Abstract
The establishment of an effective nitrogen-fixing symbiosis between Sinorhizobium meliloti and its legume host alfalfa (Medicago sativa) depends on the timely expression of nodulation genes that are controlled by LysR-type regulators. Ninety putative genes coding for LysR-type transcriptional regulators were identified in the recently sequenced S. meliloti genome. All 90 putative lysR genes were mutagenized using plasmid insertions as a first step toward determining their roles in symbiosis. Two new LysR-type symbiosis regulator genes, lsrA and lsrB, were identified in the screening. Both the lsrA and lsrB genes are expressed in free-living S. meliloti cells, but they are not required for cell growth. An lsrA1 mutant was defective in symbiosis and elicited only white nodules that exhibited no nitrogenase activity. Cells of the lsrA1 mutant were recovered from the white nodules, suggesting that the lsrA1 mutant was blocked early in nodulation. An lsrB1 mutant was deficient in symbiosis and elicited a mixture of pink and white nodules on alfalfa plants. These plants exhibited lower overall nitrogenase activity than plants inoculated with the wild-type strain, which is consistent with the fact that most of the alfalfa plants inoculated with the lsrB1 mutant were short and yellow. Cells of the lsrB1 mutant were recovered from both pink and white nodules, suggesting that lsrB1 mutants could be blocked at multiple points during nodulation. The identification of two new LysR-type symbiosis transcriptional regulators provides two new avenues for understanding the complex S. meliloti-alfalfa interactions which occur during symbiosis.
In the absence of fixed nitrogen, Sinorhizobium meliloti establishes an effective nitrogen-fixing symbiosis with its legume partner, alfalfa (Medicago sativa), through a series of intricate signal exchanges and reciprocal structural changes (10, 22, 33, 40, 51, 57). The earliest known signal exchanges between the two organisms consist of the flavonoids secreted from alfalfa roots and the S. meliloti nodulation factor produced in response to the alfalfa flavonoids (33). This exchange of signals results in the formation of tightly curled alfalfa root hairs that are colonized by S. meliloti cells (14). S. meliloti cells elicit the formation of infection threads inside these curled root hairs, possibly through a different set of signal exchanges that involve succinoglycan and nodulation factor (14, 17, 37, 43). The infection threads elongate toward the base of root hairs, penetrate layers of alfalfa root cells, and reach developing nodule primordia. Live bacterial cells are released from infection threads into the plant cells inside the nodule primordia (17, 55). These S. meliloti cells, which are surrounded by a plant membrane in the cytoplasm of plant cells, differentiate into bacteroids (38). Successful differentiation of the S. meliloti cells may depend on another set of signal exchanges between S. meliloti and alfalfa involving proteins like BacA (19), which is also required for the intracellular survival of the animal pathogen Brucella abortus, a close relative of S. meliloti (30). The function of the BacA protein is unknown, but BacA could be a membrane receptor that senses signals for the start of the S. meliloti differentiation into bacteroids (31). The complexity of the S. meliloti-alfalfa interaction raises the possibility that many more signal exchanges between the two organisms remain unidentified.
Production of the nodulation factor that is part of the signal exchanges that initiate symbiosis is positively controlled by three nodD genes, nodD1, nodD2, and nodD3, and the syrM gene (23, 36, 52). Nodulation factor production is also negatively regulated by the nolR gene (27). Both nodD1 and nodD2 interact with plant flavonoids with the help of GroESL (59). The nodD3 and syrM genes are required for the production of nodulation factor with N-acylated (omega-1)-hydroxylated fatty acids (12). The nolR gene encodes a repressor that negatively regulates the expression of the nodD1 and nodABC operons by binding to the divergent promoter between them (9). All of these genes belong to the family of LysR transcriptional regulators.
LysR transcriptional regulators may also be involved in regulating signal exchanges for the formation of infection threads. The formation of infection threads inside a curled root hair colonized by S. meliloti requires the production of the bacterial exopolysaccharide succinoglycan (6) in addition to nodulation factor (17). The production of succinoglycan is regulated by the ExoS/ChvI two-component regulatory system (7), the exoR gene (45), the LysR-type transcriptional regulator gene syrM (52), and other genes, including syrA (2), exoX, and exoD (44, 46, 61). The involvement of the S. meliloti bioS gene in perceiving plant signals which regulate S. meliloti biotin production is one of the most recent examples of a LysR family transcriptional regulator involved in the S. meliloti-alfalfa signal exchange (20).
The family of LysR transcriptional factors is also involved in other microbe-plant and microbe-animal interactions (49). A LysR-type regulator, GbpR, induces the expression of the chromosomal virulence gene chvE in the presence of sugar in Agrobacterium tumefaciens (13). Another LysR-type regulator, MvfR, induces expression of a set of Pseudomonas aeruginosa virulence genes (5). A third LysR family transcriptional regulator, AphB, regulates expression of the Vibrio cholerae ToxR virulence cascade (28).
The structure and function of LysR family transcriptional regulators are highly conserved. These regulators are typically 300 amino acids long with an N-terminal DNA binding domain and a C-terminal sensing domain for signal molecules (49). They can function as activators or repressors. They typically regulate genes with promoters that are divergent from their own promoter. The promoters of the target genes are highly conserved and typically have at least one TN11A motif (49). The promoters of the nod genes contain two inverted ATCN9GAT repeats (39, 48). The conserved promoter and the close proximity of target genes to the lysR genes facilitate the quick recognition of the genes regulated by most of the lysR genes.
The genomic sequence of S. meliloti Rm1021 revealed 90 putative genes encoding LysR family transcriptional regulators (18). The large number of putative lysR genes, the fact that nod genes are regulated by LysR-type regulators, the potential for quick identification of downstream target genes, and the availability of genetic tools have made LysR proteins good targets for increasing our understanding of nodulation. In this paper we describe our efforts to systematically interrupt each of the putative lysR genes and to characterize the role of putative lysR genes in nodulation. Through this process, we identified two new LysR-type transcriptional regulators that are required for nodulation. Our findings provide new avenues for understanding the symbiosis better.
MATERIALS AND METHODS
Strains and bacterial media.
The Escherichia coli strains used in this study were DH5α, which was used for cloning, and MT616(pRK600) (Cmr), which was used as the helper for conjugation (29). S. meliloti Rm1021 (Strr) was used as the wild-type strain (29). The S. meliloti Rm1354 (nifA) mutant was used as a negative control for the nitrogenase assay (25). The S. meliloti phage used for general transduction was φM12 (32).
Luria-Bertani (LB) medium was used for growth of the E. coli strains, and LB medium supplemented with 2.5 mM MgSO4 and 2.5 mM CaCl2 (LB/MC) was used for all S. meliloti strains (29). Minimal medium Z-MGS was used to examine the growth requirements of the mutants (60). Agar (1.5%) was used to solidify media. Antibiotics were used at the following concentrations: chloramphenicol, 10 μg/ml; kanamycin, 25 μg/ml; neomycin, 200 μg/ml; and streptomycin, 500 μg/ml.
Construction of a suicide plasmid for mutagenesis.
To perform plasmid integration mutagenesis in S. meliloti, the suicide vector pK19mob2ΩHMB was constructed. The PstI-HindIII fragment of pHP45Ω (Km) (16) that carries the transcription-termination sequence (hairpin) was cloned between the PstI-HindIII sites of plasmid pK19mob2 (53). The HindIII site on the resulting plasmid was removed by digesting the plasmid with HindIII, filling the overhang with the Klenow fragment, and religating the plasmid. Three restriction sites (HindIII, MfeI, and BsrGI) were added between the EcoRI and PstI sites. Linker fragment 1U/1L carrying HindIII, MfeI, and BsrGI restriction sites was constructed by hybridizing oligonucleotides 1U (AATTCAAGCTTAACTTCGTCAATTGGGACCTAAATGTACACTGCA) and 1L (GTGTACATTTAGGTCCCAATTGACGAAGTTAAGCTTG). This linker fragment was cloned between the EcoRI and PstI restriction sites of the plasmid. The resulting plasmid was designated pK19mob2ΩHMB.
Plasmid insertion mutagenesis.
Suicide plasmid pK19mob2ΩHMB was prepared using the Wizard Plus Midipreps DNA purification system (Promega, Madison, WI), digested with restriction enzymes HindIII and BsrGI (New England BioLabs, Beverly, MA), and purified with a QIAquick PCR purification kit (QIAGEN, Valencia, CA). The DNA fragments (∼0.3 kb) containing the middle part of each putative lysR gene were produced using one primer containing a HindIII site and another primer containing a BsrGI site. For example, the primers used to produce part of the lsrA gene (the smc00037 open reading frame) were GGTTCCACGTAAGCTTCCGTGCGATCGGAACTGCTGC(HindIII site underlined) and GCGATTACCCTGTACACCAAAGGCGCCACCAGAGACC(BsrGI site underlined), and the primers used to produce part of the lsrB gene (the smc01225 open reading frame) were GGTTCCACGTAAGCTTCCGACGACGGACAAGCCAAGC(HindIII site underlined) and GCGATTACCCTGTACACCAGATAGTTGGGGGCGGGCT(BsrGI site underlined). The 0.3-kb DNA fragments were digested with both HindIII and BsrGI, ligated with a similarly treated suicide plasmid pK19mob2ΩHMB DNA fragment, and transformed into DH5α. The resulting plasmid constructs were conjugated into wild-type S. meliloti Rm1021 using helper MT616 in a triparental mating. The cells from the mating plates were used to select for plasmid insertion into putative lysR genes on solid LB/MC containing streptomycin and neomycin. The single colonies formed on the selective medium were restreaked to obtain single colonies on the same selective medium. A bacterial culture started from a single colony on the selective medium was used to prepare a phage lysate using S. meliloti phage φM12. The lysate was used to transduce the plasmid insertion in a putative lysR gene into a fresh Rm1021 background to ensure that the mutant strain carried a single plasmid insertion in one of the putative lysR genes.
To confirm that the mutant S. meliloti cells isolated from root nodules of alfalfa plants still carried the plasmid insertion in the targeted genes, genomic DNA was isolated from bacterial cells recovered from the nodules using a Wizard genomic DNA purification kit (Promega, Madison, WI) and used as a template in a PCR performed with one primer (primer 5) that annealed to the suicide plasmid and another primer (primer 7 for the lsrA gene) that annealed to the promoter region of the putative lysR gene, as shown in Fig. 1A. When the plasmid was inserted into the putative S. meliloti lysR gene, a DNA fragment of a predictable size was generated by PCR. Failure to generate a PCR fragment of the predicted size indicated that the plasmid did not integrate into the putative lysR gene.
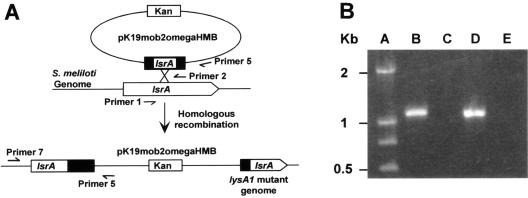
FIG. 1.
Strategies for plasmid insertion mutagenesis and conformation of the insertion in the targeted gene. (A) Schematic representation of the location of the genomic and plasmid-borne genes before and after integration of the plasmid into the genome. The locations of primers used to amplify the middle parts of targeted genes and the junction between the targeted gene and the suicide plasmid are also indicated. (B) Agarose gel with a DNA size marker and DNA fragments amplified with different combinations of primers and genomic DNA. Lane A, primers for amplifying the junction of the lsrA gene and the inserted suicide plasmid mixed with genomic DNA from the lsrA mutant; lane B, primers for amplifying the junction of the lsrA gene and the inserted suicide plasmid mixed with genomic DNA from wild-type strain Rm1021; lane C, primers for amplifying the junction of the lsrB gene and the inserted suicide plasmid mixed with genomic DNA from the lsrB1 mutant; lane D, primers for amplifying the junction of the lsrB gene and the inserted plasmid mixed with genomic DNA from strain Rm1021.
The following PCR primers were used: primer 1 (GGTTCCACGTAAGCTTCCGACGACGGACAAGCCAAGC) (lsrB gene fragment), primer 2 (GCGATTACCCTGTACACCAGATAGTTGGGGGCGGGCT) (lsrB gene fragment), primer 3 (GGTTCCACGTAAGCTTCCGTGCGATCGGAACTGCTGC) (lsrA gene fragment), primer 4 (GCGATTACCCTGTACACCAAAGGCGCCACCAGAGACC) (lsrA gene fragment), primer 5 (CCTGGCCTTTTGCTGGCCT) (suicide plasmid), primer 6 (CCTCCGATGTCGATCTCGG) (lsrB promoter), and primer 7 (GCTCGAAAGCGTCAAGGCC) (lsrA promoter).
Nodulation assays.
Alfalfa (M. sativa cv. Iroquois) seeds were surface sterilized and germinated as previously described (29). Bacterial cells (0.1 ml) were plated on the surface of a solid plant growth medium, Jensen's agar (34). Ten alfalfa seedlings were spread evenly across each plate. A stack of 10 plates was wrapped with aluminum foil and placed in a plant growth chamber on one side so that the shoots of alfalfa seedlings pointed up. Plants were examined once a week, and the growth of the plants was documented using a Kodak digital camera.
To test the nodulation efficiency of the mutants in a greenhouse, alfalfa seeds were surface sterilized and soaked for 15 min in 5 ml of a saline solution containing 2 × 109 cells of wild-type strain Rm1021 or one of the lysR mutants. A pot was filled with a mixture of vermiculite and perlite (2:1) and autoclaved. Alfalfa seeds coated with bacterial cells were placed on top of the vermiculite-perlite (2:1) mixture inside the pot and watered with 0.2 liter Jensen's liquid medium. The seeds were covered with another layer of autoclaved perlite (depth, 0.5 cm). The pot was wrapped with a piece of black plastic film, covered with a piece of paper, and incubated at 28°C for 2 days for seed germination. Pots with seedlings were transferred into the greenhouse and watered with 100 ml of autoclaved Jensen's liquid medium every 3 days. The alfalfa plants in the pots were examined once a week, and the average height of the plants was determined.
Nitrogenase assay.
Nitrogenase activity was analyzed using the C2H2 reduction method (58). A gas chromatograph (type 102G; 220 V, 50 Hz, 1.5 kW; Shanghai Analytic Instrument Factory, Shanghai, China) was filled with PORAPAKN gas chromatography column packing material (Waters Associates Inc.). The volume of C2H2 used was 1.5 ml for every reduction reaction, which was completed in 28 to 30 min. The amplifying ratios were 250 for C2H4 and gas and 62.5 for C2H2 (4). Typically, 10 root systems of alfalfa plants were first cleaned and then sealed in a glass test tube to determine the nitrogenase activities of the plants inoculated with different strains of S. meliloti.
Nodule occupation assay.
To recover bacterial cells from nodules, nodules of the alfalfa plants inoculated with mutants were collected, surface sterilized, crushed, and plated on nonselective rich medium (29). Bacterial colonies on the nonselective medium were replica plated onto LB/MC with neomycin, which allowed only the cells with the plasmid insertion to grow. After replica plating, none of the wild-type colonies grew on the selective medium, while every colony formed by the two mutants recovered from the nodules grew on the selective medium.
Detection of gene expression using RT-PCR.
Total RNA was collected from cells of S. meliloti Rm1021 and its mutants using an RNA extraction kit and RNAex reagent (Huashun Biotechnology Co., Ltd., Shanghai, China). Briefly, bacterial cells were collected and mixed with RNAex reagent, and then the RNA was extracted with chloroform. Total RNA was precipitated with isopropanol, washed with ethanol, dried, resuspended, and stored at −70°C. Gene expression was measured by reverse transcription (RT)-PCR using an RT-PCR kit (TaKaRa Biotechnology, Dalian, China) according to the instructions of the manufacturer. The forward and reverse primers for the lsrA gene were 5′GGTTCACCTGAACGGATTGC3′ and 5′CCGAGATCGACATCGGAGG3′; the forward and reverse primers for the lsrB gene were 5′CGACGATATAGTCCGGAAGC3′ and 5′GCTCGAAAGCGTCAAGGCC3′; and the forward and reverse primers for the rpsF gene were 5′GGACCACGGTCGCCAAAC3′ and 5′GACGCTCTCGTCGAGCAGTA3′.
RESULTS
Identifying putative lysR genes.
A total of 80 open reading frames were identified as possible genes coding for LysR-type transcriptional regulators by the Genome Sequence Project (18), and 92 putative lysR genes were identified in our lab using the SMART sequence analysis software provided online by the European Bioinformatics Institute. Further analysis of the 12 extra putative LysR proteins showed that 10 of them have helix-turn-helix DNA binding domains and typical LysR substrate binding domains, suggesting that they could be LysR-type transcriptional regulators. All 90 putative lysR genes (Table 1) were selected for further analysis using plasmid insertion mutagenesis.
TABLE 1.
Phenotypes of the lysR mutants
| Strain | Gene | Free-living phenotypes
|
Nodulation phenotypes
|
|
||
|---|---|---|---|---|---|---|
| Growth | Swarming | Pink nodules (%) | Plant growth | |||
| Rm1021 | Wild type | +++ | ++ | 100 | +++ | |
| SMc00037 | lsrA | +++ | ++ | 18a | − | |
| SMc01225 | lsrB | + | + | 45 | +/− | |
| SMc00275 | +++ | ++ | 93 | +++ | ||
| SMc00283 | +++ | ++ | 100 | +++ | ||
| SMc00370 | +++ | ++ | 100 | +++ | ||
| SMc00504 | +++ | ++ | 100 | +++ | ||
| SMc00766 | +++ | ++ | 86 | +++ | ||
| SMc00780 | +++ | ++ | 100 | +++ | ||
| SMc00818 | +++ | ++ | 98 | +++ | ||
| SMc00820 | +++ | ++ | 91 | +++ | ||
| SMc00831 | +++ | ++ | 100 | +++ | ||
| SMc00929 | +++ | ++ | 100 | +++ | ||
| SMc01092 | +++ | ++ | 76 | +++ | ||
| SMc01330 | +++ | ++ | 96 | +++ | ||
| SMc01570 | +++ | ++ | 81 | +++ | ||
| SMc01651 | +++ | ++ | 100 | +++ | ||
| SMc01664 | +++ | ++ | 77 | +++ | ||
| SMc01817 | +++ | ++ | 79 | +++ | ||
| SMc01968 | +++ | ++ | 91 | +++ | ||
| SMc02030 | +++ | ++ | 100 | +++ | ||
| SMc02429 | +++ | ++ | 100 | +++ | ||
| SMc02523 | +++ | ++ | 90 | +++ | ||
| SMc02596 | +++ | ++ | 98 | +++ | ||
| SMc02893 | +++ | ++ | 86 | +++ | ||
| SMc02984 | +++ | ++ | 84 | +++ | ||
| SMc03122 | +++ | ++ | 90 | +++ | ||
| SMc03186 | +++ | ++ | 73 | +++ | ||
| SMc03975 | +++ | ++ | 94 | +++ | ||
| SMc04055 | +++ | ++ | 100 | +++ | ||
| SMc04163 | +++ | ++ | 100 | +++ | ||
| SMc04224 | +++ | ++ | 100 | +++ | ||
| SMc04315 | +++ | ++ | 88 | +++ | ||
| SMf00020 | +++ | ++ | 97 | +++ | ||
| SMa0039 | +++ | ++ | 88 | +++ | ||
| SMa0097 | +++ | ++ | 100 | +++ | ||
| SMa0179 | +++ | ++ | 100 | +++ | ||
| SMa0303 | +++ | ++ | 100 | +++ | ||
| SMa0307 | +++ | ++ | 100 | +++ | ||
| SMa0310 | +++ | + | 92 | +++ | ||
| SMa0347 | +++ | ++ | 100 | +++ | ||
| SMa0353 | ++ | ++ | 80 | +++ | ||
| SMa0355 | +++ | ++ | 92 | +++ | ||
| SMa0372 | +++ | ++ | 79 | +++ | ||
| SMa0498 | + | + | 84 | +++ | ||
| SMa0557 | +++ | + | 78 | +++ | ||
| SMa0750 | +++ | ++ | 81 | +++ | ||
| SMa0985 | +++ | ++ | 91 | +++ | ||
| SMa1387 | +++ | ++ | 81 | +++ | ||
| SMa1480 | +++ | ++ | 94 | +++ | ||
| SMa1493 | +++ | ++ | 89 | +++ | ||
| SMa1602 | +++ | + | 96 | +++ | ||
| SMa1625 | +++ | ++ | 100 | +++ | ||
| SMa1632 | ++ | ++ | 90 | +++ | ||
| SMa1720 | +++ | ++ | 85 | +++ | ||
| SMa1736 | +++ | ++ | 89 | +++ | ||
| SMa1782 | +++ | ++ | 98 | +++ | ||
| SMa1828 | +++ | ++ | 83 | +++ | ||
| SMa1893 | +++ | ++ | 88 | +++ | ||
| SMa1933 | +++ | ++ | 100 | +++ | ||
| SMa1954 | +++ | ++ | 97 | +++ | ||
| SMa1956 | +++ | ++ | 89 | +++ | ||
| SMa1966 | ++ | ++ | 83 | +++ | ||
| SMa1979 | + | + | 85 | +++ | ||
| SMa1987 | +++ | + | 81 | +++ | ||
| SMa2014 | +++ | ++ | 100 | +++ | ||
| SMa2015 | +++ | ++ | 95 | +++ | ||
| SMa2027 | +++ | ++ | 92 | +++ | ||
| SMa2107 | +++ | ++ | 98 | +++ | ||
| SMa2287 | +++ | ++ | 94 | +++ | ||
| SMa2293 | +++ | ++ | 100 | +++ | ||
| SMa2341 | +++ | ++ | 100 | +++ | ||
| SMb20004 | +++ | ++ | 100 | +++ | ||
| SMb20009 | +++ | ++ | 100 | +++ | ||
| SMb20123 | +++ | ++ | 100 | +++ | ||
| SMb20203 | +++ | ++ | 100 | +++ | ||
| SMb20211 | +++ | ++ | 100 | +++ | ||
| SMb20285 | +++ | ++ | 100 | +++ | ||
| SMb20494 | +++ | ++ | 87 | +++ | ||
| SMb20580 | +++ | ++ | 100 | +++ | ||
| SMb20582 | +++ | ++ | 100 | +++ | ||
| SMb20683 | +++ | ++ | 88 | +++ | ||
| SMb20715 | +++ | ++ | 100 | +++ | ||
| SMb20847 | +++ | ++ | 100 | +++ | ||
| SMb20870 | +++ | ++ | 100 | +++ | ||
| SMb20896 | +++ | ++ | 100 | +++ | ||
| SMb21093 | +++ | ++ | 100 | +++ | ||
| SMb21180 | +++ | ++ | 100 | +++ | ||
| SMb21291 | +++ | ++ | 100 | +++ | ||
| SMb21434 | +++ | ++ | 100 | +++ | ||
| SMb21535 | +++ | ++ | 100 | +++ | ||
In the alfalfa plants inoculated with the lsrA1 mutant about 18% of the nodules were slightly pink, which was very different from the pink nodules on plants inoculated with Rm1021.
Mutagenizing the putative lysR genes.
Each of the 90 putative lysR genes was mutagenized by targeted plasmid insertion. Suicide plasmid pK19mob2ΩHMB was specifically constructed to systematically mutagenize the putative lysR genes. To mutagenize a targeted putative lysR gene, a 0.3-kb DNA fragment containing the middle part of the lysR gene was generated by PCR and cloned directionally into suicide plasmid pK19mob2ΩHMB. The suicide plasmids carrying parts of putative lysR genes were conjugated into wild-type strain Rm1021. The Rm1021 cells with plasmids inserted into the putative lysR genes were selected on LB/MC with streptomycin and neomycin. A single colony was randomly selected for each of the putative mutations and purified by restreaking to obtain single colonies on the same selective medium. The mutagenized putative lysR gene in the mutant was retransduced into wild-type strain Rm1021 to regenerate the mutant. This ensured that the mutant had a single insertion mutation in its genome. Mutations in three open reading frames, smc00037, smc01225, and smc04224, were difficult to transduce to the wild-type background. The plasmid insertions in smc00037 and smc01225 were confirmed directly by PCR using appropriate primers as shown in Fig. 1. Mutations in all other open reading frames were successfully transduced into the wild-type background.
Phenotypes of the free-living lysR mutants.
LysR transcriptional regulators are often involved in regulating the expression of genes involved in cellular metabolism and other cellular functions. All 90 lysR mutants were tested for growth on minimal medium Z-MGS and for the ability to swarm to screen out the LysR transcriptionally regulated genes that are involved in regulating the expression of genes required in free-living S. meliloti cells. All 90 lysR mutants were able to grow on rich medium (LB/MC) as well as wild-type strain Rm1021. On the minimal growth medium (Z-MGS), five mutants, SMc01225, SMa0353, SMa0498, SMa1632, and SMa1979, formed smaller colonies than Rm1021 formed and took a longer time to form saturated liquid batch cultures, suggesting that they grew more slowly than the wild-type strain in minimal medium.
The motility of rhizobial cells often influences their nodulation efficiency. All 90 lysR mutants were examined on swarm plates with rich medium (LB/MC with 0.3% agar) using the wild type as a control. Mutants SMc01225, SMa0498, and SMa1979, which grew more slowly on minimal medium, also formed smaller colonies than wild-type strain Rm1021. Four other mutants, SMa0310, SMa0557, SMa1602, and SMa1987, formed colonies smaller than wild-type colonies on swarm plates, although they grew as fast as the wild-type strain on minimal medium. The other 81 lysR mutants formed colonies whose sizes were similar to the sizes of colonies formed by the wild-type strain. These results suggest that some of the LysR regulators might influence cell motility either directly or indirectly.
Symbiotic phenotypes of the putative lysR mutants.
Each of the 90 lysR mutants was used to inoculate 10 alfalfa plants growing in a square petri dish. After 3 weeks, the alfalfa plants that were not inoculated with any S. meliloti cells were short and turning yellow. The alfalfa plants that were inoculated with Rm1021 were tall and green. The alfalfa plants inoculated with the lysR mutant SMc00037 were short and turning yellow just like the alfalfa plants that were not inoculated with any bacterial cells. Most of the alfalfa plants inoculated with the SMc01225 mutant were short and light green and showed signs of nitrogen deficiency, although the height of a few plants was close to 50% of the height of the plants inoculated with wild-type strain Rm1021 (Fig. 2). The alfalfa plants inoculated with all other lysR mutants were tall and green and therefore had established an efficient symbiosis.
FIG. 2.
Symbiotic phenotypes of the mutants: alfalfa plants growing on plates inoculated with Rm1021 (+wt) (A), no bacterial cells (B), the lsrA1 mutant (E), or the lsrB1 mutant (F) and root systems of alfalfa plants inoculated with Rm1021 (C), no bacterial cells (D), the lsrA1 mutant (G), or the lsrB1 mutant (H).
The alfalfa plants that were not inoculated with any bacterial cells had no nodules (Fig. 2). The plants inoculated with Rm1021 had an average of six nodules per plant, and most of the nodules were pink and cylindrical after 6 weeks; 73 to 100% of the nodules on alfalfa plants inoculated with the other 88 lysR mutants were pink. The plants inoculated with the SMc00037 mutant had an average of 11 nodules of various sizes and shapes per plant, and 18% of the nodules were slightly pink. The color of these slightly pink nodules remained unchanged throughout the experiments, which suggests that the normal development of the nitrogen-fixing nodules was interrupted and that these nodules could not fix nitrogen. The plants inoculated with the SMc01225 mutant had an average of seven nodules of various sizes and shapes per plant, and 45% of the nodules were pink. Some of the nodules had an unusually thin senescent zone, which may or may not have been the reason that these pink nodules did not support the growth of the plants.
To further confirm the nodulation deficiency of the SMc00037 and SMc01225 mutants over a longer period, cells of these two mutants were used to inoculate alfalfa plants growing in pots in the greenhouse. After 2 months, the alfalfa plants inoculated with Rm1021 were tall and green, and the plants that were not inoculated with any bacterial cells were short and yellow, a clear sign of nitrogen deficiency. The alfalfa plants inoculated with mutant SMc00037 were short and light green, suggesting that the growth of the plants was limited by the lack of fixed nitrogen. The alfalfa plants inoculated with the SMc01225 mutant were 50 to 80% shorter than the plants inoculated with Rm1021, and they were light green, suggesting that the growth of the plants was limited by the lack of fixed nitrogen.
The smc00037 open reading frame was designated the lsrA gene (for “LysR-type symbiosis regulator”), and the plasmid insertion mutant was designated the lsrA1 mutant. The smc01225 open reading frame was designated the lsrB gene, and the plasmid insertion mutant was designated the lsrB1 mutant.
To ensure that the symbiotic phenotypes of the lsrA1 and lsrB1 mutants were the results of plasmid insertions in the lsrA and lsrB genes, cosmids plsrA+ and plsrB+ carrying genomic regions covering the lsrA and lsrB genes, respectively, were isolated from an S. meliloti genomic cosmid library and conjugated into either the lsrA1 or lsrB1 mutant. Alfalfa plants inoculated with the lsrA1 mutant carrying the complementing cosmid, plsrA+, were as tall and green as the plants inoculated with the wild-type strain (data not shown). The same was true for alfalfa plants inoculated with the lsrB1 mutant carrying the complementing plasmid (data not shown). All these findings suggest that both the lsrA1 and lsrB1 mutants were the result of plasmid insertions in the prospective genes.
Nitrogenase assays of plants inoculated with the lsrA1 and lsrB1 mutants.
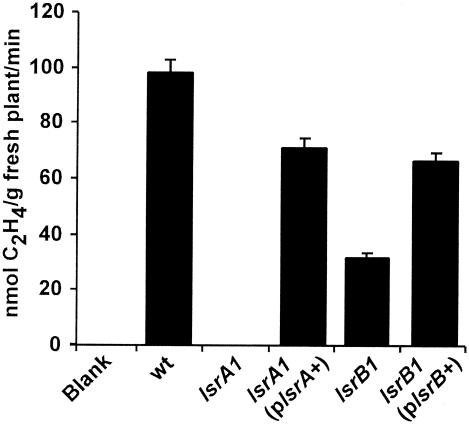
The growth of alfalfa plants inoculated with either the lsrA1 or lsrB1 mutant appeared to be limited by the lack of nitrogen. A lack of nitrogen could have arisen because no nitrogen was fixed by the bacteroids inside nodules or because the fixed nitrogen could not reach the plants. To quantitatively determine the amount of nitrogen fixed by bacteroids inside the nodules, 10 whole alfalfa root systems were used to measure the specific nitrogenase activities of the nodules on plants inoculated with different bacterial strains (58). As shown in Fig. 3, the blank control showed no nitrogenase activity, and alfalfa roots inoculated with wild-type strain Rm1021 showed high levels of nitrogenase activity. Alfalfa roots inoculated with the lsrA1 mutant showed no nitrogenase activity. This is consistent with the white or light pink nodules since the lack of leghemoglobin makes it impossible for bacteroids to have active nitrogenase molecules. The presence of the complementing plsrA+ cosmid in the lsrA1 mutant restored the nitrogenase activity to a level close to that of the wild-type strain. The alfalfa roots from the plants inoculated with the lsrB1 mutant, however, showed low levels of nitrogenase activity, which was reconfirmed by four independent experiments. This is also consistent with the fact that 45% of the nodules were pink. The presence of the complementing plsrB+ cosmid in the lsrB1 mutant restored the nitrogenase activity to a level close to that of the wild-type strain.
FIG. 3.
Nitrogenase activities of wild-type strain Rm1021 (wt), the lsrA1 mutant, the lsrA1 mutant carrying complementing cosmid plsrA+, the lsrB1 mutant, and the lsrB1 mutant carrying complementing cosmid plsrB+.
Bacterial occupation of the nodules.
To determine whether the lsrA1 and lsrB1 mutants invaded the white nodules and to determine how stable the plasmid insertion in the genome was, white and pink nodules from alfalfa plants inoculated with these two mutants were crushed. Similar numbers of bacterial cells were recovered from the pink nodules from plants inoculated with wild-type strain Rm1021 and pink and white nodules from the plants inoculated with the mutants. All of the cells recovered from the pink and white nodules from plants inoculated with the mutants still carried the neomycin antibiotic marker that was part of the suicide plasmid. These findings suggest that the lsrA1 and lsrB1 mutants were able to invade nodules and that the plasmid insertion mutations in the genes were stably maintained.
To further confirm the stability of the mutations, the recovered cells of the lsrA1 and lsrB1 mutants were used to inoculate alfalfa growing on Jensen's agar in petri dishes. Plants inoculated with the recovered and purified mutants developed both white and pink nodules, findings which were similar to the original findings.
To directly confirm that the inserted suicide plasmid remained in the middle of the targeted lsrA gene, a primer annealing to the promoter region of the lsrA gene (primer 6) and a primer annealing to the suicide plasmid (primer 5) were used in PCRs performed with different genomic DNAs as templates (Fig. 1B). No PCR product was detected when genomic DNA from Rm1021 was used (Fig. 1B, lane C). A 1.1-kb DNA fragment was detected when genomic DNA of the lsrA1 mutant was used as the template (lane B). The size of the DNA fragment matched the distance between primer 6 and primer 5. Similarly, the size of the DNA fragment generated by PCR when genomic DNA of the lsrB1 mutant was used matched the distance between primer 7, which annealed to the lsrB promoter, and primer 5. Altogether, these findings confirmed that the plasmid insertions in the lsrA and lsrB genes were stably maintained.
Both the lsrA and lsrB genes are expressed in free-living S. meliloti cells.
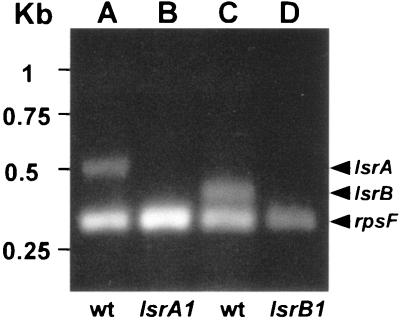
To ensure that the nodulation-defective phenotypes of the lsrA1 and lsrB1 mutants resulted from loss of the LsrA and LsrB proteins, the expression of both genes was checked for the wild type and the two mutants. Total RNA was extracted from wild-type strain Rm1021 and both mutants. A set of DNA primers for amplifying the S. meliloti rpsF gene as a positive control and a set of primers for amplifying the lsrA gene were used in RT-PCRs with total RNA from either the wild type or a mutant. The S. meliloti rpsF gene is predicted to encode the ribosome S6 protein based on sequence homology (50). The products of the RT-PCRs were resolved on an agarose gel (Fig. 4). Two DNA fragments, one matching the size of the predicted rpsF gene and the other matching the predicted size of the lsrA gene, were detected when total RNA from the wild-type cells was used (Fig. 4, lane A). Only one DNA fragment matching the predicted size of the rpsF gene was detected (lane B). These results suggested that the lsrA gene was expressed in the wild-type cells but not in the lsrA1 mutant cells. Similar experimental results suggested that the lsrB gene was expressed in the wild-type cells but not in the lsrB1 mutant cells. The control lane showed no DNA fragment (data not shown). Altogether, these results confirmed that lsrA and lsrB are real genes and that they are expressed in free-living cells.
FIG. 4.
Expression of the lsrA and lsrB genes in free-living cells: agarose gel with gel fragments generated by RT-PCR using different combinations of primers and genomic DNA templates. Lane A, primers for the rpsF and lsrA genes mixed with Rm1021 (wt) total RNA; lane B, primers for the rpsF and lsrA genes mixed with total RNA of the lsrA1 mutant; lane C, primers for the rpsF and lsrB genes mixed with Rm1021 total RNA; lane D, primers for the rpsF and lsrB genes mixed with total RNA of the lsrB1 mutant.
Homologs and neighboring genes of the LsrA and LsrB genes.
The proposed lsrA gene encodes a 302-amino-acid protein with an N-terminal conserved DNA binding domain and a C-terminal sensing domain, a structure highly conserved among LysR-type transcriptional regulators. The LsrA protein exhibited higher homology with the Enterobacter cloacae AmpR protein (98 of 286 residues [34%] were identical; 142 of 286 residues [49%] were positive) (49). The ampR gene has been found in E. cloacae, Citrobacter freundii, and Rhizobacter capsulatus, and it encodes a transcription activator that regulates the expression of the ampC gene in the presence of the inducer (49). Other members of this subfamily are the Staphylococcus aureus perR regulatory gene involved in peroxide resistance in stationary-phase cells (24) and the Pseudomonas syringae trpI gene, which regulates trpB and trpA gene expression. In the absence of inducer, TrpI binds upstream of the trpAB operon, overlapping its own promoter region (1).
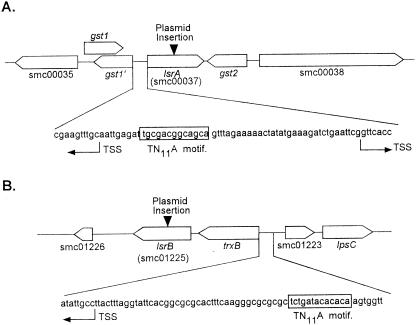
The lsrA gene is located on the chromosome between nucleotides 1051802 and 1052707 and is flanked on the downstream side by a glutathione S-transferase gene, gst2 (Fig. 5A). The gst2 gene is most likely transcribed from its own promoter in the opposite direction of the lsrA gene promoter so that it is unlikely that the plasmid insertion in the lsrA gene had a polar effect on expression of the gst2 gene. Indeed, RT-PCR verified that there was no change in expression of the gst2 gene in the lsrA mutant compared to the wild type (data not shown). Two possible overlapping gst genes are upstream of the lsrA gene (Fig. 5A). The gst1 gene was predicted by the S. meliloti genomic sequencing project, and it is transcribed from its own promoter in the same direction as the lsrA gene. The gst1′ gene was identified by us through use of the DNA analysis software Vector NTI based on a high level of homology to the glutathione S-transferase gene. The predicated gst1′ gene is transcribed from its own promoter, which is opposite and overlaps the promoter for the lsrA gene. There is a putative LysR binding site (TN11A) in the lsrA promoter, and it is also located in the −10 region of the promoter for the gst1′ gene so that LsrA not only may autoregulate its own expression but also probably regulates expression of the gst1′ gene. Further studies are needed to analyze these possibilities.
FIG. 5.
Locations and surrounding genes of the lsrA and lsrB genes, showing predicted binding sites for LysR-type transcriptional regulators and predicted transcription start sites (TSS).
The lsrB gene encodes a 302-amino-acid peptide, and the LsrB protein has all of the signature structures of a LysR-type transcriptional regulator. The LsrB protein exhibited relatively high homology to the Neisseria meningitidis CrgA protein (90 of 301 residues [29%] were identical; 154 of 301 residues [51%] were positive) (11). The N. meningitidis CrgA protein is a LysR-type transcriptional regulator that down-regulates the production of pili and capsules when N. meningitidis cells are in direct contact with animal epithelial cells (11).
The lsrB gene is located between nucleotide 1700052 and 1699147 on the chromosome (Fig. 5B). It is 110 bp downstream of the trxB gene for thioredoxin reductase, which also participates in the reduction of hydrogen peroxide and other oxygen radicals. The trxB gene is transcribed from its own promoter in the same direction as the lsrB gene. The −35 region of the trxB promoter contains a nearly perfect recognition site (TN11A) for a LysR regulator so it is possible that LsrB regulates the expression of both the trxB and lsrB genes. Transcribed divergently from the trxB gene is the smc01223 open reading frame. The putative protein encoded by the smc01223 open reading frame exhibits a high level of homology with the leucine-responsive regulatory protein (Lrp), although S. meliloti also has a putative lrp gene on the second symbiotic plasmid. The lrp gene regulates the synthesis of glutamate synthase, which controls the nitrogen response, presumably through its regulatory effect on intracellular glutamine. Guanosine tetraphosphate appears to control lipopolysaccharide biosynthesis (47). Further downstream of the smc01223 open reading frame are the lpsCDE genes, which are not involved in the rhizobium-legume symbiosis (8). The gene arrangements and previous findings suggest that it is unlikely that LsrB is related to lipopolysaccharide synthesis or the intracellular concentration of guanosine tetraphosphate. On the downstream side of the lsrB gene, there is a putative open reading frame, smc01226, which encodes a putative 96-amino-acid regulatory protein, which exhibits little homology with any other known regulatory protein. The 0.6-kb intergenic region between the lsrB gene and the smc01226 open reading frame should contain the promoter for the smc01226 open reading frame. It is extremely unlikely that the plasmid insertion in the lsrB gene has a polar effect on expression of the protein encoded by the smc01226 open reading frame. Expression of the smc01226 gene was not affected by the insertion in lsrB, as shown by RT-PCR (data not shown).
DISCUSSION
S. meliloti and alfalfa undergo reciprocal cellular and morphological changes during nodulation, which require continuous exchange of symbiosis signals. Identification of signal transduction pathways that lead to these changes would provide insights into the symbiotic process. Identification of the S. meliloti nodABCD genes led to a breakthrough in understanding the S. meliloti-alfalfa symbiosis. The involvement of the LysR-type transcriptional regulators, NodD1, NodD2, NodD3, NolR, and SyrM, in the S. meliloti symbiosis and the identification of 90 putative genes for LysR-type transcriptional regulators offer promising approaches for investigating this symbiosis through identification of new signal transduction pathways.
It would be ideal to first eliminate the putative lysR genes that are not expressed in free-living cells to reduce the number of genes to be analyzed. This is technically possible but time-consuming and expensive, and more importantly, eliminating lysR genes not expressed in free-living cells could potentially exclude the lysR genes expressed only during symbiosis. Eliminating the lysR genes that are not expressed during symbiosis is also very difficult and could potentially eliminate the lysR genes expressed in the free-living cells that are required for starting symbiosis. On balance, systematically mutagenizing and screening every putative lysR gene is still a more efficient approach.
Of the 90 putative lysR genes, 5, including lsrB, appear to influence the cell growth rate directly or indirectly, although the lsrB gene is the only gene required for symbiosis. Three of the five slow growers formed colonies on swarming plates that were smaller than the colonies formed by wild-type strain Rm1021, suggesting that the lower growth rate could explain the smaller colonies. Another set of four other lysR mutants that grow as well as Rm1021 formed colonies on swarming plates that were smaller than the colonies formed by Rm1021. All four mutants did form an effective nitrogen symbiosis with alfalfa, although the nodule occupancy was slightly lower than that of nodules from plants inoculated with the wild type. The role of these four putative LysR-type transcriptional regulators in cell motility is being investigated further.
Two of the lysR mutants, the lsrA1 and lsrB1 mutants, consistently failed to establish an efficient nitrogen fixation symbiosis with alfalfa. A series of experiments were performed to confirm that the insertion of the suicide plasmid into both the lsrA and lsrB genes was stably maintained by mutant cells and that disruption of either of the two genes prevented S. meliloti cells from establishing an efficient nitrogen symbiosis with their host plant, alfalfa. The difficulty of transducing both the lsrA1 and lsrB1 mutations raised the possibility that other genes linked to these two genes are required for symbiosis. Altogether, these findings suggest that both the lsrA and lsrB genes are involved in the S. meliloti-alfalfa symbiosis.
Both the lsrA and lsrB genes were found to be expressed in the free-living wild-type strain but not in either the lsrA1 or lsrB1 mutant. Loss of the lsrA gene did not change the growth rate of the bacterial cells in minimal medium or impair cell motility, while loss of the lsrB gene did slow cell growth and reduced cell motility. These findings suggest that neither LsrA nor LsrB is required for cell growth in the free-living state. The presence of these two regulators in the free-living state would allow S. meliloti cells to regulate expression of certain genes during symbiosis based on intracellular or extracellular signals.
The symbiosis-deficient phenotype of the lsrA1 mutant is more clear-cut. Alfalfa plants inoculated with this mutant were short and yellow with only white nodules. These plants did not have detectable levels of nitrogenase activity, which is consistent with the white color of the nodules. Since live bacterial cells could be recovered from the white nodules, the lsrA1 mutant is most likely capable of invading nodules but is blocked at some point before induction of leghemoglobin biosynthesis. These findings suggest that LsrA might regulate the expression of the genes required for nitrogen fixation. To better understand the role of LsrA in symbiosis, studies are being carried out to determine the precise step at which the lsrA1 mutant is blocked in establishing an effective nitrogen fixation symbiosis.
The homologs of LsrA and the genes around the lsrA gene on the chromosome were analyzed in detail to help determine the role of LsrA in symbiosis. LsrA exhibits the highest homology with the C. freundii AmpR protein (3). The AmpR protein regulates expression of the ampC gene, which encodes a beta-lactamase (3, 21). A putative S. meliloti ampC gene is located on the chromosome about 90 kb from the lsrA gene. It is unlikely that expression of the ampC gene is essential for the symbiosis. The great distance between the two genes also reduces the possibility that LsrA regulates expression of the ampC gene. The homology between LsrA and the AmpR protein is most pronounced in the N-terminal DNA binding region of the proteins. It is unlikely that LsrA and AmpR respond to the same stimuli in regulating the expression of their target genes.
The most prominent genes flanking the lsrA gene are several glutathione S-transferase genes, gst1′, gst1, and gst2. These genes are 3 of the 17 putative S. meliloti gst genes in the genome. The gst1′ gene is transcribed from a promoter that is divergent from and overlaps the lsrA promoter. The LysR motif in the lsrA promoter could be used for lsrA autoregulation and regulation of gst1′ expression. S. meliloti also has a putative gstR gene on symbiotic plasmid A but no gstA gene. The Rhizobium leguminosarum gstA gene is regulated by GstR, a LysR-type transcriptional regulator. The R. leguminosarum gstA gene is transcribed from a promoter divergently from the gstR gene. The loss of either gstA or gstR did not block efficient symbiosis between R. leguminosarum and its legume host (54). This could have been due to the presence of multiple copies of the gstA gene in its genome.
The thiol tripeptides glutathione and homoglutathione are very abundant in legume root nodules, and their synthesis is catalyzed by the enzymes glutamylcysteine synthetase, glutathione synthetase, and homoglutathione synthetase (35). The glutathione transferases are a family of multifunctional proteins that catalyze the conjugation of glutathione to a large variety of hydrophobic electrophilic compounds, resulting in their detoxification (54). They also carry out the transfer and reduction of the peroxide molecule (41). Further purification and analysis of the two R. leguminosarum glutathione S-transferases showed that they have a high affinity to herbicides (15), which is consistent with the notion that they might be involved in plant defense responses (41). Efforts are under way to determine if expression of the S. meliloti gst genes is blocked by the lsrA mutation to determine if any of the glutathione transferase genes is regulated by LsrA.
Compared to the symbiotic phenotype of the lsrA1 mutant, the symbiotic phenotype of the lsrB1 mutant is a bit more complicated. The height of the plants and the color of the leaves indicated that the lsrB1 mutant did not form an efficient nitrogen fixation symbiosis with the plants, which is consistent with the low nitrogenase activity of the plants inoculated with the lsrB1 mutant. A low percentage of nodules on plants inoculated with the lsrB1 mutant were pink, and the abnormal shapes of the pink nodules suggest that the symbiosis between the lsrB1 mutant and alfalfa might be blocked at multiple points. These findings could suggest that LsrB is required in both an early stage and a later stage of nodule development. Efforts are currently under way to determine more precisely at which points the lsrB1 mutant-alfalfa symbiosis is blocked.
The LsrB protein has high homology with the N. meningitidis CrgA protein, which regulates meningococcal adhesion by regulating the expression of pilus biosynthesis genes (pilE, for example) (11). The homology, however, is mostly limited to the DNA binding domain of the protein. The putative S. meliloti pilA gene that might encode a pilin subunit is located on the chromosome 1,538 kb from the lsrB gene. It is unlikely that LsrB is involved in regulating pilus biosynthesis in S. meliloti.
The S. meliloti lsrB gene is located on the chromosome and is flanked by a short downstream open reading frame (smc01226), which encodes an unknown regulatory protein, and by the upstream trxB gene, which encodes a thioredoxin reductase. The trxB gene is 110 bp upstream of the lsrB gene, and it is transcribed in the same direction as the lsrB gene. Since there is no obvious signature for a promoter in the region between the trxB and lsrB genes and both genes are transcribed in the same direction, it is possible that the lsrB gene is transcribed from the trxB promoter. A nearly perfect LysR recognition motif is located in the trxB promoter, which further raises the possibility that LsrB regulates the expression of both trxB and lsrB itself. E. coli trxB expression is regulated by OxyR, a LysR-type transcriptional regulator (42). The OxyR protein is directly sensitive to oxidation and becomes an active transcriptional activator when it is oxidized (56). This allows OxyR to mediate bacterial sensing of the intracellular redox potential (26). The intracellular redox potential in plant cells must be carefully controlled by the nodule cells to ensure efficient nitrogen fixation. Blocking the expression of the trxB gene could result in abnormal development of nodules and disrupt other nitrogen fixation processes. Expression of the trxB gene in the wild-type and lsrB1 mutant backgrounds is currently being characterized.
Both the lsrA and lsrB genes were identified based on the inability of lsrA1 and lsrB1 mutants to establish an effective nitrogen-fixing symbiosis with alfalfa. This screening might not have been able to detect the lysR mutants that are less efficient in various stages of nodulation. Mutants that are slightly inefficient in root hair invasion, for example, might not have been detected in these assays since many bacterial cells are used in a typical inoculation. We are currently investigating the efficiency of root hair invasion by the four lysR mutants that formed small colonies on swarming plates.
Direct screening of single lysR mutations has its limitations. For example, it does not identify signal transduction pathways that have redundant signal transduction proteins for one of the steps. Double mutations or even triple mutations would be required for detecting such signal transduction pathways. Even though it is technically feasible to construct double lysR mutations using different antibiotic markers, it would not be prudent to use this approach to screen the putative lysR genes for their role in symbiosis without some other means to narrow the list of candidates for study. It is still possible some of these putative LysR-type transcriptional regulators are indeed involved in symbiosis and will be discovered in the future using other approaches.
In summary, all 90 previously uncharacterized putative S. meliloti LysR-type transcriptional regulators were examined to determine their role(s) in symbiosis. Two of these LysR-type regulators, LsrA and LsrB, appeared to play important roles in establishing an efficient nitrogen-fixing symbiosis between S. meliloti and its plant host, alfalfa. Identification of these two new symbiosis genes provides two new avenues to identify additional symbiosis genes and signal transduction pathways involved in symbiosis.
Acknowledgments
We thank Huasong Zou for providing cosmids carrying the S. meliloti lsrA or lsrB gene.
This work was supported by grant 5506GM08225 for the NIH; by grants 617320030 and 632140032 from PSC-CUNY to H.-P.C.; by national grants 0311752 and 031U213D from Bundesministerium für Forschung und Technologie, Germany, and by the “Bioinformatik Initiative” of Deutsche Forschungsgemeinschaft to A.B. and S.R.; and by grant 2001AA214211from The National High Technology (863) International Research Program and grant 30170512 from the National Science Foundation of China to G.-Q.Y. and J.-B.Z.
REFERENCES
- 1.Auerbach, S., J. Gao, and G. N. Gussin. 1993. Nucleotide sequences of the trpI, trpB, and trpA genes of Pseudomonas syringae: positive control unique to fluorescent pseudomonads. Gene 123:25-32. [DOI] [PubMed] [Google Scholar]
- 2.Barnett, M. J., J. A. Swanson, and S. R. Long. 1998. Multiple genetic controls on Rhizobium meliloti syrA, a regulator of exopolysaccharide abundance. Genetics 148:19-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartowsky, E., and S. Normark. 1991. Purification and mutant analysis of Citrobacter freundii AmpR, the regulator for chromosomal AmpC beta-lactamase. Mol. Microbiol. 5:1715-1725. [DOI] [PubMed] [Google Scholar]
- 4.Burns, R. C., and R. W. Hardy. 1972. Purification of nitrogenase and crystallization of its Mo-Fe protein. Methods Enzymol. 24:480-496. [DOI] [PubMed] [Google Scholar]
- 5.Cao, H., G. Krishnan, B. Goumnerov, J. Tsongalis, R. Tompkins, and L. G. Rahme. 2001. A quorum sensing-associated virulence gene of Pseudomonas aeruginosa encodes a LysR-like transcription regulator with a unique self-regulatory mechanism. Proc. Natl. Acad. Sci. USA 98:14613-14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng, H. P., and G. C. Walker. 1998. Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J. Bacteriol. 180:5183-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, H. P., and G. C. Walker. 1998. Succinoglycan production by Rhizobium meliloti is regulated through the ExoS-ChvI two-component regulatory system. J. Bacteriol. 180:20-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clover, R. H., J. Kieber, and E. R. Signer. 1989. Lipopolysaccharide mutants of Rhizobium meliloti are not defective in symbiosis. J. Bacteriol. 171:3961-3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cren, M., A. Kondorosi, and E. Kondorosi. 1994. An insertional point mutation inactivates NolR repressor in Rhizobium meliloti 1021. J. Bacteriol. 176:518-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cullimore, J. V., R. Ranjeva, and J. J. Bono. 2001. Perception of lipo-chitooligosaccharidic Nod factors in legumes. Trends Plant Sci. 6:24-30. [DOI] [PubMed] [Google Scholar]
- 11.Deghmane, A. E., D. Giorgini, M. Larribe, J. M. Alonso, and M. K. Taha. 2002. Down-regulation of pili and capsule of Neisseria meningitidis upon contact with epithelial cells is mediated by CrgA regulatory protein. Mol. Microbiol. 43:1555-1564. [DOI] [PubMed] [Google Scholar]
- 12.Demont, N., M. Ardourel, F. Maillet, D. Prome, M. Ferro, J. C. Prome, and J. Denarie. 1994. The Rhizobium meliloti regulatory nodD3 and syrM genes control the synthesis of a particular class of nodulation factors N-acylated by (omega-1)-hydroxylated fatty acids. EMBO J. 13:2139-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doty, S. L., M. Chang, and E. W. Nester. 1993. The chromosomal virulence gene, chvE, of Agrobacterium tumefaciens is regulated by a LysR family member. J. Bacteriol. 175:7880-7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Downie, J. A., and S. A. Walker. 1999. Plant responses to nodulation factors. Curr. Opin. Plant Biol. 2:483-489. [DOI] [PubMed] [Google Scholar]
- 15.Faraone, A., M. Petrucci, D. Paludi, A. Aceto, and B. Dainelli. 2003. Purification and characterisation of two GST's forms from Rhizobium leguminosarum with a high affinity to herbicides. Int. J. Immunopathol. Pharmacol. 16:55-60. [DOI] [PubMed] [Google Scholar]
- 16.Fellay, R., J. Frey, and H. Krisch. 1987. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene 52:147-154. [DOI] [PubMed] [Google Scholar]
- 17.Gage, D. J., and W. Margolin. 2000. Hanging by a thread: invasion of legume plants by rhizobia. Curr. Opin. Microbiol. 3:613-617. [DOI] [PubMed] [Google Scholar]
- 18.Galibert, F., T. M. Finan, S. R. Long, A. Puhler, P. Abola, F. Ampe, F. Barloy-Hubler, M. J. Barnett, A. Becker, P. Boistard, G. Bothe, M. Boutry, L. Bowser, J. Buhrmester, E. Cadieu, D. Capela, P. Chain, A. Cowie, R. W. Davis, S. Dreano, N. A. Federspiel, R. F. Fisher, S. Gloux, T. Godrie, A. Goffeau, B. Golding, J. Gouzy, M. Gurjal, I. Hernandez-Lucas, A. Hong, L. Huizar, R. W. Hyman, T. Jones, D. Kahn, M. L. Kahn, S. Kalman, D. H. Keating, E. Kiss, C. Komp, V. Lelaure, D. Masuy, C. Palm, M. C. Peck, T. M. Pohl, D. Portetelle, B. Purnelle, U. Ramsperger, R. Surzycki, P. Thebault, M. Vandenbol, F. J. Vorholter, S. Weidner, D. H. Wells, K. Wong, K. C. Yeh, and J. Batut. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293:668-672. [DOI] [PubMed] [Google Scholar]
- 19.Glazebrook, J., A. Ichige, and G. C. Walker. 1993. A Rhizobium meliloti homolog of the Escherichia coli peptide-antibiotic transport protein SbmA is essential for bacteroid development. Genes Dev. 7:1485-1497. [DOI] [PubMed] [Google Scholar]
- 20.Heinz, E. B., D. A. Phillips, and W. R. Streit. 1999. BioS, a biotin-induced, stationary-phase, and possible LysR-type regulator in Sinorhizobium meliloti. Mol. Plant-Microbe Interact. 12:803-812. [DOI] [PubMed] [Google Scholar]
- 21.Higgins, C. S., M. B. Avison, L. Jamieson, A. M. Simm, P. M. Bennett, and T. R. Walsh. 2001. Characterization, cloning and sequence analysis of the inducible Ochrobactrum anthropi AmpC beta-lactamase. J. Antimicrob. Chemother. 47:745-754. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch, A. M. 1999. Role of lectins (and rhizobial exopolysaccharides) in legume nodulation. Curr. Opin. Plant Biol. 2:320-326. [DOI] [PubMed] [Google Scholar]
- 23.Honma, M. A., and F. M. Ausubel. 1987. Rhizobium meliloti has three functional copies of the nodD symbiotic regulatory gene. Proc. Natl. Acad. Sci. USA 84:8558-8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horsburgh, M. J., M. O. Clements, H. Crossley, E. Ingham, and S. J. Foster. 2001. PerR controls oxidative stress resistance and iron storage proteins and is required for virulence in Staphylococcus aureus. Infect. Immun. 69:3744-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huala, E., and F. M. Ausubel. 1989. The central domain of Rhizobium meliloti NifA is sufficient to activate transcription from the R. meliloti nifH promoter. J. Bacteriol. 171:3354-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim, S. O., K. Merchant, R. Nudelman, W. F. Beyer, Jr., T. Keng, J. DeAngelo, A. Hausladen, and J. S. Stamler. 2002. OxyR: a molecular code for redox-related signaling. Cell 109:383-396. [DOI] [PubMed] [Google Scholar]
- 27.Kondorosi, E., M. Pierre, M. Cren, U. Haumann, M. Buire, B. Hoffmann, J. Schell, and A. Kondorosi. 1991. Identification of NolR, a negative transacting factor controlling the nod regulon in Rhizobium meliloti. J. Mol. Biol. 222:885-896. [DOI] [PubMed] [Google Scholar]
- 28.Kovacikova, G., and K. Skorupski. 1999. A Vibrio cholerae LysR homolog, AphB, cooperates with AphA at the tcpPH promoter to activate expression of the ToxR virulence cascade. J. Bacteriol. 181:4250-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leigh, J. A., E. R. Signer, and G. C. Walker. 1985. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc. Natl. Acad. Sci. USA 82:6231-6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LeVier, K., R. W. Phillips, V. K. Grippe, R. M. Roop, 2nd, and G. C. Walker. 2000. Similar requirements of a plant symbiont and a mammalian pathogen for prolonged intracellular survival. Science 287:2492-2493. [DOI] [PubMed] [Google Scholar]
- 31.LeVier, K., and G. C. Walker. 2001. Genetic analysis of the Sinorhizobium meliloti BacA protein: differential effects of mutations on phenotypes. J. Bacteriol. 183:6444-6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long, S., J. W. Reed, J. Himawan, and G. C. Walker. 1988. Genetic analysis of a cluster of genes required for synthesis of the calcofluor-binding exopolysaccharide of Rhizobium meliloti. J. Bacteriol. 170:4239-4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long, S. R. 2001. Genes and signals in the rhizobium-legume symbiosis. Plant Physiol. 125:69-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meade, H. M., S. R. Long, G. B. Ruvkun, S. E. Brown, and F. M. Ausubel. 1982. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J. Bacteriol. 149:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moran, J. F., I. Iturbe-Ormaetxe, M. A. Matamoros, M. C. Rubio, M. R. Clemente, N. J. Brewin, and M. Becana. 2000. Glutathione and homoglutathione synthetases of legume nodules. Cloning, expression, and subcellular localization. Plant Physiol. 124:1381-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulligan, J. T., and S. R. Long. 1989. A family of activator genes regulates expression of Rhizobium meliloti nodulation genes. Genetics 122:7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niehaus, K., and A. Becker. 1998. The role of microbial surface polysaccharides in the Rhizobium-legume interaction. Subcell. Biochem. 29:73-116. [DOI] [PubMed] [Google Scholar]
- 38.Oke, V., and S. R. Long. 1999. Bacteroid formation in the Rhizobium-legume symbiosis. Curr. Opin. Microbiol. 2:641-646. [DOI] [PubMed] [Google Scholar]
- 39.Okker, R. J., H. P. Spaink, B. J. Lugtenberg, and H. R. Schlaman. 2001. Mutants in the nodFEL promoter of Rhizobium leguminosarum bv. viciae reveal a role of individual nucleotides in transcriptional activation and protein binding. Arch. Microbiol. 175:152-160. [DOI] [PubMed] [Google Scholar]
- 40.Patriarca, E. J., R. Tate, and M. Iaccarino. 2002. Key role of bacterial NH4+ metabolism in Rhizobium-plant symbiosis. Microbiol. Mol. Biol. Rev. 66:203-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pickett, C. B., and A. Y. Lu. 1989. Glutathione S-transferases: gene structure, regulation, and biological function. Annu. Rev. Biochem. 58:743-764. [DOI] [PubMed] [Google Scholar]
- 42.Prieto-Alamo, M. J., J. Jurado, R. Gallardo-Madueno, F. Monje-Casas, A. Holmgren, and C. Pueyo. 2000. Transcriptional regulation of glutaredoxin and thioredoxin pathways and related enzymes in response to oxidative stress. J. Biol. Chem. 275:13398-13405. [DOI] [PubMed] [Google Scholar]
- 43.Pühler, A. M., W. Arnold, A. Becker, A. Roxlau, M. Keller, D. Kapp, A. Lagares, J. Lorenzen, and K. Niehaus. 1993. The role of Rhizobium meliloti surface polysaccharides in nodule development, p. 207-212. In R. Palacios, J. Mora, and W. E. Newton (ed.), New horizons in nitrogen fixation. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 44.Reed, J. W., M. Capage, and G. C. Walker. 1991. Rhizobium meliloti exoG and exoJ mutations affect the exoX-exoY system for modulation of exopolysaccharide production. J. Bacteriol. 173:3776-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reed, J. W., J. Glazebrook, and G. C. Walker. 1991. The exoR gene of Rhizobium meliloti affects RNA levels of other exo genes but lacks homology to known transcriptional regulators. J. Bacteriol. 173:3789-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reed, J. W., and G. C. Walker. 1991. The exoD gene of Rhizobium meliloti encodes a novel function needed for alfalfa nodule invasion. J. Bacteriol. 173:664-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reitzer, L. 2003. Nitrogen assimilation and global regulation in Escherichia coli. Annu. Rev. Microbiol. 57:155-176. [DOI] [PubMed] [Google Scholar]
- 48.Rostas, K., E. Kondorosi, B. Horvath, A. Simoncsits, and A. Kondorosi. 1986. Conservation of extended promoter regions of nodulation genes in Rhizobium. Proc. Natl. Acad. Sci. USA 83:1757-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47:597-626. [DOI] [PubMed] [Google Scholar]
- 50.Schnier, J., M. Kitakawa, and K. Isono. 1986. The nucleotide sequence of an Escherichia coli chromosomal region containing the genes for ribosomal proteins S6, S18, L9 and an open reading frame. Mol. Gen. Genet. 204:126-132. [DOI] [PubMed] [Google Scholar]
- 51.Stougaard, J. 2001. Genetics and genomics of root symbiosis. Curr. Opin. Plant Biol. 4:328-335. [DOI] [PubMed] [Google Scholar]
- 52.Swanson, J. A., J. T. Mulligan, and S. R. Long. 1993. Regulation of syrM and nodD3 in Rhizobium meliloti. Genetics 134:435-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tauch, A., Z. Zheng, A. Puhler, and J. Kalinowski. 1998. Corynebacterium striatum chloramphenicol resistance transposon Tn5564: genetic organization and transposition in Corynebacterium glutamicum. Plasmid 40:126-139. [DOI] [PubMed] [Google Scholar]
- 54.Tawfi Qalkafaf, N. K., K. H. Yeoman, M. Wexler, H. Hussain, and A. W. Johnston. 1997. Analysis of a Rhizobium leguminosarum gene encoding a protein homologous to glutathione S-transferases. Microbiology 143:813-822. [DOI] [PubMed] [Google Scholar]
- 55.Timmers, A. C., M. C. Auriac, and G. Truchet. 1999. Refined analysis of early symbiotic steps of the Rhizobium-Medicago interaction in relationship with microtubular cytoskeleton rearrangements. Development (Cambridge) 126:3617-3628. [DOI] [PubMed] [Google Scholar]
- 56.Toledano, M. B., I. Kullik, F. Trinh, P. T. Baird, T. D. Schneider, and G. Storz. 1994. Redox-dependent shift of OxyR-DNA contacts along an extended DNA-binding site: a mechanism for differential promoter selection. Cell 78:897-909. [DOI] [PubMed] [Google Scholar]
- 57.van Rhijn, P., and J. Vanderleyden. 1995. The Rhizobium-plant symbiosis. Microbiol. Rev. 59:124-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang, S. P., J. B. Zhu, G. Q. Yu, Y. F. Wu, and S. J. Shen. 1991. Studies on heterologous expression of Rhizobium meliloti nifA gene and oxygen sensitivity of its product. Sci. China Ser. B 34:71-77. [PubMed] [Google Scholar]
- 59.Yeh, K. C., M. C. Peck, and S. R. Long. 2002. Luteolin and GroESL modulate in vitro activity of NodD. J. Bacteriol. 184:525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zevenhuizen, L. P. T. M., and A. R. W. vanNeerven. 1983. (1,2)-β-d-Glucan and acidic oligosaccharides produced by Rhizobium meliloti. Carbohydr. Res. 118:127-134. [Google Scholar]
- 61.Zhan, H., and J. A. Leigh. 1990. Two genes that regulate exopolysaccharide production in Rhizobium meliloti. J. Bacteriol. 172:5254-5259. [DOI] [PMC free article] [PubMed] [Google Scholar]