Abstract
Acetic acid bacteria are obligate aerobes able to oxidize ethanol, sugar alcohols, and sugars into their corresponding acids. Among them, Acetobacter and Gluconacetobacter species have very high ethanol oxidation capacity, leading to accumulation of vast amounts of acetic acid outside the cell. Since these bacteria are able to grow in media with high concentrations of acetic acid, they must possess a specific mechanism such as an efflux pump by which they can resist the toxic effects of acetic acid. In this study, the efflux pump of Acetobacter aceti IFO 3283 was examined using intact cells and membrane vesicles. The accumulation of acetic acid/acetate in intact cells was increased by the addition of a proton uncoupler and/or cyanide, suggesting the presence of an energy-dependent efflux system. To confirm this, right-side-out and inside-out membrane vesicles were prepared from A. aceti IFO 3283, and the accumulation of acetic acid/acetate in the vesicles was examined. Upon the addition of a respiratory substrate, the accumulation of acetic acid/acetate in the right-side-out vesicles was largely decreased, while its accumulation was very much increased in the inside-out vesicles. These respiration-dependent phenomena observed in both types of membrane vesicles were all sensitive to a proton uncoupler. Acetic acid/acetate uptake in the inside-out membrane vesicles was dependent not on ATP but on the proton motive force. Furthermore, uptake was shown to be rather specific for acetic acid and to be pH dependent, because higher uptake was observed at lower pH. Thus, A. aceti IFO 3283 possesses a proton motive force-dependent efflux pump for acetic acid.
Acetic acid bacteria are obligate aerobes that belong to the α-Proteobacteria and have a strong ability to oxidize ethanol, sugar alcohols, and sugars into their corresponding organic acids. Such oxidation reactions are traditionally called oxidative fermentation, since they involve incomplete oxidation of these compounds. These bacteria accumulate the corresponding incomplete oxidation products in large quantities in their surrounding environment. Acetic acid fermentation is a typical oxidative fermentation and is used industrially to produce vinegar.
Acetic acid bacteria were divided into five to six genera (16, 21, 22), of which Acetobacter and Gluconacetobacter species can tolerate high concentrations of acetic acid, which explains their use in vinegar production. However, in addition to their ability to oxidize ethanol, Acetobacter and Gluconacetobacter species can further oxidize acetic acid, a phenomenon called acetate overoxidation. In ethanol culture, these bacteria exhibit three growth phases; they first grow by completely oxidizing ethanol into acetic acid, then stop growing for a period before they resume growing by utilizing the accumulated acetic acid during the overoxidation phase (1, 13, 14). This overoxidation had led to confusion in understanding the mechanism responsible for acetic acid resistance, because it may also cause acetic acid resistance by consuming acetic acid.
For Acetobacter aceti, the isolation of acetic acid-sensitive mutants (2, 3) and proteome analysis of acetic acid-induced proteins (10) have been performed without regard for this overoxidation-dependent phenomenon. The overoxidation of acetic acid is caused by increased activity of tricarboxylic acid cycle enzymes and acetyl-coenzyme A synthetase (14) and also by increased NADH oxidase activity in the respiratory chain (unpublished data). The acetate resistance genes isolated from A. aceti mutants encode citrate synthase and a protein involved in acetate assimilation (2, 3), and aconitase is increased in the presence of acetic acid in the culture (10). The genes and proteins are involved as part of the acetate assimilating system active during the overoxidation phase. However, since there is no acetate utilization during ethanol oxidation and the diauxic phase of Acetobacter spp. (1, 13, 14), another mechanism must be responsible for acetic acid resistance. The most plausible mechanism is an efflux pump in the cytoplasmic membrane that pumps acetic acid from the cytoplasm to the outside. However, no candidate efflux protein has been detected in acetic acid bacteria, although as many as 50 protein species have been observed by proteomic analysis to increase after acetic acid adaptation in A. aceti (5, 17).
To examine whether an efflux pump is responsible for acetic acid resistance in acetic acid bacteria, we measured acetic acid and acetate transport in intact cells of A. aceti IFO 3283. In addition, we prepared right-side-out (RSO) and inside-out (ISO) membrane vesicles and measured acetic acid or acetate accumulation or uptake. The results suggested that A. aceti IFO 3283 has an efflux pump specific for acetic acid which is driven by a proton motive force but not by ATP.
MATERIALS AND METHODS
Materials.
[1-14C] sodium acetate (9.25 MBq, 50 mCi/mmol, 200 μCi/ml) was purchased from Amersham, and 3,3′-diisopropylthiodicarbocyanine [diS-C3-(5)] and bis(1,3-diethylbarbituric acid) pentamethin oxonol [diBA-C2(5)] were purchased from Molecular Probes Inc. 9-Aminoacridine (9-AA) was from Sigma. All other chemicals were commercial products of guaranteed grade.
Bacterial strains and growth conditions.
A. aceti IFO 3283 was used in this study. Cells were maintained on an agar slant which was prepared by adding 1.7% agar and 0.5% CaCO3 to a potato medium (6) consisting of 5 g of glucose, 20 g of glycerol, 10 g of yeast extract, 10 g of polypeptone, and 100 ml of potato extract in 1 liter of tap water. Cells from the slant were inoculated in 5 ml of the potato medium and cultivated at 30°C with rotary shaking at 200 rpm until a Klett unit (Klett Summerson photoelectric colorimeter, Klett MFG Co.) of 250 was reached. The seed culture was then transferred to 100 ml of glycerol medium consisting of 0.2% each of glycerol, yeast extract, and polypeptone in a 500-ml Erlenmeyer-flask and then grown at 30°C with rotary shaking at 200 rpm to the late exponential phase.
Preparation of membrane vesicles.
Cells were collected by centrifugation at 7,000 × g for 10 min and washed twice with 50 mM potassium phosphate buffer (KPB, pH 6.5) including 5 mM MgCl2. Spheroplasts were prepared by essentially the same procedure used for Acetobacter lovaniensis (originally called A. aceti) IFO 3284 (7). The washed cells were resuspended in 0.1 M Tris-HCl (pH 8.0) containing 10% sucrose and 1% sodium EDTA (pH 10.6), and then lysozyme and DNase were added to final concentrations of 1 mg/ml and 6 μg/ml, respectively. The suspension was incubated for 1 h at 30°C with shaking and then centrifuged at 7,000 × g for 10 min to isolate spheroplasts.
To prepare RSO membrane vesicles, the precipitated spheroplasts were suspended with a minimal volume (0.7 g/ml) of 50 mM KPB (pH 6.5) containing 10% sucrose and rapidly diluted into 200 ml of 50 mM KPB (pH 6.5) containing 5 mM MgSO4 and 6 μg/ml of DNase to lyse by osmotic shock. After incubation for 30 min at room temperature, the solution was centrifuged twice at 7,000 × g for 10 min, and the supernatant was centrifuged at 48,000 × g for 30 min. The precipitate was suspended in 50 mM KPB (pH 6.5) containing 5 mM MgSO4 at 0.5 g wet weight/ml and stored at −80°C as RSO membrane vesicles.
To prepare ISO membrane vesicles, spheroplasts were suspended in 50 mM KPB (pH 6.5) containing 5 mM MgSO4 and 6 μg/ml of DNase at a concentration of 0.7 g wet spheroplasts/ml. The suspension was passed through a French pressure cell at 5,000 lb/in2, and after removing cell debris by centrifugation at 9,000 × g for 10 min, the supernatant was centrifuged at 105,000 × g for 90 min. The precipitate was suspended in 50 mM KPB (pH 6.5) containing 5 mM MgSO4 at 0.2 g wet weight/ml and stored at −80°C as ISO membrane vesicles.
Measurement of the membrane potential (Δψ) and pH gradient (ΔpH).
Generation of Δψ with RSO and ISO membrane vesicles was monitored by following fluorescence quenching of diS-C3-(5) for inside negative potential and diBA-C2(5) for inside positive potential, respectively (8). Measurement of Δψ (inside negative) was performed at 25°C in a reaction mixture (total, 1 ml) consisting of 50 mM KPB (pH 6.5), 5 mM MgSO4, 1 μM diS-C3-(5), and RSO membrane vesicles (≈100 μg of protein), and fluorescence emission was monitored at 670 nm with excitation at 622 nm. For the measurement of Δψ (inside positive), the reaction mixture (1 ml, total volume) contained 50 mM KPB (pH 6.5), 5 mM MgSO4, 2 μM diBA-C2-(5), and ISO membrane vesicles (≈500 μg of protein). The reaction was carried out at 25°C with fluorescence emission at 614 nm and excitation at 588 nm. In either case, the reaction was started by the addition of an electron donor, such as lactate, followed by the addition of 0.1 μM nigericin, and stopped by the addition of 1 μM valinomycin. The Δψ (inside positive) generated with ISO vesicles was quantified by comparison to valinomycin-induced potassium diffusion potentials (Kout+→Kin+).
ΔpH (inside acidic) was measured with ISO vesicles by following the fluorescence quenching of 9-AA (20). The fluorescence was monitored by emission at 480 nm with excitation at 400 nm in the reaction mixture (1 ml total) consisting of 50 mM KPB (pH 6.5), 5 mM MgSO4, 5 μM 9-AA, and ISO membrane vesicles (≈100 μg of protein). Data were quantified by comparison to an artificial ΔpH established by suspending the vesicles (pH 6.0) in 50 mM KPB buffer of different pHs from 6.0 to 8.0.
Measurement of oxidase activity.
Oxidase activity was measured using a Clark-type oxygen electrode at 25°C with 10 mM lactate, 10 mM succinate, or 0.5 mM NADH as the substrate. The reaction mixture (0.6 ml total) contained ISO membrane vesicles, 50 mM KPB (pH 6.5), and substrate, and the reaction was started by the addition of substrate. One unit was defined as the amount of enzyme which reduced 1 natom of O per min.
Transport assay.
For the transport assay with intact cells, cells were collected at the late exponential phase, washed once with 50 mM KPB (pH 6.5), and suspended in glycerol medium containing 1.0% acetic acid (pH 3.5) to a final protein concentration of about 1.0 mg/ml. For the assay with membrane vesicles, RSO or ISO membrane vesicles were suspended with 1 ml of 50 mM KPB (pH 6.5) containing 5 mM MgSO4 at a final protein concentration of 10 to 500 μg/ml. The reaction was started by adding acetic acid containing [1-14C]sodium acetate (8 μCi, 6.25 μCi/mmol) to a final concentration of 0.4 mM into 1 ml of the cell or membrane suspension. Samples of cells or membrane vesicles, 50 μl in volume, were withdrawn at intervals and filtered through 0.45-μm-pore-size cellulose-acetate filters. The filters were quickly washed with 3 ml of cold 50 mM KPB (pH 6.5). After each filter membrane was put into a vial and 5 ml of scintillator solution (ACS II Scintillation Cocktail, Amersham) was added, retained radioactivity was counted by a scintillation counter.
Protein content.
Protein content was determined by the amido black method (15). Each sample was precipitated with trichloroacetic acid in the presence of sodium dodecyl sulfate, filtered on a Millipore membrane, and stained with amido black 10B. The protein-dye complex that formed was then extracted with eluant solution, and the absorbance of the extract was measured at 630 nm. Bovine serum albumin was used as a standard protein.
RESULTS
Acetic acid and acetate accumulation in cells of A. aceti IFO 3283.
The most plausible mechanism for acetic acid resistance in acetic acid bacteria is the operation of an efflux pump in the cytoplasmic membrane which pumps out toxic acetic acid and enables the cell to grow in the presence of high concentrations of it. In fact, it has been observed that an A. aceti strain adapted to high levels of acetic acid exhibits lower intracellular acetate concentrations than does a nonadapted strain (18).
To confirm the findings, acetic acid/acetate transport in A. aceti IFO 3283 was examined using [1-14C]sodium acetate and intact cells. When the labeled acetate was added to the cell suspension containing 1% acetic acid, the pH of the suspension being held at 3.5, only marginal acetate accumulation was observed, but it was increased by the addition of either carbonylcyanide m-chlorophenylhydrazone (CCCP) or KCN and more by the addition of both inhibitors (data not shown). The results suggested that at such a lower external pH, acetate can be passively accumulated but is pumped out by an efflux system which is inhibited by CCCP and/or KCN. However, since the metabolic conversion of acetate may occur inside the cell, the results obtained with the intact cells may lead to some under- or overestimation of the ability to pump acetate out of the cell.
Preparation of membrane vesicles from A. aceti IFO 3283.
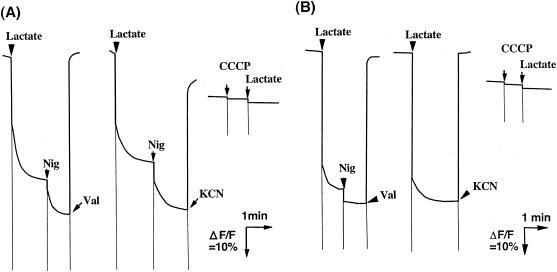
To overcome the uncertainty of the results obtained using intact cells, we tried to measure acetate accumulation or uptake with membrane vesicles from A. aceti IFO 3283. As described in Materials and Methods, we prepared RSO and ISO membrane vesicles by osmotic lysis of spheroplasts and by passage through a French pressure cell at relatively low applied pressures. As shown in Fig. 1, the RSO and ISO membrane vesicles thus prepared generated a lactate-dependent Δψ of inside negative and inside positive, respectively. The Δψ generated in opposite directions in the two membrane vesicle types was completely dissipated by the addition of nigericin and valinomycin, 2 mM KCN, or 10 μM CCCP (Fig. 1). The magnitude of the Δψ was dependent on the concentration of the membrane vesicles but was saturated at high concentrations (data not shown for RSO membrane vesicles and see Fig. 2A for ISO membrane vesicles). In ISO membrane vesicles, Δψ generation was also induced by succinate or NADH as well as by lactate, suggesting that it is generated by respiration.
FIG. 1.
Membrane potential generation in RSO (A) and ISO (B) membrane vesicles of A. aceti IFO 3283. The reaction mixture contained RSO membrane vesicles (A: 38.5 μg of protein) or ISO membrane vesicles (B: 415 μg of protein) in 50 mM KPB (pH 6.5) containing 5 mM MgSO4. The reaction was started by the addition of 10 mM lactate, followed by the addition of 0.025 μM nigericin (Nig) and 1 μM valinomycin (Val) or 2 mM KCN. Ten micromolar CCCP was also added before the addition of lactate. Fluorescence quenching of diS-C3-(5) (A) and diBA-C2-(5) (B) was monitored as described in Materials and Methods.
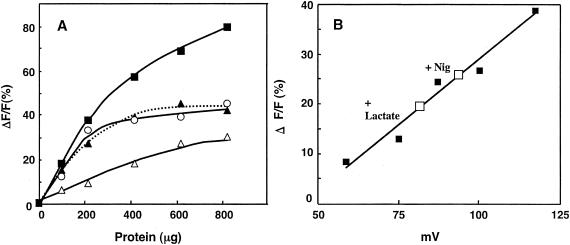
FIG. 2.
Membrane potential generation with different respiratory substrates and quantification of lactate-dependent membrane potential in ISO membrane vesicles. (A) The reaction mixtures contained different concentrations of ISO membrane vesicles in 50 mM KPB (pH 6.5) containing 5 mM MgSO4 and 2 μM diBA-C2-(5). The reactions were started by the addition of 10 mM lactate (▪), 10 mM succinate (▴), 0.5 mM NADH (○), or 3 mM ATP (pH 7.0) (▵), followed by the addition of nigericin (Nig) and valinomycin. ΔF/F indicates the rate (%) of fluorescence quenched per total unit of fluorescence. (B) ISO membrane vesicles were washed twice with 50 mM sodium phosphate buffer (NaPB, pH 6.5) to replace KPB with NaPB. The ISO membrane vesicles (10 μl; 101 μg of protein) were used for valinomycin-induced potassium diffusion potential measurement (▪), as described in Materials and Methods, and also for lactate-dependent Δψ (□) without Nig (+Lactate) or with Nig (+Nig) in 1 ml of the same buffer system as A.
The ISO membrane vesicles exhibited lactate, succinate and NADH oxidase activities of 0.120, 0.079, and 0.063 units/mg, respectively, and the activity was correlated with the magnitude of the Δψ generated (Fig. 2A). Furthermore, ATP could also be used to energize the vesicles (Fig. 2A), suggesting that the ISO vesicles still retained F1-ATPase with at least some level of exposure to the outside of the membrane vesicles. In RSO membrane vesicles, NADH, a membrane-nonpermeating substrate, could not generate any Δψ (inside positive or inside negative) (data not shown) despite its ability to generate a relatively large Δψ, inside positive, in ISO membrane vesicles, as described. Thus, the RSO membrane vesicles seemed not to have any substantial contamination by ISO membrane vesicles. The ISO membrane vesicles also seemed not to be contaminated by RSO vesicles, because lactate-dependent generation of Δψ (inside negative) was not observed (data not shown).
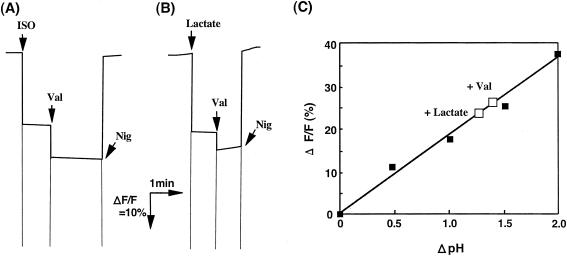
The lactate-dependent Δψ (inside positive) generation with ISO membrane vesicles was quantified based on comparison with potassium diffusion potential measurements (Fig. 2B). As shown, the Δψ generated with lactate was estimated to be 82 mV and 95 mV without and with nigericin, respectively. Lactate-dependent ΔpH generation with the ISO membrane vesicles was also estimated by 9-AA fluorescence measurement (Fig. 3). As shown, ISO membrane vesicles generated a ΔpH (inside acidic) of 1.33 and 1.46 without and with valinomycin, respectively. These data suggest that ISO membrane vesicles could generate a proton motive force consisting of a Δψ of 82 mV and a ΔpH of 1.33, total proton motive force of 161 mV, and also that the addition of either nigericin or valinomycin to ISO membrane vesicles could not completely compensate for the ΔpH or Δψ, respectively, so that ISO membrane vesicles produced a proton motive force of 95 mV and 86 mV in the presence of nigericin and valinomycin, respectively.
FIG. 3.
ΔpH measurement in ISO membrane vesicles of A. aceti IFO 3283. 9-AA fluorescence was measured with ISO membrane vesicles (101 μg of protein) prepared with 50 mM KPB (pH 6.0) as described in Materials and Methods. (A) The reaction was started by the addition of ISO vesicles to 50 mM KPB (pH 8.0), followed by the addition of 1 μM valinomycin (Val) and 0.1 μM nigericin (Nig) as indicated. (B) The reaction was started by the addition of lactate to the assay mixture containing ISO vesicles in 50 mM KPB (pH 6.0). (C) The quenching data (▪) obtained as shown in A by suspending the vesicles (pH 6.0) in 50 mM KPB buffer of pH 6.0, 6.5, 7.0, 7.5, and 8.0 were plotted against ΔpH, and lactate-dependent quenching data (□) without Val (+Lactate) or with Val (+Val) obtained in B were also plotted.
Acetic acid and acetate accumulation in RSO membrane vesicles.
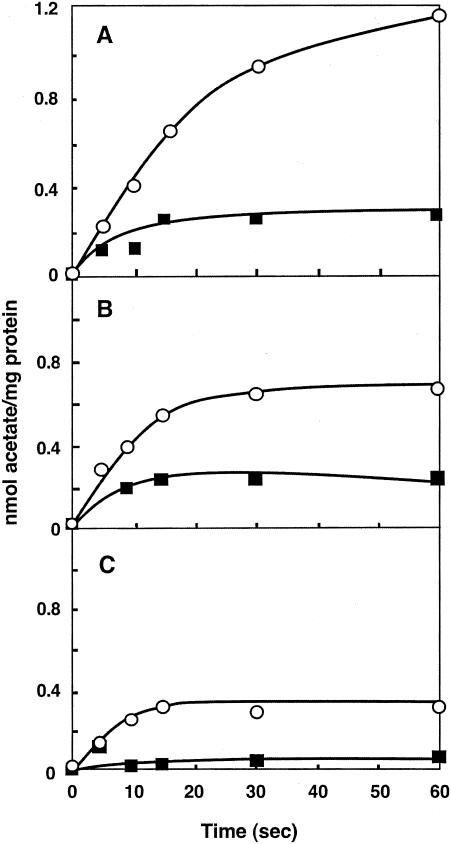
Accumulation of acetic acid and acetate in RSO membrane vesicles was examined. In contrast to intact cells, RSO membrane vesicles accumulated a relatively large amount of acetic acid/acetate, which was higher in the presence of a higher ΔpH between the vesicle inside (pH 6.5 or 5.0) and outside (pH 5.0 or 3.5) (Fig. 4). In spite of the presence or absence of a ΔpH, accumulation was almost completely eliminated by addition of the respiratory substrate lactate. Thus, as occurred with the intact cells having endogenous respiration, RSO membrane vesicles inhibited acetic acid/acetate accumulation as long as respiration was functioning.
FIG. 4.
Accumulation of acetic acid or acetate in RSO membrane vesicles of A. aceti IFO 3283. Accumulation of [1-14C]acetate was measured, as described in Materials and Methods, in the absence (○) and the presence (▪) of 10 mM lactate. The reactions were performed with RSO membrane vesicles (38.5 μg of protein) prepared with 50 mM KPB (pH 6.5) in McIlvaine buffer (pH 3.5) (A), in 50 mM MacIlvaine buffer (pH 5.0) (B), and also with vesicles prepared with 50 mM KPB (pH 5.0) in 50 mM KPB (pH 5.0) (C).
Acetic acid and acetate uptake in ISO membrane vesicles.
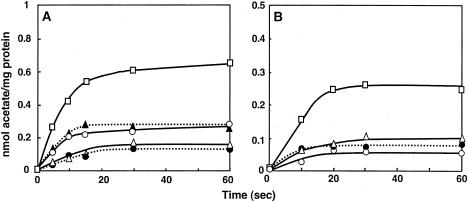
Uptake of acetic acid and acetate into ISO membrane vesicles was also examined. There was a weak acetic acid/acetate uptake into the vesicles when no substrate was present, but the uptake was stimulated in the presence of the respiratory substrate lactate, and this activity was completely blocked by the addition of CCCP (Fig. 5 and 6). As shown in Fig. 5A, the lactate-dependent acetic acid/acetate uptake was partially inhibited by either nigericin or valinomycin and completely inhibited by both ionophores together. The partial inhibition seems to be related to the findings that conversion of Δψ or ΔpH was not fully achieved upon addition of valinomycin or nigericin, respectively (Fig. 2 and 3). Furthermore, acetic acid/acetate uptake was observed after the addition of ATP instead of a respiratory substrate, although the intensity was lower than that for lactate (Fig. 5B). The ATP-dependent uptake was inhibited by dicyclohexylcarbodiimide, an inhibitor of F0-ATPase, indicating that it also occurs via a proton motive force generated by ATP hydrolysis with ATPase but not by ATP itself.
FIG. 5.
Acetic acid or acetate uptake in ISO membrane vesicles of A. aceti IFO 3283. The transport of [1-14C]acetate was measured with ISO membrane vesicles at 537 μg (A) or 540 μg (B) of protein in 50 mM KPB (pH 6.5) containing 5 mM MgSO4, as described in Materials and Methods. When indicated, 0.025 μM nigericin (Nig), 1 μM valinomycin (Val), 10 μM CCCP, or 100 μM DCCD was added to the ISO membrane vesicles suspension and incubated before the addition of [1-14C]acetate. (A) The reaction was carried out in the absence (•) or presence of 10 mM lactate (□), lactate and Nig (▴), lactate and Val (○), or lactate, Nig and Val (▵). (B) The reaction was done in the absence (•) or presence of 3 mM ATP (□), ATP and CCCP (○), or ATP and DCCD (▵).
FIG. 6.
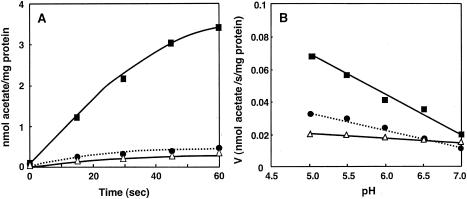
Effect of pH on acetic acid or acetate uptake in ISO membrane vesicles of A. aceti IFO 3283. The reactions were carried out using ISO membrane vesicles (545 μg of protein) as described in Materials and Methods. (A) The reaction was carried out in 50 mM MacIlvaine buffer (pH 5.0) in the absence of lactate and CCCP (•) or in the presence of 10 mM lactate (▪) or 10 mM lactate and 10 μM CCCP (▵). (B) The reactions were carried out under different buffer conditions (MacIlvaine buffer at pH 5.0 and 5.5 and 50 mM KPB at pH 6.0, 6.5, and 7.0) with lactate (▪) and with lactate and CCCP (▵) and also in the absence of the substrate and the inhibitor (•).
Substrate specificity in acetic acid and acetate uptake of ISO membrane vesicles.
There is some uncertainty whether acetic acid or acetate is the substrate for the efflux pump, because sodium acetate equilibrates between protonated acetic acid and deprotonated acetate in solution. Since the ratio of the two species is pH dependent, we may expect which species is the substrate for the pump by examining uptake at different pHs. As can be compared in Fig. 5A and Fig. 6A, uptake was largely increased at pH 5 relative to pH 6.5. And also, as shown in Fig. 6B, there was a clear pH dependence for acetic acid/acetate uptake in ISO membrane vesicles, being higher at lower pH. Although the passive diffusion of acetic acid that occurs without a respiratory substrate was also increased by decreasing the pH outside, the uptake of acetic acid was very much increased by the addition of lactate, and the increased acetic acid accumulation also exhibited pH dependence.
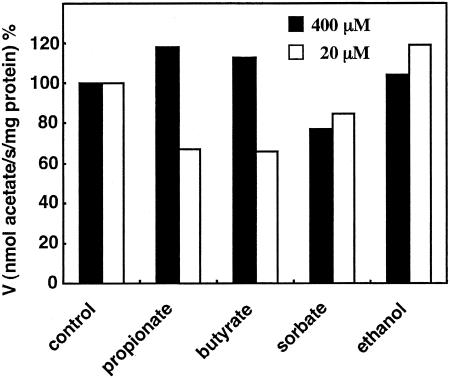
To further characterize the substrate specificity, acetic acid/acetate uptake in ISO membrane vesicles was examined in the presence of 2.5- and 50-fold molar excesses of other substrates, such as propionic acid, butyric acid, sorbic acid, and ethanol (Fig. 7). All these compounds could dissipate the Δψ generated in RSO membrane vesicles at a millimolar concentration except for propionate, which does so at a concentration of 200 mM or more, like acetic acid (data not shown). Thus, the uptake experiments were performed with 20 μM and 400 μM acetate in the presence of 1 mM of other acids or ethanol. As shown, propionic acid and butyric acid inhibited acetic acid/acetate uptake a little at a 50-fold molar excess but not at a 2.5-fold molar excess. Sorbic acid and ethanol did not have any effect on uptake, although sorbic acid had some inhibitory activity by exhibiting some uncoupling ability.
FIG. 7.
Effect of other substrates on acetic acid or acetate uptake in ISO membrane vesicles of A. aceti IFO 3283. By adding acetic acid containing [1-14C]sodium acetate to a final concentration of 20 μM (open bar) or 0.4 mM (solid bar), the transport assay was carried out at pH 5.0 with ISO membrane vesicles (380 μg of protein) in the absence (control) or presence of 1 mM propionate, butyrate, sorbate, or ethanol.
DISCUSSION
Acetic acid resistance is a crucial ability allowing acetic acid bacteria to stably produce large amounts of acetic acid. In Acetobacter and Gluconacetobacter species, spontaneous mutations occur at high frequencies and sometimes lead to simultaneous defects in their acetate-producing (ethanol-oxidizing) and acetic acid resistance abilities (11, 19). Furthermore, alcohol dehydrogenase (ADH) disruption in Acetobacter pasteurianus has also been shown to lead to loss of acetic acid resistance (1). Since ADH deficiency causes defects in ethanol respiration, loss of acetic acid resistance may be related to respiration, from which a proton motive force is generated, via ubiquinol oxidase (9) (see Fig. 8B). Therefore, if the acetic acid resistance mechanism requires energy, ethanol respiration and the energy it produces may be important. An energy-requiring mechanism responsible for acetic acid resistance is expected to be an efflux pump in the cytoplasmic membrane which pumps out acetic acid and enables the cell to grow in the presence of high concentrations of acetic acid. For example, Pdr12 in Saccharomyces cerevisiae acts as an ATP-dependent acetate (but not acetic acid) efflux pump, allowing the cell to adapt to high acetic acid concentrations (4, 12).
FIG. 8.
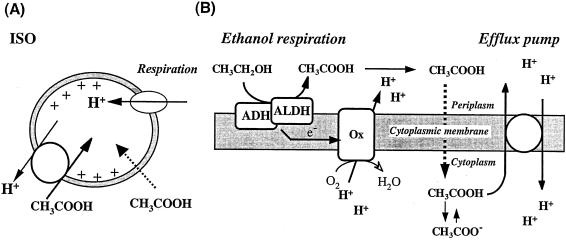
Schematic representation of acetic acid uptake in ISO membrane vesicles and model of the efflux pump present in the cytoplasmic membrane of A. aceti. (A) ISO membrane vesicles generate a proton motive force, inside positive and acidic, by respiration (lactate oxidation), which then drives acetic acid uptake. The stoichiometry of protons and acetic acid in the pump is unknown. There is also a passive diffusion of acetic acid, which is ΔpH dependent. (B) In intact cells, ethanol respiration produces acetic acid from ethanol via the activity of alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH), which also donate electrons to a terminal ubiquinol oxidase (Ox) that in turn generates a proton motive force. The acetic acid produced can passively penetrate into the cytoplasm but is pumped out by an efflux pump which is energized by the proton motive force.
To examine this hypothesis, we first measured acetic acid and acetate accumulation in intact cells and in RSO membrane vesicles of A. aceti IFO 3283. Acetate accumulation was low in intact cells having endogenous respiration, but it was increased by the addition of protonophores and/or a respiratory inhibitor. In contrast, RSO membrane vesicles accumulated a relatively high concentration of acetic acid in a ΔpH-dependent passive diffusion manner, but accumulation was decreased almost completely by respiration. The results suggest that cells or RSO membrane vesicles actively pump out acetic acid which has passively penetrated into the cell by using energy produced by respiration. Such an acetic acid efflux mechanism in A. aceti was also demonstrated using ISO membrane vesicles. The ISO membrane vesicles, able to generate a large enough inside-positive Δψ by respiration, could accumulate acetic acid/acetate in the presence of respiratory substrates in a proton uncoupler-sensitive manner (Fig. 8A). The results obtained for the vesicles also indicated that the energy source required for uptake is the proton motive force but not ATP (Fig. 5).
Another question associated with the efflux pump was the identity of the substrate for this transporter, since the acetate anion is expected to be a substrate for the Pdr12 efflux pump in yeast (4, 12). In ISO membrane vesicles of A. aceti, lactate-dependent acetic acid/acetate uptake was proportionally increased by decreasing the pH (Fig. 6). Since lactate oxidase activity has an optimum pH of 6.5, the higher uptake at lower pH seems not to be related to the degree of energy generation. Although it is not definitive evidence, this result suggests that acetic acid but not acetate is transported inside the vesicles. It also suggested that the efflux pump is relatively specific for acetic acid, though it may work for high concentrations of short-chain acids such as propionic acid and butyric acid (Fig. 7).
The efflux pump may not work for ethanol, although Acetobacter species also exhibit resistance to high concentrations of alcohol. In yeast cells, acetic acid is expected to be pumped out by coupling the Pdr12 ABC transporter, which transports acetate anions, with the Pma1 plasma membrane H+-ATPase (12). However, acetic acid bacteria such as A. aceti seem not to have any proton efflux system such as Pma1, and the acetic acid efflux system we observed seems to be an H+-antiporter but not an ABC transporter (Fig. 8A). Thus, it is reasonable to expect that the efflux system of acetic acid bacteria is pumping out “protonated” acetic acid, much the same as a proton motive force-dependent multidrug transporter.
The results obtained in this study therefore suggest that a proton motive force-dependent acetic acid efflux pump exists in A. aceti IFO 3283. Although cells grown in glycerol medium were used in this study, much higher levels of efflux activity are expected to occur in ethanol culture, where Acetobacter species produce and accumulate a large amount of acetic acid outside of the cell and thus cannot survive in such a culture without machinery able to eliminate acetic acid. Therefore, as shown in Fig. 8B, the acetic acid efflux pump seems to be highly expressed and expels acetic acid by using the proton motive force produced by ethanol respiration. We have found much higher activity of the efflux pump in cells in the ethanol culture compared to the glycerol culture used in this study (unpublished observation). Now, we are examining the activity change of the efflux pump in the different growth phases of ethanol cultures of A. aceti IFO 3283.
REFERENCES
- 1.Chinnawirotpisan, P., G. Theeragool, S. Limtong, H. Toyama, O. Adachi, and K. Matsushita. 2003. Quinoprotein alcohol dehydrogenase is involved in catabolic acetate production, while NAD-dependent alcohol dehydrogenase in ethanol assimilation in Acetobacter pasteurianus SKU1108. J. Biosci. Bioeng. 96:564-571. [DOI] [PubMed] [Google Scholar]
- 2.Fukaya, M., H. Takemura, H. Okumura, Y. Kawamura, S. Horinouchi, and T. Beppu. 1990. Cloning of genes responsible for acetic acid resistance in Acetobacter aceti. J. Bacteriol. 172:2096-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukaya, M., H. Takemura, K. Tayama, H. Okumura, Y. Kawamura, S. Horinouchi, and T. Beppu. 1993. The aarC gene responsible for acetic acid assimilation confers acetic acid resistance on Acetobacter aceti. J. Ferment. Bioeng. 76:270-275. [Google Scholar]
- 4.Hatzixanthis, K., M. Mollapour, L. Seymour, B. E. Bauer, G. Krapf, C. Schuller, K. Kuchler, and P. W. Piper. 2003. Moderately lipophilic carboxylate compounds are the selective inducers of the Saccharomyces cerevisiae Pdr12p ATP-binding cassette transporter. Yeast 20:575-585. [DOI] [PubMed] [Google Scholar]
- 5.Lasko, D. R., C. Schwerdel, J. E. Bailey, and U. Sauer. 1997. Acetate-specific stress response in acetate-resistant bacteria: an analysis of protein patterns. Biotechnol. Prog. 13:519-523. [DOI] [PubMed] [Google Scholar]
- 6.Matsushita, K., and M. Ameyama. 1982. d-Glucose dehydrogenase from Pseudomonas fluorescens, membrane-bound. Methods Enzymol. 89: 149-153. [DOI] [PubMed] [Google Scholar]
- 7.Matsushita, K., M. Nonobe, E. Shinagawa, O. Adachi, and M. Ameyama. 1985. Isolation and characterization of outer and cytoplasmic membranes from spheroplasts of Acetobacter aceti. Agric. Biol. Chem. 49:3519-3526. [Google Scholar]
- 8.Matsushita, K., and H. R. Kaback. 1986. d-Lactate oxidation and generation of the proton electrochemical gradient in membrane vesicles from Escherichia coli GR19N and in proteoliposomes reconstituted with purified d-lactate dehydrogenase and cytochrome o oxidase. Biochemistry 25:2321-2327. [DOI] [PubMed] [Google Scholar]
- 9.Matsushita, K., H. Toyama, and O. Adachi. 1994. Respiratory chain and bioenergetics of acetic acid bacteria. Adv. Microb. Physiol. 36:247-301. [DOI] [PubMed] [Google Scholar]
- 10.Nakano, S., M. Fukaya, and S. Horinouchi. 2004. Enhanced expression of aconitase raises acetic acid resistance in Acetobacter aceti. FEMS Microbiol. Lett. 235:315-322. [DOI] [PubMed] [Google Scholar]
- 11.Ohmori, S., T. Uozumi, and T. Beppu. 1982. Loss of acetic acid resistance and ethanol oxidizing ability in an Acetobacter strain. Agric. Biol. Chem. 46:381-389. [Google Scholar]
- 12.Piper, P., C. O. Calderon, K. Hatzixanthis, and M. Mollapour. 2001. Weak acid adaptation: the stress response that confers yeasts with resistance to organic acid food preservatives. Microbiology 147:2635-2642. [DOI] [PubMed] [Google Scholar]
- 13.Saeki, A., M. Taniguchi, K. Matsushita, H. Toyama, G. Theeragool, N. Lotong, and O. Adachi. 1997. Microbiological aspects of acetate oxidation by acetic acid bacteria, unfavorable phenomena in vinegar fermentation. Biosci. Biotechnol. Biochem. 61:317-323. [Google Scholar]
- 14.Saeki, A., K. Matsushita, S. Takeno, M. Taniguchi, H. Toyama, G. Theeragool, N. Lotong, and O. Adachi. 1999. Enzymes responsible for acetate oxidation by acetic acid bacteria. Biosci. Biotechnol. Biochem. 63:2102-2109. [DOI] [PubMed] [Google Scholar]
- 15.Schaffner, W., and C. Weissman. 1973. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal. Biochem. 56:502-514. [DOI] [PubMed] [Google Scholar]
- 16.Sievers, M., C. Gaberthuel, C. Boesch, W. Ludwig, and M. Teuber. 1995. Phylogenetic position of Gluconobacter species as a coherent cluster separated from all Acetobacter species on the basis of 16S ribosomal RNA sequences. FEMS Microbiol. Lett. 126:123-126. [DOI] [PubMed] [Google Scholar]
- 17.Steiner, P., and U. Sauer. 2001. Proteins induced during adaptation of Acetobacter aceti to high acetate concentrations. Appl. Environ. Microbiol. 67:5474-5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steiner, P., and U. Sauer. 2003. Long-term continuous evolution of acetate resistant Acetobacter aceti. Biotechnol. Bioeng. 84:40-44. [DOI] [PubMed] [Google Scholar]
- 19.Takemura, H., S. Horinouchi, and T. Beppu. 1991. Novel insertion sequence IS1380 from Acetobacter pasteurianus is involved in loss of ethanol-oxidizing ability. J. Bacteriol. 173:7070-7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toyomizu, M., K. Okamoto, Y. Akiba, T. Nakatsu, and T. Konishi. 2002. Anacardic acid-mediated changes in membrane potential and pH gradient across liposomal membranes. Biochim. Biophys. Acta 1558:54-62. [DOI] [PubMed] [Google Scholar]
- 21.Yamada, Y., K. Hoshino, and T. Ishikawa. 1997. The phylogeny of acetic acid bacteria based on the partial sequences of 16S ribosomal RNA: The elevation of subgenus Gluconoacetobacter to the generic level. Biosci. Biotechnol. Biochem. 61:1244-1251. [DOI] [PubMed] [Google Scholar]
- 22.Yamada, Y., K. Katsura, H. Kawasaki, Y. Widyastuti, S. Saono, T. Seki, T. Uchimura, and K. Komagata. 2000. Asaia bogorensis gen. nov., sp. nov., an unusual acetic acid bacterium in the alpha-Proteobacteria. Int. J. Evol. Microbiol. 50:823-829. [DOI] [PubMed] [Google Scholar]