Abstract
The growth, morphology, and life cycle of two marine myxobacterial isolates, halotolerant Myxococcus fulvus strain HW-1 and halophilic Haliangium ochraceum strain SMP-2, were studied as models to determine the living patterns of myxobacteria in the ocean. The growth, morphology, and development of halotolerant strain HW-1 shifted in response to salinity. The optimal seawater concentration for growth of HW-1 was 0 to 80% (salinity, 0.1 to 2.9%), and the strain grew poorly in media with a salinity of more than 4%. The cells became shorter as the seawater concentration increased. The fruiting body structure was complete only on agar prepared with low concentrations of seawater or salts (less than 60% seawater; salinity, 2.1%), and rudimentary structures or even simple cell mounds appeared as the seawater concentration increased. In contrast, the halophilic strain SMP-2 was unable to grow without NaCl. The cell length and the morphology of the fruiting body-like structure did not change in response to salts. In seawater liquid medium, the cells of both strains were confirmed to be able to form myxospores directly from vegetative cells, but they could not do so in medium containing a low seawater concentration (10% or less). HW-1 cells from medium containing a high concentration of seawater grew independent of cell density, while cells from medium containing a low concentration of seawater (10% or less) showed density-dependent growth. SMP-2 cells showed density-dependent growth under all salinity conditions. The results suggest that the halotolerant myxobacteria are the result of degenerative adaptation of soil myxobacteria to the marine environment, while the halophilic myxobacteria form a different evolutionary group that is indigenous to the ocean.
Gram-negative unicellular myxobacteria are phylogenetically located in the δ division of the Proteobacteria (19-21, 24), and they are notable among prokaryotes for their complicated social behavior (2, 18). Myxobacterial cells move by gliding in swarms on solid surfaces, feed by cooperatively digesting macromolecules as well as whole microorganisms, and form multicellular fruiting bodies on solid surfaces. The resting cells, or myxospores, develop within fruiting bodies. Fruiting bodies develop only on solid surfaces (18), and terrestrial myxobacteria do not usually tolerate an NaCl concentration greater than 1% (15). The myxobacteria are typically considered to be soil microorganisms and are common in many terrestrial environments (1, 14). On the other hand, myxobacteria can be isolated from marine samples (6, 7, 8, 9, 13, 14, 25), as proven by the 16S rRNA gene fragments amplified from marine samples (4, 11) and permanently cold marine sediments (12). Depending on their adaptation to salinity, the marine myxobacterial isolates include three types: nonhalotolerant isolates, halotolerant isolates, and halophilic isolates. The nonhalotolerant isolates are the isolates that are unable to grow with a salt concentration greater than 1.0% (15). They are presumed to have germinated from myxospores of soil myxobacteria that were washed into seawater (9, 13). The halotolerant isolates are capable of tolerating a wide range of salt water concentrations and grow with or without sodium chloride. They have nearly the same 16S rRNA gene sequences as terrestrial myxobacteria (9) and are thought to have evolved from the terrestrial myxobacterial strains by acclimating to the conditions of seawater. The halophilic isolates are unable to grow in the absence of sodium chloride. The halophilic myxobacteria are both phylogenetically distant and morphogenetically different from the normal terrestrial myxobacteria and have been classified as a novel myxobacterial group (6, 7, 25).
Halophilic and halotolerant myxobacteria have been isolated from various marine samples, including seawater, marine sands, mud, and sediments on marine animals and plants (6, 7, 8, 9, 25), which suggests that myxobacteria might be indigenous to the ocean. However, the natural marine environments do not favor the morphogenesis of the fruiting body structure, which not only protects myxospores but also ensures the cell density for a new life cycle (18). So how could these marine myxobacterial isolates live in the ocean?
In the present paper, a halotolerant myxobacterial strain, Myxococcus fulvus HW-1 (= ATCC BAA-855) (9), and a halophilic myxobacterial strain, Haliangium ochraceum SMP-2 (3), were employed as models to study the characteristics of the marine myxobacteria. From the results obtained we suggest possible living patterns of myxobacteria in the ocean.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Halotolerant strain M. fulvus HW-1 (= ATCC BAA-855) was isolated from a seawater sample (11), and halophilic strain H. ochraceum SMP-2 (9) was kindly furnished by T. Iizuka (Ajinomoto Co., Inc., Japan). The strains were routinely cultured on VY/2 agar (15) prepared with seawater or diluted seawater (HVY agar). The salinity of the seawater was 3.6%. VY/2 medium prepared with distilled water supplemented with 0.5 g liter−1 CaCl2 and 0.5 g liter−1 MgCl2 is referred to below as DVY. In some experiments, instead of seawater, a sodium chloride (NaCl) solution was used for medium preparation. The cells of HW-1 from a 3-day DVY culture and the cells of SMP-2 from a 7-day 50% HVY agar (VY/2 agar in 50% diluted seawater) culture were gently homogenized with glass beads (3 mm in diameter), and the dispersed cells in the supernatant were collected by centrifugation (12,000 × g, 10 min). The cell mass was then suspended in distilled water, and the final cell concentration was adjusted to about 1 × 107 cells ml−1 for immediate inoculation. All the cultures were incubated at 30°C. The strains were kept on 50% HVY agar slants at room temperature for routine use and on dried filter paper for long-term storage.
Determination of growth and morphology.
Growth and morphology were routinely observed with a phase-contrast microscope or a dissection microscope. The cell length was the average for about 200 cells measured under the microscope with a micrometer. The vegetative cells and spherical cells were counted separately, and the latter cells were defined as the cells with a length/width ratio of <2:1.
The growth rate was determined from the generation time of a strain under different culture conditions. Cells in the exponential growth phase were collected at two different times, gently homogenized with glass beads (3 mm in diameter), and counted with a Helber Bacteria counting chamber under a phase-contrast microscope. The generation time (G) was calculated as follows:
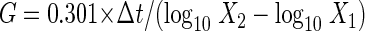 |
where Δt is the interval between the two collection times (in hours) and X1 and X2 are the cell densities (in cells/ml) of the two samples.
Sporulation frequencies, which reflected the proportion of cells in starving populations that had formed heat-resistant spores after 168 h of development, were measured by the method described by Velicer et al. (22), with minor modifications. Cultures were centrifuged (12,000 × g, 10 min, 4°C), and the cells were resuspended in TPM (22) buffer at 5 × 109 cells/ml. Then 100 μl of the suspension was inoculated onto 1.5% modified TPM (10 mM Tris-hydrochloride [pH 7.6] supplemented with different combinations of NaCl, MgCl2, and CaCl2, as shown in Table 1) agar developmental plates and starved for 168 h. The number of viable spores was estimated after heat selection (2 h at 50°C) or dispersion of spores by sonication (5 min in a horn sonicator at an output of 600 W). The spores were then diluted 10−1 to 10−6, and each 1-ml dilution was mixed with 2.5 ml soft CTT (15) agar at 50°C. The soft CTT agar mixture was immediately overlaid onto CTT hard agar plates to prevent migration of colonies after spore germination, thereby allowing accurate counting of distinct colonies.
TABLE 1.
Growth and fruiting body formation for M. xanthus strain HW-1 on VY/2 agar containing different combinations of the three main seawater salts
| NaCl concn (%) | MgCl2 concn (%) | CaCl2 concn (%) | Generation time (h) | Fruiting body formationa | Sporulation frequency (per 104 cells)b |
|---|---|---|---|---|---|
| 0-0.5 | 0 | 0 | 10 | ++ | 20 |
| 1.0-1.5 | 0 | 0 | 8 | + | 10 |
| 2.0-2.5 | 0 | 0 | 15 | ± | 0.04 |
| 3.0 | 0 | 0 | NGc | ||
| 0-0.5 | 0.05 | 0.05 | 8 | ++ | 32 |
| 1.0-1.5 | 0.05 | 0.05 | 7 | + | 16 |
| 2.0-2.5 | 0.05 | 0.05 | 16 | + | 2.5 |
| 3.0 | 0.05 | 0.05 | 18 | ± | 0.2 |
| 4.0 | 0.05 | 0.05 | 24 | − | 0 |
| 0 | 0 | 0.05 | 11 | ± | 0.5 |
| 0 | 0.1-2.5 | 0.05 | 10 | ++ | 50 |
| 0 | 0.05 | 0-0.1 | 10 | ++ | 12.6 |
| 0 | 0.05 | 0.5 | 18 | + | 4.0 |
| 0 | 0.05 | 1.0-1.5 | 23 | ± | 0.04 |
| 0 | 0.05 | 2.0 | NG | ||
| Seawater (100% HVY) | 21 | ± | 2.0 |
++, complete fruiting bodies; +, rudimentary fruiting bodies; ±, cell mounds; −, no fruiting bodies.
The sporulation frequencies reflect the proportion of cells in starving populations that formed heat-resistant spores after development. For details see Materials and Methods.
NG, no growth or weak growth.
For electron microscopy, the harvested cells were immediately fixed in 2.5% glutaraldehyde at room temperature and incubated for more than 2 h. The fixed cells used for scanning electron microscopy were dehydrated in a graded ethanol series to 100% ethanol, and this was followed by drying at the critical point and coating with gold.
Tolerance capacity of the cells.
Because no suitable medium was found for both strain HW-1 and strain SMP-2 for the formation of colonies from single cells, the studies on the characteristics of the spherical cells were performed in rather descriptive ways. In old liquid cultures (20 days after inoculation), HW-1 and SMP-2 cells all turned into spherical types. To test the tolerance capacity of the transformative cells, the old cultures were collected, washed with distilled water, and centrifuged (12,000 × g, 10 min) twice to remove adhering matter. The cells were then suspended in distilled water, gently homogenized, and collected by centrifugation (12,000 × g, 10 min). The cell mass was then resuspended, and the concentration was adjusted to about 1 × 107 cells ml−1. The suspension was either heat treated at different temperatures ranging from 40 to 100°C with 5°C steps for 5 min or treated with ultrasound in an ice bath with an ultrasonicator (JY92-2 type s; Scientz Co.) at different frequencies for 5 min (with alternate working and resting periods of 10 s). After treatment, the cells were inoculated onto 50% HVY agar to determine their viability. Because individual cells of HW-1 or SMP-2 were unable to grow into colonies on whole yeast cell agar, sufficient cells were inoculated; i.e., 0.2 ml of a suspension containing 1 × 107 cells ml−1 was spread on a 90-mm-diameter plate. Four replicate plates were made. After ultrasonic treatment, the cell integrity was also determined with a microscope.
The transformative cells from old cultures, together with the fruiting bodies that developed on agar, were tested for desiccation tolerance by transferring them onto small pieces of sterilized filter paper for preservation by using the method described by Reichenbach and Dworkin (15). The tubes containing the paper pieces were stored in a desiccator at room temperature. After storage for a period of time, the cells were inoculated onto 50% HVY agar to determine their viability. The assay was done every month in the first 6 months after storage began and then every 6 months. The cell germination process was observed by scanning electron microscopy at 6-h intervals.
Cell-density-dependent growth.
Myxobacteria are social bacteria, and a single cell cannot form a colony on whole yeast cell medium (10, 17). Because the cells in marine environments cannot form fruiting bodies (see below), cell-density-dependent growth was determined in liquid culture or on agar. Strains HW-1 and SMP-2 were inoculated into DVY and 10% to 100% HVY and then shaken at 30°C for 3 to 4 days to obtain vegetative cells or for 20 to 30 days to obtain transformative cells. The collected cells were washed and gently homogenized, and then the dispersed cells in the supernatant were collected by centrifugation. The cell mass was then resuspended, counted, and diluted. After dilution, the cell solutions (1 × 108, 1 × 107, 1 × 106, 1 × 105, 1 × 104, 1 × 103, 1 × 102, and 10 cells per ml) were immediately inoculated (1 ml per flask) into 100 ml DVY, 50% HVY, and 100% HVY and spread (0.2 ml per plate) on DVY, 50% HVY, and 100% HVY agar to assay the growth limitation by cell concentration. Three replicates were prepared. The incubation time was 20 days. Cell-density-dependent growth was determined by determining the greatest dilution of the inoculum that grew in liquid culture or by counting the numbers of swarm colonies that appeared on the plate.
Effects of the main salts in seawater on growth and morphogenesis.
Because the fruiting bodies could not form well in synthetic medium, yeast medium was used to determine the effects of salts on growth and morphogenesis. The main components in seawater (NaCl, MgCl2, and CaCl2) were added at different concentrations to DVY (Table 1). The characteristics of the cells, such as the growth rate and sporulation frequency, were assayed as described above.
RESULTS
Salt-tolerant growth.
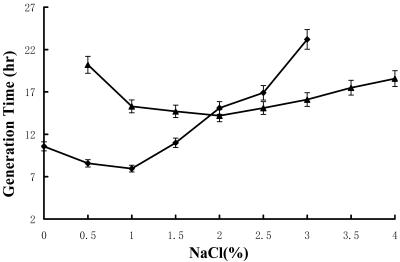
M. fulvus strain HW-1 grew in media containing from 0% seawater (DVY) to 130% seawater (seawater-containing medium was prepared by diluting or air evaporating the seawater before use; the salinity in 130% HVY was determined to be 4.7%). The optimal growth conditions were 0 to 80% seawater (DVY and 10 to 80% HVY [the salinity in 80% HVY was 2.9%]). In DVY or HVY containing a low concentration of seawater, the swarms on agar or the cell clumps in liquid medium were bright yellow. If the concentration of seawater was more than 70%, the color of swarms or clumps changed to light tan. To make a comparison, HW-1 and SMP-2 were both inoculated onto VY/2 agar supplemented with different concentrations of sodium chloride (NaCl) (Fig. 1). As Iizuka et al. indicated (8), H. ochraceum strain SMP-2 is an obligate halophile. This strain was unable to grow without NaCl. The NaCl concentration required for growth ranged from 0.2% to 5%, and the optimal concentration was between 1% and 3%. On the other hand, M. fulvus strain HW-1 could tolerate NaCl concentrations only as high as 3%, and the optimal concentration was 0 to 1.5%. HW-1 cells clumped substantially when they were growing in liquid DVY or HVY medium containing a low concentration of seawater, but they were homogenized in the presence of a high concentration of seawater. In contrast, SMP-2 grew in tight clumps at all salt concentrations tested.
FIG. 1.
Growth of halophilic strain SMP-2 and halotolerant strain HW-1 in liquid culture with different NaCl concentrations. ♦, HW-1; ▴, SMP-2.
The limiting salt concentration for HW-1 growth in medium containing NaCl was obviously lower than that in seawater-containing medium, which might have been due to the fact that seawater contains many other salts besides NaCl. The difference in salt tolerance of HW-1 in medium containing seawater and medium containing NaCl was considered to be caused by the balance of or interaction between the salts in seawater. Table 1 shows the tolerance of HW-1 for the main salts of seawater (NaCl, MgCl2, and CaCl2) in different combinations. It is clear that HW-1 could tolerate higher concentrations of NaCl in the presence of Mg2+. However, there was little difference in growth between 0% and 2.5% MgCl2 when NaCl was omitted.
Cellular morphological changes.
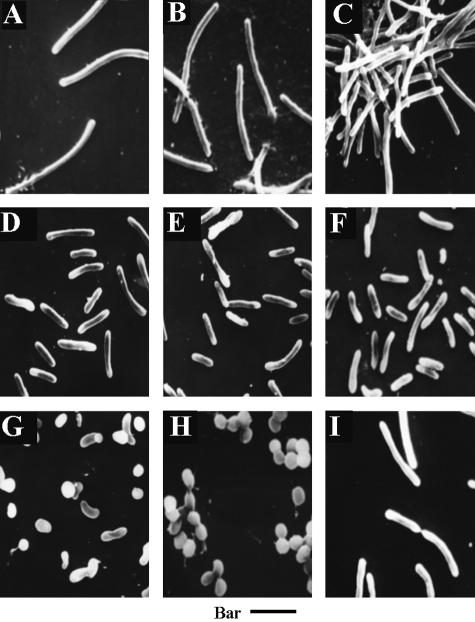
The length of HW-1 cells changed in different growth stages, both in liquid and on agar. Besides, in response to the seawater concentration, the size of the cells also changed (Fig. 2). However, after growth, almost all the cells, whether they were initially long or short, in liquid or on agar shortened into spheres or ovoids whose sizes were similar (1.2 to 1.8 μm in diameter) to the sizes of the myxospores from the fruiting bodies. By contrast, there was essentially no change in the SMP-2 cell size in response to different seawater concentrations. In the fruiting body-like structure of SMP-2 on agar or in aged liquid cultures, the cells were shortened into ovoids (8).
FIG. 2.
Cell morphologies of M. fulvus HW-1 with different seawater concentrations. (A to F) Vegetative cells in liquid DVY and 20%, 40%, 60%, 80%, and 100% HVY, respectively. The cells were all obtained from 3-day cultures. (G) Morphogenesis of myxospores in liquid 50% HVY. The cells were obtained from a 7-day culture. (H) Myxospores in liquid 50% HVY. The cells were obtained from a 12-day culture. (I) Vegetative cells of SMP-2 in liquid 50% HVY. The cells were obtained from a 7-day culture. Bar = 2.1 μm.
Fruiting body morphogenesis.
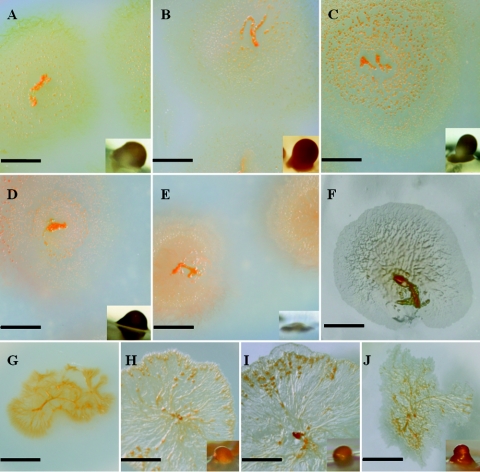
Fruiting body formation by halotolerant strain HW-1 occurred only on solid surfaces, such as agar, and was strongly affected by the salinity (Fig. 3). On DVY agar or on HVY agar containing less than 60% seawater, typical fruiting bodies, which were orange red and 100 to 150 μm in diameter with a cushion-like pedicel, appeared on the third day after inoculation. As the salinity increased, the fruiting body formation was delayed, and the final structure became more rudimentary. On medium with a salinity of 2.9% (80% HVY agar) or higher, the cells failed to produce fruiting bodies, but they still aggregated to form multicellular mounds. Examined under the microscope, the mounds contained a mixture of spherical and bacilliform cells. The myxospores that developed in the fruiting bodies or in the mounds were spherical or ovoid with diameters between 1.2 μm and 1.5 μm.
FIG. 3.
Effect of seawater concentration on growth and fruiting body formation on agar. (A to F) HW-1 on DVY and 20%, 40%, 60%, 80%, 100% HVY, respectively. (G to J) SMP-2 on 20%, 40%, 80%, and 100% HVY, respectively. The structures are the fruiting body structures on the media. There were no fruiting bodies in HW-1 cultures with 100% seawater (F) or in SMP-2 cultures with 20% seawater (G). (A, B, C, E, G, and I) Bars = 0.5 cm; (D and H) bars = 0.6 cm; (F) bar = 0.2 cm; (J) bar = 0.7 cm. The bars for the insets in panels A, B, C, D, E, H, I, and J are all 50 μm long.
To examine the effects of the salts in seawater on the development of fruiting bodies, the morphogenesis of the fruiting bodies on VY/2 agar with different combinations of the three main salts in seawater (NaCl, MgCl2, and CaCl2) was observed (Table 1). Of the three salts, MgCl2 stimulated fruiting body formation, while the other two salts inhibited the process.
In comparison, the halophilic myxobacterium SMP-2 was able to form a few fruiting body-like structures in the center of a swarm (Fig. 3). The structure changed a little in response to the shift in salinity. However, if the seawater concentration in medium was 20% or lower, the cells seldom developed.
Characteristics of the cells.
Myxobacteria cannot form fruiting bodies in liquid, including seawater environments. Therefore, it is essential to learn how marine myxobacterial isolates survive in the ocean. Considering that the cells of HW-1 in liquid DVY or HVY cultures underwent a similar morphological process (i.e., they changed from bacilliform to spherical) and that the size of the spheres was similar to the size of the myxospores that developed in the fruiting bodies formed on agar, we assayed the adversity tolerance and germination ability of the spherical cells formed both in liquid and on agar. The spherical cells of SMP-2 were also checked in parallel.
The spherical cells of HW-1 that formed on agar or in liquid HVY containing a high concentration of seawater were strongly optically refractive, while those that formed in liquid DVY or in HVY containing a low concentration of seawater were not. The spherical cells of HW-1 from agar or liquid medium containing a high concentration of seawater could survive heat treatment at 60°C for 5 min (Table 2). The spherical cells from seawater-containing media, whether they were grown in liquid or on agar, tolerated higher temperatures than the myxospores that developed in the fruiting bodies on DVY. The spherical cells from cultures in liquid DVY or HVY containing a low concentration of seawater died after 5 min of treatment at only 45°C. The HW-1 myxospores from fruiting bodies and the spherical cells from liquid HVY cultures containing a high concentration of seawater were able to germinate after at least 5 years of storage, while the spherical cells from liquid DVY cultures died after 1 month. The results strongly support the hypothesis that HW-1 is able to develop myxospores without morphogenesis of fruiting bodies in seawater conditions, while the spherical cells formed in liquid DVY were only degenerate vegetative cells. The resting form of HW-1 in terrestrial conditions was the fruiting body structure that developed on solid surfaces.
TABLE 2.
Characteristics of spherical cells formed with different seawater concentrations
| Strain | Mediuma | Heat tolerance (°C)b | Desiccation tolerancec |
|---|---|---|---|
| HW-1 | Liquid DVY or 10% HVY | 45 | <1 mo |
| Liquid 20%-100% HVY | 80-85 | >5 yr | |
| DVY and 10% HVY agar | 60-65 | >5 yr | |
| 20%-100% HVY agar | 80-85 | >5 yr | |
| SMP-2 | Liquid 10% HVY | 45 | <1 mo |
| Liquid 20%-100% HVY | 55-60 | >3 mo | |
| 10% HVY agar | 45 | <1 mo | |
| 20%-100% HVY agar | 55-60 | >3 mo |
The seawater concentrations tested were 10% to 100% at intervals of 10%.
The spherical cells were heat treated at temperatures from 45°C to 90°C at intervals of 5°C.
After storage for a period of time, the cells were transferred at intervals onto 50% HVY agar to determine viability. The intervals were 1 month for the first 6 months and then 6 months. SMP-2 has been assayed for desiccation tolerance for only 3 months since we got it.
SMP-2 developed a few fruiting body-like structures on agar. The transformative cells in the fruiting body-like structure of SMP-2 were able to tolerate 5 min of treatment at 55 to 60°C. On the other hand, the cells of SMP-2 in liquid also changed shape and size in old cultures, just like the cells that developed in the fruiting body-like structure, and these transformative cells in liquid culture were able to tolerate heat treatments at 55 to 60°C; that is, the transformative cells of SMP-2 in old liquid cultures or in fruiting body-like structures on agar were real myxospores.
Cell-density-dependent growth.
There is another serious question that has to be answered concerning marine myxobacteria. It is well known that a rather high cell density, which can easily be achieved by a multicellular fruiting body, is a unique requirement for myxobacterial growth and that a single cell cannot form a colony on whole cell agar (e.g., VY/2 agar). Rosenberg et al. explained that myxobacteria have to pool their extracellular enzymes by increasing cell density for growth on insoluble nutrient sources, like casein (16). Because myxospores can develop in the seawater environment without multicellular morphogenesis, how does HW-1 guarantee the cell density for growth? Can one cell develop a colony (swarm) in marine conditions?
Myxobacterial strains SMP-2 and HW-1 fed on the only insoluble nutrient in liquid media: intact yeast cells. The minimum cell density required for growth was determined by serially diluting the cells or myxospores and inoculating the dilutions onto agar or into liquid media. The results (Table 3) showed that HW-1 vegetative cells from DVY or 10% HVY formed zero to two colonies if 0.2 ml of a 1 × 104 cell/ml suspension was spread on a 90-mm-diameter plate containing DVY, 50% HVY, or 100% HVY agar, or they were able to grow if 1 × 104 cells per ml was inoculated into liquid media. Inoculating 0.2 ml of a 1 × 103 cell/ml suspension onto a plate did not result in colony formation. However, the cells from liquid HVY containing higher concentrations of seawater (20% or higher) were able to grow and form swarms on a 90-mm-diameter plate with only 0.2 ml of a 10-cell/ml suspension (less than 10 cells per plate) or with a concentration of 10 cells/ml of inoculum in liquid media (DVY, 50% HVY, or 100% HVY).
TABLE 3.
Cell-density-dependent growth
| Strain | Mediuma | No. of inoculated cells per plate | No. of colonies per plate on agarb
|
Minimum no. of inoculated cells in liquid media (cells/ml)c | ||
|---|---|---|---|---|---|---|
| DVY | 50% HVY | 100% HVY | ||||
| HW-1 | DVY or 10% HVY | 2 × 103 | 1 ± 1 | 1 ± 1 | 0 | 104 |
| 20%-100% HVY | <10 | 1 ± 1 | 2 ± 2 | 1 ± 1 | 10 | |
| SMP-2 | 10% HVY | 105 | 0 | 1 ± 1 | 2 ± 1 | 105 |
| 20%-100% HVY | 105 | 0 | 2 ± 2 | 1 ± 1 | 105 | |
The cells were cultivated in liquid medium containing different seawater concentrations.
The data are averages for three replicates.
The minimum cell concentration in the inoculum that resulted in growth in liquid DVY or HVY.
We also assayed the density-dependent growth of the cells grown on DVY, 50% HVY, and 100% HVY by inoculating serial dilutions of the cells onto new DVY, 50% HVY, and 100% HVY agar. The cells from 50% or 100% HVY agar still grew independently of the cell density, whether the cultures were originally inoculated from DVY or seawater-containing HVY. In contrast, the growth of cells from DVY plates, even if they were originally obtained from HVY containing a high concentration of seawater, was dependent on the cell density. Several dilution transfers confirmed the results; that is, the cells had different growth abilities when they were grown in media with different salinities, or seawater changed the cellular quorum-sensing system (5).
On the other hand, the halophilic strain SMP-2 cultured in HVY containing different concentrations of seawater, whether in liquid medium or on agar, could form swarms on a 90-mm-diameter plate with a concentration of about 1 × 105 cells per plate, but it failed to form swarms with 1 × 104 cells per plate; thus, the growth of strain SMP-2 was strongly dependent on cell density.
DISCUSSION
Myxobacteria are unicellular bacteria that are characterized by complicated multicellular behaviors, such as feeding, social movement, aggregation, and fruiting body formation (13), which make them highly unusual. Myxobacteria have usually been regarded as soil bacteria due to their special requirement for social behavior, especially the development of the multicellular resting form, the fruiting body. The fruiting body structure, which so far is the only known way for myxobacteria to resist adversity in nature, can only be generated on solid surfaces. However, myxobacteria have been found many times in various marine samples. It has been generally accepted that myxobacteria can live in the ocean. But how the “marine myxobacteria” grow and develop their resting forms is still unknown. In this paper, we conducted a series of experiments with a halotolerant strain, M. fulvus HW-1, and a halophilic strain, H. ochraceum SMP-2, to answer these questions.
In marine conditions, the most common features for microbial life are the high salinity and the water environment. Soil myxobacteria, however, are normally unable to grow with more than 1% salinity (13) or to develop resting forms in liquid conditions (18). The halotolerant strain M. fulvus HW-1 is very similar to normal soil myxobacteria on the basis of its 16S rRNA gene sequence (9), morphology, and morphogenesis in terrestrial conditions. However, in an asocial habitat, a seawater liquid culture, HW-1 exhibits individual behavior, such as direct development of myxospores without fruiting body formation, and cell-density-independent growth. Social behavior is no longer needed for the halotolerant myxobacterium HW-1 to live and survive in seawater conditions, but it is still essential if this organism migrates to land. Velicer et al. (22, 23) showed with a social evolution experiment in which Myxococcus xanthus strain DK1622 was subcultured that the social behavior is an evolutionary burden in asocial habitats and that the loss of social motility in particular directly benefited fitness in an asocial liquid environment. In fact, we have isolated many halotolerant myxobacterial strains (9), and all of them showed a similar shift between asocial behavior and social behavior, regardless of their halotolerance. Some of the 16S rRNA gene sequences of these strains have been deposited in GenBank; these sequences include the sequences of M. fulvus strain 128-7 (accession number AY032879), M. fulvus strain 125-1 (AF466191), and Myxococcus macrosporus strain 125-10-1 (AY072740). Cellular morphogenesis and multicellular morphogenesis are greatly dependent on the salinity. Among the salts in seawater, NaCl is obviously the major factor that restricts the social behavior.
Considering the different characteristics of halotolerant myxobacteria in terrestrial and marine conditions, we suggest that there are two growth strategies for myxobacteria. One is simple: myxospores form directly from vegetative cells, and the cells grow independent of the cell density. The other is complicated: fruiting body structures form from numerous cells, and the cellular growth is dependent on the cell density. A fruiting body ensures that a new life cycle is able to start in a terrestrial environment. It seems that the adaptation of the halotolerant myxobacteria to a marine environment is a degenerative procedure. The two living patterns could shift if the cells migrate between the two environments.
H. ochaceum SMP-2, a halophilic myxobacterium, uses a different living strategy. SMP-2 grew in tight clumps at all salt concentrations, and its growth was very dependent on cell density. The cells rested on solid media by constructing fruiting body-like structures containing myxospores or in liquid by directly developing myxospores. So far, all the halophilic myxobacterial strains, such as the strains of H. ochraceum (3), Plesiocystis pacifica (6), and Enhygromyxa salina (7), have been phylogenetically assigned to novel genera. Considering that no closely related myxobacteria have been found in soil yet, we suggest that the halophilic myxobacteria might form a different evolutionary group that is indigenous to the ocean.
Acknowledgments
The work was financially supported by grants 30070008 and 30270023 from the National Natural Science Foundation of China and by grant 2002CCCD1800 from Pre-973 of China.
We thank T. Iizuka (Ajinomoto Co., Inc., Japan) for the gift of H. ochraceum SMP-2. We also thank Pamela Holt, Shandong University, for helpful discussions.
REFERENCES
- 1.Dawid, W. 2000. Biology and global distribution of myxobacteria in soils. FEMS Microbiol. Rev. 24:403-427. [DOI] [PubMed] [Google Scholar]
- 2.Dworkin, M. 1996. Recent advances in the social and developmental biology of the myxobacteria. Microbiol. Rev. 60:70-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fudou, R., Y. Jojima, T. Iizuka, and S. Yamanaka. 2002. Haliangium ochraceum gen. nov., sp. nov. and Haliangium tepidum sp. nov.: novel moderately halophilic myxobacteria isolated from coastal saline environments. J. Gen. Appl. Microbiol. 48:109-116. [DOI] [PubMed] [Google Scholar]
- 4.Gray, J. P., and R. P. Herwig. 1996. Phylogenetic analysis of the bacterial communities in marine sediments. Appl. Environ. Microbiol. 62:4049-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray, K. M. 1997. Intercellular communication and group behavior in bacteria. Trends Microbiol. 5:184-188. [DOI] [PubMed] [Google Scholar]
- 6.Iizuka, T., Y. Jojima, R. Fudou, A. Hiraishi, J. W. Ahn, and S. Yamanaka. 2003. Plesiocystis pacifica gen. nov., sp. nov., a marine myxobacterium that contains dihydrogenated menaquinone, isolated from the Pacific coasts of Japan. Int. J. Syst. Evol. Microbiol. 53:189-195. [DOI] [PubMed] [Google Scholar]
- 7.Iizuka, T., Y. Jojima, R. Fudou, M. Tokura, A. Hiraishi, and S. Yamanaka. 2003. Enhygromyxa salina gen. nov., sp. nov., a slightly halophilic myxobacterium isolated from the coastal areas of Japan. Syst. Appl. Microbiol. 26:189-196. [DOI] [PubMed] [Google Scholar]
- 8.Iizuka, T., Y. Jojima, R. Fudou, and S. Yamanaka. 1998. Isolation of myxobacteria from the marine environment. FEMS Microbiol. Lett. 169:317-322. [DOI] [PubMed] [Google Scholar]
- 9.Li, Y. Z., Hu, W., Y. Q. Zhang, Z. J. Qiu, Y. Zhang, and B. H. Wu. 2002. A simple method to isolate salt-tolerant myxobacteria from marine samples. J. Microbiol. Methods 50:205-209. [DOI] [PubMed] [Google Scholar]
- 10.McBride, M. J., and D. R. Zusman. 1996. Behavioral analysis of single cells of Myxococcus xanthus in response to prey cells of Escherichia coli. FEMS Microbiol. Lett. 137:227-231. [DOI] [PubMed] [Google Scholar]
- 11.Moyer, C. L., F. C. Dobbs, and D. M. Karl. 1995. Phylogenetic diversity of the bacterial community from a microbial mat at an active, hydrothermal vent system, Loihi Seamount, Hawaii. Appl. Environ. Microbiol. 61:1555-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravenschlag, K., K. Sahm, J. Pernthaler, and R. Amann. 1999. High bacterial diversity in permanently cold marine sediments. Appl. Environ. Microbiol. 65:3982-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reichenbach, H. 1993. Biology of myxobacteria: ecology and taxonomy, p. 13-62. In M. Dworkin and D. Kaiser (ed.), Myxobacteria II. American Society for Microbiology, Washington, D.C.
- 14.Reichenbach, H. 1999. The ecology of myxobacteria. Environ. Microbiol. 1:15-21. [DOI] [PubMed] [Google Scholar]
- 15.Reichenbach, H., M. Dworkin. 1992. The myxobacteria, p. 3416-3487. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes, 2nd ed. Springer-Verlag, New York, N.Y.
- 16.Rosenberg, E., K. H. Keller, and M. Dworkin. 1977. Cell density-dependent growth of Myxococcus xanthus on casein. J. Bacteriol. 129:770-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg, E., and M. Varon. 1984. Antibiotics and lytic enzymes, p. 123-124. In E. Rosenberg (ed.), Myxobacteria. Springer-Verlag New York, N.Y.
- 18.Shimkets, L. J. 1990. Social and developmental biology of myxobacteria. Microbiol. Rev. 54:473-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimkets, L. J., and C. R. Woese. 1992. A phylogenetic analysis of the myxobacteria: basis for their classification. Proc. Natl. Acad. Sci. USA 89:9459-9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spröer, C., H. Reichenbach, and E. Stackebrandt. 1999. The correlation between morphological and phylogenetic classification of myxobacteria. Int. J. Syst. Bacteriol. 49:1255-1262. [DOI] [PubMed] [Google Scholar]
- 21.Stackebrandt, E., R. G. E. Murray, and H. G. Trüper. 1988. Proteobacteria classis nov., a name for the phylogenetic taxon that includes the ‘purple bacteria and their relatives.’ Int. J. Syst. Bacteriol. 38:321-325. [Google Scholar]
- 22.Velicer, G. J., L. Kroos, and R. E. Lenski. 1998. Loss of social behaviors by Myxococcus xanthus during evolution in an unstructured habitat. Proc. Natl. Acad. Sci. USA 95:12376-12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velicer, G. J., and K. L. Stredwick. 2002. Experimental social evolution with Myxococcus xanthus. Antonie Leeuwenhoek 81:155-164. [DOI] [PubMed] [Google Scholar]
- 24.Woese, C. R., E. Stackebrandt, T. J. Macke, and G. E. Fox. 1985. A phylogenetic definition of the major eubacterial taxa. Syst. Appl. Microbiol. 6:143-151. [DOI] [PubMed] [Google Scholar]
- 25.Zhang, J., Z. Liu, S. Wang, and P. Jiang. 2002. Characterization of a bioflocculant produced by the marine myxobacterium Nannocystis sp. NU-2. Appl. Microbiol. Biotechnol. 59:517-522. [DOI] [PubMed] [Google Scholar]