Abstract
In Escherichia coli, σE regulon functions are required for envelope homeostasis during stress and are essential for viability under all growth conditions. The E. coli genome encodes approximately 100 lipoproteins, and 6 of these are regulated by σE. Phenotypes associated with deletion of each of these lipoproteins are the subject of this report. One lipoprotein, YfiO, is essential for cellular viability. However, overexpression of this protein is not sufficient to alleviate the requirement of σE for viability, suggesting that the σE regulon provides more than one essential function. The remaining five lipoproteins in the σE regulon are nonessential; cells are viable even when all five are removed simultaneously. Deletion of three nonessential lipoprotein genes (nlpB, yraP, ygfL) results in the exhibition of phenotypes that suggest they are important for maintenance of the integrity of the cell envelope. ΔnlpB cells are selectively sensitive to rifampin; ΔyraP cells are selectively sensitive to sodium dodecyl sulfate. Such selective sensitivity has not been previously reported. Both ΔyraP and ΔnlpB are synthetically lethal with surA::Cm, which encodes a periplasmic chaperone and PPIase, suggesting that NlpB and YraP play roles in a periplasmic folding pathway that functions in parallel with that of SurA. Finally, the ΔyfgL mutant exhibits a broad range of envelope defects, including sensitivity to several membrane-impermeable agents, an altered outer membrane protein profile, synthetic lethality with both surA::Cm and ΔfkpA::Cm strains, and sensitivity to a bactericidal permeability-increasing peptide. We suggest that this lipoprotein performs a very important but as-yet-unknown function in maintaining the integrity of the cell envelope.
When exposed to denaturants such as heat or ethanol, all cells induce stress responses to restore protein homeostasis. In Escherichia coli, the alternative sigma factor σ32 responds to the accumulation of misfolded and unfolded cytoplasmic proteins (55). Two stress-response pathways, the two-component CpxAR system (reviewed in reference 34) and the σE signal transduction cascade (reviewed in reference 2), respond to stress in the envelope. In addition to mitigating envelope stress, the σE regulon provides an essential function to the cells during normal cell growth, as σE is essential under all conditions tested (4). In this report, we investigate the physiological role of the σE regulon.
To understand the general role of the σE regulon, three genomic strategies have been employed to identify its members. First, two groups (11, 35) searched for σE-dependent promoters by screening for increased expression of genomic DNA sequences fused to lacZ upon increased expression of σE. Second, whole-genome expression analysis identified genes that were up-regulated in response to σE overexpression during exponential growth (V. A. Rhodius, submitted for publication). Finally, transcription start-site mapping and bioinformatic analysis identified additional σE-dependent promoters (Rhodius, submitted). Taken together, 47 σE-dependent promoters, which control the expression of approximately 100 genes, have been identified. The majority of the promoters drive the production of envelope proteins, including periplasmic chaperones and proteases that act on misfolded periplasmic proteins, phospholipid and lipopolysaccharide biosynthesis and transport proteins, and a variety of inner and outer membrane proteins (OMP). However, σE also directs transcription of many genes of unknown function, including six lipoprotein genes, identified because their mRNAs increased after σE overexpression (Rhodius, submitted). In addition, each of these genes (except for nlpB) is preceded by promoters recognized by σE (Rhodius, submitted). These six lipoproteins are the subject of the present work.
Lipoproteins are envelope proteins whose N-terminal cysteine residues are each covalently modified with a lipid moiety (reviewed in references 29 and 49). Proteins targeted for lipid modification each have a lipobox motif encoded in their signal sequences. The consensus sequence of the lipobox is Leu (Ala, Val)−4-Leu−3-Ala (Ser)−2-Gly (Ala)−1-Cys+1, where Cys+1 designates the cysteine to be modified. A transacetylase reaction occurs at the periplasmic face of the inner membrane (IM), wherein the sulfhydryl group of Cys+1 is initially modified by addition of diacylglycerol. The signal sequence is then cleaved after the Gly−1 residue by signal peptidase II, and Cys+1 becomes the N-terminal residue of the mature lipoprotein. Finally, the amino group of Cys+1 is substituted by a fatty acid residue, usually palmitate (40). These steps take place on the periplasmic face of the inner membrane.
Lipoproteins are inserted into either the inner membrane or the outer membrane (OM) via their lipid modification. Most are localized to the inner leaflet of the outer membrane; the remainder reside on the periplasmic face of the inner membrane. The Lol system plays a critical role in the membrane specificity of lipoprotein targeting (reviewed in reference 29). An ABC transporter (LolCDE) releases newly assembled lipoproteins from the inner membrane (51), allowing them to interact with LolA, a periplasmic chaperone specific for lipoproteins (22, 27, 43). The lipoprotein is then transferred from LolA to LolB, a lipoprotein anchored to the outer membrane (23, 44). LolB facilitates the incorporation of lipoproteins into the outer membrane by an unknown mechanism. The lipoproteins associated with the inner membrane usually have an Asp residue immediately following the modified Cys+1 (52) and an Asp, Glu, Gln, or Asn at position +3 (reviewed in reference 29). These amino acids prevent a lipoprotein that is to be retained in the inner membrane from interacting with LolCDE (“lol avoidance”) (22, 51, 52).
The E. coli chromosome is predicted to encode approximately 100 lipoproteins, about 90 of which have been experimentally confirmed (26). However, few of these have been extensively characterized. Lpp, the most abundant lipoprotein in E. coli, anchors the OM to the peptidoglycan layer (reviewed in references 32 and 49). lpp mutants exhibit decreased envelope integrity, as indicated by leakage of periplasmic proteins and by hypersensitivity to both EDTA and low-osmotic-strength media (53, 54). The OM lipoprotein Pal also functions in a very important yet poorly understood process that helps to maintain the barrier function of the OM. Like Lpp, Pal anchors the OM to the peptidoglycan. However, the functions of Pal require the proton motive force of the IM in a manner that is similar to that of the TonB transport systems (18). Mutations in pal result in membrane alterations similar to those observed in lpp mutants (6). Other lipoproteins, such as PulS and PrsA, have been shown to assist in the secretion of proteins across the outer membrane and in the release of bacteriocins (31). Three inner-membrane-localized lipoproteins, AcrA (21), AcrE (21), and EmrA (19, 20, 45), function together with an RND (metal resistance, nodulation, cell division) permease to form an efflux pump that transfers its substrates across the IM and the OM to remove them from the E. coli cell (14). Several lipoproteins, including NlpE (34) and YafY (26), are potent inducers of the CpxAR two-component system, which, like σE, monitors envelope integrity (reviewed in reference 34). To date, LolB is the only essential lipoprotein to have been identified (30, 44). Given the importance of the σE regulon in maintaining envelope integrity, we thought it probable that the six lipoproteins in the σE regulon might play important roles in this process. Phenotypes associated with deletion of each of these lipoproteins are the subject of this report.
MATERIALS AND METHODS
Media.
Luria-Bertani (LB) broth, LB agar, M9 minimal medium, M9 agar, and tryptic soy broth (TSB) were prepared as described in the work of Miller (24). M9 minimal medium and M9 agar were supplemented with 0.2% glucose, 1 mM MgSO4, vitamins, and all amino acids (40 μg/ml). These media are referred to as M9 complete medium and M9 complete agar, respectively. When necessary, media were supplemented with 100 μg/ml ampicillin, 10 μg/ml tetracycline, 30 μg/ml kanamycin, or 20 μg/ml chloramphenicol.
Bacterial strains.
The bacterial strains used in this study are listed in Table 1, and plasmids are listed in Table 2. The construction of strains and plasmids is described briefly below. The chromosomal disruptions of the yeaY, yfgL, nlpB, yfeY, and yraP open reading frames were generated according to the procedure described in the work of Datsenko and Wanner (12) as follows: PCR products were generated by using several pairs of 56- to 70-nucleotide (nt)-long primers that included 36- to 50-nt regions of homology upstream and downstream of the gene of interest and 20-nt priming sequences for pKD3 or pKD4 as templates. Sequences are available upon request. The 1.1-kb PCR products were gel-purified, digested with DpnI, repurified, and suspended in elution buffer (10 mM Tris, pH 8.0). Fifty microliters of cells and 10 to 100 ng of PCR product were used in each electroporation for transformation into strain CAG49001. Strain CAG49001 was grown in 500 ml SOB medium (0.5% yeast extract, 2.0% tryptone, 10 mM NaCl, 2.5 mM KCl, 10 mM MgCl2, 10 mM MgSO4) supplemented with 100 μg/ml ampicillin and 0.2% l-arabinose at 30°C to an optical density at 600 nm (OD600) of 0.6 and then made electrocompetent by concentrating it 100-fold and washing it three times with ice-cold 10% glycerol. Electroporations were performed with Bio-Rad E. coli pulser according to the manufacturer's instructions. One milliliter of SOC medium (0.5% yeast extract, 2.0% tryptone, 10 mM NaCl, 2.5 mM KCl, 10 mM MgCl2, 20 mM MgSO4, 20 mM glucose) was added to shocked cells, which were then incubated 1 h at 37°C. The cells were then spread onto LB agar supplemented with 20 μg/ml chloramphenicol to select Cmr transformants or with 30 μg/ml kanamycin to select Kmr transformants. After primary selection, mutants were maintained on medium without an antibiotic. They were colony purified once nonselectively at 37°C and then tested for ampicillin sensitivity to test for the loss of the pKD46 plasmid. If it was not lost, then a few isolates of each mutant were colony purified once at 43°C and similarly tested. Each knockout was confirmed via PCR.
TABLE 1.
Bacterial strains
| Strain | Relevant genotype, plasmid, and/or phenotype | Source or reference |
|---|---|---|
| BW25141 | Δ(araD-araB)567 ΔlacZ4787 lacIp-4000(lacIq) Δ(phoB-phoR)580 galU95 ΔuidA3::pir+rpoS396(Am) Δend::FRT rph-1 Δ(rhaD-rhaB)568 rnD3 pKD4 Apr Kanr | 12 |
| BW25141 | Δ(araD-araB)567 ΔlacZ4787 lacIp-4000(lacIq) Δ(phoB-phoR)580 galU95 ΔuidA3::pir+rpoS396(Am) Δend::FRT rph-1 Δ(rhaD-rhaB)568 rnD3 pKD3 Apr Cmr | 12 |
| BW25113 | Δ(araD-araB)567 ΔlacZ4787 lacIp-4000(lacIq) Δ(phoB-phoR)580 rpoS396(Am) rph-1 Δ(rhaD-rhaB)568 rnD3 hsdR514 pKD46 Apr | 12 |
| BT340 | supE44 ΔlacU169 (f80 lacZDM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 pCP20 Apr | 10 |
| KM44 | argE3 his4 leuB6 proA2 thr1 ara14 galK2 lacY1 mtl1 xyl5 thi1 rpsL31 tsx33 D(recC ptr recB recD)::Ptac-bet exo kan | 28 |
| CAG12158 | MG1655 pheA18::Tn10 Tetr | 41 |
| CAG37125 | ΔlacX74 galK galU Δ(araABC-leu)7679 araD139 hsdR rpsL mcrB rpoE::ΩCm nadB51::Tn10 Cmr Tetr | De Las Peñas (unpublished) |
| CAG41241 | CAG45114 ΔyeaY::Cm Cmr | This study |
| CAG41245 | CAG45114 ΔyraP::Cm Cmr | This study |
| CAG41247 | CAG45114 ΔyeaY | This study |
| CAG41251 | CAG45115 ΔyraP | This study |
| CAG41254 | CAG45114 ΔnlpB::Cm Cmr | This study |
| CAG41258 | CAG45114 ΔnlpB | This study |
| CAG41264 | KM44 pheA18::Tn10 Tetr | This study |
| CAG41265 | CAG45114 surA::Cm Cmr | This study |
| CAG41292 | CAG45114 degP::Km Kmr | This study |
| CAG41293 | CAG45114 ΔyraP degP::Km Kmr | This study |
| CAG41295 | CAG45114 ΔnlpB degP::Km Kmr | This study |
| CAG41311 | CAG45114 ΔyraP ΔfkpA::Cm Cmr | This study |
| CAG41313 | CAG45114 ΔnlpB ΔfkpA::Cm Cmr | This study |
| CAG41318 | CAG45114 ΔfkpA::Cm Cmr | This study |
| CAG41323 | CAG45114 Δskp zae502::Tn10 Tetr | This study |
| CAG41324 | CAG45114 ΔyraP Δskp zae502::Tn10 Tetr | This study |
| CAG41326 | CAG45114 ΔnlpB Δskp zae502::Tn10 Tetr | This study |
| CAG41349 | CAG41264 lacZ::ptrc-b2595-bla Apr Tetr | This study |
| CAG41350 | CAG45114 pCO109 Apr | This study |
| CAG41351 | CAG45114 pCO10 Apr | This study |
| CAG41356 | CAG41349 ΔyfiO::Cm Cmr Apr Tetr | This study |
| CAG41408 | CAG45114 ΔyfeY::Cm Cmr | This study |
| CAG41413 | CAG45114 ΔyfeY | This study |
| CAG41434 | CAG45114 ΔyfgL::Km Cmr | This study |
| CAG41445 | CAG45114 ΔyfgL | This study |
| CAG41510 | CAG45114 pBA169 Apr | This study |
| CAG41511 | CAG45114 pCO123 Apr | This study |
| CAG41512 | CAG41251 pBA169 Apr | This study |
| CAG41513 | CAG41251 pCO123 Apr | This study |
| CAG41516 | CAG41445 pBA169 Apr | This study |
| CAG41517 | CAG41445 pCO109 Apr | This study |
| CAG41534 | CAG41258 pCO122 Apr | This study |
| CAG41535 | CAG41258 pBA169 Apr | This study |
| CAG41536 | CAG45114 pCO122 Apr | This study |
| CAG41541 | CAG45114 ΔyraP ΔnlpB ΔyeaY yfeY::Cm yfgL::Km Cmr Kmr | This study |
| CAG41543 | CAG45114 ΔyfgL degP::Km Kmr | This study |
| CAG41544 | CAG45114 ΔyfgL Δskp zae502::Tn10 Tetr | This study |
| CAG41551 | CAG45114 ΔfkpA | This study |
| CAG41552 | CAG45114 ΔfkpA::Cm Δskp zae502::Tn10 Cmr Tetr | This study |
| CAG41553 | CAG45114 ΔfkpA::Cm degP::Km Cmr Kmr | This study |
| CAG41554 | CAG45114 ΔfkpA surA::Cm Cmr | This study |
| CAG41560 | CAG45114 Δskp degP::Km Cmr Kmr | This study |
| CAG49001 | CAG45114 pKD46 Apr Cmr | This study |
TABLE 2.
Plasmids
| Plasmid | Insert/construction | Source/reference |
|---|---|---|
| pTrc99A | Expression vector, pBR322 ori, pTrc promoter, Apr | Amersham Pharmacia Biotech |
| pBA169 | pTrc99A ΔNcoI; eliminates vector ATG; Apr | Alba (unpublished) |
| pCO109 | yfgL in pBA169, Apr | This study |
| pCO110 | yfiO in pBA169, Apr | This study |
| pCO122 | nlpB in pBA169, Apr | This study |
| pCO123 | yraP in pBA169, Apr | This study |
| pCP20 | FLP+ λcI857+ λP Rep(Ts) Cmr Apr | 9 |
| pKD3 | FRT-flanked Cmr gene from pSC140 cloned into pANTSγ, Cmr | 11 |
| pKD4 | FRT-flanked Kmr gene from pCP15 cloned into pANTSγ, Kmr | 11 |
| pKD46 | araC, ParaB—γ, β, and exo in pINT(Ts), Apr | 11 |
pCP20 is an Apr and Cmr plasmid that shows temperature-sensitive replication and thermal induction of FLP synthesis. Cmr and Kmr mutants were transformed with pCP20, and Apr transformants were selected at 30°C, after which a few were colony purified once nonselectively at 43°C. The transformants were then tested for loss of all antibiotic resistances. The majority of the mutants lost the FRT-flanked Cmr gene and the FLP helper plasmid simultaneously.
To generate a strain carrying a targeted disruption of yfiO linked to the cotransducible marker pheA18::Tn10, strain KM44 was transduced with a P1 lysate grown on CAG12158 to generate CAG41264. A second copy of yfiO was inserted into the chromosomal lacZ gene of CAG41264 via the targeted insertion of a 2.26-kb PCR fragment encoding the promoter Ptrc, yfiO, and β-lactamase with its endogenous promoter. This PCR fragment was amplified from pCO110 using primers 5′-ttatttttgacaccagaccaactggtaatggtagcgaccggcgctcagctCTGACAGTTACCAATGCTTAATCA-3′ and 5′-atgaccatgattacggattcactggccgtcgttttacaacgtcgtgactgTGAGCTGTTGACAATTAATCATCCGG-3′, each of which contain 40 bp of homology to lacZ (shown in lowercase) at their 5′ ends.
The targeted disruption of lacZ was performed using methods described in the work of Murphy (28), and transformants were selected on LB plates containing 30 μg/ml ampicillin. The resultant strain CAG41439 was confirmed to carry two wild-type (WT) alleles of yfiO via PCR performed with primers of sequences flanking each gene. The endogenous chromosomal copy of yfiO in CAG41439 was then disrupted, using methods described in the work of Murphy (28), by a 1.1-kb PCR product amplified from pKD3 using primers 5′-aaacggcagctcaagggctgccgttttgtgtttcaggtttctgCATATGAATATCCTCCTTA-3′ and 5′-gagaatctcccgtattacattttgaggaaagtcaaaacgtcTGTGTAGGCTGGAGCTGCTTC-3′. Transformants were selected on LB plates containing 20 μg/ml chloramphenicol and 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), generating CAG41356. The chromosomal disruption of endogenous yfiO in CAG41356 was confirmed via PCR. Lysates were prepared from CAG41356 and used in linked marker cotransduction experiments to determine whether yfiO is essential.
Motif search.
The lipobox motifs at the N termini of YeaY, YfgL, YfiO, YfeY, and YraP polypeptides were identified using the program Motif included in version 10.3 of the Genetics Computer Group, Inc. sequence analysis software package.
Linked marker cotransductions.
The yfiO::Cm allele was linked to pheA18::Tn10 from strain CAG12158 as described above. These two genes are 95% linked. CAG45114 and CAG41351 were infected with P1 lysate grown on CAG41356 using a standard P1 transduction protocol. Tetracycline-resistant transductants were selected at 30°C on LB agar plates with 10 μg/ml tetracycline and 10 mM sodium citrate added. After approximately 16 h, tetracycline-resistant transductants were streak purified and then screened for chloramphenicol resistance at 30°C on LB agar plates supplemented with 20 μg/ml chloramphenicol. The frequency of cotransduction of yfiO::Cm with pheA18::Tn10 was calculated by dividing the number of yfiO::Cm pheA18::Tn10 transductants by the total number of pheA18::Tn10 transductants. This experiment was repeated twice and yielded similar results both times. The linked marker cotransduction assays into CAG45114 and CAG41351 using P1(rpoE::Cm nadB::Tn10) were performed as described above. P1(rpoE::Cm nadB::Tn10) was prepared from CAG37125.
Phenotypic assays.
To identify the phenotypes of the ΔyeaY, ΔyfgL, ΔnlpB, ΔyfeY, or ΔyraP strains, efficiency of plating (EOP) assays were performed as follows: overnight cultures of strains of CAG41241, CAG41434, CAG41254, CAG41408, CAG41245, the surA::Cm strain CAG41265, and WT strain CAG45114 were grown in LB at 30°C. The cultures were serially diluted 107-fold in 10-fold increments. One-hundred-microliter portions of each dilution of each culture were spread on LB agar and LB agar plates supplemented with one of the following agents: 1.5 μg/ml crystal violet, 0.15 μg/ml polymyxin B, 3.5% SDS, 1 mM EDTA, 3.5% SDS plus 1 mM EDTA, 150 μg/ml novobiocin, 5 μg/ml rifampin, and 0.1% phenethyl alcohol. The EOPs were calculated by dividing the number of CFU of a given mutant on supplemented LB plates by the number of CFU of that same mutant on LB plates. Where phenotypes were observed, EOP assays for each phenotype for each strain were performed three times with three independent isolates.
To determine whether the phenotypes of the ΔnlpB, ΔyraP, and ΔyfgL strains could be complemented, the EOP experiments were repeated as described above using strains CAG41350 and CAG41517, which carry pCO109; CAG41534 and CAG41536, which carry pCO122; CAG41511 and CAG41513, which carry pCO123; and CAG41510, CAG41512, CAG41516, and CAG41535, which carry pBA169.
Assays for sensitivity to growth on LB agar at 18, 30, 37, and 42°C and on M9 complete agar at 18, 30, 37, and 42°C were performed by diluting overnight cultures of strains CAG41241, CAG41434, CAG41254, CAG41408, CAG41245, the surA::Cm strain CAG41265, and WT strain CAG45114 105-fold in 10-fold increments and determining titers of 10 μl of each dilution of each strain on LB agar and M9 complete agar plates. Plates were placed at 18°C for 72 h or at 30, 37, or 42°C for 16 h. The mutant strains' growth under these conditions was compared to that of the WT strain, and no differences were observed. The sensitivity of these strains to growth on MacConkey agar at 30°C was also tested in this manner. Again, no differences between WT and the mutants were observed.
Sensitivities to 1 M each of CuCl2, NiCl2, CrCl2, CoCl2, FeCl2, CdCl2, and dithiothreitol (DTT) were tested as well. The solutions listed above were spotted onto sterile Whatman 3 MM filter paper disks that were allowed to dry before being placed onto lawns of CAG41241, CAG41434, CAG41254, CAG41408, and CAG41245 on LB. Zones of clearing on mutant strain lawns were compared to that of WT strain CAG45114, and no differences were observed.
The motility of the null mutants was also tested. Overnight cultures of strains were grown in LB at 30°C. The next day, the cultures were diluted 1:10 in LB, the OD600 of each culture was recorded, and the number of cells per μl of each culture was calculated. Volumes of each culture equivalent to 106 cells were then inoculated into the centers of LB soft agar plates (4 mg/ml agar in LB broth). After 24 h of incubation at 30°C and 37°C, the diameters of the swarms were measured and compared to that of WT. No differences were observed.
The sensitivities of the mutants to osmotic shock were tested in the following manner: mutant strains were grown at 30°C in low-osmolarity medium, described in the work of Lacroix et al. (17), and in low-osmolarity medium supplemented with 0.1 M NaCl, 0.2 M NaCl, 0.3 M NaCl, and 0.4 M NaCl. The OD600 for each culture was measured every 60 min for 600 min. The slopes of log10 OD600 versus time plots for each strain grown in each medium were compared to that of WT to determine whether NaCl concentration affected the mutants' growth rates. No differences were found.
σE activity assay.
σE activity was assayed by monitoring β-galactosidase activity from a chromosomal σE-dependent lacZ reporter gene Φλ[rpoHP3-lacZ] as described previously (1). Cells to be assayed were grown at 30°C in M9 complete medium (100 μg/ml ampicillin and 1 mM IPTG were added when necessary), and single point determinations were made at the indicated optical densities. The rate of β-galactosidase synthesis from the σE-dependent reporter gene increases as the cells enter mid-log phase when grown in LB (J. Mecsas and C. A. Gross, unpublished). Due to this growth phase effect, differences in σE activity were determined by comparing the slopes of the initial linear portions of the plots of β-galactosidase activity/0.5 ml cells to the OD450. Slopes were determined by linear regression analysis. The value of each slope was normalized to the WT value, taken as 1.0.
Preparation and analysis of outer membrane proteins.
Outer membrane proteins were prepared from strains grown in LB broth at 37°C (100 μg/ml ampicillin and 1 mM IPTG were added when necessary). Cultures were grown to an OD600 of 0.5 to 1.0, whereupon 10 ml of cells was harvested by centrifugation at 5,000 rpm for 10 min. The OD600 of each culture was recorded at the time of harvest. Pellets were resuspended in 100 mM Tris-HCl, pH 8, and 20% glucose and incubated on ice for 10 min. The cells were spun again at 5,000 rpm for 10 min, and pellets were resuspended in 100 mM Tris-HCl, pH 8, and 20% glucose supplemented with 10 mM EDTA. Cell walls were digested with lysozyme (10 μg/ml) on ice for 30 min. Each sample was adjusted to 10 mM MgCl2 and was then lysed by the addition of 1 ml of ice-cold water followed by three freeze-thaw cycles. Five micrograms per milliliter each of DNase I and RNase A were added to each sample, and the cell extracts were incubated on ice until they became completely fluid, indicating that the nucleic acids in the extracts had been digested. The extracts were then centrifuged for 15 min at 15,000 rpm, and the pellets were washed with 20 mM NaPO4, pH 7.0. Cytoplasmic membranes were solubilized with 1 ml of 0.5% sarcosyl in 20 mM NaPO4 at room temperature for 30 min. The insoluble outer membranes were pelleted by centrifugation at 15,000 rpm for 15 min, washed twice with 1 ml sarcosyl solution, and resuspended in 40 μl of SDS sample buffer (100 mM Tris-HCl, 200 mM DTT, 4% SDS, 0.2% bromophenol blue, 20% glycerol). The numbers of cells/μl for each sample were calculated from the OD600s recorded at harvest. A volume of each sample equivalent to 109 cells was loaded and resolved on a 12% polyacrylamide-SDS gel containing 50% wt/vol urea. (The addition of urea is necessary to resolve OmpC and OmpF.)
Assay of susceptibility to BPI-derived peptide P2.
Peptide P2, as described by Barker et al. (3) and containing residues 86 to 104 of bactericidal permeability-increasing (BPI) peptide flanked by cysteines (SKISGKWKAQKRFLKMSGNFGC), was synthesized by the Macromolecular Resources Facility at Colorado State University (Fort Collins, CO). The size and purity of the peptide were confirmed by matrix-assisted laser desorption ionization-mass spectroscopy and analytical high-performance liquid chromatography. The method of Barker et al. (3) was modified to determine the P2 susceptibility of strains CAG45114, CAG41241, CAG41434, CAG41254, CAG41408, andCAG41245. Bacteria were cultured to stationary phase without agitation in 5 ml TSB, washed twice in 0.9% NaCl, and diluted to the desired concentration of CFU/ml in 50 ml of 20 mM sodium phosphate buffer, pH 6.0. Samples were incubated with 4 μg/ml P2 peptide for 90 min at 37°C without agitation. Aliquots (20 μl) were serially diluted, plated on LB agar plates, and allowed to grow for 18 h at 37°C for enumeration of surviving CFU.
RESULTS
Identification of lipoproteins in the σE regulon.
A computational motif search of the 96 members of the σE regulon revealed that the amino acid sequences of 6 of these genes encode lipobox motifs embedded in signal sequences (Fig. 1) but did not provide any clues as to their function. The proteins encoded by these genes have each been confirmed to carry lipid modifications (26). Because these lipoproteins lack an Asp at position +2, they are all likely to interact with the Lol pathway and to be localized to the outer membrane. To determine whether any of these lipoproteins are essential, we used the methods described by Datsenko and Wanner (12) to delete the entire open reading frame of each gene encoding a lipoprotein. We were able to delete yfgL, yraP, yeaY, nlpB, and yfeY, indicating that these genes are not essential. The results for nlpB and yfgL validate previous reports wherein null alleles of these genes were generated via insertional mutagenesis (8, 15). Because YraP and OsmY exhibit 29% identity and 50% similarity, we postulated that these two genes perform redundant functions; however, a strain carrying both the ΔyraP and ΔosmY null alleles is still viable. We considered the possibility that the five genes were not essential because their encoded lipoproteins perform redundant functions. Were this so, a strain lacking all five lipoproteins would be nonviable. We constructed such a strain using methods described in the work of Datsenko and Wanner (12) and found this strain to be viable at temperatures ranging from 18°C to 42°C in both standard LB medium and M9 medium supplemented with all the amino acids. We concluded that the five nonessential lipoproteins as a group do not perform an essential function in E. coli.
FIG. 1.
The lipoboxes of the six σE-transcribed lipoproteins. Displayed are the N-terminal sequences of each lipoprotein in the σE regulon. The lipobox motifs are depicted in large bold type. The conserved cysteine residue in each lipobox, which is destined for lipid modification, is shaded. For each sequence, the number in parentheses to the right refers to the position of the cysteine from the N-terminal methionine.
Because we were unable to delete yfiO using the strategy by which we constructed the other null mutants, we considered that yfiO might be essential. To test this possibility, we first constructed a null allele of yfiO in a strain carrying a second copy of this gene inserted in the lac operon (see Materials and Methods). We then performed linked marker cotransduction to assess whether yfiO was essential. Whereas ΔyfiO::Cm was >95% cotransducible with pheA18::Tn10 in a WT strain carrying a plasmid-encoded copy of yfiO (Table 3, columns 2 to 4, row 1), ΔyfiO::Cm could not be cotransduced with pheA18::Tn10 into the WT strain alone, in which successful cotransduction would result in a lack of YfiO (Table 3, columns 2 to 4, row 2). Due to the close linkage of ΔyfiO::Cm and pheA18::Tn10, we obtained only very few Tetr transductants in the WT strain, even though both recipient strains are equally transducible by pheA18::Tn10 when it is not linked to ΔyfiO::Cm (Table 3, column 5, rows 1 and 2). We conclude that yfiO is essential.
TABLE 3.
P1 transduction experiments demonstrate that yfiO is essential
| Recipient | Donor P1(ΔyfiO::Cm; pheA18::Tn10)
|
Donor P1(pheA18::Tn10)
|
||
|---|---|---|---|---|
| Number of Tetr transductants | Number of Tetr Cmr cotransductants | Linkage (%) | Number of Tetr transductants | |
| MG1655 + pyfiO | 100 | 97 | 97 | >100 |
| MG1655 | 7 | 0 | 0 | >100 |
The essentiality of yfiO raised the possibility that σE is essential solely because it provides YfiO. We tested this idea by determining whether rpoE::Cm could be transduced into WT cells with a plasmid carrying yfiO transcribed from a Ptrc promoter. Linked marker cotransduction experiments demonstrated that such cells were not able to accept the rpoE::Cm marker even when Ptrc expression of YfiO was induced by addition of IPTG (Table 4, columns 2 to 4, row 1). Again, due to the close linkage of ΔyfiO::Cm and pheA18::Tn10, we obtained only very few Tetr transductants in the WT strain (Table 4, columns 2 to 4, row 2). Both recipient strains are equally transducible by nadB::Tn10 when it is not linked to rpoE::Cm (Table 4, column 5, rows 1 and 2). Additional control transductions established that the P1(rpoE::Cm nadB::Tn10) lysate used in these experiments was of a high titer (data not shown). Thus, the σE regulon must provide two or more proteins that are essential for cell viability.
TABLE 4.
P1 transduction experiments demonstrate that providing YfiO is not sufficient to relieve σE essentiality
| Recipient | Donor P1(rpoE::Cm; nadB::Tn10)
|
Donor P1(nadB::Tn10)
|
||
|---|---|---|---|---|
| Number of Tetr transductants | Number of Tetr Cmr cotransductants | Linkage (%) | Number of Tetr transductants | |
| MG1655 + pyfiO | 3 | 0 | 0 | >100 |
| MG1655 | 5 | 0 | 0 | >100 |
Phenotypes of strains with deletions of nonessential lipoproteins.
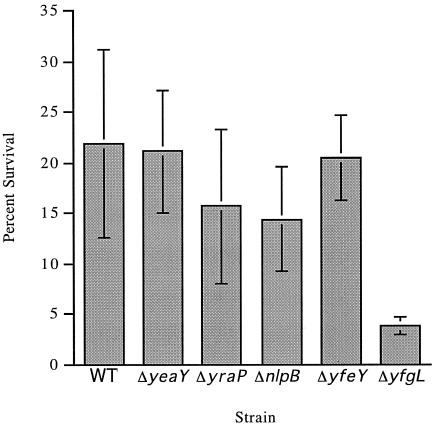
We assessed whether any of the individual nonessential lipoprotein deletion mutants altered the growth properties of cells under a variety of conditions. All strains were able to grow at temperatures ranging from 18°C to 42°C both in standard LB medium and in M9 minimal medium supplemented with all of the amino acids, indicating that none of these lipoproteins were essential for growth at temperature extremes. Because the σE regulon is induced under hypo-osmotic conditions (data not shown), we next tested whether the mutants were altered in their osmotic sensitivities. This phenotype is also a general indicator of envelope integrity (reviewed in references 32 and 49). However, all strains grew equally well in hypo-osmotic medium alone (described in reference 17) and in hypo-osmotic medium supplemented with 0.1 M, 0.2 M, 0.3 M, or 0.4 M NaCl (data not shown). All deletion strains were also unimpaired in their motilities, in their sensitivities to DTT, and in their sensitivities to a variety of metals (LB agar containing 1 M each of CuCl2, NiCl2, CrCl2, CoCl2, FeCl2, or CdCl2). The mutants exhibited growth similar to that of the WT strain on MacConkey agar at 30°C, 37°C, and 42°C. Finally, given that the σE regulon lipoproteins are all targeted to the outer membrane, we tested the lipoprotein deletion mutants for their sensitivities to a variety of agents that are normally excluded by a WT outer membrane. These agents included amphipathic antibiotics (rifampin, novobiocin), detergents (SDS, bile salts), hydrophobic dyes (crystal violet), and EDTA, which destabilizes the outer membrane by chelating divalent cations bound to lipopolysaccharide (reviewed in reference 31). Three of the mutants exhibited EOP phenotypes that were reversed in each case by supplying the missing lipoprotein on a plasmid (Table 5). ΔyfgL exhibited the broadest phenotypes: plating was eliminated on rifampin, and although cells grew on SDS and novobiocin, colony growth was dramatically slowed. This phenotype was reversed in ΔyfgL strains carrying pyfgL (Table 5 and data not shown). In contrast, ΔnlpB and ΔyraP exhibited highly specific phenotypes, with the former exhibiting sensitivity to rifampin only and the latter exhibiting sensitivity to SDS only. In contrast, a surA::Cm mutant strain, which interferes with OMP assembly and destabilizes the outer membrane, exhibits general sensitivity to hydrophobic dyes, hydrophobic amphipathic antibiotics, and detergents (38) (Table 5). The human BPI protein has potent antimicrobial activity against gram-negative bacteria (see reference 3; also reviewed in reference 48). The P2 peptide derived from BPI retains much of the antimicrobial properties of BPI itself. BPI and P2 increase outer membrane permeability and decrease cellular O2 consumption (48). The cytotoxic mechanism, though not well understood, may result from membrane rupture. We tested whether the lipoprotein deletion mutants survived more poorly than WT strains in the presence of the P2 peptide. We find that the ΔyfgL strain is significantly more sensitive to P2 than are WT cells (Fig. 2). In addition, ΔyraP and ΔnlpB are marginally more sensitive to P2 than are WT cells. This modest difference has been reproducible, but given the variability of these assays, this may not be statistically significant. We cannot determine whether the increased sensitivity of cells of the ΔyfgL strain to P2 is simply a consequence of envelope defects or whether it results instead from loss of a specific mechanism conferring resistance to the effects of the peptide. We favor the former idea because these cells have multiple alterations in their envelope.
TABLE 5.
EOP of various deletion mutant strains ± complementing plasmids on selected agents
| Strain | EOPa
|
||
|---|---|---|---|
| 3.5% SDS | 5 μg/ml rifampicin | 150 μg/ml novobiocin | |
| WT | 0.9 | 0.1 | 0.7 |
| ΔyfgL | 0.4* | 6 × 10−9 | 0.3* |
| ΔyfgL + pyfgLb | NT | 1 | NT |
| ΔnlpB | 0.8 | 4 × 10−5 | 0.7 |
| ΔnlpB + pnlpBb | NT | 0.4 | NT |
| ΔyraP | 4 × 10−7 | 0.6 | 0.8 |
| ΔyraP + pyraPb | 1 | NT | NT |
| surA::Cm | 2 × 10−6 | 1 × 10−8 | 6 × 10−5 |
Values for EOP are the averages of three determinations; almost all values differed from the mean by less than 50%. An asterisk indicates that colonies were not apparent until 48 hr at 30°C. For comparison, all other strains formed colonies after 18 hr at 30°C. NT, not tested.
All complementation experiments were performed in the presence of IPTG to induce expression.
FIG. 2.
Effects of deleting nonessential lipoprotein genes on sensitivity to BPI-derived P2 peptide. Stationary-phase bacteria grown without agitation in 5 ml TSB were diluted to ∼2 × 107 CFU/ml in 20 mM sodium phosphate buffer, pH 6.0, in a final volume of 50 μl. Samples were incubated with 4 mg/ml BPI for 90 min at 37°C. Data represent the mean CFU per ml from serial dilutions of triplicate samples plated on LB agar and incubated for 18 h at 37°C.
Effect of lipoprotein deletions on σE activity and porin content of the outer membrane.
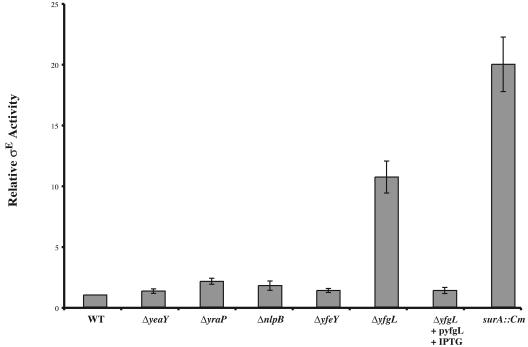
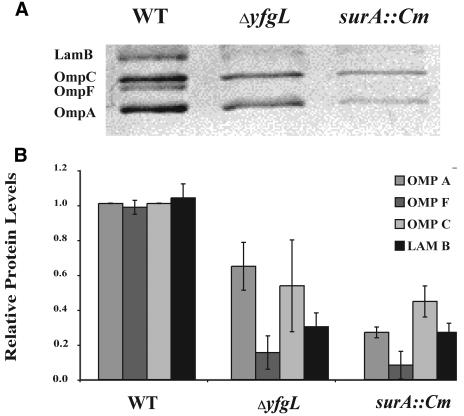
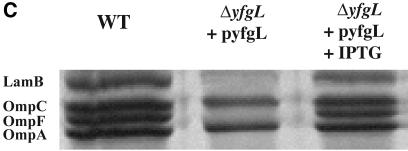
The activity of σE is a sensitive indicator of the integrity of the outer membrane (reviewed in reference 2). The assembly status of β-barrel porins is sensed directly by the signal transduction pathway controlling σE activity (47). Additionally, this pathway indirectly senses the status of LPS assembly and protein folding (2). Because several of the lipoprotein deletion strains exhibit sensitivities to agents normally excluded by the outer membrane, we thought it probable that these mutants would also exhibit increased σE activity (Fig. 3). ΔyfgL, which is sensitive to the broadest range of agents tested in Table 3, exhibited a 10-fold increase in σE activity, which is only slightly less than the induction mediated by the surA::Cm mutation, a potent inducer of σE (38). The induction phenotype of the ΔyfgL mutant was relieved by expression of YfgL from a plasmid (Fig. 3). ΔyraP also exhibited slight (approximately twofold) induction of the response. Many mutations that increase σE activity also affect OMP profiles. For example, the levels of porins OmpF, OmpC, OmpA, and LamB are significantly reduced in the surA::Cm mutant, in which σE activity is highly induced (38). We determined the levels of OmpF, OmpC, OmpA, and LamB in outer membrane preparations purified from each lipoprotein deletion mutant, as well as surA::Cm, a mutant known to exhibit severe defects in porin content in the OM, using both the surA::Cm mutant and WT strain MG1655 as a basis for comparison. The contents of ΔyfgL strains were deficient for the four porins tested, exhibiting a phenotype almost as severe in this regard as surA::Cm (Fig. 4A and B). This phenotype was complemented when YfgL was expressed from a plasmid (Fig. 4C). No other lipoprotein deletion mutant showed any OMP profile defects.
FIG. 3.
σE activity of strains in which each of the nonessential lipoproteins has been deleted. Relative σE activities in WT (CAG45114), ΔyeaY (CAG41241), ΔyraP (CAG41251), ΔnlpB (CAG41258), ΔyfeY (CAG41413), ΔyfgL (CAG41445), and surA::Cm (CAG41265) strains growing at 30°C in LB were assayed by monitoring β-galactosidase activity from a single-copy Φλ[rpoHP3-lacZ] fusion as described in Materials and Methods. The increased σE activity observed in the ΔyfgL mutant can be complemented by pyfgL.
FIG.4.
The OMP profiles of wild type, ΔyfgL, and ΔsurA::Cm strains. (A) Outer membrane fractions were prepared from cells that had been growing exponentially at 37°C in LB plus 0.2% maltose, as described in Materials and Methods. The fractions were analyzed on a 12% SDS-polyacrylamide gel with 50% urea and stained with Coomassie blue. The positions of LamB, OmpC, OmpF, and OmpA are indicated. (B) The levels of LamB, OmpC, OmpF, and OmpA were quantified via spot densitometry as described in Materials and Methods. (C) The OMP profile defect in the ΔyfgL mutant can be complemented by pyfgL.
Do these lipoproteins contribute to the protein-folding capacity of the envelope?
A number of periplasmic chaperones and periplasmic peptidyl prolyl isomerases (PPIases) facilitate the folding, assembly, and insertion of trimeric porins into the outer membrane. SurA has both chaperone and PPIase activity and is required for the proper assembly of porins (5, 37). DegP is predominantly a chaperone at low temperatures and has both chaperone and protease activity at elevated temperatures (42). Skp also exhibits chaperone activity and has been found to bind to outer membrane porin precursors (7, 9, 13). Deletion of the gene encoding any one of these three folding agents is not lethal. However Δskp surA::Cm and degP::Km surA::Cm double mutants are synthetically lethal, whereas the Δskp degP::Km double mutant is not (36). This finding led the Silhavy group to propose the existence of two parallel periplasmic folding pathways: one involving SurA and the other involving Skp and DegP (36). We have expanded this study to include our lipoprotein deletion strains, as several confer envelope phenotypes. We have also included FkpA, a periplasmic PPIase and chaperone, in this analysis, because mutations in fkpA exhibit phenotypes similar to but much less severe than those reported for surA (25).
Our studies were performed with MG1655 rather than with the MC4100 strain utilized by Rizzitello et al. (36). Therefore, we determined that the previously reported synthetic phenotypes were maintained in MG1655, although they were less severe in MG1655 than in MC4100 (Table 6). The Δskp surA::Cm and degP::Km surA::Cm double mutants were distinctly impaired, as they formed very small colonies that could be visualized only after 48 h at 30°C. We refer to this phenotype as “very small” (vs). In contrast, the single-mutant strains formed colonies after about 18 h at 30°C. Moreover, we found that ΔfkpA is not synthetically lethal with Δskp, degP::Km, or surA::Cm.
TABLE 6.
Synthetic phenotypes of strains lacking periplasmic chaperones
| Recipient | P1 donor strainsa
|
|||
|---|---|---|---|---|
| surA::Cm | degP::Km | Δskp zae::Tn10 | ΔfkpA::Cm | |
| surA::Cm | NT | vs | vs | NT |
| degP::Km | vs | NT | + | + |
| Δskp zae::Tn10 | vs | + | NT | + |
| ΔfkpA | + | + | + | NT |
vs indicates that colonies appeared after 48 hr on LB at 30°C; for comparison, single mutants formed colonies after 18 hr at 30°C. A plus sign indicates that the phenotype of the double mutant was the same as that of the single mutant. NT, not tested.
Two of the lipoprotein deletion strains, ΔnlpB and ΔyraP, exhibited a synthetic phenotype with surA::Cm, forming vs colonies on LB after 48 h. However, neither ΔnlpB nor ΔyraP exhibited any synthetic phenotypes with any of the other chaperone mutants (Table 7). Thus, ΔnlpB and ΔyraP exhibit genetic interactions very similar to those shown for Δskp and degP::Km. The simplest interpretation of these results is that the NlpB and YraP lipoproteins are part of the DegP/Skp folding pathway. A third lipoprotein deletion strain, ΔyfgL, exhibited a novel synthetic phenotype: when a ΔyfgL strain was transduced with either P1(surA::Cm) or P1(ΔfkpA::Cm), the resulting double mutants formed vs colonies. These phenotypes would be explained if YfgL functioned in more than one periplasmic protein-folding pathway.
TABLE 7.
ΔnlpB, ΔyraP, and ΔyfgL exhibit synthetic phenotypes with strains lacking periplasmic chaperones
| Recipient | P1 donor strainsa
|
|||
|---|---|---|---|---|
| surA::Cm | degP::Km | Δskp zae::Tn10 | ΔfkpA::Cm | |
| ΔnlpB | vs | + | + | + |
| ΔyraP | vs | + | + | + |
| ΔyfgL | vs | + | + | vs |
vs indicates that colonies appeared after 48 hr on LB at 30°C; for comparison, single mutants formed colonies after 18 hr at 30°C. A plus sign indicates that the phenotype of the double mutant was the same as that of the single mutant.
DISCUSSION
The cellular roles of most lipoproteins are very poorly understood. The E. coli genome is predicted to encode approximately 100 lipoproteins, making it a daunting task to characterize this entire group. In addition, very few previously characterized lipoproteins proved to have significant phenotypes. Because σE is essential for viability and plays a major role in envelope homeostasis, we hoped that choosing to study lipoproteins encoded in the σE regulon would enrich for those with roles in envelope homeostasis. Indeed, four of the six lipoproteins in the regulon are functionally important. One of these lipoproteins, YfiO, is essential, making it the second essential lipoprotein in E. coli and the most important lipoprotein in the σE regulon. The other three lipoproteins are nonessential, and their absences confer distinct envelope phenotypes.
LPS rough mutants and other mutations that alter the porin content of the outer membrane are known to destabilize the cell envelope and increase its permeability to a wide variety of detergents, dyes, and antibiotics (31). Both NlpB and YraP also appear to be involved in maintaining envelope integrity, as deleting either gene alters the barrier function of the outer membrane. Interestingly, each mutant strain is affected in its permeability to a specific class of molecules only. Cells lacking YraP become selectively sensitive to SDS but not to rifampin, whereas those lacking NlpB are sensitive to rifampin but exhibit unaltered sensitivity to SDS. Such specific sensitivity has not been previously reported. These lipoproteins may be required for processes that impart very subtle alterations to the outer membrane, thereby conferring the selective permeability we observe. This idea is consistent with the fact that each selectively permeable mutant exhibits a synthetic phenotype with surA::Cm. These data, together with the previous observations by Rizzitello et al. (36), lead us to posit that both NlpB and YraP work in the DegP/Skp pathway to facilitate assembly of porins and other outer membrane proteins.
Cells lacking YfgL exhibit a number of different phenotypes, all of which indicate a disturbance in the barrier function of the cell envelope. ΔyfgL cells are modestly pleiotropic in their sensitivities to agents normally excluded by the outer membrane and exhibit significant sensitivity to BPI-derived peptide P2. Additionally, ΔyfgL cells are defective in assembling porins in the outer membrane and exhibit synthetic phenotypes not only with surA::Cm but also with ΔfkpA::Cm. Taken together, these data indicate that YgfL participates in an important process or processes that ensure outer membrane integrity. Like SurA, YgfL could be a chaperone whose activity is integral to the insertion of outer membrane porins. Alternatively, YgfL could participate more generally in assembly of some outer membrane component. Whatever the particular function of YgfL, the large increase in σE activity exhibited by these mutants suggests that, either directly or indirectly, the lack of YgfL results in a rather large increase in the pools of unassembled porins, which then act as an inducing signal for σE.
Research performed concurrently with ours provides strong evidence that two of the nonessential lipoproteins that we implicated in porins assembly, along with the essential lipoprotein YfiO, participate together in proins assembly (39, 50). These three lipoproteins form a multiprotein complex with YaeT, another essential member of the σE regulon (39, 50). YaeT is required for insertion of β-barrel proteins, including outer membrane porins, into the outer membrane (39, 50). Moreover, orthologues of YaeT, including Omp85 (Neisseria spp.) (46), Tob55 (mitochondria) (16, 33), and Toc75 (chloroplasts) (33), are also necessary for the insertion of β-barrel proteins into the outer membrane. Taken together, this suggests that YaeT, together with three lipoproteins, is part of the machine that inserts OMPs into the outer membrane.
Many questions are unresolved. First, although YfiO, YfgL and NlpB are all are implicated in OMP assembly, deletions of each have distinct phenotypes. Clearly, the role played by each lipoprotein in OMP assembly remains to be determined. Second, although YraP was not detected in the four-protein YfiO/NlpB/YfgL/YaeT complex (39, 50), the experiments performed thus far do not rule out the possibility that YraP is a loosely associated component of that complex. Finally, we suggested that YfgL might have multiple roles in the cell because it had the unusual property of synthetic lethality with two different chaperones. Likewise, the Silhavy-Kahne groups showed that YfgL deletions had multiple effects in the cell, participating not only in OMP assembly but also in the alteration of the permeability properties of cells defective in LPS insertion (39, 50). These studies open the door to multiple lines of investigation.
Acknowledgments
This work was supported by Public Health Service grant GM036278 (to C.A.G.) and AI44486 (to F.C.F.) from the National Institutes of Health.
We thank Joyce West for helpful guidance in the performance of this work.
REFERENCES
- 1.Ades, S. E., L. E. Connolly, B. M. Alba, and C. A. Gross. 1999. The Escherichia coli σE dependent extracytoplasmic stress response is controlled by the regulated proteolysis of an anti-sigma factor. Genes Dev. 13:2449-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alba, B. M., and C. A. Gross. 2004. Regulation of the Escherichia coli σE-dependent envelope stress response. Mol. Microbiol. 52:613-619. [DOI] [PubMed] [Google Scholar]
- 3.Barker, H. C., N. Kinsella, A. Jaspe, T. Friedrich, and C. D. O'Connor. 2000. Formate protects stationary-phase Escherichia coli and Salmonella cells from killing by a cationic antimicrobial peptide. Mol. Microbiol. 35:1518-1529. [DOI] [PubMed] [Google Scholar]
- 4.Beck, B. J., L. E. Connolly, A. De Las Peñas, and D. M. Downs. 1997. A role for rseC, a gene in the rpoE cluster, in thiamine synthesis in Salmonella typhimurium. J. Bacteriol. 179:6504-6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behrens, S., R. Maier, H. de Cock, F. X. Schmid, and C. A. Gross. 2001. The SurA periplasmic PPIase lacking its parvulin domains functions in vivo and has chaperone activity. EMBO J. 20:285-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernadac, A., M. Gavioli, J. C. Lazzaroni, S. Raina, and R. Lloubes. 1998. Escherichia coli tol-pal mutants form outer membrane vesicles. J. Bacteriol. 180:4872-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bothmann, H., and A. Pluckthun. 1998. Selection for a periplasmic factor improving phage display and functional periplasmic expression. Nat. Biotechnol. 16:376-380. [DOI] [PubMed] [Google Scholar]
- 8.Bouvier, J., A. P. Pugsley, and P. Stragier. 1991. A gene for a new lipoprotein in the dapA-purC interval of the Escherichia coli chromosome. J. Bacteriol. 173:5523-5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, R., and U. Henning. 1996. A periplasmic protein (Skp) of Escherichia coli selectively binds a class of outer membrane proteins. Mol. Microbiol. 19:1287-1294. [DOI] [PubMed] [Google Scholar]
- 10.Cherepanov, P. P., and W. Wackernagel. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9-14. [DOI] [PubMed] [Google Scholar]
- 11.Dartigalongue, C., D. Missiakas, and S. Raina. 2001. Characterization of the Escherichia coli σE regulon. J. Biol. Chem. 276:20866-20875. [DOI] [PubMed] [Google Scholar]
- 12.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Cock, H., U. Schafer, M. Potgeter, R. Demel, M. Muller, and J. Tommassen. 1999. Affinity of the periplasmic chaperone Skp of Escherichia coli for phospholipids, lipopolysaccharides and non-native outer membrane proteins. Role of Skp in the biogenesis of outer membrane protein. Eur. J. Biochem. 259:96-103. [DOI] [PubMed] [Google Scholar]
- 14.Dinh, T., I. T. Paulsen, and M. H. Saier, Jr. 1994. A family of extracytoplasmic proteins that allow transport of large molecules across the outer membranes of gram-negative bacteria. J. Bacteriol. 176:3825-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eggert, U. S., N. Ruiz, B. V. Falcone, A. A. Branstrom, R. C. Goldman, T. J. Silhavy, and D. Kahne. 2001. Genetic basis for activity differences between vancomycin and glycolipid derivatives of vancomycin. Science 294:361-364. [DOI] [PubMed] [Google Scholar]
- 16.Gentle, I., K. Gabriel, P. Beech, R. Waller, and T. Lithgow. 2004. The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. J. Cell Biol. 164:19-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacroix, J. M., I. Loubens, M. Tempete, B. Menichi, and J. P. Bohin. 1991. The mdoA locus of Escherichia coli consists of an operon under osmotic control. Mol. Microbiol. 5:1745-1753. [DOI] [PubMed] [Google Scholar]
- 18.Lloubes, R., E. Cascales, A. Walburger, E. Bouveret, C. Lazdunski, A. Bernadac, and L. Journet. 2001. The Tol-Pal proteins of the Escherichia coli cell envelope: an energized system required for outer membrane integrity? Res. Microbiol. 152:523-529. [DOI] [PubMed] [Google Scholar]
- 19.Lomovskaya, O., and K. Lewis. 1992. Emr, an Escherichia coli locus for multidrug resistance. Proc. Natl. Acad. Sci. USA 89:8938-8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lomovskaya, O., K. Lewis, and A. Matin. 1995. EmrR is a negative regulator of the Escherichia coli multidrug resistance pump EmrAB. J. Bacteriol. 177:2328-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma, D., D. N. Cook, M. Alberti, N. G. Pon, H. Nikaido, and J. E. Hearst. 1993. Molecular cloning and characterization of acrA and acrE genes of Escherichia coli. J. Bacteriol. 175:6299-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuyama, S., T. Tajima, and H. Tokuda. 1995. A novel periplasmic carrier protein involved in the sorting and transport of Escherichia coli lipoproteins destined for the outer membrane. EMBO J. 14:3365-3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuyama, S., N. Yokota, and H. Tokuda. 1997. A novel outer membrane lipoprotein, LolB (HemM), involved in the LolA (p20)-dependent localization of lipoproteins to the outer membrane of Escherichia coli. EMBO J. 16:6947-6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Missiakas, D., F. Schwager, J. M. Betton, C. Georgopoulos, and S. Raina. 1996. Identification and characterization of HsIV HsIU (ClpQ ClpY) proteins involved in overall proteolysis of misfolded proteins in Escherichia coli. EMBO J. 15:6899-6909. [PMC free article] [PubMed] [Google Scholar]
- 26.Miyadai, H., K. Tanaka-Masuda, S. I. Matsuyama, and H. Tokuda. 2004. Effects of lipoprotein overproduction on the induction of DegP (HtrA) involved in quality control in the Escherichia coli periplasm. J. Biol. Chem. 279:39807-39813. [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto, A., S. Matsuyama, and H. Tokuda. 2001. Mutant of LolA, a lipoprotein-specific molecular chaperone of Escherichia coli, defective in the transfer of lipoproteins to LolB. Biochem. Biophys. Res. Commun. 287:1125-1128. [DOI] [PubMed] [Google Scholar]
- 28.Murphy, K. C. 1998. Use of bacteriophage λ recombination functions to promote gene replacement in Escherichia coli. J. Bacteriol. 180:2063-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narita, S., S. Matsuyama, and H. Tokuda. 2004. Lipoprotein trafficking in Escherichia coli. Arch. Microbiol. 182:1-6. [DOI] [PubMed] [Google Scholar]
- 30.Narita, S., K. Tanaka, S. Matsuyama, and H. Tokuda. 2002. Disruption of lolCDE, encoding an ATP-binding cassette transporter, is lethal for Escherichia coli and prevents release of lipoproteins from the inner membrane. J. Bacteriol. 184:1417-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikaido, H. 1996. Outer membrane, p. 29-47. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C.
- 32.Nikaido, H. 1994. Porins and specific diffusion channels in bacterial outer membranes. J. Biol. Chem. 269:3905-3908. [PubMed] [Google Scholar]
- 33.Paschen, S. A., T. Waizenegger, T. Stan, M. Preuss, M. Cyrklaff, K. Hell, D. Rapaport, and W. Neupert. 2003. Evolutionary conservation of biogenesis of beta-barrel membrane proteins. Nature 426:862-866. [DOI] [PubMed] [Google Scholar]
- 34.Raivio, T. L., and T. J. Silhavy. 2001. Periplasmic stress and ECF sigma factors. Annu. Rev. Microbiol. 55:591-624. [DOI] [PubMed] [Google Scholar]
- 35.Rezuchova, B., H. Miticka, D. Homerova, M. Roberts, and J. Kormanec. 2003. New members of the Escherichia coli σE regulon identified by a two-plasmid system. FEMS Microbiol. Lett. 225:1-7. [DOI] [PubMed] [Google Scholar]
- 36.Rizzitello, A. E., J. R. Harper, and T. J. Silhavy. 2001. Genetic evidence for parallel pathways of chaperone activity in the periplasm of Escherichia coli. J. Bacteriol. 183:6794-6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rouviere, P. E., A. De Las Penas, J. Mecsas, C. Z. Lu, K. E. Rudd, and C. A. Gross. 1995. rpoE, the gene encoding the second heat-shock sigma factor, σE, in Escherichia coli. EMBO J. 14:1032-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rouvière, P. G., and C. A. Gross. 1996. SurA, a periplasmic protein with peptidyl-prolyl isomerase activity, participates in the assembly of outer membrane porins. Genes Dev. 10:3170-3182. [DOI] [PubMed] [Google Scholar]
- 39.Ruiz, N., B. Falcone, D. Kahne, and T. J. Silhavy. 2005. Chemical conditionality: a genetic strategy to probe organelle assembly. Cell 121:307-317. [DOI] [PubMed] [Google Scholar]
- 40.Sankaran, K., and H. C. Wu. 1994. Lipid modification of bacterial prolipoprotein. Transfer of diacylglyceryl moiety from phosphatidylglycerol. J. Biol. Chem. 269:19701-19706. [PubMed] [Google Scholar]
- 41.Singer, M., T. A. Baker, G. Schnitzler, S. M. Deischel, M. Goel, W. Dove, K. J. Jaacks, A. D. Grossman, J. W. Erickson, and C. A. Gross. 1989. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol. Rev. 53:1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spiess, C., A. Beil, and M. Ehrmann. 1999. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97:339-347. [DOI] [PubMed] [Google Scholar]
- 43.Tajima, T., N. Yokota, S. Matsuyama, and H. Tokuda. 1998. Genetic analyses of the in vivo function of LolA, a periplasmic chaperone involved in the outer membrane localization of Escherichia coli lipoproteins. FEBS Lett. 439:51-54. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka, K., S. I. Matsuyama, and H. Tokuda. 2001. Deletion of lolB, encoding an outer membrane lipoprotein, is lethal for Escherichia coli and causes accumulation of lipoprotein localization intermediates in the periplasm. J. Bacteriol. 183:6538-6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tegos, G., F. R. Stermitz, O. Lomovskaya, and K. Lewis. 2002. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob. Agents Chemother. 46:3133-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Voulhoux, R., M. P. Bos, J. Geurtsen, M. Mols, and J. Tommassen. 2003. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science 299:262-265. [DOI] [PubMed] [Google Scholar]
- 47.Walsh, N. P., B. M. Alba, B. Bose, C. A. Gross, and R. T. Sauer. 2003. OMP peptide signals initiate the envelope-stress response by activating DegS protease via relief of inhibition mediated by its PDZ domain. Cell 113:61-71. [DOI] [PubMed] [Google Scholar]
- 48.Weiss, J. 2003. Bactericidal/permeability-increasing protein (BPI) and lipopolysaccharide-binding protein (LBP): structure, function and regulation in host defence against Gram-negative bacteria. Biochem. Soc. Trans. 31:785-790. [DOI] [PubMed] [Google Scholar]
- 49.Wu, H. C. 1996. Biosynthesis of lipoproteins, p. 1005-1014. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C.
- 50.Wu, T., J. Malinverni, N. Ruiz, S. Kim, T. J. Silhavy, and D. Kahne. 2005. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell 121:235-245. [DOI] [PubMed] [Google Scholar]
- 51.Yakushi, T., K. Masuda, S. Narita, S. Matsuyama, and H. Tokuda. 2000. A new ABC transporter mediating the detachment of lipid-modified proteins from membranes. Nat. Cell Biol. 2:212-218. [DOI] [PubMed] [Google Scholar]
- 52.Yamaguchi, K., F. Yu, and M. Inouye. 1988. A single amino acid determinant of the membrane localization of lipoproteins in E. coli. Cell 53:423-432. [DOI] [PubMed] [Google Scholar]
- 53.Yem, D. W., and H. C. Wu. 1977. Genetic characterization of an Escherichia coli mutant altered in the structure of murein lipoprotein. J. Bacteriol. 131:759-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yem, D. W., and H. C. Wu. 1978. Physiological characterization of an Escherichia coli mutant altered in the structure of murein lipoprotein. J. Bacteriol. 133:1419-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yura, T., and K. Nakahigashi. 1999. Regulation of the heat-shock response. Curr. Opin. Microbiol. 2:153-158. [DOI] [PubMed] [Google Scholar]