Abstract
Several bacterial pathogens have evolved the means to escape immune detection by mimicking host cell surface carbohydrates that are crucial for self/non-self recognition. Sialic acid, a terminal residue on these carbohydrates, inhibits activation of the alternate pathway of complement by recruiting the immune modulating molecule factors H, I, and iC3b. Sialylation of capsular polysaccharide (CPS) is important for virulence of group B streptococci (GBS), a significant human pathogen. We previously reported that cpsK, a gene within the cps locus of type III GBS, could complement a sialyltransferase deficient lst mutant of Haemophilus ducreyi, implicating its role in sialylation of the GBS capsule. To explore the function of cpsK in GBS capsule production, we created a mutant in cpsK. Immunoblot analysis and enzyme-linked immunosorbent assay using anti-type III CPS antisera demonstrated that the mutant CPS did not contain sialic acid. This was confirmed by high-performance liquid chromatography after mild acid hydrolysis of the CPS. Although increased CPS chain length was seen for this strain, CPS production was <20% of the parental isolate. An episomal cpsK copy restored synthesis of sialo-CPS to wild-type levels. These data support our hypothesis that cpsK encodes the GBS CPS sialyltransferase and provide further evidence that lack of CPS oligosaccharide sialylation reduces the amount of CPS expressed on the cell surface. These observations also imply that one or more of the components involved in synthesis or transport of oligosaccharide repeating units requires a sialo-oligosaccharide for complete activity.
Streptococcus agalactiae (group B streptococci or GBS) remains a major cause of serious neonatal bacterial infection in the developed world despite a >65% reduction in early-onset GBS disease (infection within the first week of life) due to the advent of chemoprophylactic prevention measures (37). The GBS capsular polysaccharide (CPS) is well established as a primary virulence determinant in GBS pathogenesis (16). Nine serotypes of GBS have been identified based on their unique CPS antigens (serotypes Ia and Ib and types II through VIII). The individual serotypes arise from the synthesis of distinct CPS precursor oligosaccharide repeating units (RPUs) and/or differences in the way the CPS RPUs are polymerized. The cps loci in each serotype are organized similarly with genes in the 5′ region involved in regulation and chain length, a 3′ region encoding sialic acid synthesis, and a central region containing the oligosaccharide RPU structural and polymerization genes (Fig. 1). Despite significant heterogeneity in the central structural region of their cps loci, all GBS serotypes produce RPUs with side chains terminated by N-acetylneuraminic acid (sialic acid; Neu5Ac) α2,3 linked to galactose (Gal).
FIG. 1.
Organization of the Streptococcus agalactiae serotype III CPS synthesis operon. Proposed functions of the cps gene products are indicated at the top of the diagram. Locations of the Tn916ΔE transposon and km-Ω-2 cassette insertions in strains COH1-11 (black triangle), COH1-13 (open triangle), and COH1-355 (shaded triangle) are indicated. The site of the chloramphenicol acetyltransferase allelic exchange replacement of cpsK in COH1-350 and the cps locus expressed in pLM104 are also indicated. The cps gene designations are indicated below the arrows representing each open reading frame. The locations of the cpsK and cpsL probes for Northern dot blot analysis are shown as grey or open bars, respectively, above the schematic of the cps operon.
Several bacterial species produce sialylated glycoconjugates on their surfaces. A number of gram-negative species, including Salmonella enterica, Neisseria meningitidis, Neisseria gonorrhoeae, Haemophilus influenzae, Haemophilus ducreyi, and Escherichia coli, are capable of producing sialylated forms of lipopolysaccharides (LPS) or lipooligosaccharides (LOS) (42). Certain serotypes of E. coli and N. meningitidis also produce capsules of homopolymeric polysialic acid (39). For gram-positive bacteria, only GBS and Streptococcus suis produce sialylated polysaccharides (in the form of CPS).
The genes responsible for the addition of sialic acid to prokaryotic glycoconjugates can be assigned to three separate families (39). The first group consists of the processive polymerases that produce sialic acid homopolymers on E. coli K1 and K92 and N. meningitidis serotypes B and C. These sialyltransferases are unrelated to those responsible for sialylation of LOS, LPS, and CPS synthesized by the block-type (Wzy-dependent) processes. The transferases of the latter type have been separated into two unrelated families, one composed of sialyltransferases from H. ducreyi, H. influenzae, E. coli O104, GBS, and S. suis (pfam05855.3 and Lst), and the other family which contains the Neisseria LOS sialyltransferases.
Variants of the above species lacking sialylated LPS, LOS, or CPS are generally less virulent, and a role for sialo-LOS and LPS in serum resistance and antiphagocytic activity has been demonstrated for N. gonorrhoeae and N. meningitidis (30, 44). Loss of CPS sialylation or capsule production by GBS with animal models has been correlated with decreased virulence (47, 36, 35).
It has been proposed that the presentation on the GBS cell surface of the sialylated terminal CPS RPU disaccharide, α-d-Neup5Ac(2→3)β-d-Galp, enables immune evasion through molecular mimicry (5, 24). This speculation is supported by the fact that a number of host glycoconjugates mediating self/non-self recognition, such as the blood group antigen sialyl-Lewisx and glycosphingolipids, are terminated with sialolactosamine [α-d-Neup5Ac(2→3)β-d-Galp(1→4)β-d-GlcpNAc]. Sialylation of GBS CPS has been shown to be critical for prevention of opsonophagocytosis (36, 49) through inhibition of alternative complement pathway activation (29). Since CPS plays an essential role in GBS virulence, an understanding of the mechanism of CPS sialylation is important for understanding GBS pathogenesis. We have shown that cpsK of serotype III GBS exhibited sialyltransferase activity when expressed in trans in a sialyltransferase mutant of H. ducreyi (8). This was not confirmed, however, for GBS. In this report, we explore the role of the cpsK in GBS CPS sialylation, polymerization, and production through creation of a nonpolar GBS cpsK allelic exchange mutant and complementation of the mutation in trans. Our results confirm that CpsK is the GBS sialyltransferase and provide additional insights into the effect of sialylation on CPS synthesis.
MATERIALS AND METHODS
Bacterial strains and media.
Bacterial strains and plasmids used in this work are listed in Table 1. E. coli strains were grown in Luria-Bertani broth (LB; Sigma-Aldrich, St. Louis, MO) and either 100 μg/ml ampicillin (Amp), 5 μg/ml chloramphenicol (Cm), 50 μg/ml kanamycin (Km), 100 μg/ml spectinomycin (Sp), or 400 μg/ml erythromycin (Erm) (Sigma). Strains were grown at 37°C or at 30°C for the temperature-sensitive plasmid pHY304. GBS strains were grown in Todd-Hewitt broth (THB; Difco, Becton Dickinson and Co., Sparks, MD) at 37°C or 30°C with antibiotic selection of 5 μg/ml (Cm), 1,000 μg/ml (Km), 100 μg/ml (Sp), or 5 μg/ml (Erm) as required.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype and/or phenotypea | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| MC1061 | F′ araD139 Δ(ara-leu)7696 Δ(lac)X74 galU galK hsdR2(rK− mK+) mcrB1 rpsL (Strr) | 45 |
| DH5α | F′ endA (rK− mK+) supE44 thi-1 hsdR17 gyrA (Nalr) recA1 Δ(lacZYA-orgF)U169 dcoR relA1 φ80Δlac(lacZ)M15 | 50 |
| XL1-Blue | F′::Tn10 proA+B+ lacIq Δ(lacZ)M15 recA1 endA1 gyrA96 (Nalr) thi-1 hsdR17(rK− mK+) glnV44 supE44 relA1 lac | Stratagene |
| S. agalactiae | ||
| COH1 | Wild type; serotype III; Tetr | 28 |
| COH1-11 | COH1; neuA::Tn916ΔE asialo-CPS -Tetr Ermr | 47 |
| COH1-13 | COH1; cpsE::Tn916ΔE CPS− Tetr Ermr | 35 |
| COH1-350 | COH1; ΔcpsK Tetr Cmr | This study |
| COH1-350(pLM104) | COH1; ΔcpsK pBL26 cpsK recombinant plasmid; Tetr Cmr CPS+ | This study |
| COH1-355 | COH1; cpsL::km-Ω-2 Tetr Kmr | This study |
| S. pneumoniae | ||
| NCTC11902 | Wild type; serotype 14 | 27 |
| Plasmids | ||
| pBlueScript II KS(+) | f1(+) ori, ColE1 ori; lacZα Apr; 3.0 kb | Stratagene |
| pGEM T-Easy | f1(+) ori, ColE1 ori; lacZα Apr; 3.0 kb | Promega |
| pLZ12spec | 3.7 kb; Spr | 22 |
| pBS9.0 | cpsIJKL neuBCDA; pBS derivative; Apr; 11.8 kb | 9 |
| pDC143 | cpsJKL neuB; pBlueScript II KS(+) with 5.4-kb EcoRI/NsiI fragment of pBS9.0; Apr; 8.3 kb | This study |
| pDC123 | phoZ cat; gram-positive/negative blue-white screening expression vector; Cmr; 4.5 kb | 9 |
| pDC147 | cpsJ cat cpsL neuB ΔcpsK; pDC143 with exact allelic exchange cpsK replacement with cat; Apr Cmr; 8.0 kb | This study |
| pHY304 | ori[Ts] lacZα; pBlueScript; Ermr; 4.5 kb | 26 |
| pLM103 | ori[Ts] cat; 3.9-kb pDC147 ΔcpsK amplicon cloned into pHY304 EcoRV T-vector site; Ermr Cmr; 8.5 kb | This study |
| pMut3 | gfp(mut3) Apr | 13 |
| pBL-gfp(mut3) | gfp(mut3) Spr; 4.3 kb | This study |
| pBL26 | ori[pAMβ1] gfp(mut3) cps-promoter; pLZ12spec derivative; Spr; 4.5 kb | This study |
| pLM104 | cpsK; 1.7-kb cpsK amplicon from serotype III GBS strain COH1 cloned into NcoI/HindIII-restricted pBL26; Ermr 5.6 kb | This study |
| pDC132 | cpsL; 1.9-kb cpsL amplicon from serotype III GBS strain COH1 cloned into pGEM T-Easy; Apr; 4.9 kb | This study |
| pDC150 | cpsL::BamHI; BamHI site insertion into SmaI site of pDC132; Apr; 4.9 kb | This study |
| pCIV2 | km-Ω-2; Kanr; 5.9 kb | 32 |
| pDC151 | cpsL::km-Ω-2; 2.2-kb pCIV2 km-Ω-2-containing amplicon cloned into BamHI-digested pDC150; Apr Kanr; 7.2 kb | This study |
| pDC152 | cpsL::km-Ω-2; ori[Ts]; 4.2-kb pDC151 cpsL::km-Ω-2-containing amplicon ligated to pHY304 EcoRV T-vector site; Kanr Ermr; 8.7 kb | This study |
Tetr, tetracycline resistance; Ermr, erythromycin resistance; Apr, ampicillin resistance; Cmr, chloramphenicol resistance; Kanr, kanamycin resistance; Spr, spectinomycin resistance; CPS−, capsule negative; CPS+, capsule positive; Ts, temperature sensitive.
Recombinant DNA procedures.
Restriction enzymes were from New England Biolabs (Beverly, MA). Taq DNA polymerase was obtained from Bioline (Biolase; Bioline USA, Randolph, MA). For high-fidelity DNA amplification, Expand Taq polymerase was used (Roche Applied Science, Indianapolis, IN). Other DNA modifying enzymes were from Promega (Madison, WI).
To derive a chromosomal chloramphenicol acetyltransferase gene (cat) replacement of cpsK within GBS strain COH1, pBS9.0, a genomic clone containing 9.0 kb of the COH1 cps locus, was digested with EcoRI and NsiI, and the 5.4-kb fragment containing cpsK was ligated to EcoRI/PstI restricted pBlueScript II KS(+) (Stratagene, La Jolla, CA) to form pDC143. After electroporation of the ligation mixture into E. coli DH5α, clones harboring pDC143 [DH5α(pDC143)] were selected on LB agar (LA) containing Amp. The plasmid identity was confirmed by PCR amplification and restriction enzyme digestion. A cat replacement cassette was created by amplification of cat from pDC123 using primers CG001 and CG002 (primers are listed in Table 2). The primers are complementary to the 5′ and 3′ ends of cat but have 24-bp 5′ extensions complementary to the 3′ end of cpsJ (CG001) or the 5′ end of cpsL (CG002). Strain DH5α(pDC143) was made competent, and the cat cassette was introduced by electroporation. Recombinant clones carrying the cat allelic exchange were selected by growth on LA containing 5 μg/ml Cm. The identity of the resultant plasmid (pDC147) was confirmed by PCR and restriction digestion. The insert DNA containing the cat replacement and flanking cps sequences was amplified from pDC147 by PCR using primers cps44 and cps71a and high-fidelity Taq polymerase. The cat containing PCR fragment from pDC147 was ligated to a pHY304 T-vector created by EcoRV digestion, followed by addition of deoxyribosylthymine 3′ overhangs using terminal deoxynucleotidyl transferase and dTTP (Promega). The ligation reaction mixture was introduced into DH5α by electroporation. After growth at 30°C on LA with 5 μg/ml Cm for 48 h, colonies were screened for the resultant plasmid, pLM103, by PCR and restriction digestion. GBS strain COH1 was made competent as previously described (19) and transformed with pLM103, and cpsK allelic replacement mutants were selected as previously described (51) to produce COH1-350. The chromosomal replacement of cpsK by cat was confirmed by PCR, restriction digestion, and Southern and Northern blot analyses.
TABLE 2.
PCR primers
| Primer name | Sequence |
|---|---|
| cpsK-NcoI | 5′-TTCCCATGGCAGCATTAGACGAGCGAAT-3′ |
| cpsK-HindIII | 5′-GCTAAGCTTACCACATAGCTAGCGTAACT-3′ |
| DC88 | 5′-CACAGCCACTTGCACAAGAT-3′ |
| DC89 | 5′-GTTGACTGTCTGCTGTTCCT-3′ |
| PCIV2-1 | 5′-GTGGTGTCACGCTCGTCGTT-3′ |
| PCIV2-2 | 5′-GGCGCCTGATGCGGTAT-3′ |
| CG001 | 5′-CTAATTTAGGGGAAAAATAAAATGAACTTTAATAAAATTGA-3′ |
| CG002 | 5′-GTATTCATTTAATATCCTTCAGAAGCCAGTCATTAGGCCTATCTGA-3′ |
| cps44 | 5′-GTCAAGAGCACCGTATAGTCGTAG-3′ |
| cps71a | 5′-TCTTCCATTACCGCCATACC-3′ |
| PCPSF2 | 5′-GCTGCAGTAGCGGCTTCATTCATGCTAC-3′ |
| PCPSR1 | 5′-TTTCTTCCCGGGTTGGTGACGGCGCGAAT-3′ |
| LM1 | 5′-CACGGATTATGAAACTTGGG-3′ |
| LM2-T7 | 5′-CCAAGCTTCTAATACGACTCACTATAGGGCCGCAGTGGACGAATGTGTTA-3′ |
| cps62 | 5′-CATCTGTTGCACTATCTC-3′ |
| cps65-T7 | 5′-CCAAGCTTCTAATACGACTCACTATAGGGCCTATACTTGTCTCCTCCC-3′ |
| rpsL | 5′-CTCGTGTTGTTACTATGAC-3′ |
| rpsL-T7 | 5′-CCAAGCTTCTAATACGACTCACTATAGGGCCGTTTAGCACCGTATTTAG-3′ |
To complement the COH1-350 strain in trans, a COH1 cps promoter plasmid, pBL26, was first constructed. Plasmid pLZ12spec was digested with EcoRI and SphI and then ligated to an EcoRI/SphI fragment from pMut3 containing a promoterless gfp(mut3) to form pBL-gfp. The cps promoter region from COH1 was amplified using primers PCPSF2 and PCPSR1, followed by digestion with EcoRI and SmaI; ligation to EcoRI/SmaI-cut pBL-gfp to form pBL26. Promoter activity was confirmed by fluorescence of DH5α carrying pBL26 compared to the promoterless pBL-gfp. To create a cpsK expression vector, pBL26 was digested with NcoI and HindIII. The cut vector was ligated to a similarly digested PCR fragment containing cpsK and amplified using COH1 chromosomal DNA, primers cpsK-NcoI and cpsK-HindIII, and high-fidelity Taq polymerase. E. coli DH5α was transformed with the ligation reaction, and colonies expressing the resulting plasmid pLM104 were selected on LA containing 100 μg/ml Sp. Plasmid DNA isolated from DH5α(pLM104) was used to transform competent COH1-350; colonies harboring pLM104, designated COH1-350(pLM104), were screened on THB with 100 μg/ml Sp.
A km-Ω-2 insertion mutant of cpsL was created by amplification of COH1 chromosomal DNA with high-fidelity Taq polymerase using primers DC88 and DC89, followed by ligation into pGEM-T Easy (Promega), transfer to DH5α by electroporation, and selection on LA containing 100 μg/ml Amp to produce pDC132. SmaI-digested pDC132 was ligated to BamHI linkers (Promega), cut with BamHI, and religated to introduce an internal BamHI site in cpsL to create pDC150. After electroporation of the ligated DNA into E. coli XL1-Blue (Stratagene) and selection on LA containing 100 μg/ml Amp, the presence of the inserted site was confirmed by restriction digestion with BamHI. The km-Ω-2 fragment of pCIV2 was amplified using primers PCIV2-1 and PCIV2-2, and the resulting PCR product was cut with BamHI and ligated to BamHI digested pDC150. The ligation reaction was used to transform E. coli XL1-Blue with selection on LA containing 50 μg/ml Km to form pDC151. The proper orientation of the km-Ω-2 insertion in pDC151 was confirmed by double digestion with BglII and PstI. The km-Ω-2 insertion fragment with the flanking cpsL DNA was transferred to pHY304 after PCR amplification from pDC151 with high-fidelity Taq using primers DC88 and DC89 and ligation to pHY304 T-vector prepared as above to produce pDC152. After electroporation into E. coli MC1061, colonies were selected on LA with 50 μg/ml Km. GBS strain COH1 was transformed with pDC152, and double-crossover allelic replacement mutants were selected as previously described (51) with selection on Todd-Hewitt agar containing 1,000 μg/ml Km to produce COH1-355. The replacement of cpsL by the cpsL::km-Ω-2 insertion fragment was confirmed by PCR and Southern blot analysis.
RNA isolation and dot blot hybridization.
GBS RNA was isolated from early-log-phase cells (optical density at 600 nm [OD600] = 0.3) after using disruption with glass beads the RNeasy kit (QIAGEN, Inc., Valencia, CA). Northern dot blotting was performed by spotting 4 μg and 2 μg of total cellular RNA from each strain onto a nylon membrane (MagnaGraph, Westborough, MA). Digoxigenin-labeled RNA probes for cpsK, cpsL, and rpsL were generated with primer pairs LM1 and LM2-T7, cps62 and cps65-T7, and rpsL and rpsL-T7, respectively, to amplify templates with a T7 polymerase promoter sequence appended at the 5′ end. The templates were used to direct in vitro antisense RNA synthesis with incorporation of biotin-dUTP. The probes were hybridized to the blotted RNA overnight at 68°C in Easy hyb buffer (Roche Applied Science) and washed twice for 15 min each in 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.1% sodium dodecyl sulfate (SDS) at 68°C. The blot was developed with anti-digoxigenin alkaline phosphatase-conjugated antibody (Ab) and CSPD detection reagent (Roche Applied Science).
Anti-CPS immunoblots.
Whole cell immunoblots were performed as previously described (10) using either polyclonal rabbit anti-tetanus toxoid-conjugated type III CPS Ab at a 1:30,000 dilution (the generous gift of Larry Paoletti, Channing Laboratory, Harvard Medical School, Boston, MA), mouse anti-type III sialo-CPS monoclonal antibody (MAb) S9 (34) at a 1:5,000 dilution (kindly supplied by Seth Pincus, Louisiana State University, New Orleans, LA), or anti-type 14 pneumococcal CPS Ab HASP-15 (Statens Serum Institiut, Copenhagen, Denmark) at a 1:500 dilution. For determination of secreted CPS levels, cells were grown overnight in chemically defined medium supplemented with Casamino Acids (7). The supernatants were clarified by centrifugation, filtered through a 0.22-μm filter, and concentrated 100 fold by ultrafiltration over a 10,000-Da nominal molecular-weight-cutoff (NMWCO) filter. The cell pellets were washed with phosphate-buffered saline (PBS); twofold dilutions of supernatants, concentrated supernatants, and cells in PBS were spotted onto a nitrocellulose membrane; and immunoblots were performed as previously described (10) with rabbit anti-tetanus toxoid-conjugated type III CPS polyclonal Ab. Dilutions of purified GBS type III CPS and Streptococcus pneumoniae serotype 14 (Spn14) CPS were also spotted as controls.
Isolation of capsular polysaccharides.
Capsular polysaccharides from the COH1 and COH1-350(pLM104) strains were isolated as previously described (6, 12, 46) with modification. Bacteria were grown overnight in 400 ml of THB with 5 μg/ml Cm, if necessary, diluted to 4 liters in fresh THB, and grown to an OD600 of 0.7. The culture was chilled on ice, and the cells were pelleted and washed twice with ice-cold PBS. The cells were suspended in 200 ml lysis buffer (25 mM Na phosphate buffer, pH 7.0, 10 mM MgCl2, 40% [wt/vol] sucrose, 13.3 U/ml mutanolysin [Sigma-Aldrich]) and incubated for 19 h at 37°C with end-over-end mixing. The protoplasts were removed by centrifugation, and the mutanolysin extract was dialysed extensively against distilled water (dH2O) at 4°C using a 3,500-Da NMWCO membrane. After dialysis, the sample was lyophilized, quantitatively suspended in 30 ml dH2O, and centrifuged at 10,000 × g to remove insoluble material. DNase buffer (3.5 ml, 400 mM Tris, pH 7.5, 60 mM MgCl2, 20 mM CaCl2), along with Na azide to 0.05% final concentration, was added to the extract. DNase (300 U) and RNase (200 μg) were added, and the sample was incubated 24 h at 37°C with rocking. After centrifugation at 3,200 × g for 30 min at 4°C to pellet precipitated material, pronase (0.5 mg predigested for 2 h at 50°C to destroy glycosidases in the preparation) was added along with 0.1 ml CaCl2 (10 mM final concentration), and the sample was incubated for 17 h at 37°C with rocking.
After digestion, insoluble material was removed by centrifugation; the supernatant was transferred to an Amicon Ultra-15 ultrafiltration device (Millipore, Bedford, MA), concentrated to 0.25 ml, and then perfused two times with 15 ml dH2O. The retentate from the Amicon filter was diluted to 20 ml with 0.25 N NaOH and then incubated for 38 h at 37°C to decouple the group antigen from CPS. Following neutralization with HCl and removal of salt by Amicon Ultra-15 perfusion, the CPS was re-N-acetylated with acetic anhydride in 69 mM borate buffer, pH 10.0. After concentration and perfusion as above, the CPS was separated from group carbohydrate by gel permeation chromatography over a 1.6- by 60-cm Sephacryl S-300 column (Amersham Biosciences, Piscataway, NJ) eluted with PBS buffer. Fractions were collected and assayed for group antigen by latex bead agglutination (BBL; Becton Dickinson and Co.) and for CPS by immunoblotting with anti-type III polyclonal Ab. Fractions containing CPS and no detectable group antigen were pooled, perfused extensively in an Amicon Ultra-15, and lyophilized, and the dry CPS was weighed. Total carbohydrate was determined by the phenol-sulfuric acid assay of Dubois (14, 17).
CPS was isolated from the COH1-350 and COH1-11 strains as described above for COH1 and COH1-350(pLM104) strains, except that DEAE chromatography (DEAE5, Bio-Rad, Hercules, CA) was used to separate the group carbohydrate from the CPS (47). The crude CPS was loaded on the column and then eluted with 10 ml of 10 mM Tris, pH 8.3, followed by a linear gradient to 0.2 M NaCl in 10 mM Tris, pH 8.3, over 25 ml. Fractions were collected and assayed for group antigen by agglutination and for CPS by dot blotting and enzyme-linked immunosorbent assay (ELISA) with anti-Spn14 CPS monoclonal Ab. Fractions containing CPS, but not detectable group antigen, were pooled, perfused with dH2O, lyophilized, and weighed, and total carbohydrate was determined by the phenol-sulfuric acid assay. CPS was also isolated without the use of NaOH. Instead, following DEAE separation, the CPS was exhaustively digested with 40 U of mutanolysin in 25 mM Tris, pH 7.0, and 10 mM MgCl2 and then overnight with 100 U lysostaphin in 300 μl of 50 Na phosphate, pH 7.2, and 90 mM NaCl. Besides the well-known glycine amidase activity, lysostaphin contains an endo-β-N-acetylglucosaminidase that cleaves cell wall N-acetylglucosaminyl-muramic acid bonds, producing a reducing N-acetylglucosamine (GlcNAc) (23). This is complementary to the action of mutanolysin, which cleaves between N-acetylmuramic acid and N-acetylglucosamine, producing a reducing N-acetylmuramic acid (15). Additionally, lysostaphin contains N-acetylmuramyl-l-alanine amidase activity that can release the linking peptide cross bridges from cell wall glycan strands (23). Lysostaphin is active on GBS bacterial cell walls (our unpublished observations). Following digestion, the CPS was purified as above over a Sephacryl S-400 column (Amersham).
The relative molecular mass of the purified CPS was determined by size exclusion chromatography. COH1 and COH1-350 CPS were separated over a Sephacryl S-400 column (1.6 by 65 cm) eluted with PBS at 0.5 ml/min. The apparent molecular mass of the COH1-11 CPS was determined by S-400 size exclusion chromatography under the same conditions. CPS derived from strains COH1 and COH1-350(pLM104) were compared by separation over Sephacryl S-300 (1.6- by 60-cm column) using the same elution conditions as above. The columns were calibrated with dextran standards (Fluka). Fractions (2.5 ml) were assayed for CPS by quantitative dot blots using polyclonal rabbit anti-type III CPS antisera and Alexa-680 labeled goat anti-rabbit immunoglobulin G (Molecular Probes). After detection with an LI-COR Odyssey IR imager, the amount of CPS in each fraction was determined relative to purified sialo- or asialo-CPS standards.
Spn14 CPS was prepared by lectin-affinity chromatography as previously described (40). Spn14 strain NCTC 11902 (27) was grown overnight on tryptic soy agar with 5% sheep blood (PML Biologicals, Wilsonville, OR) at 37°C with 4% CO2. Discreet colonies were transferred to 20 ml tryptic soy broth (Difco, Becton Dickinson and Co.), grown to OD600 of 0.8 at 37°C with 4% CO2, expanded into 2 liters of prewarmed tryptic soy broth, and incubated overnight at 37°C with 4% CO2. Forty milliliters of formalin was added, and the culture was stirred for 15 min. The cells were lysed by the addition of deoxycholate (0.1% [wt/vol] final concentration) with additional stirring for 15 min. Unbroken cells and debris were removed by centrifugation at 12,000 × g for 15 min at 4°C, and the supernatant was filter sterilized and then concentrated to 120 ml by ultrafiltration with a Pellicon XL (10,000-Da NMWCO, Millipore, Bedford, MA). The concentrate was dialyzed extensively against dH2O and lyophilized. Batches of 350 mg crude extract were suspended in PBS to 35 mg/ml, centrifuged to remove insoluble material, and then passed three times over a 2-ml column of Glycine max lectin conjugated to 4% cross-linked agarose beads (Sigma-Aldrich Co., St. Louis, MO). The column was washed with 100 ml PBS, and the CPS was eluted with two 4-ml aliquots of 0.1 M galactose in PBS. After concentration and extensive perfusion with an Amicon Ultra-15, the CPS was lyophilized and weighed, and total carbohydrate was determined by the phenol-sulfuric acid assay.
Quantification of cell surface-associated CPS.
Mutanolysin extracts were prepared using a procedure modified from Paoletti et al. (33). GBS were grown overnight in THB with antibiotic selection if necessary, then diluted 1:10 in 120-ml fresh medium, and grown to an OD600 of 0.8. The cells were harvested, washed once with ice-cold PBS, washed once in 50 mM Na phosphate, pH 7.0, and suspended in 5 ml 50 mM Na phosphate, pH 7.0. Four 1-ml aliquots of cells were transferred to preweighed 1.5 ml microcentrifuge tubes, pelleted by centrifugation, washed once with dH2O, lyophilized, and weighed. A 300-μl aliquot from the remaining 1.0 ml of cells was transferred to a microcentrifuge tube and diluted 1:2 with 50 mM Na phosphate, pH 7.0. From this, two 200-μl aliquots were removed to new tubes and centrifuged, the supernatants were carefully removed, and the cells were suspended in 875 μl protoplast buffer each (20% [wt/vol] sucrose, 10 mM MgCl2, 0.05% Triton X-100, 20 mM Tris, pH 7.0). Mutanolysin (125 μl, 10 U/ml) was added, and the samples were incubated at 37°C with rocking for 1 h. After centrifugation to remove protoplasts, 850 μl of the supernatant from each sample was removed to a fresh tube and frozen at −20°C.
Competitive inhibition ELISAs were performed as previously described (10) using 96-well microtiter plates coated with poly-l-lysine-coupled purified GBS type III CPS or with poly-l-lysine-coupled purified Spn14 CPS. The assays were done using fourfold serial dilutions of CPS standards (10 μg/ml type III GBS or Spn14 CPS) or CPS extracts. For quantification of CPS from the COH1 and COH1-350(pLM104) strain extracts, anti-type III GBS CPS polyclonal Ab (1:20,000) was used with type III CPS-coated plates and type III CPS standards. Anti-Spn14 CPS monoclonal Ab (1:400) was used with Spn14 CPS-coated plates and Spn14 CPS standards to determine the amount of CPS in extracts from the COH1-350 and COH1-11 strains. At least five independent assays were performed in duplicate for each strain and the values reported as milligrams of CPS per milligrams (dry weight) of cells.
Cell surface localization of CPS.
CPS was determined to be associated with either the peptidoglycan or the cell membrane by the method of Bender et al. (2). GBS were grown to an OD600 of 0.8 in 10 ml THB, rapidly chilled to 4°C, harvested by centrifugation, washed once in PBS at 4°C, resuspended in 100 μl protoplast buffer containing 5 U/μl mutanolysin, and incubated overnight at 22°C. The mutanolysin extracts were centrifuged at 8,000 × g at 4°C for 5 min, the supernatants (cell wall fraction) were removed to a fresh tube, and the pellets (protoplasts) were washed once in 500 μl protoplast buffer and resuspended in 100 μl protoplast buffer. Protoplasts and cell wall fractions (2 μl [each] of strains COH1 and COH1-350[pLM104], 20 μl [each] of the COH1-13, COH1-11, and COH1-350 strains) were separated by SDS-polyacrylamide gel electrophoresis (PAGE) with 4 to 20% gradient gels and transferred to nitrocellulose membranes. The membranes were blocked, incubated with anti-type III CPS polyclonal antisera at a 1:30,000 dilution in LI-COR (Lincoln, NE) blocking buffer, and the immunoblots were developed with Alexa-680-conjugated goat anti-rabbit fluorescent antibody. CPS was detected with a LI-COR Odyssey IR scanner.
TLC of CPS hydrolysates.
Neu5Ac was released from CPS by mild acid hydrolysis of 10 μg purified CPS in 100-μl 2 M acetic acid at 80°C for 1.0 h. The hydrolysates were dried in a SpeedVac centrifugal evaporator (Thermo Savant, Milford, MA), suspended in 2 μl 60% ethanol, and spotted on a silica gel 60 high-performance thin-layer chromatography (HPTLC) plate (Merck, Darmstadt, DE), along with Neu5Ac standards (5 μg/μl in 60% ethanol). Plates were developed twice in 1-butanol:acetic acid:H2O, 2:1:1 (47), and Neu5Ac was identified by its mobility and characteristic lavender color after being sprayed with diphenylamine-aniline-phosphoric acid detection reagent (11).
For total hydrolysis of polysaccharides, 20 μg purified CPS was incubated in 200 μl 2 M trifluoroacetic acid at 100°C for 2 h under N2 in sealed glass ampoules. After hydrolysis, the samples were evaporated under a stream of N2 at 60°C, the residue was extracted two times with 20 μl methanol, and the extracts centrifuged briefly and dried in a SpeedVac concentrator. The samples were dissolved in 10 μl methanol, and 1 μl of each hydrolysate was separated by HPTLC on silica gel 60. The plates were developed twice in ethyl acetate:pyridine:H2O, 8:3:1, and the sugars were detected with diphenylamine-aniline-phosphoric acid detection reagent and compared to authentic glucose (Glc), galactose (Gal), glucosamine (GlcNH), and N-acetylglucosamine (GlcNAc) standards.
Determination of CPS-associated Neu5Ac by HPLC.
Quantitation of the amount of Neu5Ac in each CPS was achieved by high-performance liquid chromatography (HPLC) with fluorometric detection, after mild acid hydrolysis in 2 M acetic acid and derivatization with 1,2-diamino-4,5-methylenedioxybenzene (DMB; Sigma-Aldrich) (1, 8). Assays were performed in triplicate with 2-keto-3-deoxyoctonate (KDO) added to the samples prior to hydrolysis as an internal standard. For each sample, 12.5 μg CPS spiked with 12.5 μM KDO (final concentration) was hydrolyzed in 100 μl 2 M acetic acid as above. The samples were dried, dissolved in 5 μl dH2O, and then reacted with 30 μl 7 mM DMB in 1.4 M acetic acid, 88 mM Na2SO4, and 0.75 M β-mercaptoethanol for 2 h at 50°C in the dark. After derivatization, the samples were diluted 1:25 in HPLC mobile-phase buffer (4:25:92 acetonitrile:methanol:H2O [vol/vol]) and briefly centrifuged, and 12.5 μl was injected into the HPLC. Samples were separated on an Alltech Prevail 5 μm C18 column (4.6 by 150 mm) and eluted at a rate of 0.9 ml/min with fluorescence detection at 373 nm excitation and 448 nm emission.
Quantitation of intracellular Neu5Ac.
To determine if intracellular Neu5Ac accumulated in CΟΗ1-350, 10-ml GBS cultures were grown to an OD600 of 0.8 and snap chilled in a dry-ice-ethanol bath; the cells were harvested, washed once in ice-cold PBS, and then suspended in 1.0 ml ice-cold 20 mM NH4HCO3, pH 7.8. The cells were disrupted at 4°C with glass beads (FP120; ThermoSavant); the lysates were removed; the beads washed once with 400 μl 20 mM NH4HCO3, pH 7.8; and the washes and lysates were combined. Unbroken cells and debris were removed by centrifugation at 8,000 × g and 4°C, and the supernatants were subjected to ultracentrifugation at 100,000 × g for 30 min at 4°C to pellet the cell membranes. The supernatant fraction was lyophilized a resuspended in 200 μl dH2O, 1 ml ice-cold ethanol was added, and the samples were placed at −20°C for 20 min. The samples were centrifuged at 15,000 × g and 4°C for 20 min; the supernatants were removed, dried in a SpeedVac (ThermoSavant), and resuspended in 80 μl dH2O. The KDO internal standard was added (20 μl; 200 μM), and the Neu5Ac extracts were passed over 0.3-ml Dowex 50W-X8 minicolumns. The columns were washed three times with 100 μl of dH2O, and the flowthrough and washes were combined and passed over 0.3-ml columns of Bio-Rad AG1-X8 (formate form). The columns were washed three times with dH2O, and the Neu5Ac was eluted with three 100-μl washes (each) of 2 M formic acid. The column eluates were combined, dried in a SpeedVac, and resuspended in 20 μl dH2O. A total of 5 μl of each extract was derivatized with DMB, and the amount of Neu5Ac present was determined by HPLC as above.
RESULTS
The cpsK gene is located in the cps operon of GBS (Fig. 1) with the other structural genes necessary for RPU synthesis. Because of the similar structure of the type III GBS RPU and the sialylated LOS of H. ducreyi, we hypothesized and subsequently demonstrated that the GBS cpsK could complement a mutation in the H. ducreyi sialyltransferase gene lst (8), restoring the terminal sialic acid to its LOS. This observation, as well as the homology between cpsK and the other bacterial sialyltransferases, suggested a role for cpsK in the sialylation of the RPUs of GBS CPS.
Construction of a nonpolar cpsK deletion in COH1.
To explore the role of cpsK in GBS CPS sialylation, a cpsK allelic exchange mutant was created by exact allelic replacement with a cat gene via homologous recombination. To deliver cat to the cps locus, the cat allele was flanked with cps sequences immediately 5′ and 3′ of cpsK, the chimeric fragment was ligated into the temperature-sensitive vector pHY304, and the recombinant plasmid was transformed into the type III strain COH1. Under nonpermissive conditions, the cat allele replaced cpsK via homologous recombination, producing strain COH1-350. The replacement was confirmed by Southern blotting and PCR analysis using COH1-350 chromosomal DNA (data not shown).
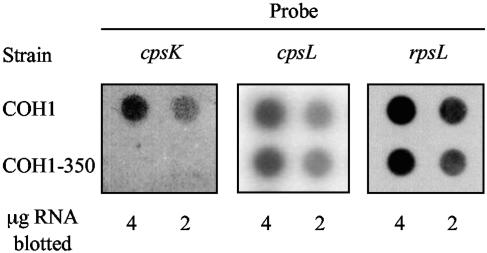
To determine if insertion of cat into the cps operon caused distal effects on downstream gene expression, RNA dot blot analysis was performed. Probes for cpsK, cpsL (Fig. 1), and rpsL, a ribosomal structural gene, were generated and hybridized to RNA isolated from the wild-type (wt) and COH1-350 strains (Fig. 2). Equivalent hybridization of the control rpsL probe to RNA from the COH1 and COH1-350 strains was observed, confirming that equal amounts of total RNA from each strain were loaded on the blots. While the cpsK probe hybridized to the wild-type RNA, there was no hybridization of the probe to RNA isolated from the COH1-350 strain. Equal amounts of hybridization with the cpsL probe were observed for both strains. These results demonstrated that insertion of the cat gene did not exert polar effects on distal genes.
FIG. 2.
RNA dot blots of total cellular RNA from the COH1 or COH1-350 strains. Total RNA was hybridized to antisense RNA probes that were generated from in vitro transcription of cpsK, cpsL, or rpsL (a ribosomal subunit housekeeping gene). Either 4 or 2 μg of cellular RNA was transferred to a nitrocellulose membrane and hybridized with the probes. Strain designations: COH1, wt; COH1-350, ΔcpsK.
Complementation of cpsK deletion.
A derivative of COH1-350 containing an episomal copy of cpsK was constructed to confirm that the results of the phenotype analysis performed below were a result of the deletion of cpsK. A wild-type copy of cpsK from COH1 was ligated into the low-copy-number vector pBL26 under control of the COH1 cps promoter. The recombinant plasmid, designated pLM104, was transformed into COH1-350 to produce COH1-350(pLM104). This strain was used along with the wild-type and mutant strains to characterize the impact of the cpsK mutation on CPS composition and expression.
Cell surface CPS expression.
The CPS produced by Spn14 is structurally and immunologically identical to that of type III GBS asialo-CPS (Fig. 3) (5, 20, 25, 27, 43). MAb HASP-14 raised against Spn14 CPS also recognized GBS serotype III asialo-CPS. To test for cell surface expression of either sialo- or asialo-CPS, HASP-14 Ab (which recognizes type III asialo-CPS), in conjunction with anti-serotype III GBS MAb S9 (recognizing only sialylated CPS) and polyclonal anti-serotype III CPS Ab (which recognizes both sialo-CPS and type III asialo-CPS), was used in immunoblot analysis of whole cells from strains COH1, COH1-350, and COH1-350(pLM104) (Fig. 4). We included COH1-11, a neuA transposon insertion mutant of COH1 that fails to express CMP-sialic acid synthase (Fig. 1) (21), and Spn14 strain NCTC11902 as control strains. The blots were reacted separately with each Ab, and the degree of binding was visualized using horseradish peroxidase-conjugated secondary Ab.
FIG. 3.
GBS serotype III and Spn14 CPS RPU structures, adapted from reference 27. Galp, galactose; Glcp, glucose; GlcpNAc, N-acetylglucosamine; NeupNAc, N-acetylneuraminic (sialic) acid. The brackets to the right delineate the portions of the structure, corresponding to the CPS RPUs produced by S. pneumoniae serotype 14 and serotype III GBS, respectively. Structural motifs recognized by anti-Spn14 monoclonal antibody HASP-15 (†), anti-type III GBS sialo-CPS monoclonal antibody S9 (§), and anti-type III GBS polyclonal antibody (*) are as indicated.
FIG. 4.
Whole cell immuno-dot blots using either anti-serotype III polyclonal (A), anti-serotype III sialo-CPS monoclonal (B), or anti-Spn14 CPS monoclonal (C) antibodies. Dilutions of cells were made in PBS and spotted on nitrocellulose membranes (1:1 = 106 CFU), and the cells were fixed and then probed with the various anti-CPS antibodies. Strains shown are COH1 (wt), COH1-350 (ΔcpsK), COH1-350(pLM104) (ΔcpsK/cpsK), COH1-11 (ΔneuA), and S. pneumoniae NCTC11902 (Spn14).
The anti-GBS type III CPS polyclonal Ab bound to the COH1 and COH1-350(pLM104) strains (Fig. 4A) but bound weakly to strains COH1-350 and COH1-11, consistent with a change in antibody affinity and/or decreased surface expressed type III CPS compared to the COH1- or cpsK-complemented mutant strains. The anti-serotype III polyclonal Ab also bound to the control pneumococcal type 14 strain, NCTC11902 with nearly the same avidity as seen for strain COH1, demonstrating its recognition of asialo-CPS. The anti-type III sialo-CPS MAb (Fig. 4B) showed strong binding to strains COH1 and COH1-350(pLM104), but binding was not seen for strains COH1-350, COH1-11, and Spn14, indicating the absence of CPS sialic acid on the latter strains. Conversely, COH1-350 and COH1-11 reacted strongly with the anti-Spn14 monoclonal Ab (Fig. 4C), consistent with an asialo phenotype for COH1-350 and COH1-11. COH1 and COH1-350(pLM104) reacted weakly with this antibody as expected, since sialic acid masks the epitope recognized by the anti-Spn14 MAb. These data suggest that COH1-350 produced an asialo-CPS and that the defect in sialylation was complemented by expression of cpsK in trans.
Biochemical analysis of mutant CPS.
CPS from COH1-350 was isolated by DEAE chromatography to determine if sialic acid was missing. Loss of sialic acid causes serotype III CPS to become neutrally charged, and thus it was not retained on DEAE. Column fractions were assayed for anti-type III CPS immunoreactive material by immuno-dot blot analysis. The immunoreactive material was observed in the void volume eluate, consistent with the loss of Neu5Ac from the CPS. The purified COH1-350 CPS was subjected to mild and strong acid hydrolysis, and the hydrolysates were analyzed by HPTLC. Mild acid hydrolysis removes sialic acid from the CPS without hydrolyzing other sugar linkages. Strong acid hydrolysis destroys sialic acid but otherwise reduces the CPS to its constituent monosaccharides. After mild acid hydrolysis, separation by HPTLC, and visualization with diphenylamine-aniline-phosphoric acid reagent, CPS purified from the COH1 and COH1-350(pLM104) strains produced a prominent band having the same characteristic lavender color and Rf seen for the Neu5Ac standard (Fig. 5, left). Some lactonization of the Neu5Ac was seen, as was previously reported with this method (47). Neu5Ac was not detected in the hydrolysates of COH1-350 or COH1-11, nor was it seen in the Spn14 CPS control (not shown). These findings are consistent with the absence of Neu5Ac. Total hydrolysis of CPS from the COH1, COH1-350, COH1-350(pLM104), and Spn14 strains, followed by HPTLC separation of the hydrolysates, revealed the presence of Glc, Gal, and GlcN (GlcNAc is converted to GlcNH under strong hydrolysis conditions), but no other sugars (Fig. 5, right). These data are consistent with the known composition of the type III GBS and Spn14 CPSs (48, 43).
FIG. 5.
Thin-layer chromatography of purified CPS acid hydrolysates. (Left) Mild acid hydrolysis of CPS from strain COH1 (wt), COH1-350 (ΔcpsK), and COH1-350(pLM104) (ΔcpsK/cpsK). The migration of pure sialic acid (Neu5Ac) is seen in the first lane (5 μg Neu5Ac). The Neu5Ac lactone is an artifact of the hydrolysis. (Right) Strong acid hydrolysis of CPS from strain COH1 (wt), COH1-350 (ΔcpsK), and COH1-350(pLM104) (ΔcpsK/cpsK), serotype 14 S. pneumoniae NCTC11902 (Spn14), or N-acetylglucosamine hydrolyzed under identical conditions. The migration distances of authentic glucosamine (GlcNH), galactose (Gal), glucose (Glc), and N-acetylglucosamine (GlcNAc) are indicated.
To quantitate the amount of Neu5Ac associated with the CPS of the strains, purified CPS from each strain was hydrolyzed under mild acid conditions, and the amount of Neu5Ac released was quantitatively determined by HPLC (Table 3). Sialic acid was not detected in hydrolyzed CPS from COH1-350 or CPS purified from Spn14 but was present in equal amounts of CPS hydrolyzed from COH1 and COH1-350(pLM104) (Table 3). The average percent composition of sialic acid in the RPU was 39% for COH1 and COH1-350(pLM104) and correlated well with the predicted 34% for type III CPS in which each repeating unit carries a single molecule of sialic acid. These observations provide compelling evidence for the loss of CPS sialylation in COH1-350 and restoration to parental levels in the cpsK mutant complemented by cpsK in trans.
TABLE 3.
Amount of CPS and degree of CPS sialylation for COH1, COH1-350, COH1-350(pLM104), and COH1-11 strains
| Strain | Amt of CPS (μg/mg cell dry wt) | % Sialic acid in CPS |
|---|---|---|
| COH1 | 8.7 ± 2.6 | 40.7 ± 2.9 |
| COH1-350(pLM104) | 6.8 ± 3.9 | 38.4 ± 3.0 |
| COH1-350 | 1.8 ± 1.1 | <0.5 |
| COH1-11 | 1.9 ± 1.2 | <0.5 |
Accumulation of intracellular sialic acid in COH1-350.
To explore the possibility that the asialo phenotype might be due to defects in sialic acid synthesis in COH1-350, intracellular sialic acid concentrations were measured by HPLC. Under the conditions of the assay, CMP-Neu5Ac was hydrolyzed to Neu5Ac, so total intracellular sialic pools were measured. COH1-350 contained threefold more intracellular sialic acid than strain COH1. Intracellular sialic acid was not detected in COH1-355, a strain containing a polar insertion in cpsL that abolishes transcription of the downstream sialic acid synthesis genes neuBCDA (Fig. 1). The accumulation of sialic acid in COH1-350 confirmed that its asialo phenotype was not due to the lack of endogenous sialic acid synthesis but was due to a block in utilization of sialic acid by the sialyltransferase.
Quantification of CPS production by COH1-350.
The amount of CPS produced by the COH1, COH1-350, COH1-11, and COH1-350(pLM104) strains was quantitated by competitive inhibition ELISA analysis of cell wall-associated CPS using anti-type III CPS polyclonal Ab (Fig. 6). ELISAs with type III CPS immobilized on microtiter plates and anti-type III CPS polyclonal antibody showed identical inhibition curves for the COH1 and COH1-350(pLM104) strains (Fig. 6A). This indicated equivalent CPS synthesis levels and identical Ab binding kinetics for both strains and suggested the CPS was immunologically indistinguishable. The COH1-350 and COH1-11 mutant strains, although having identical inhibition kinetics, showed markedly reduced competition for the antibody under these conditions when compared to the results with COH1 and COH1-350(pLM104) (Fig. 6A).
FIG. 6.
Competitive inhibition ELISA curves of mutanolysin cell wall extracts. (A) Competition between extracts (or purified GBS CPS) and immobilized GBS serotype III CPS for anti-serotype III polyclonal antibody. (B) Competition between extracts (or purified Spn14 CPS) and immobilized S. pneumoniae serotype 14 CPS for monoclonal anti-serotype 14 pneumococcal antibody. Grey diamonds, purified GBS serotype III CPS; open squares, strain COH1 CPS extract (wt); grey triangles, strain COH1-350 CPS extract (ΔcpsK); open circles, strain COH1-11 CPS extract (ΔneuA); black triangles, strain COH1-350(pLM104) CPS extract (ΔcpsK/cpsK); open diamonds, purified Spn14 CPS.
The decreased competition for anti-type III Ab by the CPS from COH1-350 and COH1-11 compared to COH1 and COH1-350(pLM104) is consistent with an asialo phenotype for the former two strains. However, another explanation for these results could be the potential decreased levels of CPS expression. To determine the absolute levels of CPS expression in these strains, ELISAs using Spn14 CPS immobilized on microtiter plates and anti-Spn14 CPS MAb were performed (Fig. 6B). Since Spn14 CPS is structurally identical to the GBS type III asialo-CPS, it was used as the standard in the ELISA to determine the concentration of CPS in the COH1-350 and COH1-11 extracts. The concentration of Spn14 control CPS used in this experiment (10 μg/ml) was equivalent to that of the control wild-type type III CPS used in the anti-type III ELISA above. COH1-350 and COH1-11 extracts demonstrated identical inhibition kinetics to each other, but they competed less effectively for the anti-Spn14 CPS antibody than the control Spn14 CPS, indicating reduced CPS production by the COH1-350 and COH1-11 strains. Quantitatively, the mutant strains produce only 20% of the CPS of the COH1 strain on the basis of millgrams of CPS per milligrams (dry weight) of cells (Table 3).
Localization of CPS in COH1-350.
We questioned if increased secretion of CPS into the media by COH1-350 might be responsible for the decrease in anti-type III polyclonal Ab binding to cell extracts observed above. To investigate this possibility, strains COH1, COH1-350, COH1-350(pLM104) and COH1-11, and the acapsular transposon mutant strain COH1-13 were grown in chemically defined medium. After clarification by centrifugation, the spent culture medium was spotted onto nitrocellulose membrane as a twofold dilution series in PBS, either directly [strains COH1 and COH1-350(pLM104)] or after 100-fold concentration over a 10-kDa NMWCO filter (strains COH1-350, COH1-11 and COH1-13). Likewise, a dilution series of washed cells harvested from the cultures was spotted on the membrane along with purified type III GBS and Spn14 CPS standards. After incubation with anti-type III CPS polyclonal antisera and visualization of Ab binding (Fig. 7) it was apparent that the 100-fold concentrated supernatants from strains COH1-350 and COH1-11 had reduced Ab binding compared to strains COH1 and COH1-350(pLM104) (panel A). The binding was only slightly more than that seen for the concentrated supernatant from the acapsular COH1-13 strain. Based on the binding observed for the purified Spn14 and type III GBS CPS standards (Fig. 7C), strains COH1-350 and COH1-11 secreted 50-fold-less CPS into the medium than the parental strain COH1. Ab binding to PBS-washed whole cells of strains COH1-350 and COH1-11 was markedly reduced compared to that seen for strains COH1 and COH1-350(pLM104) (Fig. 7B). These results demonstrated that the decrease in Ab binding seen in the COH1-350 and COH1-11 mutant strains in the previous experiments was not due to increased secretion into the bacterial culture supernatant.
FIG. 7.
Immuno-dot blots of culture supernatants and cell pellets. (A) Clarified culture supernatants, either unconcentrated (neet) [strains COH1 and COH1-350(pLM104)] or 100-fold concentrated (strains COH1-350, COH1-11, and COH1-13) versus (B) whole cells washed with PBS. (C) Purified type III GBS and Spn14 CPS. Samples were serially diluted twofold, spotted onto a nitrocellulose membrane, and tested for the presence of CPS with anti-serotype III polyclonal antiserum.
Further experiments were performed to determine the localization of the cell-associated CPS in COH1-350 compared to that of the control strains. Mutanolysin extracts of whole cells were separated by centrifugation into cell wall and protoplast fractions. Each fraction was separated by SDS-PAGE and transferred to a nitrocellulose membrane, and CPS on the membrane was detected after incubation with polyclonal anti-type III CPS Ab (Fig. 8). For all strains tested, the majority of the CPS was detected in the cell wall fraction, indicating that it had been associated with the cell wall prior to mutanolysin digestion. Only trace amounts of CPS were detected in the protoplast fractions, indicating efficient transfer of polymerized CPS to the cell wall.
FIG. 8.
SDS-PAGE of cell wall and protoplast fractions from mutanolysin extracts of whole cells. After centrifugation, the cell wall fraction (cw) or protoplasts (p) were separated on 5 to 20% gradient gels and blotted, and CPS was detected with anti-type III CPS Ab. The migration distance of the CPS is shown relative to that of protein standards run on the same gel. The arrow indicates the location of the CPS associated with the cell wall fraction in strain COH1-350.
Effect of cpsK mutation on CPS chain length.
SDS-PAGE analysis of CPS from cell wall extracts (Fig. 8), indicated a lower electrophoretic mobilty for CPS from strains COH1-350 and COH1-11 than the wild-type and the complemented COH1-350(pLM104) strains. These data suggested an increase in CPS chain length for the asialo mutants. To determine the effect of cpsK on CPS polymerization, CPS from strains COH1, COH1-350, and COH1-11 was characterized by Sephacryl S-400 size exclusion chromatography (Fig. 9). Based on dextran calibration standards, the average CPS molecular mass for COH1 was 118 ± 11 kDa (four independent determinations) which corresponded to ∼115 pentasaccharide RPUs. The COH1-350 CPS was found to be significantly larger (410 ± 107 kDa; three independent determinations) corresponding to ∼600 tetrasaccharide RPUs. The CPS from strain COH1-11 eluted at the same position as that of COH1-350, and CPS isolated using NaOH or enzymatic digestion gave similar values. Thus, the decrease in total CPS could not be attributed to decreased CPS chain length but rather suggests that the reduction in quantity is due to fewer (but longer) CPS chains on the COH1-350 strain. Similar analysis by size exclusion chromatography of the CPS from COH1-350(pLM104) revealed it to be indistinguishable in size from the CPS of COH1, demonstrating restoration of the wt phenotype when cpsK was expressed in trans.
FIG. 9.
Sephacryl S-400 size exclusion chromatograph of strain COH1 (wt) and COH1-350 (ΔcpsK) CPS. COH1 and COH1-350 CPS (300 μg and 172 μg, respectively) were fractionated over a Sephacryl S-400 column (1.6 by 65 cm). Collected fractions were assayed for CPS by quantitative immunofluorescent dot blot analysis. The molecular mass of the CPS was determined relative to dextran standards, and the number of RPUs was calculated based on the predicted molecular weight of the sialo (COH1) or asialo (COH1-350) RPUs.
DISCUSSION
GBS is among a select number of significant human and animal pathogenic bacteria expressing sialic acid on their cell surfaces. For these organisms, sialic acid enhances their virulence by interfering with the mechanisms of host innate immune recognition, specifically with opsonic components of the complement system. We previously demonstrated that GBS cpsK encodes sialyltransferase activity when expressed in H. ducreyi. In this report, we used genetic and biochemical approaches to conclusively demonstrate that cpsK is responsible for the addition of sialic acid to the GBS CPS. Additionally, we show that loss of CPS sialylation results in qualitative and quantitative differences in the nature of the CPS produced.
Creation of a cpsK in frame deletion mutant was accomplished by allelic replacement of cpsK with a cat allele. Genes distal to cpsK, including the genes responsible for sialic synthesis (neuBCDA), were transcribed normally in this strain, and determination of intracellular sialic acid levels confirmed sialic acid synthesis was retained. CPS on the surface of the cpsK deletion strain failed to bind an anti-sialo-CPS MAb. However, it did bind to an anti-type III CPS polyclonal antibody, which recognizes both asialo-CPS and native serotype III CPS, as well as to an anti-Spn14 monoclonal antibody, which only binds the asialo form of type III GBS CPS. Episomal expression of cpsK in the cpsK deletion mutant restored its antibody binding phenotype to that of the wild-type COH1 strain. These data were consistent with the absence of sialic acid on the CPS of the cpsK mutant and indicated that CPS sialylation could be restored by expression of cpsK in trans. We confirmed the above results using mild acid hydrolysates of purified CPS from the cpsK mutant, which did not contain detectable sialic acid. Glucose, galactose, and N-acetylglucosamine were observed in the expected molar ratio after strong acid hydrolysis of cpsK mutant CPS. Sialic acid was recovered from mild acid hydrolysates of CPS from the COH1-350 mutant expressing cpsK in trans, demonstrating that the asialo phenotype of COH1-350 was due to the absence of cpsK activity. These data provide compelling evidence that cpsK encodes the CPS sialyltransferase.
Of interest was the observation that loss of sialylation had a dramatic impact on the amount of CPS produced. The 80% reduction in surface associated CPS produced by both asialo mutant strains compared to the parental strain was consistent with our previously reported results for the neuA mutation (47) and suggested that sialylation was important for full synthesis of CPS by GBS. The ELISA data were corroborated by data demonstrating proportionately less CPS isolated per gram (dry weight) of cells from the cpsK mutant than from the COH1 strain. We confirmed that the decrease in cell surface-associated CPS in the cpsK and neuA mutant strains was not due to loss of surface attachment and increased secretion of CPS into the culture supernatants. Our results demonstrated that most of the CPS for the mutant and wild-type strains was located in the cell wall fraction, suggesting that it is efficiently transferred from the lipid carrier and attached to the peptidoglycan regardless of the degree of sialylation. This observation implies that the CPS muro-phosphodiester ligase activity in GBS is insensitive to the presence of sialic acid on the CPS and therefore unrelated to the reduced polysaccharide production seen in the asialo CΟΗ1-350 and CΟΗ1-11 mutant strains. That these two strains exhibit indistinguishable CPS phenotypes established that these effects are due to the loss of CPS sialylation independent of the specific lesion within the sialic acid biosynthesis and transfer pathway. This observation appears to be in contrast to that found with the lst mutations in H. ducreyi and N. meningitidis, whereby loss of sialyltransferase activity did not reduce production of the asialo-LPS backbone structure (4).
Differences in the apparent size of CPS produced by the CΟΗ1-350 strain were also evident by size exclusion chromatography. A possible explanation is that there was a significant increase in the degree of CPS polymerization in COH1-350 compared to the COH1 strain. If so, the CPS from the cpsK mutant, on average, contained five times more RPUs than CPS from COH1: 410 kDa for COH1-350 or approximately 600 RPUs, compared to 118 kDa and 120 RPUs for the COH1 CPS. In our experiments, the molecular mass of the COH1 CPS differed somewhat from that previously reported (∼250 kDa), and the CPS produced by the asialo neuA strain COH1-11 was found to be smaller than that of the parental isolate (∼100 kDa) (47). The discrepancy in size could be due to differences in CPS preparation, since group antigen and cell wall fragments were not decoupled from CPS alkaline hydrolysis in our previous report. However, when we extracted the CPS from the COH1-11 strain by only enzymatic means, we observed the same size as that seen by alkaline extraction. The greater degree of CPS polymerization in the CΟΗ1-350 strain suggested that regulation of CPS chain length is relaxed for the asialo-CPS and indicated that a decrease in overall CPS expression was not due to reduced chain length in the COH1-350 strain.
Possible reasons for the decrease in CPS production for the asialo mutant strains could include diminished production of CPS oligosaccharide precursors and/or reduced transfer of CPS precursors across the cytoplasmic membrane. If the sialyltransferase is an integral part of a membrane-associated complex of glycosyltransferases, its loss may also disrupt the functional integrity of the CPS synthesis complex. In addition, these data may indicate the role of a feedback mechanism on the functions of cpsBCD, which have been previously shown to control chain length and transport of the polysaccharide (31, 12, 3, 2). We postulate that the asialo RPUs as they are polymerized may fail to provide the maturation signal that leads to control of the overall chain length observed with the wild-type CPS. We are currently investigating the potential mechanisms behind these observations.
Despite significant differences in overall CPS structure, all known serotypes of GBS produce a sialylated CPS. Since our last report, the DNA sequence of cpsK from the remaining serotypes II, VII, and VIII have been determined. Comparison of the capsule genes from each serotype suggests that the cpsK locus is a common crossover point for insertion of DNA sequences encoding novel glycosyltransferases (12a). With the exception of types Ia and III, which differ only in their RPU polymerase alleles, the region of variable glycosyltransferases ends within cpsK. Thus, the 5′ region of cpsK differs between serotypes, whereas the 3′ end is conserved between serotypes. The variable portions of the alleles are distantly related but are phylogenetically closer to each other than to the sialyltransferases of other species. The one exception is cpsK of serotype VIII, which is unrelated to the other GBS sialytransferases and is instead homologous to the N. meningitidis and N. gonorrheae lipooligosaccharide sialyltransferase family. It remains to be determined if the heterogeneity between cpsK alleles reflects evolutionary drift alone or also functional differences in sialyltransferase specificity to accommodate the structural differences between the nine CPS serotype RPUs. The data above suggest that conservation of the sialic acid decoration among the GBS CPS structures is under strong evolutionary pressure, potentially to retain CPS epitopes crucial to immune evasion and survival in the host.
Sialylation is critical to the function of CPS (18, 38, 41) and, as shown here, is also critical to the biosynthetic process. The various GBS serotypes share virtual identity between the genes that are responsible for regulation and export of CPS production. Their capsule operons differ only in the central glycosyltransferase region responsible for synthesis of their CPS RPU oligosaccharides. The data presented here suggest that the synthesis, export, and regulatory mechanisms are highly integrated with the incorporation of sialic acid on CPS. Indeed, strains that lose the ability to synthesize sialic acid or transfer it to their CPS suffer a significant loss of surface polysaccharide, resulting in an increased sensitivity to opsonization and phagocytic killing (47). This may explain the preservation of a sialylated CPS by the different GBS serotypes in contrast to the variety of surface polysaccharides observed for other encapsulated organisms like S. pneumoniae.
Inhibition of alternative pathway of complement activation by CPS sialic acid has been demonstrated through enzymatic removal or chemical modification of sialic acid residues (18, 29) and correlation of C3 deposition and C5a production with naturally occurring variations in CPS sialylation (41). However, as recognized in early work with the neuA mutant COH1-11 (29), caution should be used in evaluating the effect of mutations in sialic acid synthesis and its presence on the capsule regarding opsonization, phagocytosis, and/or virulence, since their effects are confounded with an overall decrease in CPS production. Once the decrease in CPS expression imposed by the inability to sialylate the capsule is understood on the molecular level, it may be possible to construct strains that produce wt levels of asialo-CPS, which will allow direct evaluation of sialic acid's contribution to the ability of GBS to resist complement opsonization.
Further investigation into the mechanisms by which each GBS serotype adds sialic acid to its CPS should lead to novel insights into the poorly understood areas of CPS export and polymerization by gram-positive organisms and could help to further characterize its role in GBS pathogenesis.
Acknowledgments
This study was supported by NIH grant AI 22498 (C.E.R.).
We appreciate the antibody preparations from Seth Pincus and Larry Paoletti. We also thank Christy Ventura for the protocols on the capsule localization techniques. Anne Clancy and Christy Ventura are gratefully acknowledged for their careful review of the manuscript.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1997. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 2.Bender, M. H., R. T. Cartee, and J. Yother. 2003. Positive correlation between tyrosine phosphorylation of CpsD and capsular polysaccharide production in Streptococcus pneumoniae. J. Bacteriol. 185:6057-6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender, M. H., and J. Yother. 2001. CpsB is a modulator of capsule-associated tyrosine kinase activity in Streptococcus pneumoniae. J. Biol. Chem. 276:47966-47974. [DOI] [PubMed] [Google Scholar]
- 4.Bozue, J. A., M. V. Tullius, J. Wang, B. W. Gibson, and R. S. Munson, Jr. 1999. Haemophilus ducreyi produces a novel sialyltransferase. Identification of the sialyltransferase gene and construction of mutants deficient in the production of the sialic acid-containing glycoform of the lipooligosaccharide. J. Biol. Chem. 274:4106-4114. [DOI] [PubMed] [Google Scholar]
- 5.Brisson, J. R., S. Uhrinova, R. J. Woods, M. van der Zwan, H. C. Jarrell, L. C. Paoletti, D. L. Kasper, and H. J. Jennings. 1997. NMR and molecular dynamics studies of the conformational epitope of the type III group B Streptococcus capsular polysaccharide and derivatives. Biochemistry 36:3278-3292. [DOI] [PubMed] [Google Scholar]
- 6.Calandra, G. B., and R. M. Cole. 1980. Lysis and protoplast formation of group B streptococci by mutanolysin. Infect. Immun. 28:1033-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carey, R. B., T. K. Eisenstein, G. D. Shockman, T. F. Greber, and R. M. Swenson. 1980. Soluble group- and type-specific antigens from type III group B Streptococcus. Infect. Immun. 28:195-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaffin, D. O., K. McKinnon, and C. E. Rubens. 2002. CpsK of Streptococcus agalactiae exhibits α2,3-sialyltransferase activity in Haemophilus ducreyi. Mol. Microbiol. 45:109-122. [DOI] [PubMed] [Google Scholar]
- 9.Chaffin, D. O., and C. E. Rubens. 1998. Blue/white screening of recombinant plasmids in gram-positive bacteria by interruption of alkaline phosphatase gene (phoZ) expression. Gene 219:91-99. [DOI] [PubMed] [Google Scholar]
- 10.Chaffin, D. O., H. H. Yim, S. B. Beres, and C. E. Rubens. 2000. The serotype of type Ia and III group B streptococci is determined by the polymerase gene within the polycistronic capsule operon. J. Bacteriol. 182:4466-4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaplin, M. F. 1994. Monosaccharides, p. 14-15. In M. F. Chaplin and J. F. Kennedy (ed.), Carbohydrate analysis: a practical approach, 2nd ed. Oxford University Press, Oxford, United Kingdom.
- 12.Cieslewicz, M. J., D. L. Kasper, Y. Wang, and M. R. Wessels. 2001. Functional analysis in type Ia group B Streptococcus of a cluster of genes involved in extracellular polysaccharide production by diverse species of streptococci. J. Biol. Chem. 276:139-146. [DOI] [PubMed] [Google Scholar]
- 12a.Cieslewicz, M. J., D. Chaffin, G. Glusman, D. Kasper, A. Madan, S. Rodrigues, J. Fahey, M. R. Wessels and C. E. Rubens. 2005. Structural and genetic diversity of group B streptococcus capsular polysaccharides. Infect. Immun. 73:3096-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cormack, B. P., R. H. Valdivia, and S. Falkow. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33-38. [DOI] [PubMed] [Google Scholar]
- 14.Cuesta, G., N. Suarez, M. I. Bessio, F. Ferreira, and H. Massaldi. 2003. Quantitative determination of pneumococcal capsular polysaccharide serotype 14 using a modification of phenol-sulfuric acid method. J. Microbiol. Methods 52:69-73. [DOI] [PubMed] [Google Scholar]
- 15.De Cueninck, B. J., G. D. Shockman, and R. M. Swenson. 1982. Group B, type III streptococcal cell wall: composition and structural aspects revealed through endo-N-acetylmuramidase-catalyzed hydrolysis. Infect. Immun. 35:572-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doran, K. S., and V. Nizet. 2004. Molecular pathogenesis of neonatal group B streptococcal infection: no longer in its infancy. Mol. Microbiol. 54:23-31. [DOI] [PubMed] [Google Scholar]
- 17.Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28:350-356. [Google Scholar]
- 18.Edwards, M. S., D. L. Kasper, H. J. Jennings, C. J. Baker, and A. Nicholson-Weller. 1982. Capsular sialic acid prevents activation of the alternative complement pathway by type III, group B streptococci. J. Immunol. 128:1278-1283. [PubMed] [Google Scholar]
- 19.Framson, P. E., A. Nittayajarn, J. Merry, P. Youngman, and C. E. Rubens. 1997. New genetic techniques for group B streptococcus: high-efficiency transformation, maintenance of temperature-sensitive pWV01 plasmids, and mutagenesis with Tn917. Appl. Environ. Microbiol. 63:3539-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guttormsen, H. K., C. J. Baker, M. H. Nahm, L. C. Paoletti, S. M. Zughaier, M. S. Edwards, and D. L. Kasper. 2002. Type III group B streptococcal polysaccharide induces antibodies that cross-react with Streptococcus pneumoniae type 14. Infect. Immun. 70:1724-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haft, R. F., M. R. Wessels, M. F. Mebane, N. Conaty, and C. E. Rubens. 1996. Characterization of cpsF and its product CMP-N-acetylneuraminic acid synthetase, a group B streptococcal enzyme that can function in K1 capsular polysaccharide biosynthesis in Escherichia coli. Mol. Microbiol. 19:555-563. [DOI] [PubMed] [Google Scholar]
- 22.Husmann, L. K., J. R. Scott, G. Lindahl, and L. Stenberg. 1995. Expression of the Arp protein, a member of the M protein family, is not sufficient to inhibit phagocytosis of Streptococcus pyogenes. Infect. Immun. 63:345-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iversen, O. J., and A. Grov. 1973. Studies on lysostaphin. Separation and characterization of three enzymes. Eur. J. Biochem. 38:293-300. [DOI] [PubMed] [Google Scholar]
- 24.Jennings, H. J., E. Katzenellenbogen, C. Lugowski, F. Michon, R. Roy, and D. L. Kasper. 1984. Pure Appl. Chem. 56:893. [Google Scholar]
- 25.Jennings, H. J., K. G. Rosell, and D. L. Kasper. 1980. Structural determination and serology of the native polysaccharide antigen of type-III group B Streptococcus. Can. J. Biochem. 58:112-120. [DOI] [PubMed] [Google Scholar]
- 26.Jones, A. L., R. H. Needham, and C. E. Rubens. 2003. The delta subunit of RNA polymerase is required for virulence of Streptococcus agalactiae. Infect. Immun. 71:4011-4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolkman, M. A., D. A. Morrison, B. A. M. van der Zeijst, and P. J. M. Nuijten. 1996. The capsule polysaccharide synthesis locus of Streptococcus pneumoniae serotype 14: identification of the glycosyl transferase gene cps14E. J. Bacteriol. 178:3736-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuypers, J. M., L. M. Heggen, and C. E. Rubens. 1989. Molecular analysis of a region of the group B streptococcus chromosome involved in type III capsule expression. Infect. Immun. 57:3058-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marques, M. B., D. L. Kasper, M. K. Pangburn, and M. R. Wessels. 1992. Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of type III group B streptococci. Infect. Immun. 60:3986-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moran, A. P., M. M. Prendergast, and B. J. Appelmelk. 1996. Molecular mimicry of host structures by bacterial lipopolysaccharides and its contribution to disease. FEMS Immunol. Med. Microbiol. 16:105-115. [DOI] [PubMed] [Google Scholar]
- 31.Morona, J. K., J. C. Paton, D. C. Miller, and R. Morona. 2000. Tyrosine phosphorylation of CpsD negatively regulates capsular polysaccharide biosynthesis in Streptococcus pneumoniae. Mol. Microbiol. 35:1431-1442. [DOI] [PubMed] [Google Scholar]
- 32.Okada, N., R. T. Geist, and M. G. Caparon. 1993. Positive transcriptional control of mry regulates virulence in the group A streptococcus. Mol. Microbiol. 7:893-903. [DOI] [PubMed] [Google Scholar]
- 33.Paoletti, L. C., R. A. Ross, and K. D. Johnson. 1996. Cell growth rate regulates expression of group B Streptococcus type III capsular polysaccharide. Infect. Immun. 64:1220-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pincus, S. H., M. J. Smith, H. J. Jennings, J. B. Burritt, and P. M. Glee. 1998. Peptides that mimic the group B streptococcal type III capsular polysaccharide antigen. J. Immunol. 160:293-298. [PubMed] [Google Scholar]
- 35.Rubens, C. E., L. M. Heggen, R. F. Haft, and M. R. Wessels. 1993. Identification of cpsD, a gene essential for type III capsule expression in group B streptococci. Mol. Microbiol. 8:843-855. [DOI] [PubMed] [Google Scholar]
- 36.Rubens, C. E., M. R. Wessels, L. M. Heggen, and D. L. Kasper. 1987. Transposon mutagenesis of type III group B streptococcus: correlation of capsule expression with virulence. Proc. Natl. Acad. Sci. USA 84:7208-7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schrag, S. J., S. Zywicki, M. M. Farley, A. L. Reingold, L. H. Harrison, L. B. Lefkowitz, J. L. Hadler, R. Danila, P. R. Cieslak, and A. Schuchat. 2000. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N. Engl. J. Med. 342:15-20. [DOI] [PubMed] [Google Scholar]
- 38.Shigeoka, A. O., N. S. Rote, J. I. Santos, and H. R. Hill. 1983. Assessment of the virulence factors of group B streptococci: correlation with sialic acid content. J. Infect. Dis. 147:857-863. [DOI] [PubMed] [Google Scholar]
- 39.Steenbergen, S. M., and E. R. Vimr. 2003. Functional relationships of the sialyltransferases involved in expression of the polysialic acid capsules of Escherichia coli K1 and K92 and Neisseria meningitidis groups B or C. J. Biol. Chem. 278:15349-15359. [DOI] [PubMed] [Google Scholar]
- 40.Suarez, N., L. F. Fraguas, E. Texeira, H. Massaldi, F. Batista-Viera, and F. Ferreira. 2001. Production of capsular polysaccharide of Streptococcus pneumoniae type 14 and its purification by affinity chromatography. Appl. Environ. Microbiol. 67:969-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi, S., Y. Aoyagi, E. E. Adderson, Y. Okuwaki, and J. F. Bohnsack. 1999. Capsular sialic acid limits C5a production on type III group B streptococci. Infect. Immun. 67:1866-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai, C. M. 2001. Molecular mimicry of host structures by lipooligosaccharides of Neisseria meningitidis: characterization of sialylated and nonsialylated lacto-N-neotetraose (Galβ1-4GlcNAcβ1-3Galβ1-4Glc) structures in lipooligosaccharides using monoclonal antibodies and specific lectins. Adv. Exp. Med. Biol. 491:525-542. [DOI] [PubMed] [Google Scholar]
- 43.van Dam, J. E. G., A. Fleer, and H. Snippe. 1990. Immunologenicity and immunochemistry of Streptococcus pneumoniae capsular polysaccharides. Antonie Leeuwenhoek 58:1-47. [DOI] [PubMed] [Google Scholar]
- 44.Vogel, U., A. Weinberger, R. Frank, A. Muller, J. Kohl, J. P. Atkinson, and M. Frosch. 1997. Complement factor C3 deposition and serum resistance in isogenic capsule and lipooligosaccharide sialic acid mutants of serogroup B Neisseria meningitidis. Infect. Immun. 65:4022-4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wertman, K. F., A. R. Wyman, and D. Botstein. 1986. Host/vector interactions which affect the viability of recombinant phage lambda clones. Gene 49:253-262. [DOI] [PubMed] [Google Scholar]
- 46.Wessels, M. R., W. J. Benedi, H. J. Jennings, F. Michon, J. L. DiFabio, and D. L. Kasper. 1989. Isolation and characterization of type IV group B Streptococcus capsular polysaccharide. Infect. Immun. 57:1089-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wessels, M. R., R. F. Haft, L. M. Heggen, and C. E. Rubens. 1992. Identification of a genetic locus essential for capsule sialylation in type III group B streptococci. Infect. Immun. 60:392-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wessels, M. R., V. Pozsgay, D. L. Kasper, and H. J. Jennings. 1987. Structure and immunochemistry of an oligosaccharide repeating unit of the capsular polysaccharide of type III group B Streptococcus. A revised structure for the type III group B streptococcal polysaccharide antigen. J. Biol. Chem. 262:8262-8267. [PubMed] [Google Scholar]
- 49.Wessels, M. R., C. E. Rubens, I. V. J. Bened, and D. L. Kasper. 1989. Definition of a bacterial virulence factor: sialylation of the group B streptococcal capsule. Proc. Natl. Acad. Sci. USA 86:8983-8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woodcock, D. M., P. J. Crowther, J. Doherty, S. Jefferson, E. DeCruz, M. Noyer-Weidner, S. S. Smith, M. Z. Michael, and M. W. Graham. 1989. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 17:3469-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yim, H. H., and C. E. Rubens. 1998. Site-specific homologous recombination mutagenesis in group B streptococci. Methods Cell Sci. 20:13-20. [Google Scholar]