Abstract
The effects of substrate analogs and energy inhibitors on glucose uptake and phosphorylation by Clostridium beijerinckii provide evidence for the operation of two uptake systems: a previously characterized phosphoenolpyruvate-dependent phosphotransferase system (PTS) and a non-PTS system probably energized by the transmembrane proton gradient. In both wild-type C. beijerinckii NCIMB 8052 and the butanol-hyperproducing mutant BA101, PTS activity declined at the end of exponential growth, while glucokinase activity increased in the later stages of fermentation. The non-PTS uptake system, together with enhanced glucokinase activity, may provide an explanation for the ability of the mutant to utilize glucose more effectively during fermentation despite the fact that it is partially defective in PTS activity.
Saccharolytic clostridia are capable of utilizing a wide spectrum of carbon sources, which are transported into the cell by a variety of mechanisms. The phosphoenolpyruvate (PEP)-dependent phosphotransferase system (PTS) is the predominant transport mechanism for carbohydrates in clostridia (9), for which there is evidence that, in addition to having a role in transport, it is also involved in the regulation of gene expression (15). Nevertheless, not all carbohydrates are accumulated in this way, and in common with mechanisms of other bacteria, uptake of some carbohydrates appears to be driven by ATP hydrolysis (ABC transporters) or by ion gradients (H+ or Na+ symporters) (9). A characteristic feature of the latter systems is that substrate accumulation is prevented in the presence of uncouplers of oxidative phosphorylation which increase the proton conductance of the membrane and so collapse the electrochemical proton gradient (13). Clostridium beijerinckii NCIMB 8052 (formerly Clostridium acetobutylicum NCIMB 8052) has been shown to have a PTS for the uptake of glucose and several other substrates (8, 10, 12, 14, 16), although no PTS activity was detected for maltose or galactose (1, 8).
A typical fermentation by the solvent-producing clostridia exhibits two phases: an acidogenic phase, in which the main products are acetate and butyrate (together with H2 and CO2), which is followed by a solventogenic phase, in which some of the acids are reassimilated with the formation of butanol and acetone or isopropanol. The butanol-hyperproducing mutant strain C. beijerinckii BA101, derived from NCIMB 8052, has been shown to utilize glucose more completely than the parent strain during fermentation (4), yet it exhibited a partial defect in PTS activity for glucose and some other sugars (7). This strain did, however, show enhanced glucokinase activity during the solventogenic phase, suggesting that an alternative, non-PTS uptake system may contribute to glucose utilization by delivering unmodified sugar to the cytoplasm. In this study, we investigated the potential presence of such a transport system for glucose in both C. beijerinckii BA101 and the parent strain NCIMB 8052.
Assay of glucose phosphorylation by cell extracts.
Cell extracts were prepared from C. beijerinckii NCIMB 8052 and BA101 in order to compare the glucose phosphotransferase systems of the two strains. Spores (5 ml in distilled water) were heat shocked at 80°C for 10 min, inoculated into 10 ml reinforced clostridial medium, and incubated anaerobically for 14 h at 35°C. Reinforced clostridial medium cultures (5 ml) were transferred to 100 ml semidefined P2 medium containing 6% glucose, incubated anaerobically for 20 h at 35°C, washed, transferred to 1 liter of 6% glucose P2 medium, and incubated at 33°C (3, 7). Cells were harvested, washed with 10 mM Tris-Cl buffer (pH 7.5) by centrifugation at 8,000 × g, and disrupted by two passages through a French pressure cell at 20,000 lb/in2. Cell debris was removed by centrifugation at 12,000 × g for 10 min, and the supernatant was used as a cell extract and stored at −80°C. The protein concentrations of the extracts were measured by using a protein assay (Bio-Rad, CA).
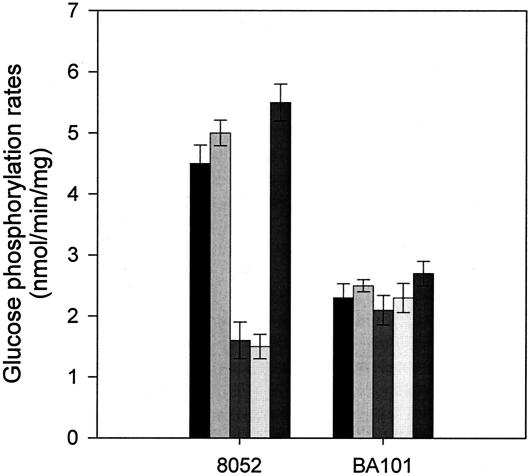
Assays of the glucose PTS were carried out as described previously (7, 10) to evaluate the effects of glucose analogs on the two strains, C. beijerinckii NCIMB 8052 and BA101. Phosphorylation rates were determined over a 3-min assay period, and where present, the analogs were at a concentration of 2 mM, an excess 20-fold greater than the concentration of the radiolabeled substrate. Rates of glucose phosphorylation in the presence of PEP by extracts prepared from cells harvested in the acidogenic phase are shown in Fig. 1. It was observed that only 2-deoxyglucose (2DG) and mannose competed with glucose in the PTS reaction in C. beijerinckii NCIMB 8052, since the glucose phosphorylation rate was significantly reduced when these analogs were present. These results are consistent with the previous finding that in this strain, both mannose and 2DG are substrates of the glucose PTS, while α-methylglucoside (MG) and 3-O-MG are not (10). However, in the case of C. beijerinckii BA101, no significant inhibition by any of the glucose analogs was observed (Fig. 1), suggesting that the substrate range of the glucose PTS in the mutant strain is altered. As previously noted (7), PTS activity in this strain was also lower than that in the wild type. This mutant was isolated following chemical mutagenesis (2), and the genetic basis of the multiple effects on the PTS remains to be established.
FIG. 1.
Effect of glucose analogs on glucose phosphorylation by C. beijerinckii NCIMB 8052 and BA101 in the presence of PEP. Shaded bars (in each group) indicate phosphorylation rates of strains with the following additions, from left to right: α-MG, 3-O-MG, mannose, 2-deoxyglucose, and no addition.
Effects of analogs on glucose uptake by whole cells.
The effects of glucose analogs on the uptake of glucose by whole cells of the two C. beijerinckii strains were examined at different stages of growth. Cells were harvested at the acidogenic (8 h) and solventogenic (24 h) stages and washed and suspended in 50 mM potassium phosphate buffer (pH 7.0). Cell density was determined at an A600 of 1.0, equivalent to 0.265 mg dry weight/ml. Glucose uptake was initiated by adding [14C]glucose to 1 ml of cell suspension, prewarmed to 37°C under anaerobic conditions (8), to give a final concentration of 0.1 mM. Samples (0.15 ml) were removed over a period of 1 min, filtered, and washed twice with 5 ml buffer. The filters were dried, and accumulated radioactivity was measured by scintillation counting (8). The effects of glucose analogs were examined by adding them to a concentration of 2 mM, an excess 20-fold greater than the glucose concentration.
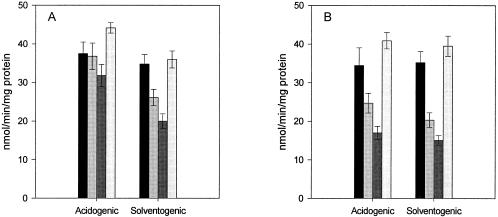
Although the glucose PTS in C. beijerinckii BA101 is defective, we found that this did not affect the rate of glucose uptake by whole cells, which was similar to that of wild-type NCIMB 8052 in both growth phases (Fig. 2). The analog 2DG, which is an inhibitor of the glucose PTS in NCIMB 8052, significantly inhibited glucose uptake by acidogenic-phase cells of this strain but was much less effective in cells harvested from the solventogenic phase. On the other hand, 3-O-MG and α-MG, neither of which is an inhibitor of the glucose PTS, had little effect on acidogenic-phase cells but inhibited glucose uptake by solventogenic-phase cells by almost 50% (Fig. 2A). In the case of strain BA101, while none of the analogs is a substrate for the PTS, both 3-O-MG and α-MG inhibited glucose uptake by more than 50% (Fig. 2B). Furthermore, unlike with the wild type, the effects of the analogs were not dependent on the growth phase, with similar inhibitions of glucose uptake observed for both acidogenic- and solventogenic-phase cells.
FIG. 2.
Effect of glucose analogs on glucose uptake at different stages of growth of C. beijerinckii NCIMB 8052 (A) and BA101 (B). Shaded bars (in each group) indicate phosphorylation rates of strains with the following additions, from left to right: α-methylglucoside, 3-O-methylglucoside, 2-deoxyglucose, and no addition.
The implication of these results is that, in addition to the glucose PTS, there is an alternative, non-PTS transport system present in both strains which plays a role in glucose uptake by intact cells. While for the wild type, this transport system is evidently important only in the solventogenic phase, the nature of the undefined mutation(s) in mutant BA101 has rendered it of significance in both the solventogenic and acidogenic phases of this strain.
Effect of energy inhibitors on glucose uptake.
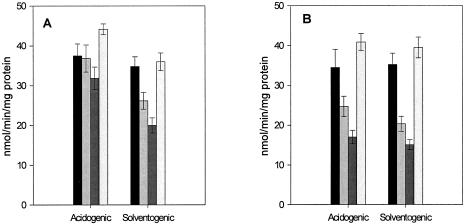
Glucose uptake in the presence of N, N′-dicyclohexylcarbodiimide (DCCD)(10 μM), valinomycin (5 μM), and carbonyl cyanide m-chlorophenylhydrazone (CCCP) (25 μM), compounds which affect the generation and maintenance of transmembrane ion gradients, was also examined. Regardless of strain or stage of growth, glucose uptake was most sensitive to CCCP and virtually unaffected by valinomycin (Fig. 3). Strain BA101 appeared to be generally more sensitive to the inhibitors than strain NCIMB 8052, with the rates of glucose uptake in the presence of DCCD and CCCP reduced by 40 to 50% and 60%, respectively, relative to that of the control, regardless of the growth stage of the cells (Fig. 3B). This observation is consistent with the operation of a non-PTS transport system in both phases. On the other hand, for strain NCIMB 8052, the effects of the inhibitors were growth stage dependent. While solventogenic-phase cells showed a similar inhibition pattern to BA101, albeit with decreased sensitivity, acidogenic-phase cells were much less affected (Fig. 3A). As described before, it would appear that a non-PTS transport system is contributing significantly to glucose uptake in solventogenic-phase cells but not acidogenic-phase cells. As this uncharacterized transport system is inhibited to a significant extent by both a proton conductor and an ATPase inhibitor, it is probably driven by a transmembrane proton gradient generated by ATP hydrolysis.
FIG. 3.
Effect of energy inhibitors on glucose uptake at different stages of growth of C. beijerinckii NCIMB 8052 (A) and BA101 (B). Shaded bars (in each group) indicate phosphorylation rates of strains with the following additions, from left to right: valinomycin, DCCD, CCCP, and no addition.
Expression of PTS and glucokinase activity.
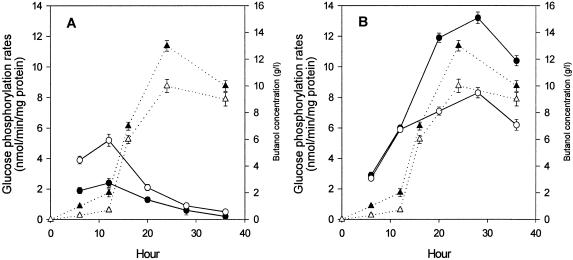
In order to examine the potential mechanisms of glucose uptake more carefully, glucose phosphorylation in cell extracts prepared from cells harvested at different points during the fermentation was measured. Butanol production in the culture was measured as described previously (11) in order to identify the times of onset of solvent formation. PTS activities associated with cell extracts of both C. beijerinckii NCIMB 8052 and BA101 were highest at 12 h (towards the end of the acidogenic phase) and decreased thereafter (Fig. 4A), and the activity of strain BA101 was 50% lower than that of NCIMB 8052, consistent with previous findings (7). On the other hand, glucokinase activity, as measured by ATP-dependent phosphorylation, increased for the first 28 h, reaching a peak in the solventogenic phase, and subsequently declined. This implies that glucose transported into the cell is phosphorylated by either the PTS or glucokinase, with the contribution of each being dependent upon the growth phase of the cells. The contribution of the PTS to glucose uptake is most significant in acidogenic-phase cells of the wild-type strain NCIMB 8052, while solventogenic-phase cells of both strains display greatly lowered PTS activity and therefore show greater reliance on a non-PTS route of glucose uptake and phosphorylation. This route of metabolism would provide an explanation for the continued utilization of glucose during the solventogenic phase (7); indeed, the lowered culture pH, which is an important factor in the onset of solventogenesis, coupled with a significant transmembrane pH gradient (5, 6, 17), would favor glucose accumulation via a proton-dependent transport system. As regards the greater solvent formation and more complete glucose utilization by BA101 relative to the parent NCIMB 8052 (3, 4), it is tempting to conclude that these characteristics may be due to differences in cellular regulation, resulting in an enhanced glucokinase activity which more than compensates for the PTS defect of this strain.
FIG. 4.
Glucose phosphorylation by cell extracts of C. beijerinckii derived from cells in different growth stages. Phosphorylation rates (left axis) in the presence of PEP (A) and ATP (B) are shown for C. beijerinckii NCIMB 8052 (○) and BA101 (•) relative to the time at which samples were removed from the culture. Butanol production in the culture (right axis) is also shown for NCIMB 8052 (Δ) and BA101 (▴).
In conclusion, by monitoring the effects of sugar analogs and energy inhibitors on glucose uptake, we have demonstrated that the transport characteristics in the two C. beijerinckii strains are different and, in the case of the wild type, are dependent on the growth phase. These results can be correlated with PTS and glucokinase activities in cell extracts. We propose that C. beijerinckii has both a PTS uptake system and a non-PTS uptake system for glucose, with their relative contributions to glucose accumulation being dependent on the physiological state of the cells.
Acknowledgments
This work was supported in part by U.S. Department of Agriculture grant no. AG98-35504-6181 and Illinois Corn Marketing Board grant no. 99-0124-02 to H. P. Blaschek.
REFERENCES
- 1.Albasheri, K. A., and W. J. Mitchell. 1995. Identification of two α-glucosidase activities in Clostridium acetobutylicum NCIB 8052. J. Appl. Bacteriol. 78:149-156. [Google Scholar]
- 2.Annous, B. A., and H. P. Blaschek. 1991. Isolation and characterization of Clostridium acetobutylicum mutants with enhanced amylolytic activity. Appl. Environ. Microbiol. 57:2544-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, C. K., and H. P. Blaschek. 1999. Acetate enhances solvent production and prevents degeneration in Clostridium beijerinckii BA101. Appl. Microbiol. Biotechnol. 52:170-173. [DOI] [PubMed] [Google Scholar]
- 4.Formanek, J., R. Mackie, and H. P. Blaschek. 1997. Enhanced butanol production by Clostridium beijerinckii BA101 grown in semidefined P2 medium containing 6% maltodextrin or glucose. Appl. Environ. Microbiol. 63:2306-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girbal, L., C. Croux, I. Vasconcelos, and P. Soucaille. 1995. Regulation of metabolic shifts in Clostridium acetobutylicum ATCC 824. FEMS Microbiol. Rev. 17:287-295. [Google Scholar]
- 6.Gottwald, M., and G. Gottschalk. 1985. The internal pH of Clostridium acetobutylicum and its effect on the shift from acid to solvent formation. Arch. Microbiol. 143:42-46. [Google Scholar]
- 7.Lee, J., W. J. Mitchell, and H. P. Blaschek. 2001. Glucose uptake in Clostridium beijerinckii NCIMB 8052 and the solvent-hyperproducing mutant BA101. Appl. Environ. Microbiol. 67:5025-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell, W. J. 1996. Carbohydrate uptake and utilization by Clostridium beijerinckii NCIMB 8052. Anaerobe 2:379-384. [Google Scholar]
- 9.Mitchell, W. J. 1998. Physiology of carbohydrate to solvent conversion by clostridia. Adv. Microb. Physiol. 39:31-130. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell, W. J., J. E. Shaw, and L. Andrews. 1991. Properties of the glucose phosphotransferase system of Clostridium acetobutylicum NCIB 8052. Appl. Environ. Microbiol. 57:2534-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qureshi, N., A. Lolas, and H. P. Blaschek. 2001. Soy molasses as fermentation substrate for production of butanol using Clostridium beijerinckii BA101. J. Ind. Microbiol. Biotechnol. 26:290-295. [DOI] [PubMed] [Google Scholar]
- 12.Reid, S. J., M. S. Rafudeen, and N. G. Leat. 1999. The genes controlling sucrose utilization in Clostridium beijerinckii NCIMB 8052 constitute an operon. Microbiology 145:1461-1472. [DOI] [PubMed] [Google Scholar]
- 13.Saier, M. H., Jr. 1985. Mechanisms and regulation of carbohydrate transport in bacteria. Academic Press Inc., Orlando, Fla.
- 14.Tangney, M., J. K. Brehm, N. P. Minton, and W. J. Mitchell. 1998. A gene system for glucitol transport and metabolism in Clostridium beijerinckii NCIMB 8052. Appl. Environ. Microbiol. 64:1612-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tangney, M., A. Galinier, J. Deutscher, and W. J. Mitchell. 2003. Analysis of the elements of catabolite repression in Clostridium acetobutylicum ATCC 824. J. Mol. Microbiol. Biotech. 6:6-11. [DOI] [PubMed] [Google Scholar]
- 16.Tangney, M., C. Rousse, M. Yazdanian, and W. J. Mitchell. 1998. Sucrose transport and metabolism in Clostridium beijerinckii NCIMB 8052. J. Appl. Microbiol. 84:914-919. [DOI] [PubMed] [Google Scholar]
- 17.Terracciano, J. S., and E. R. Kashket. 1986. Intracellular conditions required for initiation of solvent production by Clostridium acetobutylicum. Appl. Environ. Microbiol. 52:86-91. [DOI] [PMC free article] [PubMed] [Google Scholar]