sweden. Several positions of each crystal were each exposed for 10–20 images. The data were processed and scaled using the HKL package (18). Statistics from the data collection and scaling are listed in Table 1.
Table 1. Statistics from data collection and model building.
| Statistics from data collection and scaling | Statistics from model building and refinement | ||
|---|---|---|---|
| Synchrotron | MaxII, Lund | Final R-factora (%) | 20.0 |
| Wavelength (Å) | 0.927 | r.m.s.d. of bond lengths (Å) | 0.007 |
| Temperature (°C) | 5 | r.m.s.d. of bond angles (°) | 1.4 |
| Oscillation angle (°) | 0.5 | r.m.s.d. of dihedral angles (°) | 24.2 |
| No. of crystals | 4 | r.m.s.d. of improper angles (°) | 1.06 |
| No. of reflections | 161 001 | Average B-factor for protein (Å2) | 25.1 |
| Resolution (Å) | 40.0–2.85 | Average B-factor for RNA (Å2) | 69.9 |
| Scaling R-factor (%) | 12.8 | Average B-factor for water (Å2) | 36.3 |
| Completeness (%) | 67 | No. of water molecules | 156 |
| Resolution in high resolution bin (Å) | 2.95–2.85 | No. of nucleotides AB RNA/CC RNA | 15/15 |
| Scaling R-factor in high res. bin (%) | 35.8 | Occupancy AB RNA/CC RNA | 0.5/0.5 |
| Completeness in high res. bin (%) | 34 | PDB accession code | 1dzs |
aR-factor (R = Σ ||Fobs|–k|Fcalc||/ Σ|Fobs|, summation over all hkl) for all reflections in the working set (160 796) in the resolution range 40.0–2.85 Å; no cut off was used.
The structure was solved using the molecular replacement method with initial phases calculated from the model of the MS2 protein shell (19). The phases were refined using the icosahedral 10-fold non-crystallographic symmetry utilising the program RAVE (20).
The model was built using the program O (21). Positional refinement and refinement of restrained individual temperature factors were carried out using the program CNS (22). Statistics for the final model are shown in Table 1. The RNA occupancies were chosen to give temperature factors that were reasonable in relation to the temperature factors of the RNA-binding parts of the protein. The coordinates for the 4one complex have been deposited at the Protein Data Bank (ID code 1dzs).
RESULTS
Quality of the model
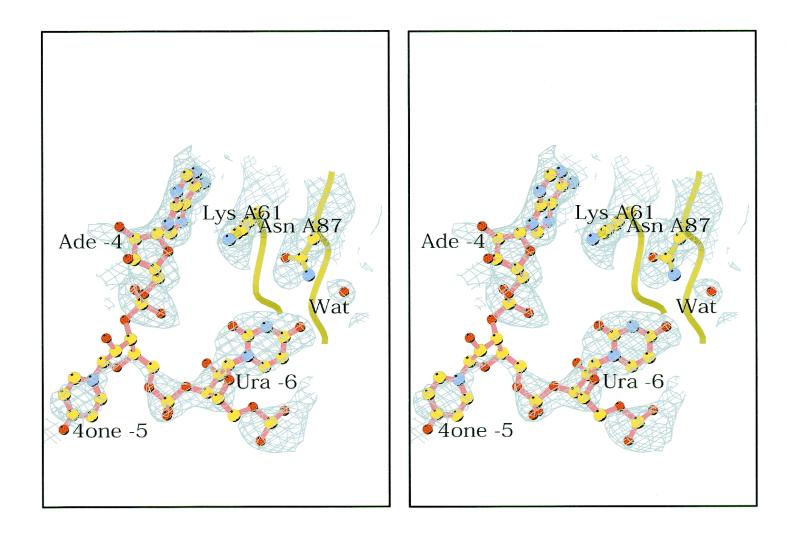
An electron density map was calculated for the 4one complex to 2.85 Å resolution. The 10-fold non-crystallographic symmetry was used to improve the phases and the resulting map was of good quality, showing density for both RNA and protein in the complex. Figure 2 shows an example. All 129 amino acids in the three protein conformers were built in the model.
Figure 2.
Stereo drawing showing the electron density for the Ura –6 base that is stacked to Tyr A85 in the 4one complex. The nucleotides Ade –4, 4one –5 and Ura –6, the side chains of Lys A61 and Asn A87 and one water (Wat) molecule are shown. At this contouring level, there is no density for the ribose of 4one –5. This drawing was made using the program Bobscript (31).
Since a single RNA molecule binds to a coat protein dimer, there are two slightly different RNA conformers, one binding to the A/B dimer and one binding to the C/C dimer. The RNA molecule binds asymmetrically to the A/B dimer, while it can bind to the C/C dimer in either of two orientations, leading to a 2-fold disorder of the electron density. Both RNA conformers were modelled in the complex. The RNA at the A/B dimer was modelled first. In the loop region of the RNA, the strong peaks in the electron density map did not correspond to the positions of the phosphates in the wt model. After rebuilding, the phosphates of the new RNA model fitted into strong density peaks (Fig. 2). At lower contouring levels, the electron density is continuous for the 4one RNA, leaving no doubt about the overall conformation. No evidence of RNA having the wt loop conformation was found in the map. The model of the RNA at the C/C dimer was based on the A/B RNA and rebuilt where motivated by the density at that position. In some regions, the electron density level for the RNA was low due to the relatively low occupancy (estimated to be 0.5) and disorder. Density for the two lowest base pairs was not present. The number of water molecules included in the model was 156. Figure 3 shows a schematic drawing of part of the A/B coat protein dimer with the bound RNA hairpin.
Figure 3.
Schematic drawing of RNA–coat protein complexes, with (a) wt and (b) 4one RNA hairpins. The bases of the RNA are shown as ball-and-stick models. Figures 3 and 4 were drawn with the program Molscript (32).
The quality of the protein model was analysed using PROCHECK (23). In the Ramachandran plot, no residues were found in disallowed regions, and one residue was found in the generously allowed region (the loop residue Asn C36 that has a similar backbone conformation as in other complexes). The torsion angles of the RNA were within the ranges observed in other RNA molecules. The final crystallographic R-factor for the 4one complex was 0.200.
The pyridin-4-one –5 complex
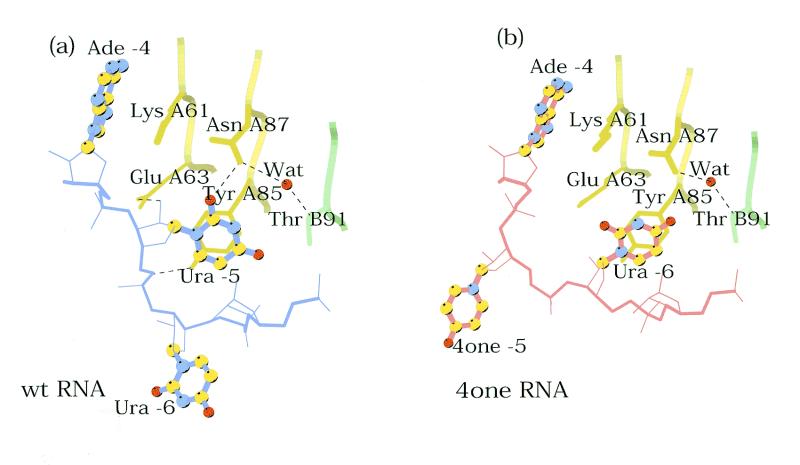
In the complex, the Ura –6 base is stacked against the side chain of Tyr A85 in contrast to all other complexes solved to date in which the –5 base makes this interaction (7,8,10,11) (Fig. 4). To accommodate this change, the 4one –5 base points away from the RNA in an analogous way to Ura –6 in all the other complexes, however, if superimposed the 4one –5 base and the –6 base in other complexes would be >10 Å apart. These conformational changes relative to wt RNA are restricted to the loop region. The only significant structural change in the protein is a small movement of the Asn A87 side chain towards the Ura –6 base. The conformational changes lead to the loss of several intermolecular hydrogen bonds: between Glu A63 and the 2′ hydroxyl of ribose –5, between the OH group of Tyr A85 and phosphate –6 and between Asn A87 and the O2 oxygen of Ura –5 (Table 2). In part, these lost contacts are compensated by van der Waal’s interactions of the 4one –5 base with a neighbouring protein dimer. In the case of the A/B bound RNA, the base is interacting with the Cβ and Cγ methylene groups of Gln C50 and the main chain carbonyl group of residue C52, and in the C/C bound RNA, the base is interacting with the side chain of Ala B53.
Figure 4.
The conformational differences of part of the loop. (a) Drawing of the wt complex showing the nucleotides –4, –5 and –6 and part of their interaction with the protein. The bases are shown as ball-and-stick models. The hydrogen bonds involving the side chains of Glu A63, Tyr A85 and Asn A87 are shown as dashed lines. (b) The corresponding drawing of the 4one complex.
Table 2. Polar protein–RNA interactions involving the loop-region of the RNA.
| Interacting atom | Interacting atom | Distance (Å) | |
|---|---|---|---|
| in the protein | in the RNA | wt | 4one |
| Lys A43 Nζ | O1P Ade-4 | 3.6 | – |
| O2P Ade | – | 3.4 | |
| Thr A45 Oγ1 | N6 Ade-4 | 3.1 | 2.6 |
| N7 Ade-4 | 2.6 | 2.9 | |
| Ser A47 Oγ | N1 Ade-4 | 2.7 | 3.1 |
| Thr A59 O | N6 Ade-4 | 2.9 | 3.1 |
| Glu A63 Oɛ2 | O2′ Ura-5 | 3.0 | – |
| Tyr A85 OH | O1P Ura-5 | 2.6 | – |
| Asn A87 Nδ2 | O2 Ura-5 | 3.0 | – |
| Ser B52 Oγ | O2P Ade-7 | 2.6 | 2.6 |
| Asn B55 Nδ2 | O1P Ura-6 | 3.2 | – |
| O2P Ade-7 | 2.9 | 2.8 | |
| Lys B57 Nζ | O1P Ade-7 | 3.2 | – |
| O1P Gua-8 | 3.6 | 2.5 | |
DISCUSSION
The 4one variant was made to test the consequences of the removal of a single hydrogen bonding atom from the RNA on binding to the MS2 coat protein. The elimination of a single oxygen atom and a ring nitrogen in the RNA has led to dramatic rearrangements in which the RNA conformation is changed, while the protein is presenting an unaltered recognition surface. The conformational change occurs in the loop region of the RNA, placing Ura –6 roughly at the position of Ura –5 in the wt complex, i.e. stacked on the sidechain of Tyr A85 and extending the 4one –5 base away from the RNA. The O4 oxygen of the stacked Ura –6 is too far away from Asn A87 (3.5 Å) to form a strong hydrogen bond.
This result highlights the flexibility of the RNA stem–loop. The conformation of hairpins with sequences similar to the MS2 hairpin has been studied by NMR spectroscopy (24,25). The loop seems to be relatively flexible, but there is no evidence for the conformation observed in the wt complex crystal structure. In solution, Ade –7 and Ura –6 appear stacked on the top GC base pair of the stem. The other two bases in the loop are not stacked; Ade –4 points into the RNA while Ura –5 is more extended. Binding to the protein therefore appears to lead to conformational changes in the RNA or alternatively selects more unusual conformations of the RNA (26). In the 4one complex, the –7 and –6 bases are stacked on the stem, and the conformation seen in this complex is thus more similar to the conformation observed in solution.
Binding studies of the 4one variant show that the affinity to MS2 coat protein dimers in solution is ~40-fold lower than that of the wt hairpin (H.Lago, N.J.Stonehouse and P.G.Stockley, unpublished results). The fact that the 4one variant binds to the capsids in spite of the relatively low affinity might partly be due to the interactions between the 4one –5 base and a neighbouring dimer. These interactions will only occur in capsids and therefore not in the binding studies, where the affinity to dimers is measured.
The 4one base would not be able to form the hydrogen bond to Asn A87 found in the wt complex. This missing hydrogen bond is probably not the main reason for the large conformational difference, since both this and several other protein–RNA contacts are lost in the 4one complex. The 4one nucleotide has been shown to strongly prefer a ‘south’ (C2′-endo) sugar puckering (27). In both the solution structures (24,25) and in the wt complex (7) the –5 sugar has a south sugar puckering, which shows that the preferred backbone conformation of the 4one nucleotide is also not the basis of the structural differences. A more likely explanation is the difference in the electrostatic properties for the 4one base when compared to the wt uracil. A more electronegative potential surrounding the 4-oxygen would lead to a more unfavourable contact with one of the oxygens of the –6 phosphate. This phosphate oxygen is found at a distance of <3.5 Å from the 4-oxygen of the base in the wt complex. In order to test this hypothesis, the electrostatic potentials of the 4one base and the uracil were calculated utilising quantum mechanical ab initio methods. The calculations were performed using the 6–31G* basis set at the HF level within the Gaussian 98 program (28) and the SPARTAN program (29). Geometries were optimised and the electrostatical potentials were then visualised using the Molden application (30) or the SPARTAN program (29). Further calculations based on the obtained geometries and charge distributions suggest that there is indeed a difference in electric potential in the vicinity of the 4-oxygen. When a negative test-charge is placed at the position of the wt –6 phosphate oxygen atom the electrostatic interaction energy between this charge and the uracil base is 6.6 kcal/mol. When the 4one base is at the same position this value will be 15 kcal/mol indicating a less favoured interaction of ~8 kcal/mol (K.Kolmodin and J.Åqvist, personal communication).
In the electron density map for the 4one complex as well as for all other complexes studied, the RNA hairpin is observed to bind only in one orientation on the A/B dimer. This asymmetrical mode of binding has been thought to be due to steric restrictions caused by a too close contact between B80 Cβ and O1P of the base –6 when binding in the other orientation (7,8). When the 4one RNA is rotated around the quasi-2-fold axis, however, the shortest distance between B80 Cβ and O2P in Ura-6 is 3.7 Å. Instead a collision is observed between the protruding 4one –5 base and residues A50 to A52 in the protein. This supports our hypothesis that sterical restrictions are responsible for the asymmetry of binding (7).
The result reported here emphasises the conformational flexibility of RNA molecules and their ability to adapt to protein recognition surfaces. The subtleties underlying the large rearrangement seen suggests that the extensive literature on the use of chemically variant RNAs needs to be interpreted with care, and preferably in combination with structural studies. These data suggest that RNA–protein complex formation is less tolerant of such ‘chemical mutations’ in the nucleic acid ligand than equivalent DNA–protein interactions.
Acknowledgments
ACKNOWLEDGEMENTS
We thank the staff of station 711 of the MAX synchrotron for their assistance and Dr Alison Ashcroft, University of Leeds, for help with mass spectroscopy. Dr Kaspars Tars, Uppsala University, is acknowledged for help with data collection. Karin Kolmodin and Dr Johan Åqvist, Uppsala University, are acknowledged for calculations and helpful discussions. This work was supported by the Swedish Natural Science Research Council, the Foundation for Strategic Research in Sweden, the UK BBSRC and MRC, the Leverhulme Trust and the Wellcome Trust.
PDB no. 1dzs
REFERENCES
- 1.Carey J., Lowary,P. and Uhlenbeck,O.C. (1983) Interaction of R17 coat protein with synthetic variants of its ribonucleic acid binding site. Biochemistry, 22, 4723–4730. [DOI] [PubMed] [Google Scholar]
- 2.Witherell G.W., Gott,J.M. and Uhlenbeck,O.C. (1991) Specific interaction between RNA phage coat proteins and RNA. Prog. Nucl. Acid Res. Mol. Biol., 40, 185–220. [DOI] [PubMed] [Google Scholar]
- 3.Peabody D.S. (1989) Translational repression by bacteriophage MS2 coat protein does not require cysteine residues. Nucleic Acids Res., 17, 6017–6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talbot S.J., Goodman,S., Bates,S.R.E., Fishwick,C.W.G. and Stockley,P.G. (1990) Use of synthetic oligoribonucleotides to probe RNA-protein interactions in the MS2 translational operator complex. Nucleic Acids Res., 18, 3521–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valegård K., Murray,J.B., Stockley,P.G., Stonehouse,N.J. and Liljas,L. (1994) Crystal structure of an RNA bacteriophage coat protein-operator complex. Nature, 371, 623–626. [DOI] [PubMed] [Google Scholar]
- 6.Valegård K., Liljas,L., Fridborg,K. and Unge,T. (1990) The three-dimensional structure of the bacterial virus MS2. Nature, 345, 36–41. [DOI] [PubMed] [Google Scholar]
- 7.Valegård K., Murray,J.B., Stonehouse,N.J., van den Worm,S., Stockley,P.G. and Liljas,L. (1997) The three-dimensional structures of two complexes between recombinant MS2 capsids and RNA operator fragments reveal sequence-specific protein-RNA interactions. J. Mol. Biol., 270, 724–738. [DOI] [PubMed] [Google Scholar]
- 8.Grahn E., Stonehouse,N.J., Murray,J.B., van den Worm,S., Valegård,K., Fridborg,K., Stockley,P.G. and Liljas,L. (1999) Crystallographic studies of RNA hairpins in complexes with recombinant MS2 capsids and implications for binding requirements. RNA, 5, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Convery M.A., Rowsell,S., Stonehouse,N.J., Ellington,A.D., Hirao,I., Murray,J.B., Peabody,D.S., Phillips,S.E.V. and Stockley,P.G. (1998) Crystal structure of an RNA aptamer-protein complex at 2.8 Å resolution. Nature Struct. Biol., 5, 133–139. [DOI] [PubMed] [Google Scholar]
- 10.Rowsell S., Stonehouse,N.J., Convery,M.A., Adams,C.J., Ellington,A.D., Hirao,I., Peabody,D.S., Stockley,P.G. and Phillips,S.E.V. (1998) Crystal structure of a series of RNA aptamers complexed to the same protein target. Nature Struct. Biol., 5, 970–975. [DOI] [PubMed] [Google Scholar]
- 11.van den Worm S., Stonehouse,N.J., Valegård,K., Murray,J.B., Walton,C., Stockley,P.G. and Liljas,L. (1998) Crystal structures of MS2 coat protein mutants in complex with wild-type RNA operator fragments. Nucleic Acids Res., 26, 1345–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim F., Spingola,M. and Peabody,D.S. (1994) Altering the RNA binding specificity of a translational repressor. J. Biol. Chem., 269, 9006–9010. [PubMed] [Google Scholar]
- 13.Johansson H.E., Dertinger,D., LeCuyer,K.A., Behlen,L.S., Greef,C.H. and Uhlenbeck,O.C. (1998) A thermodynamic analysis of the sequence-specific binding of RNA by bacteriophage MS2 coat protein. Proc. Natl Acad. Sci. USA, 95, 9244–9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray J.B., Collier,A.K. and Arnold,J.R.P. (1994) A general purification procedure for chemically synthesized oligoribonucleotides. Anal. Biochem., 218, 177–184. [DOI] [PubMed] [Google Scholar]
- 15.Matulic-Adamic J., Gonzales,C., Usman,N. and Beigelman,L. (1996) Synthesis of pyridinone ribonucleoside 3′-o-phosphoramidites and their incorporation into oligoribonucleotides. Bioorg. Med. Chem. Lett., 6, 373–378. [Google Scholar]
- 16.Mastico R.A., Talbot,S.J. and Stockley,P.G. (1993) Multiple presentation of foreign peptides on the surface of an RNA-free spherical bacteriophage capsid. J. Gen. Virol., 74, 541–548. [DOI] [PubMed] [Google Scholar]
- 17.Valegård K., Unge,T., Montelius,I., Strandberg,B. and Fiers,W. (1986) Purification, crystallization and preliminary X-ray data of the bacteriophage MS2. J. Mol. Biol., 190, 587–591. [DOI] [PubMed] [Google Scholar]
- 18.Otwinowski Z. and Minor,W. (1996) Processing of X-ray diffraction data collected in oscillation mode. In Carter,C.W.,Jr and Sweet,R.M. (eds), Methods in Enzymology. Academic Press, New York, NY, Vol. 276, pp. 307–326. [DOI] [PubMed]
- 19.Golmohammadi R., Valegård,K., Fridborg,K. and Liljas,L. (1993) The refined structure of bacteriophage MS2 at 2.8 Å resolution. J. Mol. Biol., 234, 620–639. [DOI] [PubMed] [Google Scholar]
- 20.Kleywegt G.J. and Jones,T.A. (1994) Halloween. Masks and Bones. In Bailey,S., Hubbard,R. and Waller,D. (eds), From First Map to Final Model. Proceedings of the CCP4 Study Weekend. SERC Daresbury Laboratory, Daresbury, UK, pp. 59–66.
- 21.Jones T.A., Zou,J.-Y., Cowan,S.W. and Kjeldgaard,M. (1991) Improved methods for building protein models in electron density maps and the location of errors in these model. Acta Crystallogr. A, 47, 110–119. [DOI] [PubMed] [Google Scholar]
- 22.Brünger A.T., Adams,P.D., Clore,G.M., DeLano,W.L., Gros,P., Grosse-Kunstleve,R.W., Jiang,J.-S., Kuszewski,J., Nilges,M., Pannu,N.S., Read,R.J., Rice,L.M., Simonson,T. and Warren,G.L. (1998) Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D, 54, 905–921. [DOI] [PubMed] [Google Scholar]
- 23.Laskowski R.A., MacArthur,M.W., Moss,D.S. and Thornton,J.M. (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr., 26, 283–291. [Google Scholar]
- 24.Borer P.N., Lin,Y., Wang,S., Roggenbuck,M.W., Gott,J.M., Uhlenbeck,O.C. and Pelczer,I. (1995) Proton NMR and structural features of a 24-nucleotide RNA hairpin. Biochemistry, 34, 6488–6503. [DOI] [PubMed] [Google Scholar]
- 25.Smith J.S. and Niconowicz,E.P. (1998) NMR structure and dynamics of an RNA motif common to the spliceosome branch-point helix and the RNA-binding site for phage GA coat protein. Biochemistry, 37, 13486–13498. [DOI] [PubMed] [Google Scholar]
- 26.Parrott A.M., Lago,H., Adams,C.J., Ashcroft,A.E., Stonehouse,N.J. and Stockley,P.G. (2000) RNA aptamers for the MS2 bacteriophage coat protein and the wild-type RNA operator have similar solution behaviour. Nucleic Acids Res., 28, 489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgin A.B. Jr, Gonzalez,C., Matulic-Adamic,J., Karpeisky,A.M., Usman,N., McSwiggen,J.A. and Beigelman,L. (1996) Chemically modified hammerhead ribozymes with improved catalytic rates. Biochemistry, 35, 14090–14097. [DOI] [PubMed] [Google Scholar]
- 28.Frisch M.J., Trucks,G.W., Schlegel,H.B., Scuseria,G.E., Robb,M.A., Cheeseman,J.R., Zakrzewski,V.G., Montgomery,J.A., Stratmann,R.E., Burant,J. et al. (1998) Gaussian 98. Revision A.7. Gaussian Inc., Pittsburgh, PA.
- 29. SPARTAN 5.1. (1998) Wavefunction Inc., Irvine, CA.
- 30.Schaftenaar G. and Noordik,J.H. (2000) Molden: a pre- and postprocessing program for molecular and electronic structures. J. Comput. Aided Mol. Des., 14, 123–134. [DOI] [PubMed] [Google Scholar]
- 31.Esnouf R.M. (1997) An extensively modified version of MolScript that includes greatly enhanced coloring capabilities. J. Mol. Graph., 15, 133–138. [DOI] [PubMed] [Google Scholar]
- 32.Kraulis P.J. (1991) Molscript: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr., 24, 946–950. [Google Scholar]