Abstract
Objectives
Studies show that intracoronary imaging (ICI)-guided PCI is associated with a significantly lower risk of stroke, Q-wave myocardial infarction, and death compared to angiography-guided PCI in the management of acute coronary syndromes, complex coronary lesions and left-main interventions. Despite these well-established clinical benefits, the utilization of ICI-guided PCI in Saudi Arabia remains suboptimal.
Methods
The National Heart Center (NHC) and the Saudi Arabian Cardiac Interventional Society (SACIS) gathered national experts to develop a consensus document on how to integrate ICI-guided PCI in routine clinical practice in Saudi Arabia. The consensus was based on the nominal group technique, whereby a committee of interventional cardiologists affiliated with the NHS and SACIS developed and discussed a number of statements on the clinical use of intracoronary imaging based on a systematic review of the literature.
Results
A total of 17 statements were discussed in light of scientific evidence and agreed upon. Initiatives to improve operator skills when it comes to image acquisition and interpretation are crucial in the incorporation of ICI-imaging guided PCI in Saudi Arabia. Local data on reference diameters and measurements and epidemiological data on Saudi patients being treated in catheterization laboratories are necessary.
Conclusions
Herein, we provide the first national consensus on the use of ICI-guided PCI in Saudi Arabia. We anticipate that this document contributes to a more optimal and integrative use of ICI-guided PCI in the Kingdom.
Keywords: Percutaneous coronary intervention, Intracoronary imaging, Intravascular ultrasound, Optical coherence tomography, Saudi Arabia
1. Introduction
Percutaneous coronary intervention (PCI) remains a cornerstone in the management of obstructive coronary artery disease (CAD). While angiography has traditionally been the primary imaging modality guiding PCI, its limitations in offering accurate, cross-sectional visualization of the coronary lumen and vessel wall have been recognized [1]. The management of acute coronary syndromes and complex coronary lesions and left-main interventions using angiography-guided PCI poses a considerable challenge, largely due to the high risk of major adverse cardiac events (MACE), the potential for stent thrombosis, and the rate of instent restenosis (ISR) warranting repeat revascularization [2]. In an effort to mitigate these risks, adjunctive intracoronary imaging (ICI) modalities, such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT), have emerged to provide detailed insights into plaque morphology, lumen dimensions, and stent expansion, among other parameters [3,4].
Pivotal trials advocate the use of ICI-guided PCI combined with drug-eluting stents. Long-term studies showed that IVUS-guided PCI was associated with a significantly lower risk of stroke, Q-wave myocardial infarction (MI), and death compared to angiography-guided PCI [5–9]. Likewise, meta-analyses demonstrated that ICI-guided PCI was associated with a significantly lower risk of MACE than angiography-guided PCI. Regarding revascularization, the risk of target vessel revascularization (TVR) and target lesion revascularization (TLR) was also lower in the ICI-guided patients compared to the angiography-guided respectively [10–12].
Despite the well-established clinical benefits of ICI-guided PCI, its utilization remains suboptimal in several countries, including Saudi Arabia. Recent reports suggested that the lack of national consensuses and tailored algorithms for using ICI-guided PCI contributed to its suboptimal use in modern PCI procedures [13]. Thus, the National Heart Center (NHC) in collaboration with Saudi Arabian Cardiac Interventional Society (SACIS) gathered national experts to develop a consensus document and workflow to integrate ICI-guided PCI in routine practice in Saudi Arabia.
2. Methods
This consensus is based on Nominal Group Technique (NGT). The NHC and the SACIS employed the NGT to develop a consensus statement on the utility of intracoronary imaging and the corresponding clinical pathway algorithm.
2.1. Committee development
We used a non-probability purposive sampling technique to recruit interventional cardiologists affiliated with the NHC and SACIS in Saudi Arabia. All experts are required to have an active license in the field of interventional cardiology by the Saudi Commission for Health Specialties. Eligible experts vetted by the NHC directorship and subsequently participated in the consensus development. The statements were independently reviewed by an external reviewer, with an internationally-recognized contribution to the field of ICI.
2.2. Statement development
A systematic literature search was conducted on Medline via PubMed from its inception to November 2022 to collect relevant and contemporary data by the Survey development committee. Various combinations of the following keywords were used to identify potentially eligible literature: (Saudi Arabia; Consensus; Experts opinion; ICI; IVUS; OCT; PCI). The statements were primarily extracted from studies with high quality of evidence, as classified by GRADE [14]. Additional statements were retrieved from studies with lower quality of evidence whenever deemed required by the survey development committee. The strength of recommendations was assessed using the GRADE Evidence to Decision (EtD) frameworks. This system was developed and refined to assess the certainty of evidence of effects and strength of recommendations, as shown in Table 1 [14]. All agreed upon statements are presented in Table 2.
Table 1.
Quality of evidence grades.
| Grade | Definition |
|---|---|
| High | We are very confident that the true effect lies close to that of the estimate of the effect. |
| Moderate | We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different |
| Low | Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. |
| Very Low | We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect |
Table 2.
Consensus statements on the clinical use of intracoronary imaging.
| Section | Statements | Quality of Evidence |
|---|---|---|
| I. Intracoronary imaging and PCIs outcomes | 1. ICI-guided PCI demonstrates superior safety, efficacy, and enhanced patient outcomes compared to angiography-guided PCI, especially in complex lesions. The clinical benefit of imaging guidance of PCI mainly depends on baseline planning and stent optimization. | High |
| 2. Each catheterization laboratory in Saudi Arabia should be equipped with high-resolution ICI system (s) that corresponds to catheterization lab needs. The lab should also be equipped with staff trained in image acquisition, interpretation, and measurement. | High | |
| 3. ICI-guided PCI is recommended in patients with complex coronary lesions and left-main interventions due to a reduction in MACE, revascularization, and stent thrombosis. Both IVUS and OCT-guided PCI provide comparable short and long-term benefits on stent expansion, MACE, revascularization, and stent thrombosis. The choice between both techniques should be based on the operator’s expertise, patient characteristics, and clinical scenarios. | High | |
| 4. In heavily calcified and bifurcation lesions, OCT provides valuable additional information, such as calcium thickness and three-dimensional stent views, that can better guide the step-by-step PCI optimization. When compared to standard IVUS devices, OCT may be preferred for detecting lumen or stent-related features with potential clinical impact in heavily calcified coronary lesions. | Moderate | |
| 5. In patients with renal failure undergoing PCI, IVUS is preferred over OCT. This preference is primarily driven by the significant concern of contrast-induced nephropathy in this patient population. | Moderate | |
| II. Patient selection | 6. ICI-guided PCI is recommended for patients with:
|
High |
| 7. Routine ICI-guided PCI may be considered in patients presenting with non-complex disease, especially those with diffuse disease, to allow lesion preparation, optimize stent expansion and apposition, and improve short and long-term outcomes. | Low | |
| III. Principles of Imaging Acquisition | 8. The availability and use of co-registration of ICI with coronary angiography (angio co-registration) should be considered to facilitate imaging-guided PCI. | Moderate |
| IV. Plaque composition | 9. ICI-guidance prior to stent implantation is recommended to assess plaque composition and distribution (calcification, lipid-rich plaque), allow plaque modification, and facilitate the choice of stent size (diameter and length). | High |
| 10. The low sensitivity of coronary angiography to identify high calcium content in native vessels and cases of ISR increases the risk of stent under-expansion and malapposition. Thus, pre-stenting imaging is recommended for plaque assessment in all calcified lesions or in undilatable coronary stenosis. ICI images of calcium distribution (circumferential and longitudinal) and thickness guide the selection of the calcium modification technique, allowing for better lesion preparation and stent expansion. | High | |
| V. Assessment of angiographically indeterminate coronary artery stenosis | 11. IVUS is recommended over OCT for the evaluation of angiographically indeterminate ostial left main. Either modality can be used for distal or shaft disease of the left main artery. | Low |
| 12. In indeterminate LMCA disease, a mean luminal diameter (MLD) < 2.8 mm and mean luminal area (MLA) < 5.9 mm2 correlate with significant lesions. Lesions with MLA >7.5 mm2 are not hemodynamically significant. Lesions with MLA of 6–7.5 mm2 require further physiological assessment. These cutoffs should be used cautiously and with other clinical factors to guide practice. Further studies are needed to validate these cutoffs and establish more robust criteria for identifying hemodynamically significant lesions using ICI. | Moderate | |
| 13. There are ethnic differences in coronary atherosclerosis morphology. The optimal MLA cutoff to determine significant lesions is still unknown in the Arab ethnicity, which requires future research. | Moderate | |
| 14. In non-LMCA disease, MLA <4 mm2 may be significant but requires additional physiological assessment. Lesions with MLA of >4 mm2 and MLD > 2 mm are not hemodynamically significant. These cutoffs should be used cautiously and with other clinical factors to guide practice. Further studies are needed to validate these cutoffs and establish more robust criteria for identifying hemodynamically significant lesions using ICI. | Moderate | |
| VI. Stent failure | 15. An ICI analysis of stent restenosis and stent thrombosis is strongly recommended to understand failure mechanisms, including stent malappostion, stent underexpansion and extent of neointimal hyperplasia. | Moderate |
3. Intracoronary imaging (IVUS and OCT)-guided PCI
3.1. Intracoronary imaging and PCI outcomes
The safety of ICI-guided PCI is well-established, as evidenced in pivotal trials and various meta-analyses. These studies have demonstrated a superior safety profile compared to traditional angiography-guided stent implantation. These techniques significantly reduce the risk of stent thrombosis due to more precise stent placement and sizing, addressing initial concerns about acute complications [5–12,15–17]. The comprehensive three-dimensional imaging provided by OCT and IVUS offers detailed insights into vessel wall and plaque characteristics, leading to better-informed procedural decisions and reduced short-term and long-term complications.
This has established ICI as a safe alternative and a superior approach to optimizing coronary interventions. Pivotal clinical trials, long-term studies, and meta-analyses demonstrated that ICI-guided PCI was associated with a significantly lower risk of stroke, Q-wave myocardial infarction (MI), and death compared to angiography-guided PCI [5–12]. In a real-time, updated network meta-analysis by Stone et al., it was found that the IVUS and OCT-guided PCI reduced the target lesion failure (TLF) by 29% compared to angiography-guided PCI. There were signification reductions in cardiac death (45%), target vessel myocardial infarction (18%), target lesion revascularization (28%), and stent thrombosis (48%) compared to angiography-guided PCI as well [18]. A more recent meta-analysis showed that OCT-guided PCI was associated with a significant reduction of stent thrombosis compared with angiography-guided PCI (51%) [19]. Interestingly, the ILUMIEN IV study, alongside IVUS trials, highlighted the limitations of angiography-guided PCI regarding suboptimal minimum stent area (MSA), stent under-expansion, and high rates of major dissections, malapposition, and focal plaque protrusion compared to ICI-guided PCI. The trial found no difference between OCT-and angiography-guided PCI with regard to the target-vessel failure within 2 years [20].
The evidence also shows comparable outcomes between IVUS and OCT-guided PCI. In patients with simple lesions, the OPINION and ILUMIEN III trials showed comparable outcomes of IVUS-and OCT-guided PCI in terms of stent thrombosis, MACE, and cardiac death [21,22]. Subsequent trials in patients with complex lesions, such as the OPTIMIZE PCI, OCTOBER, and OCTIVUS trials, showed similar findings regarding stent thrombosis, MACE, and cardiac death [15,22–24]. Appendices 1 and 2 present examples of IVUS-guided and OCT-guided PCI, respectively.
As such, the expert agreed that ICI-guided PCI demonstrates superior safety, efficacy, and enhanced patient outcomes compared to the angiography-guided PCI, especially in complex lesions and depends on baseline planning and stent optimization (Statement 1). Each catheterization laboratory in Saudi Arabia should be equipped with high-resolution ICI system(s) and staff trained in image acquisition, interpretation, and measurement; the number of systems depends on catheterization laboratory size and volume (Statement 2). ICI-guided PCI is recommended for patients with complex coronary lesions and left-main interventions due to its demonstrated benefits in reducing MACE, revascularization, and stent thrombosis rates. Both IVUS and OCT-guided PCI provide comparable short and long-term benefits on stent expansion, MACE, revascularization, and stent thrombosis. The choice between both techniques should be based on the operator’s expertise, patient characteristics, and clinical scenarios (Statement 3).
When compared with IVUS, OCT demonstrated a more accurate estimation of calcium thickness and plaque morphology [25]. Previous studies demonstrated superior precision of OCT in evaluating post-PCI residual dissection, plaque prolapse, incomplete stent apposition, and stent coverage over time compared to standard IVUS. (30–40 Mhz) [26,27]. OCT is also preferred for thrombus detection, ACS mechanism definition (eruptive nodules, erosion, rupture, dissection), and recrossing assessment of bifurcation, while IVUS is preferred in ostial lesions [28]. However, with the newer generations of IVUS devices, the precision of IVUS has significantly improved and become more comparable to OCT [29]. Hence, when compared to standard IVUS devices, OCT is preferred for detecting lumen or stent-related issues (such as dissection, thrombi, and incomplete stent apposition) with potential clinical impact in heavily calcified coronary lesions (Statement 4).
On the other hand, IVUS has a high reliability in assessing plaque burden [30]. Additionally, one of the primary benefits of IVUS in patients with renal failure is the reduction in the use of contrast media. OCT relies on contrast agents, which can exacerbate kidney damage, particularly in patients with pre-existing renal impairment. IVUS significantly reduces the risk of contrast-induced nephropathy by providing detailed images of the coronary arteries without the need for contrast media [31] (statement 5).
3.2. Patient selection
Several studies provided compelling evidence that some patient/lesion cohorts can benefit the most from ICI-guided PCI. These include those with unprotected left main coronary lesions (LMCA) [5,32–34], ostial lesions [15,17], bifurcation lesions with side branch diameters of 2.5 mm or more [5,35–37], multi-vessel PCI (those requiring treatment at one PCI session of 2 or more major epicardial coronary vessels) [5,35], multiple stent implantation (3 or more stents per patient) [5,35], those with calcified lesions necessitating calcium modification [5,35,38,39], those with stent thrombosis and ISR [5,35,40–43], those with long lesions (implanted stent length ≥28 mm), particularly when multiple stents are placed [44,45], those chronic total occlusion with a duration ≥3 months [46], those with unknown stent apposition/position in the LMCA [27,47,48], and those with possible stent fracture after overexpansion [48,49], Accordingly, experts suggest these patient/lesion subsets as primary candidates for ICI-guided PCI to improve cardiovascular outcomes (Statement 6).
Multiple studies have shown that ICI-guided PCI optimizes stent expansion and apposition and improves short-and long-term outcomes compared to angiography-guided PCI [22,50,51]. Specifically, in patients where the minimum lumen area achieved in the stented segment was >5.0 mm2 or >90% of the lumen area at the distal reference segments, the residual plaque burden at the stent edges (5-mm proximal or distal to the stent) was less than 50%, without major edge dissection, ICI-guided PCI led to a substantial reduction of 3-year target vessel failure (TVF) and stent thrombosis [16]. These findings suggest that ICI-guided PCI should be considered in patients presenting with a de novo culprit lesion (≥50% diameter stenosis), especially those with diffuse disease on the same vessel, to allow lesion preparation, optimize stent expansion and apposition, and improve short and long-term outcomes (Statement 7).
3.3. Co-registration of ICI and angiography
Identifying corresponding segments between intracoronary imaging and angiography during PCI is crucial for an imaging guidance procedure. Online angio co-registration (ACR) can significantly help in pinpointing the target segment [52], assist in selecting the correct stent length and landing zones, and avoid geographical misses [53]. Therefore, experts recommend the use of ACR for precise stent guidance (Statement 8).
3.4. Plaque composition
ICI-guided prior to stent implantation is recommended to assess plaque composition and distribution (calcification, lipid-rich plaque) and facilitate choice of stent size (diameter and length) (Statement 9).
3.4.1. Calcium
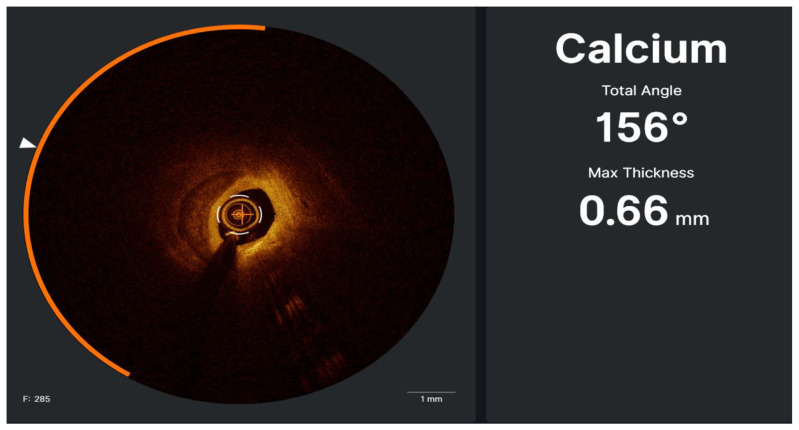
The impact of coronary calcification on adverse events following PCI has been demonstrated in multiple studies and large multiethnic registers [54,55]. Compared to standard angiography, ICI is more accurate in detecting calcium within the coronary arteries [56–58]. IVUS detected calcification in a staggering 73% of cases, while angiography detected calcification in only 40% of instances [59]. This finding underscores ICI’s high sensitivity and specificity in identifying intralesional calcium, drawing parallels with pathological studies [60,61]. ICI can accurately characterize calcium distribution, length, thickness, angle, depth, and morphology (eccentric, concentric, eruptive) [58]. Recently, an IVUS-based calcium scoring was validated to predict stent expansion, which includes superficial calcium angle >270° longer than 5 mm, 360° of superficial calcium, calcified nodule, and vessel diameter <3.5 mm [38]. There is also an elevated risk of the stent under expansion in lesions with calcium pools on OCT exhibiting characteristics such as a maximum angle greater than 180°, a maximum thickness exceeding 0.5 mm, and a length surpassing 5 mm [62]. Based on these findings, the experts recommend pre-stenting imaging for plaque assessment in calcified lesions for lesion preparation and selecting calcium modification techniques (Statement 10).
3.4.2. Lipid-rich plaque
It has been well established that the presence of residual plaque, particularly lipid-rich plaque (LRP), at the edges of the stent, as a result of an inappropriate landing zone, is associated with an increased risk of restenosis and other adverse events. An integrated analysis of the TAXUS trials showed that factors such as external elastic membrane, lumen areas, and plaque burden play a role in predicting 9-month angiographic edge restenosis post-stent implantation. However, only edge plaque burden stood as an independent predictor of stent edge restenosis [63].
3.5. Pre- and Post-PCI workflow
3.5.1. Pre-PCI imaging strategy
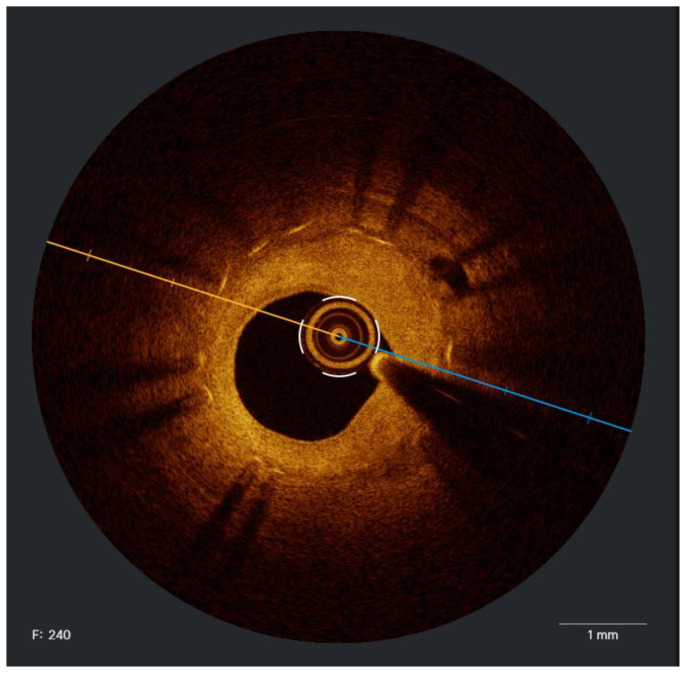
A proposed ICI-guided peri-PCI workflow is shown in Fig. 1. The assessment of lesion morphology is crucial to guide treatment decisions. In lesions with a calcific circumferential extension of more than 180° had a higher calcific burden, resulting in a diminished stent area and increased stent eccentricity [64].
Fig. 1.
Intracoronary imaging-guided peri-PCI workflow.
The next step is to specify a predefined reference segment to identify optimal landing zones and stent length. The largest lumens proximal and distal to the lesion on the lumen profile should be used to create a region of interest; defining lesions distinctly requires a separation of at least 5 mm between them. This distance ensures the greatest visibility of the arterial medial (external elastic lamina [EEL]) and prevents potential overestimation of disease burden if multiple lesions are considered single lesions [65]. If lesions are closer than 5 mm, treating the disease as a single lesion is advised. An ideal landing zone is defined as a 360° EEL visualization; the largest adjacent lumen should be selected in the case of EEL visualization <180° [66]. It should be noted that IVUS holds better ability in defining external elastic membrane (EEM) (when calcium burden is not high) and plaque burden, particularly in lipid-rich plaque; it was previously reported that OCT exhibited high discordant rates in detecting thin-cap fibroatheroma (TCFA) [67].
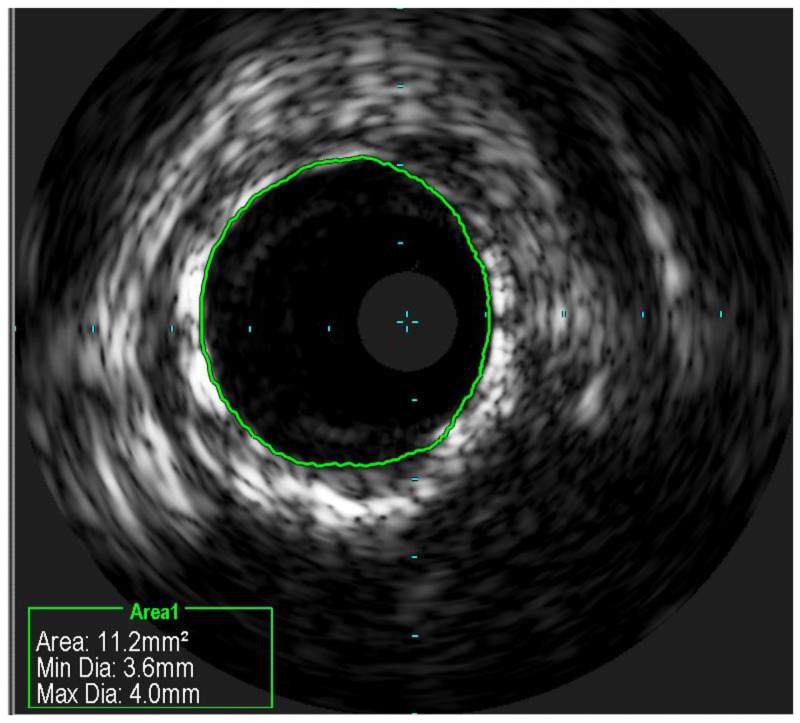
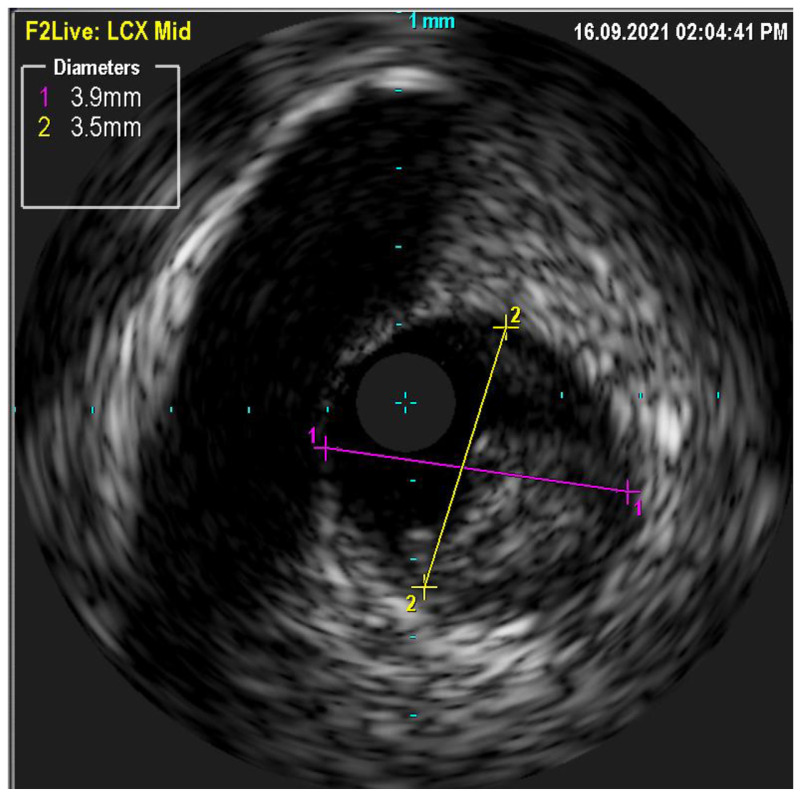
Following the selection of reference segments in ICI imaging, conducting both quantitative and qualitative evaluations is crucial to determine the stent diameter. For IVUS, the measurements are the lumen cross-sectional area (CSA) at the reference and at the lesion, defined as the area encircled by the luminal border, and the minimum and maximum lumen diameters. The lumen area stenosis is calculated as the difference between the reference lumen CSA and the minimum lumen CSA [68]. In addition, EEM and plaque measurements are performed. EEM, the interface between the media and adventitia, helps determine the vessel size and the remodeling response of the vessel to plaque accumulation [69]. Plaque measurements assist in characterizing the composition and distribution of atherosclerotic lesions within the coronary artery, offering insight into their potential vulnerability and risk for future adverse events [70].
On the other hand, for OCT assessment, an EEL-guided device sizing strategy is preferable to a lumen-guided strategy as it leads to the selection of a larger device size (≈0.5 mm) and consequently a larger lumen area without an increase in post-procedural complications. If EEL visualization is insufficient, the mean lumen diameter, recorded from the automated lumen profile feature, is utilized for device sizing.
3.5.2. Post-PCI imaging strategy
Several factors have been reported to be associated with poststent adverse events, including stent underexpansion, dissections, geographic miss, plaque or tissue prolapse, and incomplete stent apposition [71,72]. Stent underexpansion is defined as an area where the stent is inadequately expanded relative to the normal adjacent reference segment [70]. Stent expansion remains the strongest predictor of adverse events, while the current literature shows conflicting results regarding the predictive utility of other measures [73]. Despite a lack of consensus regarding the definition of adequate expansion, the MUSIC study suggested that stent expansion greater than 80%–90% of the reference cross-sectional area reduces restenosis [74]. There is significant evidence that stent underexpansion [75], significant tissue (plaque/thrombus) protrusion (more than the median that narrowed the lumen to <4 mm2), significant stent edge dissection, significant residual stenosis (>50%), and minimum lumen area of <5 mm2, were associated with increased risk of early stent thrombosis [76,77].
After stent implantation, ICI can identify abnormalities that can be related to the stent or vascular wall [78]. There are no consistent guidelines for PCI optimization in clinical practice for determining relative stent expansion. An MSA higher than the distal reference lumen area, or >80% of the average reference area, is an additional goal for stent improvement [51]. Notably, OCT excels over IVUS in detecting malapposition and stent edge dissections [22]. Moreover, OCT’s unique ability to detect thrombus, often a signal of mechanical or anticoagulation issues, emphasizes its superiority [72,79]. In the case of tissue prolapse, OCT has been shown to provide clearer and more frequent visualization than IVUS [22]. Therefore, post-PCI OCT evaluation is crucial for assessing reference segments and detecting stent malapposition; a MSA of ≥80% of the mean reference lumen area and/or >4.5mm2 is desirable for stent expansion, with reference segments outside the stent used for remeasuring vessel size, preferably via EEL guidance or lumen guidance if the EEL is not visible (See Fig. 1).
3.6. Assessment of angiographically indeterminate coronary artery stenosis
While angiography is the standard method for assessing coronary artery disease, it may have limitations in accurately determining the significance of LMCA lesions. ICI is a valuable tool for evaluating and providing treatment guidance for angiographically indeterminate LMCA disease [80,81].Along-term follow-up study showed that IVUS is a safe method to accurately assess the degree of disease in the LMCA that appears indeterminate by angiography [82]. Moreover, the role of ICI in assessing coronary stenoses of angiographically intermediate severity (50–70%) continues to evolve [80]. Based on this evidence, the panel experts recommended IVUS-guided PCI in assessing LMCA and non-LMCA lesions when the angiographic degree of stenosis is ambiguous (Statement 11).
Several investigations have illustrated that in the context of indeterminate LMCA. Reduced mean luminal diameter (MLD) and mean luminal area (MLA) values are associated with more pronounced stenosis, which in turn is linked to a higher risk of adverse cardiac events [82–85]. Based on these findings, the panel experts agreed that, in indeterminate LMCA, an MLD <2.8 mm and MLA <5.9 mm2 correlate with significant stenosis. Lesions with MLA >7.5 mm2 are not hemodynamically significant; however, 6–7.5 mm2 lesions require further physiological assessment (Statement 12). Studies have suggested that an OCT-based MLA of >5.4 mm2 correlates with not hemodynamically significant lesions [86]; however, further research is needed.
Several studies showed a significant difference between different ethnicities in terms of coronary atherosclerosis morphology, particularly in patients with LMCA disease [83,87,88], while, in Asians, the MLA cutoff appears lower at around 4.5–4.8 mm2 [87]. On the other hand, the optimal MLA cutoff to determine significant lesions is still unknown in the Arab ethnicity, and this requires future research (Statements 13).
For patients with non-LMCA disease, the majority of studies used<4 mm2 MLA cutoff point [89–93]. The panel agreed that MLA <4 mm2 might be significant but may require additional physiological assessment in non-LM disease (Statement 14). With regard to OCT, previous studies showed that OCT-derived MLA cutoffs of 1.39–1.64 mm2 had high diagnostic accuracy for predicting non-LM disease [94,95].
3.7. Stent failure
ISR and stent thrombosis remain significant problems in PCIs. The RIBS III study demonstrated the value of IVUS in treating ISR, showing larger acute gain and minimum lumen diameter at a 9-month follow-up. Still, it did not reduce the incidence of TLR at two years [41]. The iOPEN-ISR study also indicated that IVUS guidance reduced the incidence of major adverse cardiac events at 1-year follow-up compared to angiography alone guidance [40]. ICI has proven useful in identifying common causes of ISR and stent thromboses, such as stent underexpansion, under-sizing, non-overlapping stents, stent fracture, edge dissection, and increased plaque at the stent edge [75,96–98]. Recent studies reveal that identifiable leading mechanisms were found in the majority (>90%) of stent thrombosis cases [99,100]. Therefore, a customized treatment strategy based on OCT findings appears reasonable, pending confirmatory prospective data [100].
Thus, the experts strongly recommend using ICI in evaluating and treating patients with ISR and stent thrombosis (Statement 15). Emerging evidence suggest that, in patients with a high risk of future events, including those with prior acute coronary syndrome or diabetes), OCT may be beneficial to detect vulnerable plaques [101,102]. However, the current evidence is not conclusive yet.
4. Emerging directions
Emerging evidence reported that coronary computed tomography angiography (CTA) could allow for noninvasive assessment of change in coronary plaque at lower costs [103,104]. The ability of ICI to provide high-resolution, direct visualization of the plaque characterization allows a level of detail that is essential for confirming the accuracy of plaque characterization obtained from coronary CTA. By correlating the findings from noninvasive CTA with ICI, clinicians can validate the reliability of CTA in detecting and monitoring changes in plaque [105]. This correlation is particularly valuable in complex cases where the plaque characteristics determined by CTA may need further clarification or in scenarios where the risk stratification based on CTA findings requires additional validation.
In addition to advances in coronary CTA, there are emerging diagnostic tools that provide both anatomic and functional insights into coronary lesions. For example, quantitative flow ratio (QFR) is a novel method that uses angiographic images to calculate the Fractional Flow Reserve (FFR) without the need for a pressure wire. QFR has been shown to correlate well with FFR and may be useful in identifying hemodynamically significant coronary stenosis [106]. FFR can also be calculated using a technique called OCT-based FFR (OFR), which has been shown to correlate well with wire-based FFR without the need for pressure wire or induced hyperaemia. It was found that OFR had good diagnostic accuracy in the assessment of flow-limiting coronary [107]. Another tool, optical frequency domain imaging (OFDI), combines the high resolution of OCT with the ability to measure blood flow velocity, providing both anatomic and functional information. Previous reports showed that OFDI-based PCI was non-inferior to IVUS-based PCI in terms of in-segment minimum lumen area at 8 months, arterial healing, and other composite endpoints [108]. While these tools are promising, further studies are needed to establish their clinical utility and define their role in guiding decision-making.
The evolution of Artificial Intelligence (AI) in the realm of ICI and its integration with physiological parameters like FFR, Instantaneous Wave-Free Ratio (iFR), and others represents a significant advancement in interventional cardiology. AI algorithms have been increasingly employed to enhance the analysis of ICI data, such as from IVUS and OCT. These algorithms can rapidly interpret complex imaging data, identifying plaque characteristics, vessel dimensions, and optimal stent placement with greater accuracy and efficiency than traditional methods [109]. The integration of AI with physiological assessments like FFR and iFR, which measure the pressure gradient across a coronary lesion to assess its significance, has further augmented the diagnostic and therapeutic capabilities of ICI [110]. AI can process the combined imaging and physiological data to provide a more comprehensive assessment. This fusion of AI with ICI and physiological measurements not only increases the precision of PCI procedures but also significantly reduces the time required for analysis, leading to quicker and more efficient patient care [111]. Moreover, AI’s ability to learn and adapt from vast datasets continuously improves its diagnostic accuracy, potentially leading to better patient outcomes and more personalized treatment strategies in the future. This evolution signifies a transformative shift towards more data-driven, precise, and patient-specific interventions in cardiology.
Virtual stenting, resulting from advancements in medical imaging and computational techniques, has emerged as a transformative tool in coronary interventions. Virtual stenting enables patient-specific modelling of coronary arteries by harnessing high-resolution imaging modalities like IVUS and OCT in conjunction with computational fluid dynamics. This facilitates predictive analysis, allowing clinicians to anticipate complications and optimize stent positioning before actual procedures. Such a proactive approach paves the way for personalized care, potentially reducing procedural complications and promoting cost-effectiveness by minimizing repeat interventions and streamlining the stenting process for patients with coronary artery disease [112].
5. Conclusion and future recommendations
ICI has unequivocally been shown to improve procedural success with a better minimal stent area following PCI. More importantly, the totality of the evidence has noted a reduction in major adverse cardiac events and target vessel failure, particularly in more complex and high-risk lesions. The current guidelines recommend the use of imaging prior to, during, and after stent deployment to achieve optimization. The experts recognize that initiatives to improve operator skills in the field of image acquisition and interpretation are necessary. It is incumbent upon national societies and authorities to provide training opportunities for those currently in practice. Finally, there is a recognizable paucity of data from the local population on reference diameters and measurements. As such, this document encourages the generation of regional data to capture the demographics of the local population receiving therapies in the catheterization laboratory.
Acknowledgment
The authors would like to thank Dr. Giulio Guagliumi for his immense effort in reviewing the consensus draft and providing his valuable insights.
List of abbreviations
- ACR
Angio co-registration
- AI
Artificial Intelligence
- CAD
Coronary Artery Disease
- CSA
Cross-Sectional Area
- CTA
Computed Tomography Angiography
- EEL
External Elastic Lamina
- EEM
External Elastic Membrane
- EtD
Evidence to Decision
- FFR
Fractional Flow Reserve
- ICI
Intracoronary Imaging
- iFR
Instantaneous Wave-Free Ratio
- ISR
In-Stent Restenosis
- IVUS
Intravascular Ultrasound
- LCBI
Lipid Core Burden Index
- LMCA
Left Main Coronary Lesions
- LPR
Lipid-Rich Plaque
- MACE
Major Adverse Cardiac Events
- MI
Myocardial Infarction
- MLA
Mean Luminal Area
- MLD
Mean Luminal Diameter
- MSA
Minimum Stent Area
- NGT
Nominal Group Technique
- NHC
National Heart Center
- NIRS
Near-Infrared Spectroscopy
- OCT
Optical Coherence Tomography
- OFDI
Optical frequency domain imaging
- OFR
OCT-based FFR
- PCI
Percutaneous coronary intervention
- QFR
Quantitative flow ratio
- SACIS
Saudi Arabian Cardiac Interventional Society
- TLF
Target Lesion Failure
- TLR
Target Lesion Revascularization
- TVF
Target Vessel Failure
- TVR
Target Vessel Revascularization
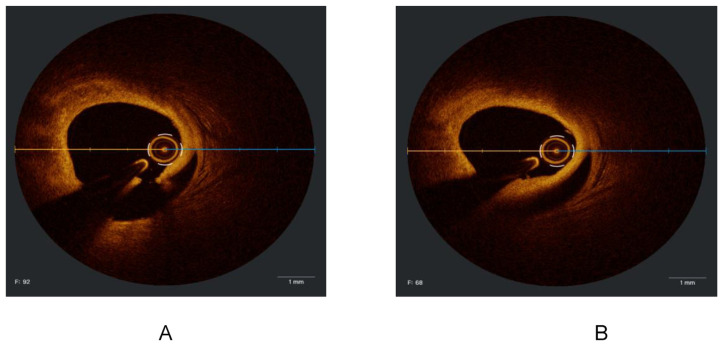
Appendix
Appendix 1
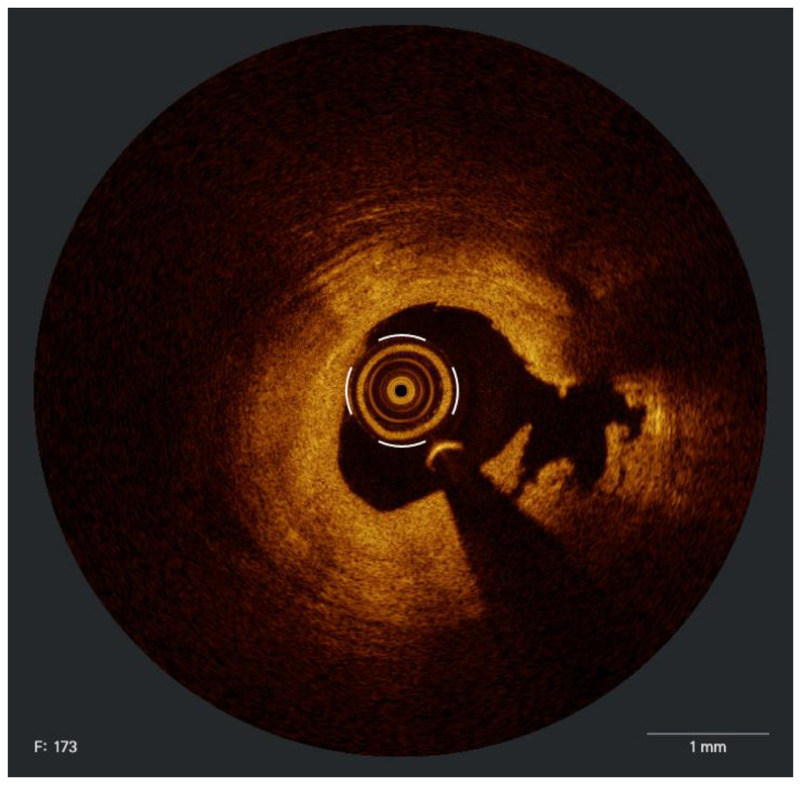
Figure 1.
IVUS image demonstrates 360° of circumferential superficial calcium.
Figure 2.
IVUS image demonstrates circumferential calcium which is cracked at the 6 O’Clock position following intra-coronary lithotripsy.
Figure 3.
Undersized stent in a larger vessel.
Figure 4.
An example of a well-apposed stent.
Figure 5.
An example of a soft eccentric ulcerated plaque (in the left circumflex artery).
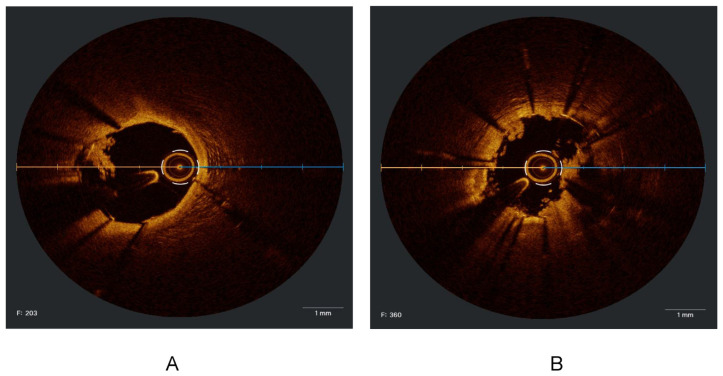
Appendix 2
Figure 1.
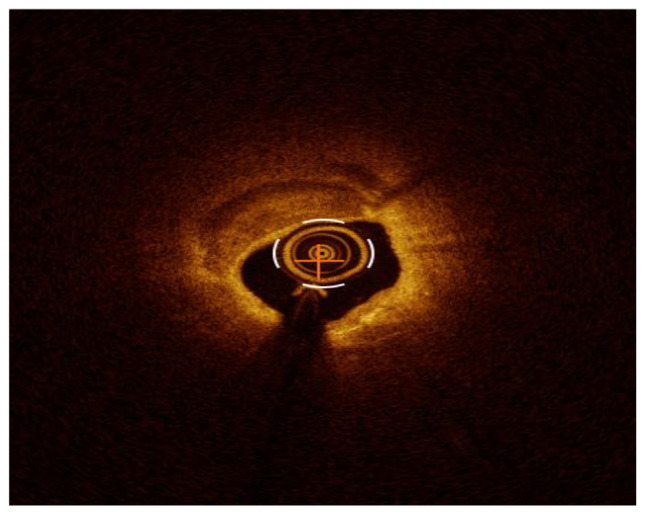
Calcified lesion from 7 to 12 o’clock characterized by well delineated border low back scattering and low attenuation. The artificial intelligence detect the arc and the maximum thickness of the calcium as shown in the right. Side of the picture.
Figure 2.
Fibrofatty plaque with calcification from 1 to 9 o’clock and calcification from 9 to 1 o’clock. Cholesterol crystals (white arrow) which is the area with high backscattering and low attenuation.
Figure 3.
Fibrotic lesion after ballooning, dissection created at 3 o’clock.
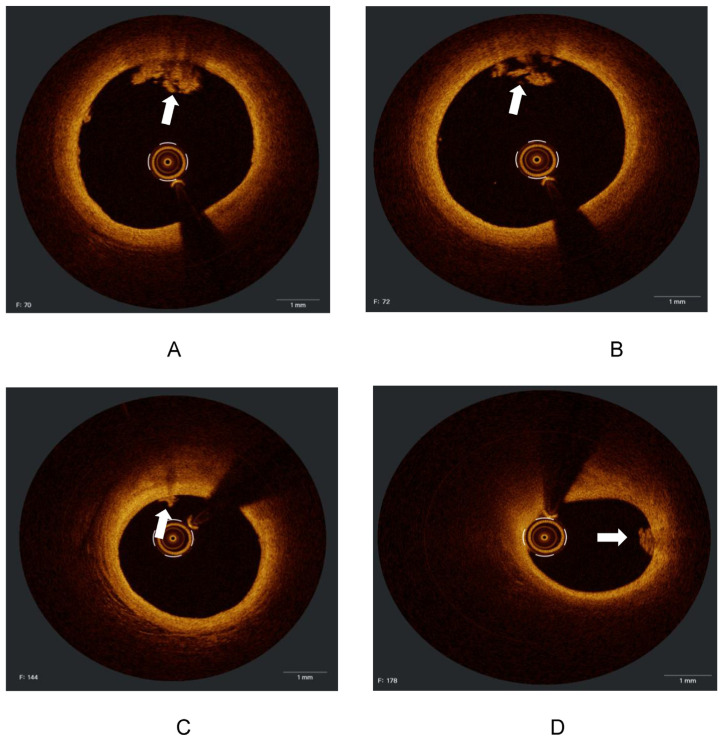
Figure 4.
(A, B, C and D). Cross-sections from the same vessel (LAD) of a patient with atrial fibrillation after stopping anticoagulant for three days showed a white thrombus (white arrows), with the absence of vessel disruption, representing an embolic event.
Figure 5.
A- Calcified lesion with about 360 calcium arch. B- Post IVL, dissections and calcium cracks at 5, 10 and 2 o’clock.
Figure 6.
Neointemal hyperplasia.
Figure 7.
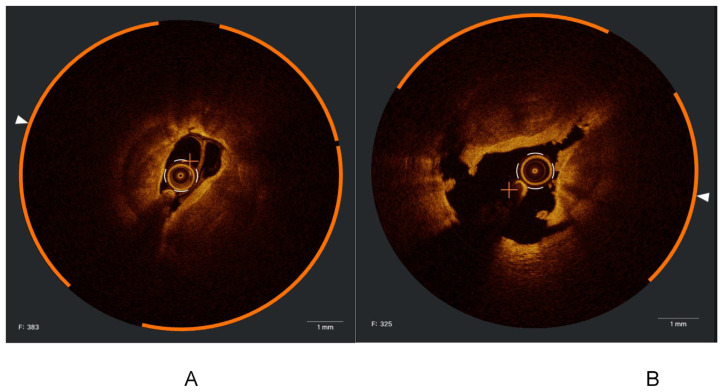
A- Iatrogenic dissection. B- Intramural hematoma.
Figure 8.
A and B. Hyperacute stent thrombosis occurred during the same index procedure.
Funding Statement
The funding was provided by Boston Scientific for the Medical Writers only. None of the authors had any financial compensation and all have no disclosures
Footnotes
Author contribution: Conception and design of Study: MA, MA, AA, HA, FA, HA, WA, AT, WHA. Literature review: MA, MA, AA, HA, FA, HA, WA, AT, WHA. Acquisition of data: MA, MA,WHA. Drafting of manuscript: MA, MA, AA, HA, FA, HA, WA, AT, WHA. Revising and editing the manuscript critically for important intellectual contents: MA, MA, AA, HA, FA, HA, WA, AT, WHA.Supervision of the research: MA, WA, AT, WHA. Research coordination and management: MA, WA, AT, WHA.
Conflict of interest: None to declare.
Funding: The funding was provided by Boston Scientific for the Medical Writers only. None of the authors had any financial compensation and all have no disclosures.
References
- 1. Mehrotra S, Mishra S, Paramasivam G. Imaging during percutaneous coronary intervention for optimizing outcomes. Indian Heart J. 2018;70:S456–65. doi: 10.1016/j.ihj.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation. 2022;145:E18–114. doi: 10.1161/CIR.0000000000001038. [DOI] [PubMed] [Google Scholar]
- 3. Ya’Qoub L, Basir MB, Soni K, Zimmet J, Yang J, Shunk K, et al. Intracoronary imaging and physiology to guide PCI: are we ready for a class i guideline recommendation? Curr Cardiol Rep. 2023;25(7):725–34. doi: 10.1007/s11886-023-01896-5. [DOI] [PubMed] [Google Scholar]
- 4. Alasnag M, Ahmed W, Al-Bawardy R, Al Shammeri O, Biswas S, Johnson TW. Optimising PCI by intracoronary image-guidance. Front Cardiovasc Med. 2022:9. doi: 10.3389/fcvm.2022.878801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kang D, Ahn J, Yun S, Park H, Cho S, Kim TO, et al. Long-term clinical impact of intravascular ultrasound guidance in stenting for left main coronary artery disease. Circ Cardiovasc Interv. 2021;14:1012–21. doi: 10.1161/CIRCINTERVENTIONS.121.011011. [DOI] [PubMed] [Google Scholar]
- 6. Hong S-J, Kim B-K, Shin D-H, Nam C-M, Kim J-S, Ko Y-G, et al. Effect of intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation: The IVUS-XPL randomized clinical trial. JAMA. 2015;314:2155–63. doi: 10.1001/jama.2015.15454. [DOI] [PubMed] [Google Scholar]
- 7. Prati F, Di Vito L, Biondi-Zoccai G, Occhipinti M, La Manna A, Tamburino C, et al. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l’Infarto-Optimisation of Percutaneous Coronary Intervention (CLIOPCI) study. EuroIntervention J Eur Collab with Work Gr Interv Cardiol Eur Soc Cardiol. 2012;8:823–9. doi: 10.4244/EIJV8I7A125. [DOI] [PubMed] [Google Scholar]
- 8. Kuku KO, Ekanem E, Azizi V, Melaku G, Bui A, Meirovich YF, et al. Optical coherence tomography-guided percutaneous coronary intervention compared with other imaging guidance: a meta-analysis. Int J Cardiovasc Imaging. 2018;34:503–13. doi: 10.1007/S10554-017-1272-2. [DOI] [PubMed] [Google Scholar]
- 9. Buccheri S, Franchina G, Romano S, Puglisi S, Venuti G, D’Arrigo P, et al. Clinical outcomes following intravascular imaging-guided versus coronary angiography-guided percutaneous coronary intervention with stent implantation: a systematic review and Bayesian network meta-analysis of 31 studies and 17,882 patients. JACC Cardiovasc Interv. 2017;10:2488–98. doi: 10.1016/j.jcin.2017.08.051. [DOI] [PubMed] [Google Scholar]
- 10. Bavishi C, Sardar P, Chatterjee S, Khan AR, Shah A, Ather S, et al. Intravascular ultrasound-guided vs angiography-guided drug-eluting stent implantation in complex coronary lesions: meta-analysis of randomized trials. Am Heart J. 2017;185:26–34. doi: 10.1016/J.AHJ.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 11. Gao X-F, Wang Z-M, Wang F, Gu Y, Ge Z, Kong X-Q, et al. Intravascular ultrasound guidance reduces cardiac death and coronary revascularization in patients undergoing drug-eluting stent implantation: results from a meta-analysis of 9 randomized trials and 4724 patients. Int J Cardiovasc Imaging. 2019;35:239–47. doi: 10.1007/s10554-019-01555-3. [DOI] [PubMed] [Google Scholar]
- 12. Ye Y, Yang M, Zhang S, Zeng Y. Percutaneous coronary intervention in left main coronary artery disease with or without intravascular ultrasound: a meta-analysis. PLoS One. 2017;12 doi: 10.1371/JOURNAL.PONE.0179756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flattery E, Rahim HM, Petrossian G, Shlofmitz E, Gkargkoulas F, Matsumura M, et al. Competency-based assessment of interventional cardiology fellows’ abilities in intracoronary physiology and imaging. Circ Cardiovasc Interv. 2020;13:E008760. doi: 10.1161/CIRCINTERVENTIONS.119.008760. [DOI] [PubMed] [Google Scholar]
- 14. Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations. 2013 [Google Scholar]
- 15. Holm NR, Andreasen LN, Neghabat O, Laanmets P, Kumsars I, Bennett J, et al. OCT or angiography guidance for PCI in complex bifurcation lesions. N Engl J Med. 2023 doi: 10.1056/NEJMoa2307770. [DOI] [PubMed] [Google Scholar]
- 16. Gao X-F, Ge Z, Kong X-Q, Kan J, Han L, Lu S, et al. 3-Year outcomes of the ULTIMATE trial comparing intravascular ultrasound versus angiography-guided drug-eluting stent implantation. JACC Cardiovasc Interv. 2021;14:247–57. doi: 10.1016/j.jcin.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 17. Lee JM, Choi KH, Bin Song Y, Lee J-Y, Lee S-J, Lee SY, et al. Intravascular imaging–guided or angiography-guided complex PCI. N Engl J Med. 2023;388:1668–79. doi: 10.1056/nejmoa2216607. [DOI] [PubMed] [Google Scholar]
- 18. Stone GW, Christiansen EH, Ali ZA, Andreasen LN, Maehara A, Ahmad Y, et al. Intravascular imaging-guided coronary drug-eluting stent implantation: an updated network meta-analysis. Lancet. 2024;403:824–37. doi: 10.1016/S0140-6736(23)02454-6. [DOI] [PubMed] [Google Scholar]
- 19. Giacoppo D, Laudani C, Occhipinti G, Spagnolo M, Greco A, Rochira C, et al. Coronary angiography, intravascular ultrasound, and optical coherence tomography for guiding of percutaneous coronary intervention: a systematic review and network meta-analysis. Circulation. 2024;149:1065–86. doi: 10.1161/CIRCULATIONAHA.123.067583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ali ZA, Landmesser U, Maehara A, Matsumura M, Shlofmitz RA, Guagliumi G, et al. Optical coherence tomography–guided versus angiography-guided PCI. N Engl J Med. 2023;389:1466–76. doi: 10.1056/NEJMoa2305861. [DOI] [PubMed] [Google Scholar]
- 21. Otake H, Kubo T, Takahashi H, Shinke T, Okamura T, Hibi K, et al. Optical frequency domain imaging versus intravascular ultrasound in percutaneous coronary intervention (OPINION Trial): results from the OPINION imaging study. JACC Cardiovasc Imaging. 2018;11:111–23. doi: 10.1016/j.jcmg.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 22. Ali ZA, Maehara A, Généreux P, Shlofmitz RA, Fabbiocchi F, Nazif TM, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. 2016;388:2618–28. doi: 10.1016/S0140-6736(16)31922-5. [DOI] [PubMed] [Google Scholar]
- 23. Kubo T, Shinke T, Okamura T, Hibi K, Nakazawa G, Morino Y, et al. Optical frequency domain imaging vs. intravascular ultrasound in percutaneous coronary intervention (OPINION trial): one-year angiographic and clinical results. Eur Heart J. 2017;38:3139–47. doi: 10.1093/eurheartj/ehx351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ali ZA, Landmesser U, Maehara A, Matsumura M, Shlofmitz RA, Guagliumi G, et al. Optical Coherence TomographyeGuided versus Angiography-Guided PCI. N Engl J Med. 2023 doi: 10.1056/NEJMoa2305861. [DOI] [PubMed] [Google Scholar]
- 25. Kume T, Okura H, Kawamoto T, Yamada R, Miyamoto Y, Hayashida A, et al. Assessment of the coronary calcification by optical coherence tomography. EuroIntervention. 2011;6(6):768–72. doi: 10.4244/EIJV6I6A130. [DOI] [PubMed] [Google Scholar]
- 26. Bouma BE, Tearney GJ, Yabushita H, Shishkov M, Kauffman CR, Gauthier DD, et al. Evaluation of intracoronary stenting by intravascular optical coherence tomography. Heart. 2003;89:317–20. doi: 10.1136/heart.89.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gudmundsdottir IJ, Adamson PD, Gray CD, Spratt JC, Behan MWH, Henriksen PA, et al. Optical coherence tomography versus intravascular ultrasound to evaluate stent implantation in patients with calcific coronary artery disease. Open Hear. 2015;2 doi: 10.1136/openhrt-2014-000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gupta A, Shrivastava A, Vijayvergiya R, Chhikara S, Datta R, Aziz A, et al. Optical coherence tomography: an eye into the coronary artery. Front Cardiovasc Med. 2022;9:854554. doi: 10.3389/fcvm.2022.854554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ishida M, Oshikiri Y, Kimura T, Sakamoto R, Shimoda Y, Ishikawa Y, et al. High-definition intravascular ultrasound versus optical frequency domain imaging for the detection of calcium modification and fracture in heavily calcified coronary lesion. Int J Cardiovasc Imaging. 2022;38:1203–12. doi: 10.1007/s10554-021-02521-8. [DOI] [PubMed] [Google Scholar]
- 30. Nagaraja V, Kalra A, Puri R. When to use intravascular ultrasound or optical coherence tomography during percutaneous coronary intervention? Cardiovasc Diagn Ther. 2020;10:1429–44. doi: 10.21037/cdt-20-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sacha J, Gierlotka M, Feusette P, Dudek D. Ultra-low contrast coronary angiography and zero-contrast percutaneous coronary intervention for prevention of contrast-induced nephropathy: step-by-step approach and review. Postep W Kardiol Interwencyjnej. 2019;15:127–36. doi: 10.5114/aic.2019.86007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kinnaird T, Johnson T, Anderson R, Gallagher S, Sirker A, Ludman P, et al. Intravascular imaging and 12-month mortality after unprotected left main stem PCI: an analysis from the British cardiovascular intervention society database. JACC Cardiovasc Interv. 2020;13:346–57. doi: 10.1016/j.jcin.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 33. Amabile N, Rangé G, Souteyrand G, Godin M, Boussaada MM, Meneveau N, et al. Optical coherence tomography to guide percutaneous coronary intervention of the left main coronary artery: the LEMON study. Euro-Intervention. 2021;17:E124–31. doi: 10.4244/EIJD-20-01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cortese B, Burzotta F, Alfonso F, Pellegrini D, Trani C, Aurigemma C, et al. Role of optical coherence tomography for distal left main stem angioplasty. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2020;96:755–61. doi: 10.1002/ccd.28547. [DOI] [PubMed] [Google Scholar]
- 35. Choi KH, Bin Song Y, Lee JM, Lee SY, Park TK, Yang JH, et al. Impact of intravascular ultrasound-guided percutaneous coronary intervention on long-term clinical outcomes in patients undergoing complex procedures. JACC Cardiovasc Interv. 2019;12:607–20. doi: 10.1016/J.JCIN.2019.01.227. [DOI] [PubMed] [Google Scholar]
- 36. Grundeken MJ, Hassell MECJ, Kraak RP, de Bruin DM, Koch KT, Henriques JPS, et al. Treatment of coronary bifurcation lesions with the Absorb bioresorbable vascular scaffold in combination with the Tryton dedicated coronary bifurcation stent: evaluation using two- and three-dimensional optical coherence tomography. EuroIntervention J Eur Collab with Work Gr Interv Cardiol Eur Soc Cardiol. 2015;11:877–84. doi: 10.4244/EIJY14M08_15. [DOI] [PubMed] [Google Scholar]
- 37. Pyxaras SA, Toth GG, Di Gioia G, Ughi GJ, Tu S, Rusinaru D, et al. Anatomical and functional assessment of Tryton bifurcation stent before and after final kissing balloon dilatation: evaluations by three-dimensional coronary angiography, optical coherence tomography imaging and fractional flow reserve. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2017;90:E1–10. doi: 10.1002/ccd.26777. [DOI] [PubMed] [Google Scholar]
- 38. Zhang M, Matsumura M, Usui E, Noguchi M, Fujimura T, Fall KN, et al. Intravascular ultrasound-derived calcium score to predict stent expansion in severely calcified lesions. Circ Cardiovasc Interv. 2021;14:e010296. doi: 10.1161/CIRCINTERVENTIONS.120.010296. [DOI] [PubMed] [Google Scholar]
- 39. Prati F, Guagliumi G, Mintz GS, Costa M, Regar E, Akasaka T, et al. Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur Heart J. 2012;33:2513–20. doi: 10.1093/eurheartj/ehs095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shlofmitz E, Torguson R, Zhang C, Mintz GS, Dheendsa A, Khalid N, et al. Impact of intravascular ultrasound on Outcomes following PErcutaneous coronary interventioN for In-stent Restenosis (iOPEN-ISR study) Int J Cardiol. 2021;340:17–21. doi: 10.1016/j.ijcard.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 41. Alfonso F, Pérez-Vizcayno MJ, Dutary J, Zueco J, Cequier A, García-Touchard A, et al. Implantation of a drug-eluting stent with a different drug (switch strategy) in patients with drug-eluting stent restenosis: results from a prospective multicenter study (RIBS III [restenosis intra-stent: balloon angioplasty versus drug-eluting stent]) JACC Cardiovasc Interv. 2012;5:728–37. doi: 10.1016/J.JCIN.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 42. Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058–72. doi: 10.1016/j.jacc.2011.09.079. [DOI] [PubMed] [Google Scholar]
- 43. Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang I-K, Schlendorf KH, et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106:1640–5. doi: 10.1161/01.cir.0000029927.92825.f6. [DOI] [PubMed] [Google Scholar]
- 44. Hong S-J, Mintz GS, Ahn C-M, Kim J-S, Kim B-K, Ko Y-G, et al. Effect of intravascular ultrasound-guided drug-eluting stent implantation: 5-year follow-up of the IVUS-XPL randomized trial. JACC Cardiovasc Interv. 2020;13:62–71. doi: 10.1016/j.jcin.2019.09.033. [DOI] [PubMed] [Google Scholar]
- 45. Palmerini T, Biondi-Zoccai G, Della Riva D, Mariani A, Sabaté M, Valgimigli M, et al. Clinical outcomes with drug-eluting and bare-metal stents in patients with ST-segment elevation myocardial infarction: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2013;62:496–504. doi: 10.1016/j.jacc.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 46. Lee SY, Choi KH, Bin Song Y, Park TK, Lee JM, Yang JH, et al. Use of intravascular ultrasound and long-term cardiac death or myocardial infarction in patients receiving current generation drug-eluting stents. Sci Rep. 2022;12:8237. doi: 10.1038/s41598-022-12339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ino Y, Kubo T, Matsuo Y, Yamaguchi T, Shiono Y, Shimamura K, et al. Optical coherence tomography predictors for edge restenosis after everolimus-eluting stent implantation. Circ Cardiovasc Interv. 2016;9 doi: 10.1161/CIRCINTERVENTIONS.116.004231. [DOI] [PubMed] [Google Scholar]
- 48. Gonzalo N, Serruys PW, Okamura T, Shen ZJ, Onuma Y, Garcia-Garcia HM, et al. Optical coherence tomography assessment of the acute effects of stent implantation on the vessel wall: a systematic quantitative approach. Heart. 2009;95:1913–9. doi: 10.1136/hrt.2009.172072. [DOI] [PubMed] [Google Scholar]
- 49. Fu Q, Suzuki N, Kozuma K, Miyagawa M, Nomura T, Kawashima H, et al. Quantitative optical coherence tomography analysis for late in-stent restenotic lesions. Int Heart J. 2015;56:13–7. doi: 10.1536/ihj.14-136. [DOI] [PubMed] [Google Scholar]
- 50. Chamié D, Costa JR, Damiani LP, Siqueira D, Braga S, Costa R, et al. Optical coherence tomography versus intravascular ultrasound and angiography to guide percutaneous coronary interventions: the iSIGHT randomized trial. Circ Cardiovasc Interv. 2021 doi: 10.1161/CIRCINTERVENTIONS.120.009452. [DOI] [PubMed] [Google Scholar]
- 51. Meneveau N, Souteyrand G, Motreff P, Caussin C, Amabile N, Ohlmann P, et al. Optical coherence tomography to optimize results of percutaneous coronary intervention in patients with non-ST-elevation acute coronary syndrome: results of the multicenter, randomized DOCTORS Study (does optical coherence tomography optimize results of stenting) Circulation. 2016;134:906–17. doi: 10.1161/CIRCULATIONAHA.116.024393. [DOI] [PubMed] [Google Scholar]
- 52. Hebsgaard L, Nielsen TM, Tu S, Krusell LR, Maeng M, Veien KT, et al. Co-registration of optical coherence tomography and X-ray angiography in percutaneous coronary intervention. the Does Optical Coherence Tomography Optimize Revascularization (DOCTOR) fusion study. Int J Cardiol. 2015;182:272–8. doi: 10.1016/J.IJCARD.2014.12.088. [DOI] [PubMed] [Google Scholar]
- 53. Van Der Sijde JN, Guagliumi G, Sirbu V, Shimamura K, Borghesi M, Karanasos A, et al. The OPTIS Integrated System: real-time, co-registration of angiography and optical coherence tomography. EuroIntervention. 2016;12:855–60. doi: 10.4244/EIJV12I7A140. [DOI] [PubMed] [Google Scholar]
- 54. Copeland-Halperin RS, Baber U, Aquino M, Rajamanickam A, Roy S, Hasan C, et al. Prevalence, correlates, and impact of coronary calcification on adverse events following PCI with newer-generation DES: Findings from a large multiethnic registry. Catheter Cardiovasc Interv. 2018;91:859–66. doi: 10.1002/ccd.27204. [DOI] [PubMed] [Google Scholar]
- 55. Hemetsberger R, Abdelghani M, Toelg R, Mankerious N, Allali A, Garcia-Garcia HM, et al. Impact of coronary calcification on clinical outcomes after implantation of newer-generation drug-eluting stents. J Am Heart Assoc. 2021:10. doi: 10.1161/JAHA.120.019815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Doan KH, Liu TL, Yun WS, Kim YS, Yun KH, Oh SK, et al. Intravascular ultrasound guided intervention in calcified coronary lesions showed good clinical outcomes during one year follow-up. J Clin Med. 2023:12. doi: 10.3390/jcm12124073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mehanna E, Dawn Abbott J, Bezerra HG. Optimizing percutaneous coronary intervention in calcified lesions: Insights from optical coherence tomography of atherectomy. Circ Cardiovasc Interv. 2018;11 doi: 10.1161/CIRCINTERVENTIONS.118.006813. [DOI] [PubMed] [Google Scholar]
- 58. Caiazzo G, Di Mario C, Kedhi E, De Luca G. Current management of highly calcified coronary lesions: an overview of the current status. J Clin Med. 2023:12. doi: 10.3390/jcm12144844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Chuang YC, et al. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995;91:1959–65. doi: 10.1161/01.CIR.91.7.1959. [DOI] [PubMed] [Google Scholar]
- 60. Abouelnour A, Gori T. Intravascular imaging in coronary stent restenosis: Prevention, characterization, and management. Front Cardiovasc Med. 2022;9:843734. doi: 10.3389/fcvm.2022.843734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kostamaa H, Donovan J, Kasaoka S, Tobis J, Fitzpatrick L. Calcified plaque cross-sectional area in human arteries: Correlation between intravascular ultrasound and undecalcified histology. Am Heart J. 1999;137:482–8. doi: 10.1016/S0002-8703(99)70496-5. [DOI] [PubMed] [Google Scholar]
- 62. Fujino A, Mintz GS, Matsumura M, Lee T, Kim SY, Hoshino M, et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 2018;13:e2182–9. doi: 10.4244/EIJ-D-17-00962. [DOI] [PubMed] [Google Scholar]
- 63. Liu J, Maehara A, Mintz GS, Weissman NJ, Yu A, Wang H, et al. An integrated TAXUS IV, V, and VI intravascular ultrasound analysis of the predictors of edge restenosis after bare metal or paclitaxel-eluting stents. Am J Cardiol. 2009;103:501–6. doi: 10.1016/J.AMJCARD.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 64. Hoffmann R, Mintz GS, Popma JJ, Satler LF, Kent KM, Pichard AD, et al. Treatment of calcified coronary lesions with Palmaz-Schatz stents. An intravascular ultrasound study. Eur Heart J. 1998;19:1224–31. doi: 10.1053/euhj.1998.1028. [DOI] [PubMed] [Google Scholar]
- 65. Jinnouchi H, Sakakura K, Yanase T, Ugata Y, Tsukui T, Taniguchi Y, et al. Impact of stent edge dissection detected by optical coherence tomography after current-generation drug-eluting stent implantation. PLoS One. 2021;16:e0259693. doi: 10.1371/journal.pone.0259693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shlofmitz E, Croce K, Bezerra H, Sheth T, Chehab B, West NEJ, et al. The MLD MAX OCT algorithm: An imaging-based workflow for percutaneous coronary intervention. Catheter Cardiovasc Interv. 2022;100:S7–13. doi: 10.1002/ccd.30395. [DOI] [PubMed] [Google Scholar]
- 67. Ueki Y, Yamaji K, Losdat S, Karagiannis A, Taniwaki M, Roffi M, et al. Discordance in the diagnostic assessment of vulnerable plaques between radiofrequency intravascular ultrasound versus optical coherence tomography among patients with acute myocardial infarction: insights from the IBIS-4 study. Int J Cardiovasc Imaging. 2021;37:2839–47. doi: 10.1007/s10554-021-02272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Keeley EC, Mehran R, Brener SJ, Witzenbichler B, Guagliumi G, Dudek D, et al. Impact of multiple complex plaques on short- and long-term clinical outcomes in patients presenting with ST-segment elevation myocardial infarction (from the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction [HORIZONS- Am J Cardiol. 2014;113:1621–7. doi: 10.1016/j.amjcard.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. König A, Klauss V. Virtual histology. Heart. 2007;93:977–82. doi: 10.1136/hrt.2007.116384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, et al. American college of cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS). A report of the American college of cardiology task force on clinical expert consensus D. J Am Coll Cardiol. 2001;37:1478–92. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 71. Fujii K, Mintz GS, Kobayashi Y, Carlier SG, Takebayashi H, Yasuda T, et al. Contribution of stent underexpansion to recurrence after sirolimus-eluting stent implantation for instent restenosis. Circulation. 2004;109:1085–8. doi: 10.1161/01.CIR.0000121327.67756.19. [DOI] [PubMed] [Google Scholar]
- 72. Hong M-K, Mintz GS, Lee CW, Park D-W, Choi B-R, Park K-H, et al. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur Heart J. 2006;27:1305–10. doi: 10.1093/eurheartj/ehi882. [DOI] [PubMed] [Google Scholar]
- 73. Lee B, Baraki TG, Kim BG, Lee YJ, Lee SJ, Hong SJ, et al. Stent expansion evaluated by optical coherence tomography and subsequent outcomes. Sci Rep. 2023:13. doi: 10.1038/s41598-023-30717-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. de Jaegere P, Mudra H, Figulla H, Almagor Y, Doucet S, Penn I, et al. Intravascular ultrasound-guided optimized stent deployment. Immediate and 6 months clinical and angiographic results from the Multicenter Ultrasound Stenting in Coronaries Study (MUSIC Study) Eur Heart J. 1998;19:1214–23. doi: 10.1053/euhj.1998.1012. [DOI] [PubMed] [Google Scholar]
- 75. Kang S-J, Ahn J-M, Song H, Kim W-J, Lee J-Y, Park D-W, et al. Comprehensive intravascular ultrasound assessment of stent area and its impact on restenosis and adverse cardiac events in 403 patients with unprotected left main disease. Circ Cardiovasc Interv. 2011;4:562–9. doi: 10.1161/CIRCINTERVENTIONS.111.964643. [DOI] [PubMed] [Google Scholar]
- 76. Choi S, Witzenbichler B, Maehara A, Lansky AJ, Guagliumi G, Brodie B, et al. Intravascular Ultrasound findings of early stent thrombosis after primary percutaneous intervention in acute myocardial infarction. Circ Cardiovasc Interv. 2011;4:239–47. doi: 10.1161/CIRCINTERVENTIONS.110.959791. [DOI] [PubMed] [Google Scholar]
- 77. Cheneau E, Leborgne L, Mintz GS, Jichi Kotani, Pichard AD, Satler LF, et al. Predictors of subacute stent thrombosis: results of a systematic intravascular ultrasound study. Circulation. 2003;108:43–7. doi: 10.1161/01.CIR.0000078636.71728.40. [DOI] [PubMed] [Google Scholar]
- 78. Mintz GS, Guagliumi G. Intravascular imaging in coronary artery disease. Lancet (London, England) 2017;390:793–809. doi: 10.1016/S0140-6736(17)31957-8. [DOI] [PubMed] [Google Scholar]
- 79. Fujii K, Carlier SG, Mintz GS, Yang Y, Moussa I, Weisz G, et al. Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimuseluting stent implantation: an intravascular ultrasound study. J Am Coll Cardiol. 2005;45:995–8. doi: 10.1016/j.jacc.2004.12.066. [DOI] [PubMed] [Google Scholar]
- 80. Mottram PM, Meredith IT. Intravascular ultrasound assessment of ambiguous coronary lesions. Heart Lung Circ. 2001;10:58–62. doi: 10.1046/j.1444-2892.2001.00084.x. [DOI] [PubMed] [Google Scholar]
- 81. Pirolo JS, Fredi JL, Shuman TA. Intracoronary ultrasound-guided CABG in patients with angiographically noncritical lesions. Cardiovascular Surgery Associates. Ann Thorac Surg. 1997;64:375–9. doi: 10.1016/s0003-4975(97)00549-3. [DOI] [PubMed] [Google Scholar]
- 82. Fassa A, Wagatsuma K, Higano ST, Mathew V, Barsness GW, Lennon RJ, et al. Intravascular ultrasound-guided treatment for angiographically indeterminate left main coronary artery disease. J Am Coll Cardiol. 2005;45:204–11. doi: 10.1016/j.jacc.2004.09.066. [DOI] [PubMed] [Google Scholar]
- 83. Jasti V, Ivan E, Yalamanchili V, Wongpraparut N, Leesar MA. Correlations between fractional flow reserve and intravascular ultrasound in patients with an ambiguous left main coronary artery stenosis. Circulation. 2004;110:2831–6. doi: 10.1161/01.CIR.0000146338.62813.E7. [DOI] [PubMed] [Google Scholar]
- 84. Park S-J, Ahn J-M, Kang S-J, Yoon S-H, Koo B-K, Lee J-Y, et al. Intravascular ultrasound-derived minimal lumen area criteria for functionally significant left main coronary artery stenosis. JACC Cardiovasc Interv. 2014;7:868–74. doi: 10.1016/j.jcin.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 85. Kang S-J, Lee J-Y, Ahn J-M, Song HG, Kim W-J, Park D-W, et al. Intravascular ultrasound-derived predictors for fractional flow reserve in intermediate left main disease. JACC Cardiovasc Interv. 2011;4:1168–74. doi: 10.1016/j.jcin.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 86. Jegere S. Use of intravascular imaging in managing coronary artery disease. World J Cardiol. 2014;6:393. doi: 10.4330/wjc.v6.i6.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rusinova RP, Mintz GS, Choi SY, Araki H, Hakim D, Sanidas E, et al. Intravascular ultrasound comparison of left main coronary artery disease between white and Asian patients. Am J Cardiol. 2013;111:979–84. doi: 10.1016/J.AMJCARD.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 88. de la Torre Hernandez JM, Hernández Hernandez F, Alfonso F, Rumoroso JR, Lopez-Palop R, Sadaba M, et al. Prospective application of pre-defined intravascular ultrasound criteria for assessment of intermediate left main coronary artery lesions results from the multicenter LITRO study. J Am Coll Cardiol. 2011;58:351–8. doi: 10.1016/j.jacc.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 89. Lee C-H, Tai B-C, Soon C-Y, Low AF, Poh K-K, Yeo T-C, et al. New set of intravascular ultrasound-derived anatomic criteria for defining functionally significant stenoses in small coronary arteries (results from intravascular ultrasound diagnostic evaluation of atherosclerosis in Singapore [IDEAS] study) Am J Cardiol. 2010;105:1378–84. doi: 10.1016/j.amjcard.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 90. Kang S-J, Ahn J-M, Song H, Kim W-J, Lee J-Y, Park D-W, et al. Usefulness of minimal luminal coronary area determined by intravascular ultrasound to predict functional significance in stable and unstable angina pectoris. Am J Cardiol. 2012;109:947–53. doi: 10.1016/j.amjcard.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 91. Brown AJ, Giblett JP, Bennett MR, West NEJ, Hoole SP. Anatomical plaque and vessel characteristics are associated with hemodynamic indices including fractional flow reserve and coronary flow reserve: a prospective exploratory intravascular ultrasound analysis. Int J Cardiol. 2017;248:92–6. doi: 10.1016/j.ijcard.2017.08.036. [DOI] [PubMed] [Google Scholar]
- 92. Takagi A, Tsurumi Y, Ishii Y, Suzuki K, Kawana M, Kasanuki H. Clinical potential of intravascular ultrasound for physiological assessment of coronary stenosis. Circulation. 1999;100:250–5. doi: 10.1161/01.CIR.100.3.250. [DOI] [PubMed] [Google Scholar]
- 93. Ben-Dor I, Torguson R, Deksissa T, Bui AB, Xue Z, Satler LF, et al. Intravascular ultrasound lumen area parameters for assessment of physiological ischemia by fractional flow reserve in intermediate coronary artery stenosis. Cardiovasc Revascularization Med. 2012;13:177–82. doi: 10.1016/j.carrev.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 94. Usui E, Yonetsu T, Kanaji Y, Hoshino M, Yamaguchi M, Hada M, et al. Efficacy of optical coherence tomography-derived morphometric assessment in predicting the physiological significance of coronary stenosis: Head-to-head comparison with intravascular ultrasound. Euro-Intervention. 2018;13:e2210–8. doi: 10.4244/EIJD-17-00613. [DOI] [PubMed] [Google Scholar]
- 95. Reith S, Battermann S, Hellmich M, Marx N, Burgmaier M. Correlation between optical coherence tomography-derived intraluminal parameters and fractional flow reserve measurements in intermediate grade coronary lesions: a comparison between diabetic and non-diabetic patients. Clin Res Cardiol. 2015;104:59–70. doi: 10.1007/s00392-014-0759-2. [DOI] [PubMed] [Google Scholar]
- 96. Francaviglia B, Capranzano P, Gargiulo G, Longo G, Tamburino CI, Ohno Y, et al. Usefulness of 3D OCT to diagnose a noncircumferential open-cell stent fracture. JACC Cardiovasc Imaging. 2016;9:210–1. doi: 10.1016/j.jcmg.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 97. Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56:1897–907. doi: 10.1016/j.jacc.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 98. Räber L, Mintz GS, Koskinas KC, Johnson TW, Holm NR, Onuma Y, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2018;39:3281–300. doi: 10.1093/EURHEARTJ/EHY285. [DOI] [PubMed] [Google Scholar]
- 99. Taniwaki M, Radu MD, Zaugg S, Amabile N, Garcia-Garcia HM, Yamaji K, et al. Mechanisms of very late drug-eluting stent thrombosis assessed by optical coherence tomography. Circulation. 2016;133:650–60. doi: 10.1161/CIRCULATIONAHA.115.019071. [DOI] [PubMed] [Google Scholar]
- 100. Souteyrand G, Amabile N, Mangin L, Chabin X, Meneveau N, Cayla G, et al. Mechanisms of stent thrombosis analysed by optical coherence tomography: insights from the national PESTO French registry. Eur Heart J. 2016;37:1208–16. doi: 10.1093/eurheartj/ehv711. [DOI] [PubMed] [Google Scholar]
- 101. Tearney GJ, Yabushita H, Houser SL, Aretz HT, Jang I-K, Schlendorf KH, et al. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003;107:113–9. doi: 10.1161/01.cir.0000044384.41037.43. [DOI] [PubMed] [Google Scholar]
- 102. Kubo T, Ino Y, Mintz GS, Shiono Y, Shimamura K, Takahata M, et al. Optical coherence tomography detection of vulnerable plaques at high risk of developing acute coronary syndrome. Eur Hear Journal Cardiovasc Imaging. 2021 doi: 10.1093/ehjci/jeab028. [DOI] [PubMed] [Google Scholar]
- 103. Schepis T, Marwan M, Pflederer T, Seltmann M, Ropers D, Daniel WG, et al. Quantification of non-calcified coronary atherosclerotic plaques with dual-source computed tomography: comparison with intravascular ultrasound. Heart. 2010;96:610–5. doi: 10.1136/hrt.2009.184226. [DOI] [PubMed] [Google Scholar]
- 104. Achenbach S, Moselewski F, Ropers D, Ferencik M, Hoffmann U, MacNeill B, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation. 2004;109:14–7. doi: 10.1161/01.CIR.0000111517.69230.0F. [DOI] [PubMed] [Google Scholar]
- 105. Taron J, Lee S, Aluru J, Hoffmann U, Lu MT. A review of serial coronary computed tomography angiography (CTA) to assess plaque progression and therapeutic effect of antiatherosclerotic drugs. Int J Cardiovasc Imaging. 2020;36:2305–17. doi: 10.1007/s10554-020-01793-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Xu B, Tu S, Qiao S, Qu X, Chen Y, Yang J, et al. Diagnostic accuracy of angiography-based quantitative flow ratio measurements for online assessment of coronary stenosis. J Am Coll Cardiol. 2017;70:3077–87. doi: 10.1016/j.jacc.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 107. Yu W, Huang J, Jia D, Chen S, Raffel OC, Ding D, et al. Diagnostic accuracy of intracoronary optical coherence tomography-derived fractional flow reserve for assessment of coronary stenosis severity. EuroIntervention. 2019;15:189–97. doi: 10.4244/EIJ-D-19-00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Muramatsu T, Ozaki Y, Nanasato M, Ishikawa M, Nagasaka R, Ohota M, et al. Comparison between optical frequency domain imaging and intravascular ultrasound for percutaneous coronary intervention guidance in biolimus A9-eluting stent implantation: a randomized MISTIC-1 non-inferiority trial. Circ Cardiovasc Interv. 2020;13:E009314. doi: 10.1161/CIRCINTERVENTIONS.120.009314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Johnson TW, O’Kane PD. Can machine learned algorithms further illuminate intracoronary imaging in PCI and improve the human touch? EuroIntervention. 2021;17:18–9. doi: 10.4244/EIJV17I1A3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Omori H, Kawase Y, Mizukami T, Tanigaki T, Hirata T, Okubo M, et al. Diagnostic accuracy of artificial intelligence-based angiography-derived fractional flow reserve using pressure wire-based fractional flow reserve as a reference. Circ J. 2023;87:783–90. doi: 10.1253/circj.CJ-22-0771. [DOI] [PubMed] [Google Scholar]
- 111. Beyar R, Davies JE, Cook C, Dudek D, Cummins PA, Bruining N. Robotics, imaging, and artificial intelligence in the catheterisation laboratory. EuroIntervention. 2021;17:537–49. doi: 10.4244/EIJ-D-21-00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gosling RC, Morris PD, Silva Soto DA, Lawford PV, Hose DR, Gunn JP. Virtual coronary intervention: a treatment planning tool based upon the angiogram. JACC Cardiovasc Imaging. 2019;12:865–72. doi: 10.1016/j.jcmg.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]