Abstract
Burkholderia cenocepacia ZmpA is expressed as a preproenzyme typical of thermolysin-like proteases such as Pseudomonas aeruginosa LasB and Bacillus thermoproteolyticus thermolysin. The zmpA gene was expressed using the pPRO-EXHTa His6 tag expression system, which incorporates a six-His tag at the N-terminal end of the protein, and recombinant ZmpA was purified using Ni-nitrilotriacetic acid affinity chromatography. Upon refolding of the recombinant His6-pre-pro-ZmpA (62 kDa), the fusion protein was autoproteolytically cleaved into 36-kDa (mature ZmpA) and 27-kDa peptides. Site-directed mutagenesis was employed to infer the identity of the active site residues of ZmpA and to confirm that the enzyme undergoes autoproteolytic cleavage. Oligonucleotide mutagenesis was used to replace H465 with G465 or A465, E377 with A377 or D377, or H380 with P380 or A380. Mutagenesis of H465, E377, or H380 resulted in the loss of both autocatalytic activity and proteolytic activity. ZmpA with either substitution in H380 was not detectable in B. cenocepacia cell extracts. The activity of the recombinant ZmpA was inhibited by EDTA and 1,10 phenanthroline, indicating that it is a zinc metalloprotease. ZmpA, however, was not inhibited by phosphoramidon, a classical inhibitor of the thermolysin-like proteases. The refolded mature ZmpA enzyme was proteolytically active against various substrates including hide powder azure, type IV collagen, fibronectin, neutrophil α-1 proteinase inhibitor, α2-macroglobulin, and gamma interferon, suggesting that B. cenocepacia ZmpA may cause direct tissue damage to the host or damage to host tissues through a modulation of the host's immune system.
Burkholderia cepacia is an important pulmonary pathogen in cystic fibrosis (CF) (reviewed in references 29 and 37). Approximately 4 to 7% of CF patients are colonized with strains of the B. cepacia complex, which consists of at least nine genetically distinct species (7, 8). Some CF patients infected with B. cenocepacia develop “cepacia syndrome” characterized by necrotizing pneumonia, fever, bacteremia, and leukocytosis (23). Sixty-nine to eighty-eight percent of clinical B. cepacia complex isolates have been reported to produce protease activity (13, 35, 41). Gotschlich et al. (14) reported that strains of B. cepacia, Burkholderia cenocepacia, and Burkholderia stabilis were positive for extracellular protease activity, whereas strains from Burkholderia dolosa (8), Burkholderia multivorans, and Burkholderia vietnamiensis did not have extracellular protease activity (14).
B. cenocepacia is an opportunistic pathogen that is difficult to treat due to its intrinsic antibiotic resistance. Thus, alternate treatment strategies must be developed to treat B. cenocepacia infections. Our laboratory is developing metalloprotease-based therapeutics for treatment of bacterial infections (48). B. cenocepacia secretes a metalloprotease, ZmpA, formerly known as PSCP (10, 25, 34). Instillation of purified ZmpA into the lungs of rats induces bronchopneumonia, characterized by polymorphonuclear leukocyte infiltration and proteinaceous exudate in the airways (34). Using a rat agar bead model of infection, a B. cenocepacia K56-2 zmpA mutant elicited significantly less lung damage than the wild-type strain (10). In most cases, the B. cenocepacia K56-2 zmpA mutant was cleared from the rat lungs, indicating that ZmpA is required for the persistence of B. cenocepacia K56-2. Neutralizing monoclonal antibodies (MAbs) raised against ZmpA cross-react with a Pseudomonas aeruginosa LasB epitope (25, 26). Immunization with a peptide (341HGFTEQNG349) corresponding to this conserved P. aeruginosa LasB epitope significantly decreases the severity of experimental B. cenocepacia lung infections (48). Although there is evidence from animal infection models that ZmpA plays a major role in the virulence of B. cenocepacia, the direct role of ZmpA in B. cenocepacia pathogenesis is not understood.
Bacterial proteases may exert tissue damage directly by cleaving cellular components such as elastin, collagen, or fibronectin (1, 30, 36). They may also cause tissue damage by affecting the balance between neutrophil elastase and the inhibitors α1-proteinase inhibitor and α2-macroglobulin. The balance between proteases and protease inhibitors may be the major factor in determining tissue integrity. P. aeruginosa LasB has been shown to cleave α1-proteinase inhibitor (39). The importance of this neutrophil elastase/inhibitor balance is demonstrated by the finding that α1-proteinase deficiency is associated with pulmonary emphysema. Bacterial proteases may also exert their effect by cleaving components of the immune system, including immunoglobulins (Ig), complement components, and cytokines such as gamma interferon (IFN-γ), and tumor necrosis factor alpha (reviewed in reference 28). P. aeruginosa LasB has been shown to degrade lactoferrin and transferrin (5). This may make more iron available for bacterial growth or promote oxidant-mediated damage to host tissues.
Many bacterial zinc metalloproteases have been classified as either clan MA or clan MB (43). B. cenocepacia ZmpA appears to belong to family M4 of the MA clan (10). Family M4, also referred to as the thermolysin-like proteases, contains only bacterial metalloproteases. Bacillus thermoproteolyticus thermolysin was the first zinc metalloprotease for which the three-dimensional structure was determined (9). Typically the thermolysin-like metalloproteases contain the HExxH and GAxNEAFSD motifs and have a neutral pH optimum (43). The thermolysin-like metalloproteases usually have specificity for hydrophobic amino acid residues and are inhibited by the zinc metalloprotease inhibitors EDTA, 1,10 phenanthroline, and phosphoramidon (43). ZmpA shares homology with preproenzymes (10), including the thermolysin-like proteases P. aeruginosa LasB, B. thermoproteolyticus thermolysin, and Vibrio cholerae hemagglutinin/protease (2, 16, 42). ZmpA has the conserved HExxH and GAxNEAFSD motifs that are found in the M4 family of proteases (10, 43). The two histidine residues in the 375HExxH380 motif and the glutamic acid in the GAxNEAFSD motif putatively act as the zinc ligands. A water molecule typically provides the fourth zinc ligand. The glutamic acid in the HExxH active site motif and a histidine further downstream are putatively required for proteolytic activity.
In this study, zmpA was expressed in Escherichia coli and the recombinant ZmpA protease was purified and characterized. Putative active site residues and a putative zinc ligand residue were mutagenized, and the effects of these mutations on the autocatalytic and proteolytic activity of ZmpA were determined. The substrate specificities of both native and recombinant ZmpA were also compared.
MATERIALS AND METHODS
Bacterial strains, plasmids, and reagents.
The bacterial strains and plasmids used in this study are described in Table 1. B. cenocepacia strains were grown on tryptic soy agar or B. cepacia selective agar (18) with 200 μg/ml tetracycline (Tc) or 100 μg/ml trimethoprim (Tp) where required. E. coli DH5α was grown in Luria-Bertani broth with 100 μg/ml ampicillin (Ap) and/or 12.5 μg/ml Tc where required. Restriction enzymes and oligonucleotide primers were purchased from Invitrogen (Burlington, Ontario, Canada). T4 DNA ligase, T4 polymerase, and the pAlter mutagenesis kit were purchased from Promega. DNA sequencing was performed at Macrogen Inc. (South Korea). All chemicals were purchased from Sigma-Aldrich except where indicated.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strain | ||
| E. coli DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) phoA supE44 thi-1 gyrA96 relA1 | Invitrogen |
| E. coli TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacΔM15 ΔlacX74 deoR recA1 araD139 Δ(ara-leu)7697 galU galK rpsL (Smr) endA1 nupG | Invitrogen |
| JM109 | endA1 recA1 gyrA96 thi hsdR17(rK− mK+) relA1 supE44 λ− Δ(lac proAB) [F′ traD36 proA+B+lacIqZΔM15] | Promega |
| ES1301 mutS | lacZ53 mutS201::Tn5 thyA36 rha-5 metB1 deoC IN(rrnD-rrnE) | Promega |
| B. cenocepacia Pc715j | Cystic fibrosis sputum isolate | 34 |
| B. cenocepacia K56-2 | Cystic fibrosis sputum isolate | 8 |
| B. cenocepacia K56-2-9 | K56-2 zmpA::tmp | 10 |
| Plasmid | ||
| pCR2.1-TOPO | TOPO-TA cloning vector, Apr Kmr | Invitrogen |
| pEX18Tc | Suicide vector, sacB Tcr | 19 |
| pUCP26 | Broad-host-range vector, Tcr | 54 |
| pCC12 | pEX18Tc with 2.6-kb PstI fragment from Pc715j containing zmpA gene, Tcr | 10 |
| pCC13 | pUCP26 with 2.6-kb PstI fragment from Pc715j containing zmpA gene, Tcr | 10 |
| pPROEX-HTa | E. coli expression vector with six-His tag, TEV protease cleavage site, and inducible trc promoter, Apr | Invitrogen |
| pCK10 | pPROEX-Hta with 1.7-kb fragment containing zmpA, Apr | This study |
| pCK11 | pPROEX-Hta with ZmpA residue H465 changed to G465 (H465G), Apr | This study |
| pCK11b | pPROEX-Hta with ZmpA residue H465 changed to A465 (H465A), Apr | This study |
| pCK12 | pPROEX-Hta with ZmpA residue E377 changed to A377 (E377A), Apr | This study |
| pCK12b | pPROEX-Hta with ZmpA residue E377 changed to D377 (E377D), Apr | This study |
| pCK13 | pPROEX-HTa with ZmpA residue H380 changed to P380 (H380P), Apr | This study |
| pCK13b | pPROEX-HTa with ZmpA residue H380 changed to A380 (H380A), Apr | This study |
| PCK15 | pPROEX-Hta with zmpA from bp 628 to bp 1695 | |
| pAlter-EX1 | Mutagenesis vector, Tcr (GenBank/EMBL accession number U47102) (repaired vector is Apr) | Promega |
| pCK20 | pAlter-EX1 with 2.6-kb fragment from pCC12 containing zmpA cloned into the PstI site, Tcr Aps | This study |
| pCK21 | pAlter-EX1 with ZmpA H465G, Tcr, Apr | This study |
| pCK22 | pAlter-EX1 with ZmpA E377A, Tcr, Apr | This study |
| pCK23 | pAlter-EX1 with ZmpA H380P, Tcr, Apr | This study |
| pCK40 | pUCP26 with ZmpA, Tcr | This study |
| pCK41 | pUCP26 with ZmpA H465G, Tcr | This study |
| pCK41b | pUCP26 with ZmpA H465A, Tcr | This study |
| pCK42 | pUCP26 with ZmpA E377A, Tcr | This study |
| pCK42b | pUCP26 with ZmpA E377D, Tcr | This study |
| pCK43 | pUCP26 with ZmpA H380P, Tcr | This study |
| pCK43b | pUCP26 with ZmpA H380A, Tcr | This study |
Expression and solubilization of recombinant forms of ZmpA.
A 1.7-kb fragment containing the zmpA gene was amplified by PCR from pCC12 (10) with the primers NPPEXF (GAATTCATGAAGAAACTGTCTCG) containing an EcoRI linker (underlined) and NPEXR (AAGCTTTCAATTCACCCCG) containing a HindIII linker (underlined). The resultant product was cloned into pCR2.1-TOPO (Invitrogen) and subcloned into pPROEX-Hta (Invitrogen) at the EcoRI and HindIII sites, resulting in pCK10 (Table 1). Genetic manipulations were generally performed as described by Sambrook et al. (45).
E. coli DH5α pCΚ10 subcultures were induced with 0.6 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at an optical density at 600 nm (OD600) of approximately 0.4. The optimum incubation time was determined by removing 1 ml of the culture at 1, 2, 3, and 4 h after induction and analyzing the normalized samples by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), after which cultures were typically harvested after 3.5 h of induction. Cultures were centrifuged at 8,000 × g for 20 min. Pellets were resuspended in 10 ml sonication buffer (SB; 50 mM sodium phosphate, pH 8.0, 300 mM NaCl), sonicated for 5 15-s bursts and centrifuged at 15,000 × g to determine if the expressed ZmpA was soluble or insoluble. The insoluble pellets were washed stepwise with SB and 2 M sodium chloride, SB and 0.25% Triton X-100, and SB and 2 M sodium chloride. The samples were centrifuged at 6,000 × g between washes. The final pellets were resuspended in SB with 6 M guanidine hydrochloride.
The gene coding for the mature ZmpA protein (bp 655 to 1695; numbering is based on the entire preprozmpA gene) was amplified by PCR with the primers NPEXF (GAATTCGCCGCCGCGACCG) containing an EcoRI linker (underlined) and NPEXR (AAGCTTTCAATTCACCCCG) containing a HindIII linker (underlined). This fragment with an additional 27 bases (bp 628 to 1695) at the 5′ end was amplified by PCR using the primers NPEXF2 (GAATTCCTCGATGCACAGGACC) containing an EcoRI linker (underlined) and NPEXR (AAGCTTTCAATTCACCCCG) containing a HindIII linker (underlined) and cloned as above, resulting in plasmid pCK15. Native ZmpA was purified from culture supernatants as previously described (34).
Refolding of recombinant mature ZmpA and preproZmpA.
The solubilized mature ZmpA or preproZmpA was bound to Ni-nitrilotriacetic acid (NTA) (QIAGEN), which had been preequilibrated in SB, containing 6 M guanidine hydrochloride, for at least 1 h at 4°C. The bound ZmpA was washed with 5 column volumes (CV) of 20 mM Tris-HCl, pH 7.4, 6 M guanidine hydrochloride (buffer A), and 5 CV 50 mM Tris-HCl, pH 7.4, 20% glycerol, 500 mM sodium chloride, and 6 M urea (buffer B), followed by 50 CV buffer B to 50 mM Tris-HCl, pH 7.4, 20% glycerol, 500 mM sodium chloride, 1 M urea (buffer C) linear gradient. The bound ZmpA was washed with 5 CV buffer C, 5 CV 50 mM Tris-HCl, pH 7.4, 10% glycerol, 50 mM NaCl (buffer D), and 5 CV SB. The refolded ZmpA was eluted from the Ni-NTA with 100 mM imidazole. The eluted ZmpA was typically dialyzed against 10 mM Tris-HCl, pH 7.2, prior to proteolytic analysis of the enzyme. Proteolytic activity was determined using 7.5 mg hide powder azure (Sigma-Aldrich) in 1.5 ml 10 mM Tris-HCl (43). One unit of activity was defined as the amount of protease required to cause a change in the OD595 of 0.1 in 1 h (43).
SDS-PAGE and Western immunoblot analysis of ZmpA.
Proteins were separated by SDS-12.5% (except where indicated) PAGE by the method of Laemmli (27). The gels were stained using Coomassie brilliant blue R-250 or transferred to polyvinylidene difluoride membrane (Millipore) (51). Immunoblots were reacted with either MAb 36-6-6 (25) to ZmpA or mouse anti-His tag antibody (GE Healthcare).
N-terminal amino acid sequencing of the recombinant ZmpA.
Partial N-terminal amino acid sequencing was performed on polyvinylidene difluoride-electroblotted proteins at the University of Victoria (UVic-Genome BC Proteomics Center) or at the University of British Columbia (NAPS unit, Protein Microchemistry).
Mutagenesis of zmpA.
A 2.6-kb PstI fragment containing the zmpA gene was subcloned into the pAlter-EX1 vector (Promega), resulting in the vector pCK20. Mutagenic oligonucleotides were designed to change H465 to G465 (ZmpA H465G), E377 to A377 (ZmpA E377A), and H380 to P380 (ZmpA H380P). The primers employed were NPH465G, CCGCGCCACGATCCCGGGTTCACGTCGGG; NPE377A, GTCGCCGGGCATGCGATGAGCCACGGC; and NPH380P, CGAGATGAGCCCCGGGGTGACCGAGGCC. These oligonucleotides were designed to introduce a restriction site (underlined; SmaI, SphI, and SmaI, respectively) for use in subsequent screening. Mutagenic oligonucleotide pairs (CCGCGCCACGATCCGGCTTTCACGTCGGGGGTC and GACCCCCGACGTGAAAGCCGGATCGTGGCGCGG; GACGTCGCCGGGCACGACATGAGCCACGGCGTG and CACGCCGTGGCTCATGTCGTGCCCGGCGACGTC; and GGGCACGAGATGAGCGCCGGCGTGACCGAGGCC and GGCCTCGGTCACGCCGGCGCTCATCTCGTGCCC) were designed to change H465 to A465 (ZmpA H465A), E377 to D377 (ZmpA E377D), and H380 to A380 (ZmpA H380A) in pCK10, respectively, using the QuikChange system (Stratagene).
Mutagenesis was conducted and transformants were screened by PCR with the primers NPPEXF and NPEXR, to ensure that an intact zmpA gene was present. Mutations were confirmed by sequence analysis. Recombinant zmpA genes were PCR amplified with the NPPEXF and NPEXR primers, cloned into pCR2.1-TOPO, excised with EcoRI and HindIII, and ligated into the corresponding sites in pPROEX-Hta. Expression, solubilization, purification, and characterization of the ZmpA mutant proteins were performed as described above.
Complementation of the zmpA deletion mutant K56-2-9.
To determine if ZmpA H465G, ZmpA H465A, ZmpA E377A, ZmpA E377D, ZmpA H380P, and ZmpA H380A had proteolytic activity in B. cenocepacia, SunI-HindIII fragments encompassing the mutated sites from the wild-type (pCK10) or mutant plasmids (pCK11-pCK13b) were cloned into the SunI/HindIII site in pCC12. PstI-HindIII fragments from the resulting constructs containing the zmpA gene with 642 bp of upstream DNA were subsequently cloned into pUCP26, resulting in pCK40 (ZmpA) and plasmids containing mutant zmpA genes (pCK41-pCK43b; Table 1). These constructs were electroporated into B. cenocepacia K56-2-9 as previously described (12). Protease activity was determined on dialyzed brain heart infusion milk agar as previously described (49). Accumulation of the wild-type and mutant ZmpA proteins was determined in cell lysates by Western immunoblotting of SDS-PAGE reacted with rat anti-ZmpA serum.
Protease inhibitor profile of the recombinant ZmpA.
Recombinant ZmpA and native ZmpA (4.0 units) in a volume of 1 ml of 10 mM Tris-Cl, pH 7.2, were incubated with either 1 mM 1,10 phenanthroline, 10 mM EDTA, 10 μM phosphoramidon, 50 μM 4-(amidinophenyl)methanesulfonyl fluoride (APMSF), 0.5 mM phenylmethanesulfonyl fluoride (PMSF), 0.1 mM di-isopropylfluorophosphate (DFP), 5 μM l-trans-epoxysuccinyl-leucylamide-(4-guanidino)-butane (E-64), or 1 μM pepstatin A for 30 min at 37°C. Native and recombinant ZmpA reactions without inhibitor were included as controls. For the phosphoramidon inhibition experiments, P. aeruginosa LasB (10 ng) (Nagase) and B. thermoproteolyticus thermolysin (50 ng) were included as additional controls. The proteolytic activity of the reaction mixtures was measured using the hide powder azure assay. Neutralization by MAb 36-6-6 was tested as previously described (25).
Substrate analysis of the recombinant ZmpA.
Approximately 50 ng native and recombinant ZmpA was incubated with hide powder azure or azocasein in Tris-HCl, pH 7.2, 1 mM CaCl2, and 1 mM MgSO4 for 2 and 24 h, respectively. After the incubation, the samples were centrifuged for 10 min at 10,000 × g and the ODs at 595 nm and 440 nm, respectively, were measured. One unit of activity was defined as the amount of protease required to cause a change in the OD595 of 0.1 per hour at 37°C in the hide powder azure assay. For the analysis of subsequent substrate digestions, 6.0 units of native and recombinant ZmpA activity was used in each assay.
To optimize the substrate digestions by ZmpA, various buffers were tested at a range of pHs in hide blue azure assays, as described above (44). Buffers tested were 25 mM morpholinoethanesulfonic acid, pH 5.6 to 6.7 (MES), 25 mM sodium succinate, pH 4.0 to 5.6, sodium acetate, pH 4.0 to 5.3, 25 mM sodium citrate, pH 3.5 to 6.0, 25 mM piperazine-1,4-bis(2-ethanesulfonic acid) (PIPES), pH 6.5 to 7.2, 25 mM 3-(N-morpholino)propanesulfonic acid (MOPS), pH 6.5 to 7.5, 25 mM Tris-maleate, pH 5.2 to 6.0, and 25 mM Tris-HCl, pH 7.0 to 9.0.
Native and recombinant ZmpA (6 U) was incubated for 24 to 48 h at 37°C with α-1 proteinase inhibitor (Bayer), human fibronectin, type IV collagen, lactoferrin, transferrin, immunoglobulins A, G, and M, rat α2-macroglobulin, and recombinant rat gamma interferon (Sigma) in 25 mM MES, pH 6.5. Native ZmpA, recombinant ZmpA, and substrate only controls were included. In some experiments, P. aeruginosa LasB was included as a positive control for substrate digestions. Digested substrates were separated by SDS-12.5% PAGE by the method of Laemmli (27) or by Tricine-SDS-16% PAGE (46).
RESULTS
Expression and purification of the recombinant mature and preproZmpA.
Attempts to amplify the region corresponding to the mature protein (bp 655 to 1695) were unsuccessful. This is probably due to the high GC content of the DNA encoding the N terminus of the mature ZmpA protein. Thus, a DNA fragment encoding the mature ZmpA with nine additional amino acids at the N terminus was amplified and cloned into the pPROEX-HTa His6 tag expression system. Expression of a 44-kDa protein with the predicted molecular mass was obtained after IPTG induction for 3 h (data not shown). This protein was solubilized in 6 M guanidine-HCl, refolded, and eluted from a Ni-NTA column. A single polypeptide with an approximate molecular mass of 44 kDa was eluted from the column, most likely corresponding to the fusion protein (His6 nine amino acids [LDAQDLIKT]-mature ZmpA, data not shown). This polypeptide was proteolytically inactive, suggesting that it was improperly refolded (data not shown). This inactivity may also be due to the presence of an additional nine amino acids at the N terminus (from the pro region) or to the absence of a full-length pro sequence.
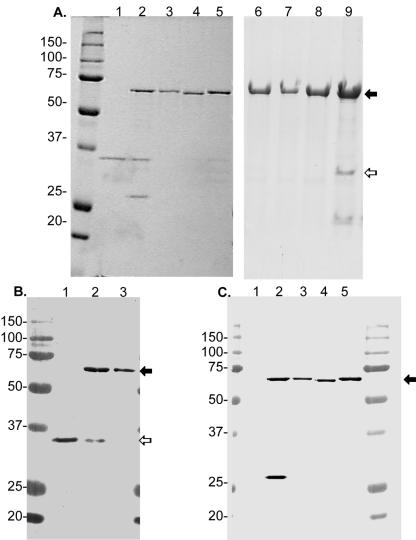
To determine if the prosequence is necessary for the correct folding and processing of ZmpA, the entire preproZmpA gene was expressed using the pPROEX-HTa His6 tag expression system. The His preproZmpA was maximally expressed at 3 to 4 h post-IPTG induction as a 62-kDa protein (Fig. 1A), which is the predicted molecular mass of this product. Following centrifugation of the cultures, the protease was not found in the cell culture supernatant but was located in the cell pellets (data not shown). Fractionation of E. coli cells demonstrated that the recombinant ZmpA was insoluble. The insoluble cell lysates containing ZmpA were washed with a series of salt and detergents with final solubilization in 50 mM sodium phosphate, pH 8.0, 300 mM NaCl, and 6 M guanidine hydrochloride. As shown in Fig. 1B, the recombinant His preproZmpA remained intact in the crude cell extract and in 6 M guanidine hydrochloride. When the protein was refolded on a Ni-NTA column using a 6 M to 1 M urea gradient followed by elution with 100 mM imidazole, the His preproZmpA was cleaved into two proteins with molecular masses of approximately 36 and 27 kDa (Fig. 1B). Attempts to significantly increase the amount of autocatalysis by increasing temperature or by salt activation (22) were unsuccessful (data not shown). The 36-kDa cleavage product has the same Mr as the native ZmpA (Fig. 2A and B). N-terminal amino acid sequencing was performed on the 36- and 27-kDa proteins. The sequence of the N terminus of the 36-kDa peptide was AAATG, which is identical to the N terminus of the mature ZmpA (10). The sequence of the N terminus of the 27-kDa peptide was SYYHHH, which corresponds to the vector sequence that precedes the pre sequence. The anti-His tag Ab reacted with both the 62- and 27-kDa peptides on Western blots but not with the 36-kDa peptide (Fig. 2C). MAb 36-6-6 to ZmpA (25) reacted with the recombinant 62-kDa and 36-kDa proteins but not the 27-kDa protein, suggesting that the 62-kDa protein contains the 36-kDa mature enzyme (Fig. 2B).
FIG. 1.
Solubilization and purification of preproZmpA by Ni-NTA chromatography. A. SDS-12.5% PAGE analysis of cell extracts of DH5α (pCK10) expressing preproZmpA. Lane 1, uninduced; lanes 2 to 5, induced with 0.6 mM IPTG for various times; lane 2, 1 h; lane 3, 2 h; lane 4, 3 h; lane 5, 4 h. The solid arrow indicates preproZmpA. B. SDS-12.5% PAGE of preproZmpA solubilized from inclusion bodies and purified using Ni-NTA chromatography. Lane 1, inclusion bodies solubilized in 6 M guanidine-HCl; lane 2, crude cell extract induced with 0.6 mM IPTG for 3 h; lanes 3 to 5, first three fractions of preproZmpA eluted from the Ni-NTA column with 100 mM imidazole.
FIG. 2.
Comparison of native, recombinant, and mutant forms of preproZmpA. A. Coomassie-stained SDS-12.5% PAGE gel. Lane 1, native ZmpA; lane 2, recombinant ZmpA; lane 3, ZmpA H465G; lane 4, ZmpA E377A; lane 5, ZmpA H380P; lane 6, ZmpA H465A; lane 7, ZmpA E377D; lane 8, ZmpA H380A; and lane 9, recombinant ZmpA. B. Western blot reacted with MAb 36-6-6 to native ZmpA. Lane 1, native ZmpA; lane 2, recombinant preproZmpA; and lane 3, ZmpA H465G. C. Western blot reacted with anti-His tag antibody. Lane 1, native ZmpA; lane 2, recombinant ZmpA; lane 3, ZmpA H465G; lane 4, ZmpA E377A; and lane 5, ZmpA H380P. The solid arrows indicate preproZmpA, and the open arrows indicate ZmpA.
Mutational analysis of zmpA.
Previous analysis of the deduced amino acid sequence of ZmpA revealed a zinc metalloprotease active site motif at amino acids 376-HExxH-380 (10). A number of amino acids appear to be highly conserved with the thermolysin-like family, including E377, H380, and H465. These amino acids in P. aeruginosa LasB and B. thermoproteolyticus thermolysin are required for proteolytic activity with E377 and H465 involved in cleavage and H380 involved in binding zinc. To test whether these residues are required for ZmpA activity, E377 was replaced with an alanine and aspartic acid, H380 was replaced with a proline and alanine, and H465 was replaced with a glycine and alanine. Wild-type preproZmpA, ZmpA H465G, ZmpA H465A, ZmpA E377A, ZmpA E377D, ZmpA H380P, and ZmpA H380A were expressed in E. coli and refolded using Ni-NTA chromatography. In the extracts from E. coli with the plasmids containing the mutant constructs, ZmpA H465G, ZmpA H465A, ZmpA E377A, ZmpA E377D, and ZmpA H380P, only the 62-kDa protein preproZmpA was detected, whereas the wild-type recombinant preproZmpA protein was processed into 36-kDa and 27-kDa polypeptides upon elution from the Ni-NTA column (Fig. 2A). These ZmpA mutant proteins displayed no proteolytic activity with the substrate hide powder azure.
To determine whether the mutant zmpA alleles are able to complement a B. cenocepacia zmpA mutant, these genes were introduced into K56-2-9 (10). The constructs pCK40, pCK41, pCK41b, pCK42, pCK42b, pCK43, and pCK43b express wild-type ZmpA, ZmpA H465G, ZmpA H465A, ZmpA E377A, ZmpA E377D, ZmpA H380P, and ZmpA H380A, respectively. Only pCK40 expressing wild-type ZmpA restored proteolytic activity in the zmpA mutant, K56-2-9 (Table 2). H465 and E377 mutant ZmpA proteins were present in the cell extracts but were not processed into a mature form. H380 mutant ZmpA proteins were not detectable in cell extracts (Fig. 3), suggesting that mutations at this site reduced the stability of ZmpA.
TABLE 2.
Ability of mutant forms of ZmpA to complement a B. cenocepacia K56-2 zmpA mutant
| Strain | Description | Zone of clearing (mm)a |
|---|---|---|
| K56-2(pUCP26) | Wild type with vector | 6.1 ± 0.5 |
| K56-2-9(pUCP26) | K56-2 zmpA with vector | 0.6 ± 0.3 |
| K56-2-9(pCK40) | K56-2 zmpA with ZmpA | 2.5 ± 0.4* |
| K56-2-9(pCK41) | K56-2 zmpA with ZmpA H465G | 0.3 ± 0.0 |
| K56-2-9(pCK41b) | K56-2 zmpA with ZmpA H465A | 0.3 ± 0.2 |
| K56-2-9(pCK42) | K56-2 zmpA with ZmpA E377A | 0.8 ± 0.5 |
| K56-2-9(pCK42b) | K56-2 zmpA with ZmpA E377D | 1.1 ± 0.3 |
| K56-2-9(pCK43) | K56-2 zmpA with ZmpA H380P | 0.3 ± 0.0 |
| K56-2-9(pCK43b) | K56-2 zmpA with ZmpA H380A | 0.4 ± 0.1 |
Values represent means ± standard deviations of six replicates. *, significantly different than K56-2-9 pUCP26 (P < 0.05, ANOVA).
FIG. 3.
Expression of wild-type and mutant zmpA proteins in K56-2-9. Western immunblot of whole cell lysates reacted with anti-ZmpA antibody. Lane 1, K56-2 (pUCP26); lane 2, K56-2-9 (pUCP26); lanes 3 to 9, K56-2-9 with wild-type or mutant zmpA in trans; lane 3, ZmpA; lane 4, ZmpA H465G; lane 5, ZmpA H465A; lane 6, ZmpA E377A; lane 7, ZmpAE377D; lane 8, ZmpA H380P; lane 9, ZmpAH380A; lane 10, native ZmpA. The solid arrow indicates preproZmpA and the open arrow indicates ZmpA.
Characterization of the recombinant ZmpA.
Several properties of the recombinant ZmpA were compared to those of native ZmpA isolated from culture supernatants. Both native ZmpA and the recombinant ZmpA were proteolytically active using hide powder azure as a substrate, although the native enzyme was slightly more active. Native ZmpA had 3.7 ± 0.4 units activity per 50 ng protein compared to 1.9 ± 0.1 units for recombinant ZmpA. Both native and recombinant ZmpA had poor activity against azocasein with OD440 values of 0.054 ± 0.001 and 0.006 ± 0.007, respectively. In contrast, P. aeruginosa LasB (3 ng) had an OD440 value of 1.508 ± 0.011 against azocasein in this assay.
To confirm that ZmpA is a zinc metalloprotease, the effect of a number of protease inhibitors on the activity of the recombinant ZmpA was examined. The recombinant ZmpA had a protease inhibitor profile identical to that of the native ZmpA (Table 3). Proteolytic activity was inhibited by EDTA and 1,10 phenanthroline, indicating that ZmpA is a metalloprotease (Table 3). The serine, cysteine, or aspartic protease inhibitors did not inhibit ZmpA proteolytic activity. Neither the native nor recombinant ZmpA was inhibited by the specific thermolysin inhibitor, phosphoramidon, which did abrogate the proteolytic activity of both P. aeruginosa LasB and B. thermoproteolyticus thermolysin (Table 4). MAb 36-6-6 has previously been shown to neutralize native ZmpA activity (25). Using hide powder azure as the substrate, MAb 36-6-6 completely neutralized the proteolytic activity of both native (OD595 0.0 ± 0.001) and recombinant ZmpA (OD595 0.02 ± 0.01) compared to the effect of control ascites on native ZmpA (OD595 0.46 ± 0.09) and recombinant ZmpA (OD595 0.38 ± 0.01).
TABLE 3.
Effect of protease inhibitors on ZmpA activity
| Inhibitor | Class | Protease activity (units)a
|
|
|---|---|---|---|
| Native ZmpA | Recombinant ZmpA | ||
| None | 4.0 ± 0.15 | 4.0 ± 0.4 | |
| 1,10 Phenanthroline | Metallo | 0.3 ± 0.05* | 0 ± 0.05* |
| EDTA | Metallo | 1.0 ± 0.05* | 0 ± 0.05* |
| APMSF | Serine | 4.2 ± 0.1 | 4.0 ± 0.7 |
| PMSF | Serine | 4.3 ± 0.2 | 4.2 ± 0.7 |
| DFP | Serine | 4.7 ± 0.3 | 4.7 ± 1.2 |
| E-64 | Cysteine | 4.1 ± 0.1 | 3.7 ± 0.3 |
| Pepstatin | Aspartic | 4.4 ± 0.4 | 4.0 ± 0.6 |
Protease activity (OD595) determined using hide powder azure as a substrate. Values represent means ± standard deviations of three replicates and are representative of three independent experiments. *, significantly different than control without inhibitor (P < 0.05, ANOVA).
TABLE 4.
Effect of phosphoramidon on ZmpA activity
| Protease | Protease activity (units)a
|
|
|---|---|---|
| No inhibitor | Phosphoramidon | |
| Native ZmpA | 1.3 ± 0.3 | 1.3 ± 0.3 |
| Recombinant ZmpA | 2.0 ± 0.2 | 1.6 ± 0.3 |
| Thermolysin | 3.2 ± 0.6 | 0.2 ± 0.1* |
| LasB | 2.9 ± 0.05 | 0.4 ± 0.2* |
Protease activity (OD595) determined using hide powder azure as a substrate. Values represent means ± standard deviations of three replicates and are representative of three independent experiments. *, significantly different than control without inhibitor (P < 0.05, ANOVA).
Previously, native ZmpA has been shown to digest gelatin and collagen (34). To determine if ZmpA has a broader range of substrates, digestion of various substrates with the recombinant and native ZmpA was tested. Both native and recombinant ZmpA cleaved α-1 proteinase inhibitor resulting in a 49-kDa polypeptide (Fig. 4A). The partial N-terminal amino acid sequence of this 49-kDa peptide is DTSHHDQD. ZmpA specifically cleaved α2-macroglobulin into 95- and 86-kDa products (Fig. 4B). Both native and recombinant ZmpA digested type IV collagen, human fibronectin (Fig. 4C), and gamma interferon (Fig. 4D). In order to ensure that this cleavage was not due to the presence of a contaminating E. coli protease, these substrates were digested with a recombinant mutant ZmpA that was purified in parallel with the recombinant wild-type ZmpA. ZmpA H465G did not cleave α-1 proteinase inhibitor, α2-macroglobulin, type IV collagen, human fibronectin, or gamma interferon (data not shown).
FIG. 4.
Ability of recombinant ZmpA and native ZmpA to cleave biologically important substrates. Substrates were digested with native ZmpA (N), recombinant ZmpA (R), or no protease (−) for 24 to 48 h. Controls included native ZmpA only (n) and recombinant ZmpA (r) only digestions. Samples were separated by SDS-PAGE and stained with Coomassie blue. A. SDS-12.5% PAGE of α-1 proteinase inhibitor. B. SDS-7.5% PAGE of α2-macroglobulin. C. SDS-7.5% PAGE of collagen (lanes 1 to 5) and fibronectin (lanes 6 to 10). D. Tricine 16%-PAGE of IFN-γ. E. SDS-12.5% PAGE of lactoferrin (lanes 1 to 3) and transferrin (lanes 4 to 6). F. SDS-12.5% PAGE of IgA (lanes 1 to 3), IgG (lanes 4 to 6), and IgM (lanes 7 to 9).
Recombinant wild-type ZmpA did not digest human lactoferrin, transferrin, IgA, IgG, and IgM under the conditions employed in this study (Fig. 4E and F). To ensure that the lack of activity against these substrates was not due to the presence of an inhibitory substance in the substrate, parallel digestions were conducted with P. aeruginosa LasB. LasB digested lactoferrin, transferrin, IgG, and IgM but not IgA (data not shown).
DISCUSSION
B. cenocepacia ZmpA is expressed as a preproenzyme (10). Expression of the ZmpA pro sequence appears to be necessary for the correct folding and autocatalytic processing of the ZmpA protease as has been shown previously for P. aeruginosa LasB and B. thermoproteolyticus thermolysin (32, 42). ZmpA residues E377 and H465 were necessary for the autocatalytic activity of the preproZmpA and for proteolytic activity in B. cenocepacia. Mutagenesis of the corresponding residues in P. aeruginosa LasB, E338 (residue 141 on the mature LasB sequence), and H420 (residue 223 on the mature LasB sequence) resulted in the loss of both autoproteolytic processing (24) of the proLasB and proteolytic activity of LasB (31, 33). Mutagenesis of the corresponding residues E375 and H463 (E143 and H231 in the mature protease) of a B. thermoproteolyticus thermolysin homologue, Bacillus subtilis neutral protease, also inactivated the enzyme (50). Mutagenesis of a putative zinc-binding ligand in ZmpA (H380) also resulted in defective autocatalytic processing in E. coli. Changing H380 to either an alanine or a proline appeared to alter the structure or stability of ZmpA, since these mutant ZmpAs were not detectable in Western blots of B. cenocepacia cell extracts.
Sequence analysis suggested that ZmpA belongs to the metalloprotease clan MA, family M4 (i.e., the thermolysin-like metalloproteases) and not to the serralysins that belong to the metalloprotease clan MB, family M10 (10). Although ZmpA activity was inhibited by EDTA and 1,10-phenanthroline, it was not inhibited by the specific thermolysin inhibitor, phosphoramidon. Phosphoramidon is a peptide that mediates its inhibition by binding to the active site region of thermolysin-like proteases typified by B. thermoproteolyticus thermolysin (15, 53). Thermolysin-like proteases that are more phylogenically related to thermolysin are sensitive to phosphoramidon. Phosphoramidon was specifically designed for thermolysin, and sequence differences in thermolysin-like proteases correlate with decreased phosphoramidon sensitivity (11).
Previously, it has been shown that ZmpA cleaves collagen, gelatin, and hide powder azure (34). Bacterial proteases may cause tissue damage by directly degrading host tissues. In this study, both recombinant and native ZmpAs were found to cleave the tissue components fibronectin and type IV collagen, which are also cleaved by P. aeruginosa LasB (1, 17). Proteolytic degradation of collagen and fibronectin in the tissue matrix in the CF lung leads to loss of normal tissue morphology. Repeated cycles of bacterial infection and inflammation lead to bronchial wall thickening and bronchiectasis (reviewed in reference 47). Thus, proteases secreted by B. cenocepacia and/or P. aeruginosa may directly contribute to tissue pathology that occurs in the CF lung.
ZmpA may also contribute to virulence through the modulation of host defenses. ZmpA was found to specifically cleave α-1 proteinase inhibitor, which inhibits neutrophil elastase, and α2-macroglobulin, a universal protease inhibitor. P. aeruginosa LasB, has been shown to cleave α-1 proteinase inhibitor specifically at P357-M358 (39), whereas ZmpA cleaves α-1 at T11-D12. Whether this cleavage inactivates neutrophil α-1 proteinase inhibitor is yet to be determined. It has been indicated that bacterial proteases, such as P. aeruginosa LasB, may play a role in host-derived damage to tissues by causing an imbalance between neutrophil elastase and the host protease inhibitors, α1-proteinase inhibitor, and α2-macroglobulin (38, 40). Neutrophil elastase degrades tissue components including elastin, type III and IV collagens, and fibronectin. Without the control of α1-proteinase inhibitor and α2-macroglobulin, neutrophil elastase proteolysis may go unchecked, resulting in increased tissue damage. In the healthy lung, neutrophil elastase is balanced by the inhibitors α1-proteinase inhibitor, secretory leukocyte protease inhibitor and elafin. In the cystic fibrosis lung, the balance of antiproteases is tipped towards proteases (reviewed in reference 3), resulting in tissue damage. Aerosolized α1-proteinase inhibitor can reduce the neutrophil elastase burden in P. aeruginosa experimental infections, resulting in reduced lung inflammation (6). Thus, ZmpA may contribute to lung pathology through the direct degradation of host tissues (e.g., collagen, fibronectin) or through modulation of host defenses (e.g., α-1 protease inhibitor).
Interestingly, ZmpA specifically cleaved α2-macroglobulin, which is known as a universal protease inhibitor since it can inhibit proteases of all classes. α2-Macroglobulin binds to and “traps” proteases, resulting in inhibition of their proteolytic activity (4). α2-Macroglobulin has also been shown to be cleaved by a Lysobacter enzymogenes proteinase (52); however, very few bacterial proteases have been reported to cleave α2-macroglobulin. The repercussions of possible inactivation of α2-macroglobulin could be catastrophic to the host. Whether B. cenocepacia ZmpA can inactivate α2-macroglobulin in vivo is currently unknown.
Bacterial metalloproteases may also contribute to pathogenesis by the ability to interrupt the immune response. ZmpA did not cleave immunoglobulins under the conditions employed; however, ZmpA was found to cleave IFN-γ, a cytokine that enhances T-cell immunity. P. aeruginosa, Serratia, and Streptococcus proteases degrade IFN-γ (reviewed in reference 28). Degradation of IFN-γ may allow a pathogen to establish a chronic infection, thereby aiding in the pathogenesis of the organism (20, 21). Thus, the potential for ZmpA to contribute to pathogenesis through mechanisms other than direct host tissue proteolysis exists.
This study demonstrates the importance of residues E377 and H465 in the autocatalytic and proteolytic activity of ZmpA and suggests that H380 is important for structural stability as well as autocatalytic activity. The data suggest that the active site predicted using sequence comparison to other M4 thermolysin-like metalloproteases is correct. The conservation of the active site residues and similarity to other M4 zinc metalloproteases suggest that ZmpA conducts catalysis by a mechanism similar to that of other M4 zinc metalloproteases. ZmpA can cleave a number of biologically relevant substrates, indicating that ZmpA may contribute to the pathogenesis of B. cenocepacia through the direct degradation of host tissues or through a modulation of the host immune system.
Acknowledgments
This study was supported by a grant from the Canadian Institutes of Health Research to P.A.S.
We thank R. Chen for critical reading of the manuscript and helpful discussions.
REFERENCES
- 1.Bejarano, P. A., J. P. Langeveld, B. G. Hudson, and M. E. Noelken. 1989. Degradation of basement membranes by Pseudomonas aeruginosa elastase. Infect. Immun. 57:3783-3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bever, R. A., and B. H. Iglewski. 1988. Molecular characterization and nucleotide sequence of the Pseudomonas aeruginosa elastase structural gene. J. Bacteriol. 170:4309-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birrer, P. 1995. Proteases and antiproteases in cystic fibrosis: pathogenetic considerations and therapeutic strategies. Respiration 62(Suppl. 1):25-28. [DOI] [PubMed]
- 4.Borth, W. 1992. Alpha 2-macroglobulin, a multifunctional binding protein with targeting characteristics. FASEB J. 6:3345-3353. [DOI] [PubMed] [Google Scholar]
- 5.Britigan, B. E., M. B. Hayek, B. N. Doebbeling, and R. B. Fick, Jr. 1993. Transferrin and lactoferrin undergo proteolytic cleavage in the Pseudomonas aeruginosa-infected lungs of patients with cystic fibrosis. Infect. Immun. 61:5049-5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantin, A. M., and D. E. Woods. 1999. Aerosolized prolastin suppresses bacterial proliferation in a model of chronic Pseudomonas aeruginosa lung infection. Am. J. Respir. Crit. Care Med. 160:1130-1135. [DOI] [PubMed] [Google Scholar]
- 7.CFF. 2002. Cystic Fibrosis Foundation patient registry annual data report. CFF, Bethesda, Md.
- 8.Coenye, T., P. Vandamme, J. J. LiPuma, J. R. Govan, and E. Mahenthiralingam. 2003. Updated version of the Burkholderia cepacia complex experimental strain panel. J. Clin. Microbiol. 41:2797-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colman, P. M., J. N. Jansonius, and B. W. Matthews. 1972. The structure of thermolysin: an electron density map at 2-3 A resolution. J. Mol. Biol. 70:701-724. [DOI] [PubMed] [Google Scholar]
- 10.Corbett, C. R., M. N. Burtnick, C. Kooi, D. E. Woods, and P. A. Sokol. 2003. An extracellular zinc metalloprotease gene of Burkholderia cepacia. Microbiology 149:2263-2271. [DOI] [PubMed] [Google Scholar]
- 11.de Kreij, A., G. Venema, and B. van den Burg. 2000. Substrate specificity in the highly heterogeneous M4 peptidase family is determined by a small subset of amino acids. J. Biol. Chem. 275:31115-31120. [DOI] [PubMed] [Google Scholar]
- 12.Dennis, J. J., and P. A. Sokol. 1995. Electrotransformation of Pseudomonas. Methods Mol. Biol. 47:125-133. [DOI] [PubMed] [Google Scholar]
- 13.Gessner, A. R., and J. E. Mortensen. 1990. Pathogenic factors of Pseudomonas cepacia isolates from patients with cystic fibrosis. J. Med. Microbiol. 33:115-120. [DOI] [PubMed] [Google Scholar]
- 14.Gotschlich, A., B. Huber, O. Geisenberger, A. Togl, A. Steidle, K. Riedel, P. Hill, B. Tummler, P. Vandamme, B. Middleton, M. Camara, P. Williams, A. Hardman, and L. Eberl. 2001. Synthesis of multiple N-acylhomoserine lactones is wide-spread among the members of the Burkholderia cepacia complex. Syst. Appl. Microbiol. 24:1-14. [DOI] [PubMed] [Google Scholar]
- 15.Hangauer, D. G., A. F. Monzingo, and B. W. Matthews. 1984. An interactive computer graphics study of thermolysin-catalyzed peptide cleavage and inhibition by N-carboxymethyl dipeptides. Biochemistry 23:5730-5741. [DOI] [PubMed] [Google Scholar]
- 16.Hase, C. C., and R. A. Finkelstein. 1993. Bacterial extracellular zinc-containing metalloproteases. Microbiol. Rev. 57:823-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heck, L. W., K. Morihara, W. B. McRae, and E. J. Miller. 1986. Specific cleavage of human type III and IV collagens by Pseudomonas aeruginosa elastase. Infect. Immun. 51:115-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry, D., M. Campbell, C. McGimpsey, A. Clarke, L. Louden, J. L. Burns, M. H. Roe, P. Vandamme, and D. Speert. 1999. Comparison of isolation media for recovery of Burkholderia cepacia complex from respiratory secretions of patients with cystic fibrosis. J. Clin. Microbiol. 37:1004-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 20.Horvat, R. T., M. Clabaugh, C. Duval-Jobe, and M. J. Parmely. 1989. Inactivation of human gamma interferon by Pseudomonas aeruginosa proteases: elastase augments the effects of alkaline protease despite the presence of alpha 2-macroglobulin. Infect. Immun. 57:1668-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horvat, R. T., and M. J. Parmely. 1988. Pseudomonas aeruginosa alkaline protease degrades human gamma interferon and inhibits its bioactivity. Infect. Immun. 56:2925-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inouye, K., K. Kuzuya, and B. Tonomura. 1998. Sodium chloride enhances markedly the thermal stability of thermolysin as well as its catalytic activity. Biochim. Biophys. Acta 1388:209-214. [DOI] [PubMed] [Google Scholar]
- 23.Isles, A., I. Maclusky, M. Corey, R. Gold, C. Prober, P. Fleming, and H. Levison. 1984. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J. Pediatr. 104:206-210. [DOI] [PubMed] [Google Scholar]
- 24.Kawamoto, S., Y. Shibano, J. Fukushima, N. Ishii, K. Morihara, and K. Okuda. 1993. Site-directed mutagenesis of Glu-141 and His-223 in Pseudomonas aeruginosa elastase: catalytic activity, processing, and protective activity of the elastase against Pseudomonas infection. Infect. Immun. 61:1400-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kooi, C., A. Cox, P. Darling, and P. A. Sokol. 1994. Neutralizing monoclonal antibodies to an extracellular Pseudomonas cepacia protease. Infect. Immun. 62:2811-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kooi, C., R. S. Hodges, and P. A. Sokol. 1997. Identification of neutralizing epitopes on Pseudomonas aeruginosa elastase and effects of cross-reactions on other thermolysin-like proteases. Infect. Immun. 65:472-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 28.Maeda, H. 1996. Role of microbial proteases in pathogenesis. Microbiol. Immunol. 40:685-699. [DOI] [PubMed] [Google Scholar]
- 29.Mahenthiralingam, E., A. Baldwin, and P. Vandamme. 2002. Burkholderia cepacia complex infection in patients with cystic fibrosis. J. Med. Microbiol. 51:533-538. [DOI] [PubMed] [Google Scholar]
- 30.Matsushita, O., K. Yoshihara, S. Katayama, J. Minami, and A. Okabe. 1994. Purification and characterization of Clostridium perfringens 120-kilodalton collagenase and nucleotide sequence of the corresponding gene. J. Bacteriol. 176:149-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McIver, K., E. Kessler, and D. E. Ohman. 1991. Substitution of active-site His-223 in Pseudomonas aeruginosa elastase and expression of the mutated lasB alleles in Escherichia coli show evidence for autoproteolytic processing of proelastase. J. Bacteriol. 173:7781-7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McIver, K. S., E. Kessler, J. C. Olson, and D. E. Ohman. 1995. The elastase propeptide functions as an intramolecular chaperone required for elastase activity and secretion in Pseudomonas aeruginosa. Mol. Microbiol. 18:877-889. [DOI] [PubMed] [Google Scholar]
- 33.McIver, K. S., J. C. Olson, and D. E. Ohman. 1993. Pseudomonas aeruginosa lasB1 mutants produce an elastase, substituted at active-site His-223, that is defective in activity, processing, and secretion. J. Bacteriol. 175:4008-4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKevitt, A. I., S. Bajaksouzian, J. D. Klinger, and D. E. Woods. 1989. Purification and characterization of an extracellular protease from Pseudomonas cepacia. Infect. Immun. 57:771-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKevitt, A. I., and D. E. Woods. 1984. Characterization of Pseudomonas cepacia isolates from patients with cystic fibrosis. J. Clin. Microbiol. 19:291-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyoshi, S., and S. Shinoda. 2000. Microbial metalloproteases and pathogenesis. Microbes Infect. 2:91-98. [DOI] [PubMed] [Google Scholar]
- 37.Mohr, C. D., M. Tomich, and C. A. Herfst. 2001. Cellular aspects of Burkholderia cepacia infection. Microbes Infect. 3:425-435. [DOI] [PubMed] [Google Scholar]
- 38.Molla, A., K. Matsumoto, I. Oyamada, T. Katsuki, and H. Maeda. 1986. Degradation of protease inhibitors, immunoglobulins, and other serum proteins by Serratia protease and its toxicity to fibroblast in culture. Infect. Immun. 53:522-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morihara, K., H. Tsuzuki, M. Harada, and T. Iwata. 1984. Purification of human plasma alpha 1-proteinase inhibitor and its inactivation by Pseudomonas aeruginosa elastase. J. Biochem. (Tokyo) 95:795-804. [DOI] [PubMed] [Google Scholar]
- 40.Morihara, K., H. Tsuzuki, and K. Oda. 1979. Protease and elastase of Pseudomonas aeruginosa: inactivation of human plasma alpha 1-proteinase inhibitor. Infect. Immun. 24:188-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakazawa, T., Y. Yamada, and M. Ishibashi. 1987. Characterization of hemolysin in extracellular products of Pseudomonas cepacia. J. Clin. Microbiol. 25:195-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Donohue, M. J., and A. Beaumont. 1996. The roles of the prosequence of thermolysin in enzyme inhibition and folding in vitro. J. Biol. Chem. 271:26477-26481. [DOI] [PubMed] [Google Scholar]
- 43.Rawlings, N. D., and A. J. Barrett. 1995. Evolutionary families of metallopeptidases. Methods Enzymol. 248:183-228. [DOI] [PubMed] [Google Scholar]
- 44.Rinderknecht, H., M. C. Geokas, P. Silverman, and B. J. Haverback. 1968. A new ultrasensitive method for the determination of proteolytic activity. Clin. Chim. Acta 21:197-203. [DOI] [PubMed] [Google Scholar]
- 45.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 46.Schagger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 47.Shute, J., L. Marshall, K. Bodey, and A. Bush. 2003. Growth factors in cystic fibrosis—when more is not enough. Paediatr. Respir. Rev. 4:120-127. [DOI] [PubMed] [Google Scholar]
- 48.Sokol, P. A., C. Kooi, R. S. Hodges, P. Cachia, and D. E. Woods. 2000. Immunization with a Pseudomonas aeruginosa elastase peptide reduces severity of experimental lung infections due to P. aeruginosa or Burkholderia cepacia. J. Infect. Dis. 181:1682-1692. [DOI] [PubMed] [Google Scholar]
- 49.Sokol, P. A., D. E. Ohman, and B. H. Iglewski. 1979. A more sensitive plate assay for detection of protease production by Pseudomanas aeruginosa. J. Clin. Microbiol. 9:538-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toma, S., S. Campagnoli, E. De Gregoriis, R. Gianna, I. Margarit, M. Zamai, and G. Grandi. 1989. Effect of Glu-143 and His-231 substitutions on the catalytic activity and secretion of Bacillus subtilis neutral protease. Protein Eng. 2:359-364. [DOI] [PubMed] [Google Scholar]
- 51.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Leuven, F., P. Marynen, L. Sottrup-Jensen, J. J. Cassiman, and H. Van den Berghe. 1986. The receptor-binding domain of human alpha 2-macroglobulin. Isolation after limited proteolysis with a bacterial proteinase. J. Biol. Chem. 261:11369-11373. [PubMed] [Google Scholar]
- 53.Weaver, L. H., W. R. Kester, and B. W. Matthews. 1977. A crystallographic study of the complex of phosphoramidon with thermolysin. A model for the presumed catalytic transition state and for the binding of extended substances. J. Mol. Biol. 114:119-132. [DOI] [PubMed] [Google Scholar]
- 54.West, S. E., H. P. Schweizer, C. Dall, A. K. Sample, and L. J. Runyen-Janecky. 1994. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene 148:81-86. [DOI] [PubMed] [Google Scholar]