Abstract
Bacteriophage p2 belongs to the most prevalent lactococcal phage group (936) responsible for considerable losses in industrial production of cheese. Immunization of a llama with bacteriophage p2 led to higher titers of neutralizing heavy-chain antibodies (i.e., devoid of light chains) than of the classical type of immunoglobulins. A panel of p2-specific single-domain antibody fragments was obtained using phage display technology, from which a group of potent neutralizing antibodies were identified. The antigen bound by these antibodies was identified as a protein with a molecular mass of 30 kDa, homologous to open reading frame 18 (ORF18) of phage sk1, another 936-like phage for which the complete genomic sequence is available. By the use of immunoelectron microscopy, the protein is located at the tip of the tail of the phage particle. The addition of purified ORF18 protein to a bacterial culture suppressed phage infection. This result and the inhibition of cell lysis by anti-ORF18 protein antibodies support the conclusion that the ORF18 protein plays a crucial role in the interaction of bacteriophage p2 with the surface receptors of Lactococcus lactis.
Lactococcus lactis is a gram-positive lactic acid bacterium used for the manufacture of fermented dairy products (2). The milk fermentation process is susceptible to infection by bacteriophages found in raw milk (3, 19, 32-34) or by induction of prophages from lysogenic starter strains (19). The phage infection results in lysis of the bacteria, leading to production delays, variations in the taste and texture of the products, or even complete failure of fermentation. To minimize economic losses by phage infections, a variety of precautions are used (35, 36). Lactococcal phages fall into three prevalent groups of DNA homology, 936-, c2- and P335-like phages (32-34). Characteristics of these phages include a double-stranded DNA genome and a long noncontractile tail. The 936 and P335 groups have a small isometric head, while members of the c2 group have a prolate head.
We describe here the generation of phage-neutralizing monoclonal single-domain antibody fragments (VHH) derived from cameloid heavy-chain antibodies. In the blood of Camelidae, a high proportion of the immunoglobulins consists of homodimers of only heavy chains, devoid of light chains (17). As described in this and other papers, it is possible to elicit good immune response in camelids against complex protein mixtures, phages, or even whole organisms (26). Genes encoding VHH fragments that bind to these complex protein mixtures can be selected easily. In such libraries of binders, there is a high probability of finding VHHs that block essential biological processes, mainly because of the long CDR3, which can block active centers (27).
It was demonstrated that after immunization of a llama with lactococcal bacteriophage p2 (936 group) the fraction of heavy-chain antibodies contained about 10-fold higher neutralizing activity than conventional antibodies. We generated a phage display library (31, 39) from which binding and neutralizing single-domain fragments were selected. Nanomolar concentrations of one of these VHHs efficiently neutralized lactococcal bacteriophage p2 even in milk fermentation on a semi-industrial scale (28). Here we show that the antigen open reading frame 18 (ORF18) turned out to be a structural protein, and it was demonstrated that this protein is located at the tip of the phage tail. The methods developed and the knowledge generated by this study will lead ultimately to a better understanding of the molecular mechanism of phage-host interactions and to new effective ways to prevent viral infections by application of llama antibody fragments.
MATERIALS AND METHODS
Selection and screening of lactococcal bacteriophage-specific single-domain antibody fragments from a llama immune phage display library.
Phage p2 was purified, amplified, and concentrated as described previously (1). A llama was immunized at days 0, 30, 58, and 86 with 3 × 107 PFU of L. lactis bacteriophage p2 as described previously (10). The immune response was followed by titration of serum samples in an enzyme-linked immunosorbent assay (ELISA) with phage p2 coated at a titer of 1010 PFU/ml in phosphate-buffered saline (PBS) following the protocol described before (10).
Peripheral blood lymphocytes were isolated from a 150-ml blood sample, taken 7 days after the last immunization, via a Ficoll-Paque gradient yielding about 108 blood cells and comprising about 107 B cells. Total RNA (between 250 and 400 μg) was extracted (5) and used for the preparation of random primed cDNA (8), which served as the template for amplification of the VHH genes with oligonucleotide primers VH-2B, Lam-07 (priming to the short hinge region), and Lam-08 (long hinge specific) (10, 45). PCR was performed as described by De Haard and colleagues (8).
The amplified products were digested with PstI and NotI and cloned in phagemid vector pUR5068, which is identical to pHEN1 (21) but contains a hexahistidine tail for immobilized metal affinity chromatography (20) and a c-myc-derived tag for detection. Ligation and transformation were performed as described previously (8).
The rescue with helper phage VCS-M13 and polyethylene glycol precipitation was performed as described previously (30). Selections were done via the biopanning method (30) by coating of phage p2 (1010 PFU/ml at round 1 and 109 PFU/ml at round 2) or via the in-solution selection method (18, 46) with in vitro biotinylated phage (3 × 1011 PFU/ml at the first round and 3 × 1010 PFU/ml at the second round).
Soluble VHH was produced by individual clones as described previously (30). Culture supernatants were tested in ELISA using immobilized phage p2; bound VHH was detected with a mixture of the mouse anti-myc monoclonal antibody 9E10 (500 ng/ml) and anti-mouse horseradish peroxidase conjugate (DAKO; 4,000-fold diluted). Fingerprint analysis (46) with the restriction enzyme HinFI (New England Biolabs) was performed on all clones.
Production of soluble VHH fragments by inducing 50-ml cultures and preparation of periplasmic fractions, which were used for ELISA experiments, were done as described previously (8). DNA sequencing was performed at Baseclear B.V. (Leiden, The Netherlands).
Production of VHH fragments in Escherichia coli and Saccharomyces cerevisiae.
For large-scale production (400 ml) in E. coli, the VHH-encoding gene fragments were recloned via PstI/BstEII digestion in vector pUR5850 (Fig. 1A). This vector is identical to phagemid vector pUR5068 but lacks gene 3 and contains an additional carboxy-terminally located tag sequence of 15 amino acids, which encodes an in vivo biotinylation signal (41). Alternatively, an E. coli production vector was used encoding a different peptide sequence of five amino acid residues (TAG) recognized by a monoclonal antibody instead of the c-myc tag.
FIG. 1.
Plasmids used in this study. (A) Expression vector for large-scale production of VHH fragments in E. coli. PlacZ = IPTG-inducible promoter; pelB = signal sequence; myc and HIS6 encode tags; biot = biotinylation site. (B) Expression/integration vector used to transform S. cerevisiae and to express the various VHH fragments. Pgal7 = galactose-inducible promoter, SUC2 = invertase signal sequence, Tpgk = the PGK terminator. LEU2 is the LEU2 gene behind a defective promoter, which ensures, together with the 2 micron sequence, the multicopy integration of this vector on the rDNA locus.
After induction of VHH gene expression (8), a soluble protein fraction was prepared by disruption of cells with a French press using a volume of 3.5 ml of cell suspension in 0.1 mol/liter phosphate buffer (pH 7) in an FA-003 minicell at 20,000 lb/in2 (American Instrument Company). This process was followed by removal of the insoluble proteins via high-speed centrifugation (30 min at 13,000 × g at 4°C). The antibody fragments were purified from the lysate via their hexahistidine tail using Talon column material (Clontech).
For secretion by S. cerevisiae, the fragments were recloned in episomal vector pUR4547 (Fig. 1B), which is identical to previously described pUR4548 (10) but does not encode any tag sequences. The host strain used, VWK18gal1, was a gal1 derivative of CEN.PK102-3A (MATa leu2 ura3) obtained by disruption of the GAL1 gene by integration of the S. cerevisiae URA3 gene (40) at this locus. Production on a 0.5-liter (shake flasks) or a 10-liter scale was performed at BAC B.V. (Naarden, The Netherlands) as described previously (43). The VHH fragments were purified by ion-exchange chromatography with Mono-S-Sepharose (Pharmacia) after concentrating the culture supernatant by ultrafiltration. The purification yield was determined by measuring the optical density at 280 nm (OD280), using the molar extinction coefficients calculated from the encoded amino acid sequence (program ProtParam-tools at www.expasy.ch). The purity was analyzed on Coomassie-stained 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels.
Bihead molecules were produced in S. cerevisiae by introduction of an XhoI site (instead of PstI) in the FR1-encoded primer and cloning of the PCR product as an XhoI/BstEII fragment in an adapted version of episomal vector pUR4547. This vector, pJS9, allows the insertion of another VHH gene downstream of the first one via digestion with PstI/HindIII.
The avidity of the purified VHH fragments was analyzed by gel filtration using a Superose 12 column combined with mass spectroscopy using matrix-assisted laser desorption ionization-time of flight mass spectrometry. In addition, a bispecificity ELISA was performed according to the protocol described above using p2 phage as coating. Detection was accomplished with a mixture of in vitro biotinylated phage p2 (at 108 PFU/ml) and streptavidin-horseradish peroxidase conjugate (DAKO; 1,000-fold diluted).
Determination of neutralizing capacity with plaque titration and small- and large-scale neutralization assays.
Plaque titration was performed according to Terzaghi and Sandine (42). Indicator strains L. lactis subsp. cremoris LM0230 and C2 (7, 13) were used for titration of phages p2 and sk1.
The small-scale neutralization assay was performed by culturing L. lactis LM0230 or C2 (1% inoculum overnight culture) in microtiter plates using 100 μl of sterilized semiskim milk containing 0.35% peptone (Difco), 0.35% yeast extract (Difco), 1% glucose, 0.5% polymyxin B (Oxoid), 1% bromophenol red (Sigma), and 103 PFU/ml of phage p2 in the presence of variable concentrations of antibody fragments (in culture supernatant of E. coli, in llama sera, or as purified antibody fragments). After overnight incubation at 30°C, growth of the cells was visualized by the yellow color of the indicator, whereas a purple color indicated repressed growth due to lysis of the cells.
The large-scale assay was performed by culturing in 20 ml LM17 medium at 30°C containing variable titers of phage p2 and concentrations of purified VHH fragment or ORF18. The pH and OD600 of 1-ml samples were measured.
Characterization of recognized antigens.
For immunoelectron microscopy, 150-mesh nickel grids were coated with Formvar, followed by carbon evaporation. The grids, with their coated side up, were glow discharged for 30 s in air at a pressure of about 0.1 torr. Thereafter, the grids were put two times for 10 s with the coated side down on a droplet of distilled water, followed by floating for 10 min on a 20-μl droplet of phage solution in 100 mM PBS (pH 7.2) with a titer of 1010 PFU/ml. Excess phage suspension was removed with filter paper. All incubations were performed at room temperature. Antibody fragments VHH#2 and VHH#5 were diluted in 1% bovine serum albumin in PBS (BPBS) to a concentration of about 20 μg/ml and incubated for 15 min with the grids. After five washes for 1 min by floating on droplets of BPBS, the different grids were placed, sample side down, for 15 min on a 50-μl droplet of rabbit anti-VHH polyclonal antibody solution (50-fold dilution in BPBS). After washing with BPBS (as before), each grid was incubated for 15 min in a 50-μl droplet of goat anti-rabbit polyclonal immunoglobulin G antibodies conjugated to 10-nm gold particles (Aurion B.V., Wageningen, The Netherlands) diluted 10-fold in BPBS. After five washes by floating in droplets of distilled water, the labeled phages were negatively stained on a 100-μl droplet of 2% aqueous uranyl acetate solution for 1 min, followed by removal of the excess stain with filter paper. After drying at room temperature for 30 min, the labeled phages were examined with an EM420 transmission electron microscope (Philips) and micrographs were taken at an acceleration voltage of 80 kV.
Epitope mapping was performed with the lambda gt11 system using the method described by Mondelli and colleagues (37), in which random fragments were generated from the genomic DNA of phage p2 and cloned in lambda gt11 (Promega). For screening of the expression library, approximately 5 × 104 plaques per plate (14 cm diameter) were analyzed. The inserts from phage clones which bound to the VHH were amplified with gt11 forward and gt11 reverse primers (Promega) and cloned into the pGEM-T easy vector (Promega), which subsequently was sequenced with T7 and M13 reverse primers (Promega). The epitope recognized was localized further by the pepscan method (15) using overlapping 15-mer peptides derived from the major structural protein (msp) gene product from phage sk1 as described previously (4).
For amino-terminal sequencing, 1011 phage p2 particles were loaded on a 15% polyacrylamide electrophoresis gel and blotted on a polyvinylidene difluoride membrane (ProBlott; Perkin-Elmer). The blot was stained with Coomassie brilliant blue, the band of interest was excised, and the amino-terminal sequence determined with an LF-3000 Protein Sequencer (Beckman). The individual phenylthiohydantoin amino acid derivatives from the Edman degradation were monitored online and analyzed with a System Gold high-performance liquid chromatography system (Beckman).
Purification of phage p2-encoded gene products expressed in E. coli.
All the primers used were based on the genomic sequence of sk1 (4). The gene encoding the major structural protein (msp; ORF11) was amplified from phage p2 with mcp 5′ primer 5′-GTC CTC GCA ACT GCC GTC TCC CAT GAA ATT AGA TTA TAA TTC ACG TGA GAT-3′ and mcp 3′ primer 5′-GAG TCA TTC TCG ACT TGC GGC CGC TGA ATG GTC AGT TAC TGA AAC TCC TGC GGT-3′ using AmpliTaq Gold (Perkin-Elmer). The lysin gene (ORF20) was obtained by amplification with lys 5′ primer 5′-GTC CTC GCA ACT GCC GTC TCC CAT GAA TAT AAC TAA TGC TGG CGT-3′ and lys 3′ primer 5′-GAG TCA TTC TCG ACT TGC GGC CGC TTT TTT AGC AAT GAT TGG TTT GT-3′. Finally, ORF18 was amplified with ORF18 5′ primer 5′-GTC CTC GCA ACT GCC GTC TCC CAT GAC AAT TAA AAA CTT CAC GTT TTT CA-3′ and ORF18 3′ primer 5′-GAG TCA TTC TCG ACT TGC GGC CGC TTT AAT GAA GTA ACT TCC GTT ACC-3′. All 5′-end primers contain a BsmBI site (shown in bold characters), which enables cloning in the NcoI site of vector pET28a (Novagen), thereby creating the ATG start codon. The 3′-end primers have a NotI site (also in bold), which gives an in-frame fusion to the hexahistidine tail when ligated to the corresponding site of pET28a. The PCR products were purified from gel, digested with BsmBI and NotI, and after spin dialysis against water, ligated to NcoI/NotI-digested pET28a and electroporated into E. coli BL21-CodonPlus(DE3) cells (Novagen).
The ORF18-containing constructs were made from two individual PCR products and sequenced with the T7 and T7rev primers at BaseClear B.V. (Leiden, The Netherlands), thereby excluding errors introduced by amplification.
For production of the protein, transformants were grown at 37°C in 400 ml 2TY medium containing kanamycin (100 μg/ml) until a late-log-phase culture was obtained (OD600, approximately 0.9). The culture was induced by addition of isopropyl-β-d-thiogalactopyranoside (IPTG; 1 mM), and growth was continued for 4 h. Cells were harvested and disrupted in a French press as described previously. The hexahistidine-tagged proteins were purified from the soluble protein fraction with Talon (Clontech). Purity was analyzed on Coomassie-stained polyacrylamide electrophoresis gels.
Affinity measurements with surface plasmon resonance.
Binding kinetics were analyzed by surface plasmon resonance on a Biacore-3000 (Biacore) using a surface-containing anti-TAG monoclonal antibody (approximately 9,000 response units) covalently coupled to a CM5 chip (Biacore). A fixed amount of tagged antibody fragment VHH#5 was captured (120 and 300 response units), and a variable concentration of purified ORF18 (between 30 nM and 5 μM) was injected at a flow rate of 10 μl/min. For an accurate determination of the off rate, the low-density surfaces were used to avoid rebinding as could be concluded from the monophasic dissociation. The on rate was determined from those measurements, which showed no mass transport limitation.
RESULTS
Isolation of lactococcal bacteriophage p2-specific single-domain antibodies via phage display.
A llama was immunized with purified bacteriophage particles from L. lactis phage p2. The antigen-specific immune response was followed by ELISA. During these titrations, the total content of serum antibodies was measured and no discrimination was made between the response of the “classical” antibodies (i.e., containing a heavy and a light chain) and the heavy-chain antibodies. After 3 weeks, the sera contained high titers of anti-p2 antibodies.
An important objective of this study was to identify VHH fragments capable of preventing lactococcal phage infection by monovalent binding to bacteriophage particles. In addition to analyzing individual phage-binding antibody fragments in ELISA, a phage microtiter plate neutralization assay was used that permitted the analysis of large numbers of clones.
The performance of the assay was evaluated with immune sera from the llama. The serum taken after the fourth immunization had high titers of neutralizing antibodies, since complete inhibition of infection at a phage titer of 105 PFU/ml was obtained even at a serum dilution of 10−4. As expected, the preimmune serum did not show neutralization. The heavy-chain and classical double-chain antibodies were purified from the postimmune serum and tested in serial dilutions. The long-hinge-containing heavy-chain antibodies inhibited infection with phage titers of 105 PFU/ml at an antibody concentration of 620 ng/ml and the short-hinge heavy-chain antibodies at a concentration of 960 ng/ml. The classical antibodies were less efficient and gave only partial neutralization at 9.5 μg/ml. Following RNA isolation from B lymphocytes, the gene segments encoding the VHHs were amplified and cloned to obtain a phage display library with approximately 107 clones. After two rounds of biopanning or selection with in vitro biotinylated L. lactis phage p2, an increasing number of phage clones was eluted, indicating successful selection. We used both selection methods as antibodies with different binding characteristics are selected by these methods (46). Several hundred clones sampled from the unselected library and after both rounds of selections were screened in an ELISA for the production of p2-specific VHH fragments. A large fraction of clones bound p2 positive, increasing from 60% after round 1 to 95% after round 2, while no binding antibodies were found in 32 analyzed clones from the unselected library. Furthermore, many different phage p2-specific VHH-encoding clones were selected from the library, as demonstrated by HinFI fingerprint analysis (details not shown).
Identification of phage p2 neutralizing antibodies with acidification screenings assay.
The culture supernatants of the VHH-producing clones were tested in phage neutralization assays, which showed that 6 out of 250 (2.4%) antibody fragments analyzed inhibited phage infection completely and 36 out of 250 (14.4%) gave some degree of neutralization. It should be noted that the most potent neutralizing VHH fragments gave poor signals in the ELISA screening and would have been missed if only a limited number of ELISA-positive clones were analyzed with plaque-forming assays.
A panel of four nonneutralizing (VHH#1 to VHH#4) and three neutralizing (VHH#5 to VHH#7) antibody fragments with different HinFI fingerprint patterns was studied in more detail. The antibody-encoding genes were sequenced; whereas the nonneutralizing VHH fragments have divergent sequences, the neutralizing VHH fragments were very similar, indicating that they all recognized the same epitope (Fig.2). The genes encoding these VHH fragments were recloned in an episomal vector designed for secretion of VHHs by S. cerevisiae (10, 46) (Fig. 1B). The purified antibodies were diluted and tested in the small-scale acidification assay, in the plaque formation assay, and in the ELISA. None of the four nonneutralizing antibodies prevented infection of phage p2, although they have a good antigen-binding capacity. The three neutralizing antibodies completely inhibited phage infection (data not shown).
FIG. 2.
Amino acid sequences of nonneutralizing (A) and neutralizing (B) bacteriophage-specific VHH fragments. Residues are numbered in accordance with Kabat et al. (23). The primer-encoded hinge region (long hinge) is also shown.
Efficiency of neutralization of VHH#5 and cross-reactivity against other lactococcal phage groups.
The ability to neutralize phage was studied in more detail by regularly measuring the pH of small-scale cultures (20 ml). A fixed titer of phage (103 PFU/ml) was combined with one concentration (667 nM) of the antibody fragment. The acidification curve from the phage-infected culture containing neutralizing antibody fragment VHH#5 was indistinguishable from the noninfected control, thus showing the normal drop of pH from 6.6 to 4.6 after 10 h of cultivation. The infected culture combined with nonneutralizing fragments VHH#2 or VHH#3 showed no change in pH, indicating that most lactococcal cells were lysed as a consequence of phage infection (data not shown). Antibody fragment VHH#5 prevented phage infection, even at concentrations as low as 2.25 nM, as can be seen from the acidification and the cell density profiles of the cultures (Fig. 3A). Higher phage titers (>105 PFU/ml) could also be neutralized with higher concentrations of antibody (Fig. 3B).
FIG. 3.
Acidification profiles and growth kinetics of phage-infected cultures as a function of the concentration of VHH#5. (A) Determination of the minimal concentration of VHH to neutralize an infection of 103 PFU/ml phage p2 by measuring pH and OD600. (B) Growth kinetics determined with OD600 for a culture containing 105 PFU/ml phage p2 and variable amounts of VHH.
The cross-reactivity against other lactococcal phages was investigated with the microtiter plate acidification assay. Phage sk1, a 936-like phage, was neutralized by VHH#5 as efficiently as phage p2. Three nanomolar VHH#5 completely protected the L. lactis host cells against phage infection, whereas a 10-fold lower concentration (0.3 nM) failed to do so. Preimmune llama serum showed no inhibition, while postimmune serum also gave identical inhibition profiles for phages p2 and sk1 (phage neutralization at a serum dilution of 10−3, no inhibition at 10−4). However, two members of the c2 group, phages Q38 and c2, were not neutralized by VHH#5, although postimmune llama serum slightly inhibited infection at a 10-fold dilution.
Localization of recognized antigens with immunoelectron microscopy and analysis of fine specificity by epitope mapping.
Immunoelectron microscopy was performed to localize the proteins recognized by the different VHH fragments on the phage structure. Using immunogold labeling, it was shown that both nonneutralizing antibodies VHH#2 (Fig. 4A) and VHH#3 (not shown) bound to proteins located on the phage capsid. In contrast, neutralizing antibody VHH#5 recognizes a protein at the tip of the tail (Fig. 4B), which is the site involved in host recognition during the phage adsorption process.
FIG. 4.
Immunoelectron microscopy with heavy-chain antibody fragments and 10-nm gold-labeled detection antibodies on bacteriophage p2. (A) Phage detected with nonneutralizing VHH#2. (B) Phage recognized by neutralizing fragment VHH#5.
To identify the epitopes of the antibodies, a lambda gt11 expression library was constructed containing random genomic DNA fragments from phage p2 approximately 50 to 250 bp in length (37). Screening of approximately 100,000 lambda plaques with VHH#2 resulted in the identification of six positive clones. The inserts were subcloned, and sequencing demonstrated that the antibody recognizes the carboxy-terminal domain of the major structural protein (msp) of phage p2 (Fig. 5A). The epitope was localized further by pepscan analysis of the 79-amino-acid peptide shared by the six gt11 clones (Fig. 5B) and was shown to consists of the sequence NGQLAPGVYIVTFSA, corresponding to residues 271 to 285 of the msp. This result also demonstrated, for the first time, that the msp (ORF11) of lactococcal 936-like phages is the predominant protein of the capsid.
FIG. 5.
Epitope mapping of anti-msp antibody fragment VHH#2. (A) Inserts of the VHH#2-recognized lambda gt11 clones (coded #1 to #6), which encode a shared segment of the carboxy-terminal region of the major structural protein. Numbering has been done as in the deposited sk1 genomic sequence (GenBank accession number AF011378). The earlier observed differences (25) between the shown p2-derived sequence and the sk1 sequence are indicated with boxes. (B) Pepscan results obtained with overlapping 15-mer peptides based on the shared sequence segment of the lambda gt11 clones. The peak corresponds with the peptide having the sequence NGQLAPGVYIVTFSA and therefore represents the epitope of VHH#2.
Upon screening of the gt11 expression library with neutralizing antibody VHH#5, no binding clones were obtained, thereby prompting an alternative approach to identify the antigen.
Characterization of antigen recognized by neutralizing antibody by Western blot analysis and amino-terminal sequencing.
To exclude a mechanism of neutralization based on avid binding of multimerized antibody fragments leading to inactivation of phage by aggregation, the valency of purified VHH#5 was determined by gel filtration (16) and mass spectrometry (Fig. 6). Gel filtration revealed a molecular mass of 12.1 kDa and mass spectroscopy 13.5 kDa for VHH#5, suggesting that the antibody fragment indeed has a monomeric appearance. A bihead fragment (6) containing head-to-tail-linked VHH#3 and VHH#2 (3-2) emerged from the column in a single peak corresponding to the dimeric product (molecular mass of 26.6 kDa as determined with mass spectroscopy; data not shown). Furthermore, a bispecificity ELISA was developed in which phage p2 was coated to capture the bivalent antibody fragment that could be detected with in vitro biotinylated p2 phages. No response was obtained with VHH#5 when detection was performed with biotinylated p2 phages, while positive signals were found upon incubation with the anti-myc antibody, thereby confirming the monovalent character of this antibody fragment. The bihead molecule 3-2, which was used as a positive control in this assay, gave high signals with biotinylated p2 phage or anti-myc. From these experiments it was concluded that the neutralizing capacity of VHH#5 is not caused by aggregation of phage particles but that the nature of the phage-derived antigen recognized by the antibody is crucial.
FIG. 6.
Size exclusion chromatography of VHH#5. VHH#5 and a mixture of marker proteins were analyzed on a Superdex 75 column, and the measured OD214 was plotted against the retention time. The peaks of the marker proteins (13.7, 25, 43, 67, and 2,000 kDa) are shown.
The structural protein recognized by neutralizing antibody fragment VHH#5 was identified by Western blot analysis (Fig. 7). Here, phage sk1 was used instead of phage p2 because its complete genomic sequence is available (4). A clear band was visible on blot after incubation with VHH#5, and comparison with a Coomassie stained blot showed that the recognized antigen migrates somewhat faster than the msp. The amino terminus of the structural protein recognized by VHH#5 is identical to the hypothetical gene product of ORF18 of phage sk1. The molecular mass (30 kDa), as estimated from a gel, suggests the presence of the complete ORF18 product (containing 264 amino acid residues) in the bacteriophage particle.
FIG. 7.
Specificity of neutralizing VHH#5 determined by Western blot analysis. Phage sk1 was loaded (lane 1, 6 × 109 PFU; lane 2, 3 × 1010 PFU) onto a 15% gel, blotted, and incubated with VHH#5. The other part of the gel (lane 3, 1 × 1010 PFU; lane 4, 2 × 1010 PFU) was stained with Coomassie brilliant blue (CBB). The experimentally determined amino-terminal sequence (ThrIleLysAsnPheThrPhePheSerProAsnSerThrGluPhe; the expected methionine as start residue has been clipped off) of the recognized protein (arrows) corresponds to the hypothetical gene product of ORF18. MW, molecular mass.
To confirm the observed specificity the ORF18 protein, the major structural protein (msp) (ORF11) and lysin (ORF20) expressed in E. coli, as well as complete phage sk1 particles, were tested in Western blot assays with VHH#5 (Fig. 8). The neutralizing antibody VHH#5 indeed recognized bacterially expressed ORF18, which comigrated with the phage-bound protein. As a control, fragment VHH#2 was included in the experiment and as expected, this antibody reacted with the mcp produced in E. coli and the phage-associated antigen. In contrast, anti-mcp fragment VHH#3 also reacted with the bacterially expressed msp (containing the phage p2 gene) but failed to recognize the corresponding product of phage sk1 (data not shown).
FIG. 8.
Western blot analysis with phage p2-derived proteins expressed in E. coli or complete bacteriophage particles of sk1. The antibody fragments VHH#2 and VHH#5 and postimmune llama serum (llama pAB blot) were used for detection. Purified ORF18, msp, lysin (lys), and a bacterial extract of E. coli cells containing the expression vector used (−) and phage sk1 (Φ) were loaded. The hexahistidine-tagged proteins were detected with anti-His monoclonal antibody (α-His).
The affinity of VHH#5 to purified ORF18 was determined by analysis of the kinetics of the antibody-antigen interaction using surface plasmon resonance. The association rate ka was (3.49 ± 0.51) × 105 M−1 s−1, and the dissociation rate kd was (4.82 ± 1.09) × 10−4 s−1, resulting in a KD of (1.40 ± 0.21) × 10−9 M or 1.40 nM.
Competition assays with purified ORF18.
Since binding of an antibody fragment to ORF18 prevents infection, it was hypothesized that this gene product might have an important function during phage adsorption to the L. lactis host receptor. This hypothesis was evaluated by adding E. coli-produced ORF18 to phage-infected L. lactis cultures. We speculated that the added ORF18 would compete with the ORF18 bound to phage p2 particles for binding to the cellular receptor and thereby prevent phage infection. At relatively high concentrations of ORF18 (approximately 500 nM) and low titers of p2 phage (102 and 103 PFU/ml), phage infection of L. lactis cells was completely prevented, while at lower concentrations of ORF18, the phage infection process was only retarded (Fig. 9). This addition of purified mcp had no effect on phage infection (data not shown).
FIG. 9.
Effect of ORF18 protein on infection as measured by acidification and the kinetics of growth. (A) Two concentrations of ORF18 were included in cultures of L. lactis C2 containing 102 PFU/ml bacteriophage p2. Infected and noninfected cultures were used as controls, while purified msp was added to another infected culture. (B) Partial protection by addition of ORF18 against higher phage titers (103 PFU/ml) as measured by the kinetics of growth (graph on the left). The dose-dependent neutralizing effect of ORF18 on cultures with phage titers of 102 PFU/ml was studied in more detail with a new batch of purified ORF18 (growth curves on the right).
DISCUSSION
The vulnerability of lactic acid bacteria to phage attack still is the biggest problem associated with industrial manufacture of fermented dairy products (2). Phage p2 is a typical member of the most frequently isolated lactococcal phage group, while heavy-chain antibodies of Camelidae have a number of very interesting properties and applications (10, 26, 43, 44, 46). We decided to use these antibodies to develop a new powerful route to neutralize phage and simultaneously to elucidate unknown mechanisms of phage infection of lactic acid bacteria. Evaluation of the neutralizing capacity of antibodies purified from postimmune llama serum showed that the heavy-chain antibodies inhibited phage infections more efficiently than classical antibodies. Overall, these data indicate that camelid heavy-chain antibodies can eliminate viral infections efficiently, most probably due to the strong interaction between the unusual long CDR3 regions of heavy-chain antibodies and functional centers of proteins (9, 27). As has been found with enzyme inhibiting heavy-chain antibodies, the extended CDR3 loops might protrude into clefts or cavities present on the surface of viral or phage particles and block them (48, 49). A large panel of different p2-specific VHH fragments was selected from a phage display library and with a specially developed assay, a limited number of phage-neutralizing antibodies were identified.
Sequence analysis revealed that all the antibody fragments examined have the characteristics of the heavy-chain variable region of single-domain antibodies (47). The four analyzed ELISA-positive, but nonneutralizing, VHHs contain completely different sequences, and in particular, the variability seems to be localized in the CDRs. The length of the CDR3 is highly diverse, varying from 5 residues for VHH#2 to 15 residues for VHH#1 and VHH#4. Antibodies VHH#2 and VHH#3, which both react with the msp of p2, are also entirely different in sequence, suggesting that both fragments might recognize different epitopes. Indeed VHH#2 recognizes an epitope shared by phages p2 and sk1, while VHH#3 reacts with a distinct antigenic site, which exclusively occurs within the mcp of phage p2. Alignment of the amino acid sequences using previously published data (25) revealed that only four amino acid differences exist between the msp of phage p2 and sk1. Two of these changes are separated by only nine residues, thereby making this region a prominent candidate for the epitope of VHH#3. This area is located 15 residues upstream of the epitope for VHH#2.
The three analyzed neutralizing antibodies VHH#5, VHH#6, and VHH#7 are less variable in sequence and have a CDR3 of the same length (14 amino acids), with only a few differing residues within this important region. This indicates that this group of antibodies recognizes the same antigen and probably also an identical epitope.
The phage-inhibiting capacity of the antibody fragment turned out to be dependent on the titer of the phage contamination. When titers of 103 to 105 PFU/ml were tested, which in a production plant would result in failed fermentation, an antibody concentration as low as 2.25 nM gave complete neutralization. This corresponds well to the measured affinity (KD = 1.40 nM) of the antibody fragment. This means that the proportion of antigen not bound by antibodies must be rather high. This high efficiency may be explained in terms of competition between antibody (with a high affinity for the phage) and cell-bound receptor (with a lower affinity) for binding to phage particles. The antibody concentration used, 2 nM, corresponds to 1012 molecules/ml. From Western blot data, we estimate that 10 copies of ORF18 protein are present per phage particle, which implies 104 to 106 ORF18 molecules per ml, while 1012 VHH molecules are present with a high affinity (1.4 nM) for ORF18, whereas only about 1010 ORF18 receptor molecules are present per ml of bacterial cultures, with a moderate affinity for ORF18 (a few hundred nanomolar).
The antibody fragment prevents infection with the only other 936-like phage examined (sk1) but failed to neutralize two members of the c2 group, which is the second most prevalent lactococcal phage group and is genetically distinct from the 936-like phages. This indicates that VHH#5 will only protect against 936-like phages. The efficacy of fragment VHH#5 was evaluated in cheese production experiments using a 200-liter vessel (28). The results were in line with those described for the small-scale cultures; addition of VHH#5 to a final concentration of 7 or 70 nM completely neutralized phage infections of 103 to 105 PFU/ml, and acidification profiles identical to those of noninfected control were obtained. Using the above-determined values for interactions and lysis and a set of differential equations, we mimicked cell lysis by phages in the presence and absence of VHH#5 and ORF18 at various concentrations of L. lactis cells. Depending on the number of ORF18 proteins per phage cell, lysis can be predicted reasonably well (data not shown).
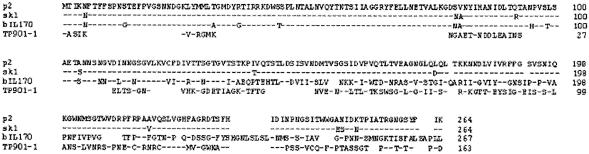
It was demonstrated that antibody fragment VHH#5 recognizes the ORF18-encoded gene product, for which no function was known (4). The antigen is a constituent of the tip of the phage tail, as shown by immunoelectron microscopic studies. FASTA analysis with the amino acid sequence of ORF18 gave the expected hits with the corresponding gene product of phage sk1 and the gene 120 protein from Lactococcus phage bIL170, which both belong to the 936 group.
Interestingly, some similarity was found with the baseplate protein (orf bpp) from the temperate lactococcal bacteriophage TP901-1, a P335-like phage (38) (Fig. 10). The homology with orf bpp was found within the carboxy-terminal domain of the gene product, of which the localization within the phage particle was established by immunogold labeling (22). Comparison of the late regions of phages sk1 and lambda by gene size and the predicted isoelectric points of their ORFs (4) previously hinted toward a relationship of ORF18 with phage lambda gene K, which belongs to the cluster of tail genes.
FIG. 10.
Alignment of ORF18-encoded amino acid sequences of 936 bacteriophages p2, sk1, and bIL170. FASTA analysis revealed homology with the baseplate protein from temperate phage TP901-1 (residues 53 to 163).
This conclusion is in agreement with the proposed function of orf bpp from phage TP901-1. Knocking out almost the complete gene of orf bpp in the lysogenic phage resulted in the production of noninfectious phage particles (38). The authors mentioned the possibility that this might be achieved by binding of orf bpp directly to the cells or indirectly by binding with other proteins involved in the phage-host interaction. However, our competition experiments strongly suggest a direct interaction.
The L. lactis-encoded receptor (pip) for c2-like phages has been identified (7, 12-14) and was shown not to be responsible for the entrance of 936-like (11) or P335-like (24) phages. During FASTA analysis, no homology of ORF18 of phage p2 was found with genes from phage c2 (29). Since phage c2 binds to another type of receptor, it can be assumed that the receptor-binding proteins of phage c2 and p2 are different, explaining why VHH#5 does not neutralize c2 infections. As a potential function of ORF18 has now been elucidated, this protein can be used for the characterization of a Lactococcus receptor, which is most likely to be a cell surface glycoprotein.
During this study, we have also proven that camelid antibodies can be raised against proteins of a complete biological entity, which opens the opportunity to develop protein arrays based on these antibodies (26). Our electron microscopy studies showed that VHHs can be used to study protein complexes in biological systems. Moreover, based on the knowledge gained and the technologies developed in this study, the development of VHHs that neutralize other prokaryotic or eukaryotic viruses can be envisioned.
ADDENDUM
Following acceptance of the manuscript, Dupont et al. (K. Dupont, F. K. Vogensen, H. Neve, J. Bresciani, and J. Josephsen, Appl. Environ. Microbiol. 70: 5818-5828, 2004) also showed that ORF18 is the receptor-binding protein of 936-like lactococcal phages.
Acknowledgments
We are grateful to S. Muyldermans (Vrije Universiteit Brussels, Belgium) for useful suggestions concerning the experimental work. We are grateful to N. van Riel (Technical University of Eindhoven) for developing a model for cell lysis and to D. Tremblay and I. Boucher for technical assistance in lactococcal phage purification. We thank W. Bos, C. Bulkmans, J. van Heemskerk, P. Hermans, I. Schaffers, and C. van Vliet for technical assistance and J. Chapman, M. van Egmond, and M. van der Vaart, all from Unilever Research Laboratories Vlaardingen, for discussions.
S. Moineau was supported by the Natural Sciences and Engineering Research Council of Canada.
REFERENCES
- 1.Azaïez, S. R. C., I. Fliss, R. E. Simard, and S. Moineau. 1998. Monoclonal antibodies raised against native major capsid proteins of lactococcal c2-like bacteriophages. Appl. Environ. Microbiol. 64:4255-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bissonnette, F., S. Labrie, H. Deveau, M. Lamoureux, and S. Moineau. 2000. Characterization of mesophilic mixed starter cultures used for the manufacture of aged cheddar cheese. J. Dairy Sci. 83:620-627. [DOI] [PubMed] [Google Scholar]
- 3.Bruttin, A., F. Desiere, N. d'Amico, J.-P. Guérin, J. Sidoti, B. Huni, S. Lucchini, and H. Brüssow. 1997. Molecular ecology of Streptococcus thermophilus bacteriophage infections in a cheese factory. Appl. Environ. Microbiol. 63:3144-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandry, P. S., S. C. Moore, J. D. Boyce, B. E. Davidson, and A. J. Hillier. 1997. Analysis of the DNA sequence, gene expression, origin of replication and modular structure of the Lactococcus lactis lytic bacteriophage sk1. Mol. Microbiol. 26:49-64. [DOI] [PubMed] [Google Scholar]
- 5.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 6.Conrath, K. E., M. Lauwereys, L. Wyns, and S. Muyldermans. 2001. Camel single-domain antibodies as modular building units in bispecific and bivalent antibody constructs. J. Biol. Chem. 276:7346-7350. [DOI] [PubMed] [Google Scholar]
- 7.Cords, B. R., and L. L. McKay. 1974. Characterization of lactose-fermenting revertants from lactose-negative Streptococcus lactis C2 mutants. J. Bacteriol. 119:830-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Haard, H. J., N. van Neer, A. Reurs, S. E. Hufton, R. C. Roovers, P. Henderikx, A. P. de Bruine, J. W. Arends, and H. R. Hoogenboom. 1999. A large non-immunised human Fab fragment phage library that permits rapid isolation and kinetic analysis of high affinity antibodies. J. Biol. Chem. 274:18218-18230. [DOI] [PubMed] [Google Scholar]
- 9.Desmyter, A., T. R. Transue, M. A. Ghahroudi, M. H. Thi, F. Poortmans, R. Hamers, S. Muyldermans, and L. Wyns. 1996. Crystal structure of a camel single-domain VH antibody fragment in complex with lysozyme. Nat. Struct. Biol. 3:803-811. [DOI] [PubMed] [Google Scholar]
- 10.Frenken, L. G., R. H. van der Linden, P. W. Hermans, J. W. Bos, R. C. Ruuls, B. de Geus, and C. T. Verrips. 2000. Isolation of antigen specific llama VHH antibody fragments and their high level secretion by Saccharomyces cerevisiae. J. Biotechnol. 78:11-21. [DOI] [PubMed] [Google Scholar]
- 11.Garbutt, K. C., J. Kraus, and B. L. Geller. 1997. Bacteriophage resistance in Lactococcus lactis engineered by replacement of a gene for a bacteriophage receptor. J. Dairy Sci. 80:1512-1519. [Google Scholar]
- 12.Garvey, P., D. van Sinderen, D. P. Twomey, C. Hill, and G. F. Fitzgerald. 1995. Molecular genetics of bacteriophages and natural defense systems in the genus Lactococcus. Int. Dairy J. 5:905-947. [Google Scholar]
- 13.Geller, B. L., R. G. Ivey, J. E. Trempy, and B. Hettinger-Smith. 1993. Cloning of a chromosomal gene required for phage infection of Lactococcus lactis subsp. lactis C2. J. Bacteriol. 175:5510-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geller, B. L., N. Wade, T. D. Gilberts, D. E. Hruby, R. Johanson, and L. Topisirovic. 2001. Surface expression of the conserved C repeat region of streptococcal M6 protein within the Pip bacteriophage receptor of Lactococcus lactis. Appl. Environ. Mircobiol. 67:5370-5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geysen, H. M., R. H. Meloen, and S. J. Barteling. 1984. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc. Natl. Acad. Sci. USA 81:3998-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffiths, A. D., S. C. Williams, O. Hartley, I. M. Tomlinson, P. Waterhouse, W. L. Crosby, R. Kontermann, P. T. Jones, N. M. Low, T. J. Allison, T. D. Prospero, H. R. Hoogenboom, A. Nissim, J. P. L. Cox, J. L. Harrison, M. Zaccolo, E. Gherardi, and G. Winter. 1994. Isolation of high affinity human antibodies directly from large synthetic repertoires. EMBO J. 13:3245-3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamers-Casterman, C., T. Atarhouch, S. Muyldermans, G. Robinson, C. Hamers, E. B. Songa, N. Bendahman, and R. Hamers. 1993. Naturally occurring antibodies devoid of light chains. Nature 363:446-448. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins, R. E., S. J. Russell, and G. Winter. 1992. Selection of phage antibodies by binding affinity. Mimicking affinity maturation. J. Mol. Biol. 226:889-896. [DOI] [PubMed] [Google Scholar]
- 19.Heap, H. A., and R. C. Lawrence. 1977. The contribution of starter strains to the level of phage infection in a commercial cheese factory. N. Z. J. Dairy Sci. Technol. 12:213-218. [Google Scholar]
- 20.Hochuli, E., W. Bannwarth, H. Döbeli, R. Gentz, and D. Stüber. 1988. Genetic approach to facilitate purification of recombinant proteins with a novel metal chelate adsorbent. Bio/Technology 6:1321-1325. [Google Scholar]
- 21.Hoogenboom, H. R., A. D. Griffiths, K. S. Johnson, D. J. Chiswell, P. Hudson, and G. Winter. 1991. Multi-subunit proteins on the surface of filamentous phage: methodologies for displaying antibody (Fab) heavy and light chains. Nucleic Acids Res. 19:4133-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnsen, M. G., H. Neve, F. K. Vogensen, and K. Hammer. 1995. Virion positions and relationships of lactococcal temperate bacteriophage TP901-1 proteins. Virology 212:595-606. [DOI] [PubMed] [Google Scholar]
- 23.Kabat, E. A., T. T. Wu, H. M. Perry, K. S. Gottesman, and C. Foeller. 1991. Sequences of proteins of immunological interest, 5th ed. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, Bethesda, Md.
- 24.Kraus, J., and B. L. Geller. 1998. Membrane receptor for prolate phages is not required for infection of Lactococcus lactis by small or large isometric phages. J. Dairy Sci. 81:2329-2335. [Google Scholar]
- 25.Labrie, S., and S. Moineau. 2000. Multiplex PCR for detection and identification of lactococcal bacteriophages. Appl. Environ. Microbiol. 66:987-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landa, I. 2004. Immune responses and antibody selection against complex protein mixtures. Ph.D. thesis. Utrecht University, Utrecht, The Netherlands.
- 27.Lauwereys, M., M. Arbabi Ghahroudi, A. Desmyter, J. Kinne, W. Hölzer, E. de Genst, L. Wyns, and S. Muyldermans. 1998. Potent enzyme inhibitors derived from dromedary heavy-chain antibodies. EMBO J. 17:3512-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ledeboer, A. M., S. Bezemer, J. J. de Haard, I. M. Schaffers, C. T. Verrips, C. van Vliet, E. M. Dusterhoft, P. Zoon, S. Moineau, and L. G. Frenken. 2002. Preventing phage lysis of Lactococcus lactis in cheese production using a neutralising heavy-chain antibody fragment from llama. J. Dairy Sci. 85:1376-1382. [DOI] [PubMed] [Google Scholar]
- 29.Lubbers, M. W., N. R. Waterfield, T. P. J. Beresford, R. W. F. Le Page, and A. W. Jarvis. 1995. Sequencing and analysis of the prolate-headed lactococcal bacteriophage c2 genome and identification of the structural genes. Appl. Environ. Microbiol. 61:4348-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marks, J. D., H. R. Hoogenboom, T. P. Bonnert, J. McCafferty, A. D. Griffiths, and G. Winter. 1991. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J. Mol. Biol. 222:581-597. [DOI] [PubMed] [Google Scholar]
- 31.McCafferty, J., A. D. Griffiths, G. Winter, and D. J. Chiswell. 1990. Phage antibodies: filamentous phage displaying antibody variable domains. Nature 348:552-554. [DOI] [PubMed] [Google Scholar]
- 32.McIntyre, K., H. A. Heap, G. P. Davey, and G. K. Y. Limsowtin. 1991. The distribution of lactococcal bacteriophage in the environment of a cheese manufacturing plant. Int. Dairy J. 1:183-197. [Google Scholar]
- 33.Moineau, S., M. Borkaev, B. J. Holler, S. A. Walker, J. K. Kondo, E. R. Vedamuthu, and P. A. Vandenbergh. 1996. Isolation and characterization of lactococcal bacteriophages from cultured buttermilk plants in the United States. J. Dairy Sci. 79:2104-2111. [Google Scholar]
- 34.Moineau, S., J. Fortier, H. W. Ackermann, and S. Pandian. 1992. Characterization of lactococcal phages from Québec cheese plants. Can. J. Microbiol. 38:875-882. [Google Scholar]
- 35.Moineau, S. 1999. Applications of phage resistance in lactic acid bacteria. Antonie Leeuwenhoek 76:377-382. [PubMed] [Google Scholar]
- 36.Moineau, S., D. Tremblay, and S. Labrie. 2002. Phages of lactic acid bacteria: from genomics to industrial applications. ASM News 68:388-393. [Google Scholar]
- 37.Mondelli, M. U., A. Cerino, P. Boender, P. Oudshoorn, J. Middeldorp, C. Fipaldini, N. La Monica, and W. Habets. 1994. Significance of the immune response to a major, conformational B-cell epitope on the hepatitis C virus NS3 region defined by a human monoclonal antibody. J. Virol. 68:4829-4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pedersen, M., S. Ostergaard, J. Bresciani, and F. K. Vogensen. 2000. Mutational analysis of two structural genes of the temperate lactococcal bacteriophage TP901-1 involved in tail length determination and baseplate assembly. Virology 276:315-328. [DOI] [PubMed] [Google Scholar]
- 39.Persson, M. A., R. H. Caothien, and D. R. Burton. 1991. Generation of diverse high-affinity human monoclonal antibodies by repertoire cloning. Proc. Natl. Acad. Sci. USA 88:2432-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rothstein, R. J. 1983. One-step gene disruption in yeast. Methods Enzymol. 101:202-211. [DOI] [PubMed] [Google Scholar]
- 41.Schatz, P. J. 1993. Use of peptide libraries to map the substrate specificity of a peptide-modifying enzyme: a 13 residue consensus peptide specifies biotinylation in Escherichia coli. Biotechnology (New York) 11:1138-1143. [DOI] [PubMed] [Google Scholar]
- 42.Terzaghi, B. E., and W. E. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomassen, Y. E., W. Meijer, L. Sierkstra, and C. T. Verrips. 2002. Large scale production of VHH antibody fragments by Saccharomyces cerevisiae. Enzyme Microb. Technol. 30:273-278. [Google Scholar]
- 44.Van der Linden, R. H. J., L. G. J. Frenken, B. de Geus, M. M. Harmsen, R. C. Ruuls, W. Stok, L. de Ron, S. Wilson, P. Davis, and C. T. Verrips. 1999. Comparison of physical chemical properties of llama VHH antibody fragments and mouse monoclonal antibodies. Biochim. Biophys. Acta 1431:37-46. [DOI] [PubMed] [Google Scholar]
- 45.Van Koningsbruggen, S., J. J. de Haard, P. Kievit, R. W. Dirks, A. van Remoortere, A. J. Groot, B. G. van Engelen, J. T. den Dunnen, C. T. Verrips, R. R. Frants, and S. M. van der Maarel. 2003. Llama-derived phage display antibodies in the dissection of the human disease oculopharyngeal muscular dystrophy. J. Immunol. Methods 279:149-161. [DOI] [PubMed] [Google Scholar]
- 46.Verheesen, P., M. R. ten Haaft, N. Lindner, C. T. Verrips, and J. J. de Haard. 2003. Beneficial properties of single domain antibody fragments for application in immunoaffinity purification and immuno-perfusion chromatography. Biochim. Biophys. Acta 1624:21-28. [DOI] [PubMed] [Google Scholar]
- 47.Vu, K. B., M. A. Ghahroudi, L. Wyns, and S. Muyldermans. 1997. Comparison of llama VH sequences from conventional and heavy chain antibodies. Mol. Immunol. 34:1121-1131. [DOI] [PubMed] [Google Scholar]
- 48.Ward, E. S., D. Gussow, A. D. Griffiths, P. T. Jones, and G. Winter. 1989. Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature 341:544-546. [DOI] [PubMed] [Google Scholar]
- 49.Weis, W., J. H. Brown, S. Cusack, J. C. Paulson, J. J. Skehel, and D. C. Wiley. 1988. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature 333:426-431. [DOI] [PubMed] [Google Scholar]