Abstract
Microsporidia are ubiquitous opportunistic parasites in nature infecting all animal phyla, and the zoonotic potential of this parasitosis is under discussion. Fecal samples from 124 pigeons from seven parks of Murcia (Spain) were analyzed. Thirty-six of them (29.0%) showed structures compatible with microsporidia spores by staining methods. The DNA isolated from 26 fecal samples (20.9%) of microsporidia-positive pigeons was amplified with specific primers for the four most frequent human microsporidia. Twelve pigeons were positive for only Enterocytozoon bieneusi (9.7%), 5 for Encephalitozoon intestinalis (4%), and one for Encephalitozoon hellem (0.8%). Coinfections were detected in eight additional pigeons: E. bieneusi and E. hellem were detected in six animals (4.8%); E. bieneusi was associated with E. intestinalis in one case (0.8%); and E. hellem and E. intestinalis coexisted in one pigeon. No positive samples for Encephalitozoon cuniculi were detected. The internally transcribed spacer genotype could be completed for one E. hellem-positive pigeon; the result was identical to the genotype A1 previously characterized in an E. hellem Spanish strain of human origin. To our knowledge, this is the first time that human-related microsporidia have been identified in urban park pigeons. Moreover, we can conclude that there is no barrier to microsporidia transmission between park pigeons and humans for E. intestinalis and E. hellem. This study is of environmental and sanitary interest, because children and elderly people constitute the main visitors of parks and they are populations at risk for microsporidiosis. It should also contribute to the better design of appropriate prophylactic measures for populations at risk for opportunistic infections.
Microsporidia are intracellular obligate parasites mainly considered as opportunistic pathogens (58), ubiquitous in nature, infecting all animal phyla (6, 59). Although initially associated with AIDS patients, they are being detected in increasing numbers in immunocompetent patients and thus are gaining attention as emerging pathogens (25, 33, 35, 53, 56). The phylum Microsporidia contains over 144 genera and 1,200 species (59). The number of genera implicated in human microsporidiosis has increased at the same rate as the improvements in diagnostic techniques, and the interest in this group of parasites has grown accordingly. To date, eight genera are recognized as human pathogens: Nosema, Vittaforma, Pleistophora, Encephalitozoon, Enterocytozoon, Brachiola, Trachipleistophora, and Microsporidium. Among these, Enterocytozoon bieneusi is the species of microsporidia that most frequently causes infection in humans, followed by Encephalitozoon intestinalis, Encephalitozoon hellem, and Encephalitozoon cuniculi. The majority of reported cases of human microsporidiosis involve diarrhea. However, the spectrum of diseases caused by these parasites has expanded to include infection in almost all organ systems, including keratoconjunctivitis, hepatitis, myositis, cerebritis, sinusitis, and disseminated infection (59).
In the last few years, studies related to the epidemiology of human microsporidiosis have shown that although AIDS patients continue to be the main group at risk for this parasitosis, other individuals are also at risk. In relation to other immunosuppressed patients, microsporidiosis is becoming increasingly recognized as an opportunistic infection after transplantation (43). In other studies, travelers have recently emerged as a risk group (33, 39). Microsporidiosis has also been detected in elderly people and children who, due to their special immune status, may also be considered as groups at risk (35).
Many questions about microsporidial transmission remain unanswered but, in recent years, new data have begun to change the general conceptions about this parasitosis. For example, E. bieneusi, E. intestinalis, and E. hellem were considered only human parasites, but the first two have been identified in a wide range of domestic and wild animals (1, 4, 11, 14, 17, 22, 26, 32, 34, 36, 41, 45), and E. hellem has been found in avian hosts (3, 7, 21, 37, 42, 48, 49, 51). Consequently, the zoonotic potential of human-related microsporidia is a focus of discussion (59) and efforts have been made towards the development of a series of genetic markers for some species (16, 19, 20, 27, 37, 46, 48, 50, 60, 61). E. cuniculi is the best known species of microsporidia in molecular terms. Its genome has been completed (29); different genotypes have been described, and its zoonotic potential was recognized several years ago (6, 19). However, the zoonotic potential of other species, such as E. bieneusi and E. hellem, is still under discussion. In the latter, a strong intraspecies variability has been described after the analysis of different genetic markers (27, 37). Therefore, studies focusing on the transmission of human microsporidiosis and its propagation though animal contact have both a phylogenetic and prophylactic interest, due to the characteristics of patients affected.
To contribute to the knowledge of the epidemiology of microsporidiosis and focusing on its zoonotic potential, we have studied park pigeons for the presence of microsporidia. Urban park pigeons closely interact with humans and may be considered an invasive species in some cities. Moreover among park visitors, elderly people and children are highly represented and considered two populations that may be at risk for the acquisition of opportunistic parasites.
MATERIALS AND METHODS
Animal specimens, search for microsporidia, and parasite cultures.
A total of 124 pigeons were captured in seven public parks of Murcia (Spain). Pigeons were placed in individual cages after capture in order to obtain fecal samples. These animals and samples were further analyzed within a multidisciplinary study related to the utilization of pigeons as bioindicators of pollution in urban areas. Thin smears were made from all fecal samples, and a small portion of samples were frozen (−20°C) to perform molecular techniques. Microsporidia were investigated by Weber's chromotrope- and Gram chromotrope-based stains (38, 57).
E. cuniculi (ECLD), E. hellem (CDC V257), and E. intestinalis (CDC V297) were cultured on E6 monolayers and used as controls, as described previously (12, 55).
Molecular analyses. (i) DNA isolation.
DNA from fecal samples found to be positive for microsporidia in the staining methods was extracted by bead disruption of spores using the Fast-DNA-Spin kit, according to the manufacturer's instructions (Bio 101, Carlsbad, Calif.). PCR inhibitors were removed using the QIAquick PCR kit (QIAGEN, Chatsworth, CA).
(ii) Species characterization by PCR.
Microsporidial small subunit rRNA (SSU-rRNA) coding regions were amplified using the following species-specific primers: EBIEF1/EBIER1 for E. bieneusi (9), SINTF/SINTR for E. intestinalis (10), ECUNF/ECUNR for E. cuniculi, and EHELF/EHELR for E. hellem (54). PCR amplification was done with the GenAmp kit (Perkin-Elmer Cetus, Norwalk, CT) according to manufacturer's procedures, and the conditions for the reaction were described previously (13). Purified samples were tested for the presence of PCR inhibitors by spiking the samples with the corresponding cloned SSU-rRNA coding region, as described previously (13).
(iii) Polymorphism analysis by PCR.
The internally transcribed spacer (ITS) region between the 16S and 5.8S rRNA genes was analyzed. PCR was only performed on the E. hellem-positive sample by using the primer pair A/B designed by Hollister et al. (28), which amplifies a fragment of 208 bp including the ITS region. The forward primer A (5′ TTGTACACACCGCCCGTCG) was designed on the basis of the sequence positions 1186 to 1204 of the small subunit rRNA genes and the reverse primer B (5′ CCGATAATGCCAATCAATCC), on the sequence of the nucleosides from positions 1375 to 1394 of the large-subunit rDNA. PCR amplification was done by using a 25-μl reaction mix containing 50 ng of DNA, 0.2 mM of each deoxynucleoside triphosphate, 0.2 μM each of the A and B primers, 1 unit of Taq DNA polymerase (Roche Molecular Biochemicals), and the PCR buffer, including a final concentration of 1.5 mM MgCl2. After an initial step of 5 min at 80°C, a total of 35 cycles were performed as follows: 30 s at 98°C (except for the first cycle, which was made at 94°C), 30 s at 55°C, and 1.5 min at 72°C; the reaction products were kept at 4°C until the next protocol. Each isolate was analyzed by a minimum of four PCRs. The quality of the PCR amplifications was checked in routine 2% agarose gel electrophoresis.
(iv) Polyacrylamide gel electrophoresis and SSCA.
Eight percent polyacrylamide gels (19 acrylamide:1 bisacrylamide) were prepared in TBE buffer (Tris-borate 45 mM, 0.1 mM EDTA, pH 8.3) in vertical cuvettes (Hoefer SE400, Amersham Pharmacia Biotech), using 175 μl of 0.1% ammonium persulfate (APS) and 8.8 μl of N,N,N′,N′-tetramethylethylene diamine (TEMED) as activators. Bands were resolved by electrophoresis during 23 h at 130 V at room temperature. Single strand conformation analysis (SSCA) was performed following the method described by Myers et al. (40), with some modifications: the PCR products were denatured by mixing with 95% deionized formamide and 5% gel loading buffer (GenSura Laboratories, San Diego, CA) and incubated for 2 min at 95°C, followed by rapid cooling in an ice bath. TBE 8% polyacrylamide gels were prepared in a Hoefer SE600 apparatus, both at 99:1 and 49:1 acrylamide-bisacrylamide proportions, using 8.8 μl TEMED and 175 μl of 0.1% APS as activators. The electrophoresis was performed for 15 h at 10°C and 300 V. The gels were revealed after silver staining by standard methods modified as described by Haro et al. (27).
(v) DNA sequencing.
PCR products were prepared following the manufacturer's instructions using the Bioclean Columns kit (21.016 Biotools). Sequencing was carried out in both directions by the Sequencing Service of the Centro de Investigaciones Biológicas (Madrid, Spain). For the new alleles detected, two or three different PCR products were also sequenced in both directions.
Nucleotide sequence accession numbers.
GenBank accession numbers for the rRNA gene ITS sequences from E. bieneusi-positive samples are AY668952 and AY668953.
RESULTS
Stool samples from 36 pigeons (29%) showed a variable number of spores that stained pinkish red with the use of Weber's chromotrope-based stain and also took the Gram chromotrope stain with the characteristics of microsporidia. These spores measured 0.9 to 1.6 μm and were ovoid in shape with a clear vacuole-like polar zone. The typical belt-like stripe of these organisms was also displayed by some of the spores.
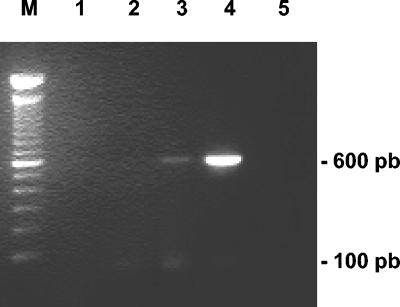
Amplification of DNAs isolated from positive samples in the staining technique with specific primers for the four most common microsporidia infecting humans allowed us to confirm 26 pigeons (20.9%) as positive by both techniques. Single-species infection was detected in the fecal samples of 12 pigeons (9.7%) for E. bieneusi, showing the diagnostic band of 607 bp in agarose gels (Fig. 1), 5 pigeons (4%) for E. intestinalis, and one case (0.8%) for E. hellem. Coinfections were also detected in eight additional pigeons: E. bieneusi coexisted with E. hellem in six animals (4.8%); E. bieneusi was also associated with E. intestinalis in one case (0,8%); and finally, E. hellem and E. intestinalis were jointly detected in 1 pigeon. No positive samples for E. cuniculi were detected.
FIG. 1.
Agarose gel analysis of PCR-amplified products with species-specific primers diagnostic for E. bieneusi. PCR performed with DNA extracted from a positive pigeon. Lanes 1 to 3, 5 μl of sample extract as well as 1/10 and 1/100 dilutions, respectively; lane 4, 0.1 μl of sample extract including a positive control; M, 100-bp ladder standard.
Due to the scarce amount of DNA available from fecal samples, the genotyping for the ITS region, between rRNA genes, could only be completed for one E. hellem-positive sample. Polyacrylamide gel electrophoresis and SSCA of PCR products revealed a pattern identical to that of the human 1A genotype (60), which was confirmed by sequencing (Fig. 2).
FIG. 2.
Comparison of the three ITS genotypes of E. hellem usually considered from human isolates (G I, G II, G III), and the sequence found from a park pigeon isolate (Pal), showing an obviously closer relationship with genotype I. The dots denote identity with the sequence of genotype III, and dashes depict nucleotide deletions.
The rRNA gene ITS could also be sequenced for two E. bieneusi-positive samples. The sequences (GenBank accession numbers AY668952 and AY668953) differ from any of the genotypes so far described (5, 14, 36, 44, 46, 47, 50). Since most of these correspond to North American wild animals, the significance of our genotypes from an epidemiological point of view requires the analysis of further samples.
DISCUSSION
The knowledge of the epidemiology of human microsporidiosis has evolved notably in the last decade with the improvement of diagnostic methods and with the development of molecular markers. However, many questions remain unanswered, and sources of infection as well as transmission routes are not well understood. Microsporidia-infected humans and animals eliminate spores with feces, urine, and secretions, which contaminate the environment and probably constitute the main source of infection. In this line, microsporidia spores have recently been detected in water sources and foods, including vegetables and bivalves (18, 41, 52). Therefore, in the last few years several authors have focused on animal microsporidiosis, mainly in mammalians, in order to elucidate the possible zoonotic origin of human microsporidiosis (1, 4, 11, 14, 17, 22, 26, 32, 34, 36, 41, 45, 47). Since studies on birds close to humans are much scarcer (3, 7, 21, 37, 42, 48, 49, 51), we decided to investigate the possible presence of human-related microsporidia in park pigeons.
Urban park pigeons closely interact with humans, mainly children and elderly people. These are two special groups of population because, for opposite reasons, they have a diminished immune response. The importance of a competent immunosystem for protection against microsporidiosis is well documented, and children as well as elderly people may be considered as populations at risk for opportunistic infections, including this one (35). Aging involves a process known as “immunosenescence” and is mainly associated with a dysfunction in cell-mediated immunity (2), correlating with the great importance that cell immunity has in the control of microsporidial infection. This is seen not only in animal models (30), but also in patients with AIDS (8).
Among the fecal samples from 124 pigeons from seven parks of Murcia (Spain), 29.0% showed structures compatible with microsporidia spores by staining methods. They appeared to be pinkish red with the Weber's chromotrope-based stain, and also showed the characteristics of microsporidia with the Gram chromotrope stain. From the DNA isolated from 26 fecal samples, PCR amplifications with specific primers confirmed single-species infection with E. bieneusi (12 pigeons, 9.7%), E. intestinalis (5 pigeons, 4%), or E. hellem (1 pigeon, 0.8%). Eight additional coinfections with E. bieneusi and E. hellem were detected in six animals (4.8%); E. bieneusi associated with E. intestinalis in 1 case (0.8%); and E. hellem and E. intestinalis coexisted in 1 pigeon. It is important to highlight that when applying the PCR technique to fecal sample analysis, one should be cautious of false-negative results. The concentration of parasitic DNA may be low, and the amplification will only be observed when high reaction volumes of sample are used. On the other hand, PCR inhibitors are frequently present in fecal samples, so that amplification is possible only after convenient sample dilution or the application of purification techniques for the elimination of PCR inhibitors (9). The purification method employed in this study assayed using three different dilutions plus an internal PCR control, which should support the interpretation of the results. The criteria for considering a sample negative by PCR implied no amplification in all dilutions assayed, with similar amplification in the positive control and in the sample added with cloned DNA (ensuring that there are no PCR inhibitors). However, and in addition to the above difficulties, it is necessary to consider that animal samples positive by the staining methods may not necessarily amplify with the specific primers used, because of the possible presence of microsporidia other than the species studied, the overload of extruded spores in samples with low parasitic load, etc.
To date, E. cuniculi is the microsporidia with clear, widely recognized zoonotic implications, and several genotypes have been described (15). However, we were unable to find this species in our material. On the other hand, E. intestinalis, the second most frequent human microsporidia (23, 31, 61), has also been found in various mammals such as the donkey, dog, pig, cow, and goat (4, 23), and its possible zoonotic potential has been previously mentioned (4). To our knowledge, the present study constitutes the first identification of E. intestinalis in birds and, more accurately, in pigeons. This finding significantly broadens the zoonotic potential of this microsporidia. It is interesting that no genetic heterogeneity has been found for E. intestinalis, suggesting that either the adequate markers are still to be found or there are no transmission barriers between the species to date identified as hosts.
E. hellem infection in humans is mainly extraintestinal (58); however it has been found in the feces of different animal hosts. It was initially detected by PCR in psittacine bird feces in 1997 (3). More recently, this microsporidia infection has been identified in some bird species close to humans, such as parrots (3, 42, 51), ostriches (24, 48), peach-faced lovebirds (49), and the Gouldian finch (7). Together with our work, all these studies may suggest that such infections are more common than previously suspected. Intraspecies genotype variability of E. hellem was initially based on the sequence of the ITS of the rRNA genes (37), but later also characterized in two intergenic spacers (IGS-TH and IGS-HZ) and in the polar tube protein gene (PTP), justifying the description of new genotypes (27, 61). It is interesting that from seven E. hellem isolates obtained from human immunodeficiency virus-positive patients, six of them (of Spanish, Italian, and American origin) showed the ITS genotype 1A (27), the same one obtained for the E. hellem isolate of the park pigeon. This means that the ITS genotype found in the park pigeon strain is a common one with a wide geographical distribution including Europe and America, and unequivocally shared by pigeons and humans in Spain, Italy and the United States.
From our point of view, it is also significant that the level of park pigeons in which intestinal microsporidiosis was confirmed by PCR was high (20.9%). Bearing in mind their abundance in urban and periurban areas and their close relationship to humans, the penetration of the microsporidia spores from this source by inhalation, direct contact with mucosa, or ingestion seems a feasible possibility. Pigeons move together in groups and when they approach the visitor to eat what is offered, the flapping of their wings produces a notable particle suspension in which earth mixes with debris and their own feces. Therefore, microsporidia spores, if present in this situation, may easily penetrate through ocular mucosa, by inhalation, or by accidental ingestion after resting on hands or toys.
To our knowledge, this is the first time that human-related microsporidia have been isolated in park pigeons. It is noteworthy that E. bieneusi is the most frequent species of human microsporidia and was also the most frequent in park pigeons (12%). As shown in recent phylogenetic studies, the lack of a transmission barrier of E. bieneusi between humans and animals points to a zoonotic origin of this parasitosis (14). Moreover, E. bieneusi was detected for the first time in nonmammalian hosts (chickens) (44), and our report of a second avian host broadens its zoonotic potential. As previously mentioned, E. intestinalis, the second most frequent species of human microsporidia, has also been detected for the first time in birds, constituting the second most frequent species of microsporidia in park pigeons, while E. hellem, which, although previously identified in birds, has never before been isolated in pigeons. We should finally highlight that all microsporidia species found in pigeons have been detected both alone and as coinfections. The most frequent is the combination E. bieneusi and E. hellem.
We may therefore conclude that this study is of environmental and sanitary interest because opportunistic parasites such as human-associated microsporidia have been identified in park pigeons. Such animals have a frequent, intense relationship with humans, mainly with children and elderly people. Other immunosuppressed individuals may also visit them, all constituting populations at risk for microsporidiosis. Accurate information about the dangers of an intense contact with these animals should be offered to these high-risk populations in order to design appropriate prophylactic measures.
Acknowledgments
We are indebted to Linda Hamalainen for helpful revision of the manuscript.
This work was supported by grants from Fundación San Pablo-CEU (1/01 and 11/03).
REFERENCES
- 1.Akerstedt, J., K. Nordstoga, A. Mathis, E. Smeds, and P. Deplazes. 2002. Fox encephalitozoonosis: isolation of the agent from an outbreak in farmed blue foxes (Alopex lagopus) in Finland and some hitherto unreported pathologic lesions. J. Vet. Med. B Infect. Dis. Vet. Public Health 49:400-405. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Yehuda, A., and M. E. Weksler. 1992. Immune senescence: mechanisms and clinical implications. Cancer Investig. 10:525-531. [DOI] [PubMed] [Google Scholar]
- 3.Black, S. S., L. A. Steinohrt, D. C. Bertucci, L. B. Rogers, and E. S. Didier. 1997. Encephalitozoon hellem in budgerigars (Melopsittacus undulatus). Vet. Pathol. 34:189-198. [DOI] [PubMed] [Google Scholar]
- 4.Bornay-Llinares, F. J., A. J. da Silva, H. Moura, D. A. Schwartz, G. S. Visvesvara, N. J. Pieniazek, A. Cruz-Lopez, P. Hernandez-Jauregui, J. Guerrero, and F. J. Enriquez. 1998. Immunologic, microscopic, and molecular evidence of Encephalitozoon intestinalis (Septata intestinalis) infection in mammals other than humans. J. Infect. Dis. 178:820-826. [DOI] [PubMed] [Google Scholar]
- 5.Breitenmoser, A. C., A. Mathis, E. Burgi, R. Weber, and P. Deplazes. 1999. High prevalence of Enterocytozoon bieneusi in swine with four genotypes that differ from those identified in humans. Parasitology 118:447-453. [DOI] [PubMed] [Google Scholar]
- 6.Canning, E. U., and J. Lom. 1986. The microsporidia of vertebrates. Academic Press, San Diego, Calif.
- 7.Carlisle, M. S., K. Snowden, J. Gill, M. Jones, P. O'Donoghue, and P. Prociv. 2002. Microsporidiosis in a Gouldian finch (Erythrura [Chloebia] gouldiae). Aust. Vet. J. 80:41-44. [DOI] [PubMed] [Google Scholar]
- 8.Conteas, C. N., O. G. Berlin, C. E. Speck, S. S. Pandhumas, M. J. Lariviere, and C. Fu. 1998. Modification of the clinical course of intestinal microsporidiosis in acquired immunodeficiency syndrome patients by immune status and anti-human immunodeficiency virus therapy. Am. J. Trop. Med. Hyg. 58:555-558. [DOI] [PubMed] [Google Scholar]
- 9.da Silva, A. J., D. A. Schwartz, G. S. Visvesvara, H. de Moura, S. B. Slemenda, and N. J. Pieniazek. 1996. Sensitive PCR diagnosis of Infections by Enterocytozoon bieneusi (microsporidia) using primers based on the region coding for small-subunit rRNA. J. Clin. Microbiol. 34:986-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Da Silva, A. J., S. B. Slemenda, G. S. Visvesvara, D. A. Schwartz, C. M. Wilcox, S. Wallace, and N. J. Pieniazek. 1997. Detection of Septata intestinalis (microsporidia) Cali et al. 1993 using polymerase chain reaction primers targeting the small subunit ribosomal RNA coding region. Mol. Diagn. 2:47-52. [DOI] [PubMed] [Google Scholar]
- 11.del Aguila, C., F. Izquierdo, R. Navajas, N. J. Pieniazek, G. Miro, A. I. Alonso, A. J. Da Silva, and S. Fenoy. 1999. Enterocytozoon bieneusi in animals: rabbits and dogs as new hosts. J. Eukaryot. Microbiol. 46:8S-9S. [PubMed] [Google Scholar]
- 12.del Aguila, C., R. Lopez-Velez, S. Fenoy, C. Turrientes, J. Cobo, R. Navajas, G. S. Visvesvara, G. P. Croppo, A. J. Da Silva, and N. J. Pieniazek. 1997. Identification of Enterocytozoon bieneusi spores in respiratory samples from an AIDS patient with a 2-year history of intestinal microsporidiosis. J. Clin. Microbiol. 35:1862-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.del Aguila, C., R. Navajas, D. Gurbindo, J. T. Ramos, M. J. Mellado, S. Fenoy, M. A. Munoz Fernandez, M. Subirats, J. Ruiz, and N. J. Pieniazek. 1997. Microsporidiosis in HIV-positive children in Madrid (Spain). J. Eukaryot. Microbiol. 44:84S-85S. [DOI] [PubMed] [Google Scholar]
- 14.Dengjel, B., M. Zahler, W. Hermanns, K. Heinritzi, T. Spillmann, A. Thomschke, T. Loscher, R. Gothe, and H. Rinder. 2001. Zoonotic potential of Enterocytozoon bieneusi. J. Clin. Microbiol. 39:4495-4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deplazes, P., A. Mathis, R. Baumgartner, I. Tanner, and R. Weber. 1996. Immunologic and molecular characteristics of Encephalitozoon-like microsporidia isolated from humans and rabbits indicate that Encephalitozoon cuniculi is a zoonotic parasite. Clin. Infect. Dis. 22:557-559. [DOI] [PubMed] [Google Scholar]
- 16.Deplazes, P., A. Mathis, C. Muller, and R. Weber. 1996. Molecular epidemiology of Encephalitozoon cuniculi and first detection of Enterocytozoon bieneusi in faecal samples of pigs. J. Eukaryot. Microbiol. 43:93S. [DOI] [PubMed] [Google Scholar]
- 17.Deplazes, P., A. Mathis, and R. Weber. 2000. Epidemiology and zoonotic aspects of microsporidia of mammals and birds. Contrib. Microbiol. 6:236-260. [DOI] [PubMed] [Google Scholar]
- 18.Didier, E. S., M. E. Stovall, L. C. Green, P. J. Brindley, K. Sestak, and P. J. Didier. 2004. Epidemiology of microsporidiosis: sources and modes of transmission. Vet. Parasitol. 126:145-166. [DOI] [PubMed] [Google Scholar]
- 19.Didier, E. S., G. S. Visvesvara, M. D. Baker, L. B. Rogers, D. C. Bertucci, M. A. De Groote, and C. R. Vossbrinck. 1996. A microsporidian isolated from an AIDS patient corresponds to Encephalitozoon cuniculi III, originally isolated from domestic dogs. J. Clin. Microbiol. 34:2835-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Didier, E. S., C. R. Vossbrinck, M. D. Baker, L. B. Rogers, D. C. Bertucci, and J. A. Shadduck. 1995. Identification and characterization of three Encephalitozoon cuniculi strains. Parasitology 111:411-421. [DOI] [PubMed] [Google Scholar]
- 21.Fayer, R., M. Santin, R. Palmer, and X. Li. 2003. Detection of Encephalitozoon hellem in feces of experimentally infected chickens. J. Eukaryot. Microbiol. 50(Suppl.):574-575. [DOI] [PubMed] [Google Scholar]
- 22.Fayer, R., M. Santin, and J. M. Trout. 2003. First detection of microsporidia in dairy calves in North America. Parasitol. Res. 90:383-386. [DOI] [PubMed] [Google Scholar]
- 23.Graczyk, T. K., J. Bosco-Nizeyi, A. J. da Silva, I. N. Moura, N. J. Pieniazek, M. R. Cranfield, and H. D. Lindquist. 2002. A single genotype of Encephalitozoon intestinalis infects free-ranging gorillas and people sharing their habitats in Uganda. Parasitol. Res. 88:926-931. [DOI] [PubMed] [Google Scholar]
- 24.Gray, M. L., M. Puette, and K. S. Latimer. 1998. Microsporidiosis in a young ostrich (Struthio camelus). Avian Dis. 42:832-836. [PubMed] [Google Scholar]
- 25.Gumbo, T., I. T. Gangaidzo, S. Sarbah, A. Carville, S. Tzipori, and P. M. Wiest. 2000. Enterocytozoon bieneusi infection in patients without evidence of immunosuppression: two cases from Zimbabwe found to have positive stools by PCR. Ann. Trop. Med. Parasitol. 94:699-702. [DOI] [PubMed] [Google Scholar]
- 26.Harcourt-Brown, F. M., and H. K. Holloway. 2003. Encephalitozoon cuniculi in pet rabbits. Vet. Rec. 152:427-431. [DOI] [PubMed] [Google Scholar]
- 27.Haro, M., C. Del Aguila, S. Fenoy, and N. Henriques-Gil. 2003. Intraspecies genotype variability of the microsporidian parasite Encephalitozoon hellem. J. Clin. Microbiol. 41:4166-4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollister, W. S., E. U. Canning, and C. L. Anderson. 1996. Identification of microsporidia causing human disease. J. Eukaryot. Microbiol. 43:104S-105S. [DOI] [PubMed] [Google Scholar]
- 29.Katinka, M. D., S. Duprat, E. Cornillot, G. Metenier, F. Thomarat, G. Prensier, V. Barbe, E. Peyretaillade, P. Brottier, P. Wincker, F. Delbac, H. El Alaoui, P. Peyret, W. Saurin, M. Gouy, J. Weissenbach, and C. P. Vivares. 2001. Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature 414:450-453. [DOI] [PubMed] [Google Scholar]
- 30.Khan, I. A., J. D. Schwartzman, L. H. Kasper, and M. Moretto. 1999. CD8+ CTLs are essential for protective immunity against Encephalitozoon cuniculi infection. J. Immunol. 162:6086-6091. [PubMed] [Google Scholar]
- 31.Liguory, O., S. Fournier, C. Sarfati, F. Derouin, and J. M. Molina. 2000. Genetic homology among thirteen Encephalitozoon intestinalis isolates obtained from human immunodeficiency virus-infected patients with intestinal microsporidiosis. J. Clin. Microbiol. 38:2389-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lobo, M. L., A. Teles, M. B. da Cunha, J. Henriques, A. M. Lourenco, F. Antunes, and O. Matos. 2003. Microsporidia detection in stools from pets and animals from the zoo in Portugal: a preliminary study. J. Eukaryot. Microbiol. 50(Suppl.):581-582. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Velez, R., M. C. Turrientes, C. Garron, P. Montilla, R. Navajas, S. Fenoy, and C. del Aguila. 1999. Microsporidiosis in travelers with diarrhea from the tropics. J. Travel Med. 6:223-227. [DOI] [PubMed] [Google Scholar]
- 34.Lores, B., C. del Aguila, and C. Arias. 2002. Enterocytozoon bieneusi (microsporidia) in faecal samples from domestic animals from Galicia, Spain. Mem. Inst. Oswaldo Cruz 97:941-945. [DOI] [PubMed] [Google Scholar]
- 35.Lores, B., I. Lopez-Miragaya, C. Arias, S. Fenoy, J. Torres, and C. del Aguila. 2002. Intestinal microsporidiosis due to Enterocytozoon bieneusi in elderly human immunodeficiency virus-negative patients from Vigo, Spain. Clin. Infect. Dis. 34:918-921. [DOI] [PubMed] [Google Scholar]
- 36.Mathis, A., A. C. Breitenmoser, and P. Deplazes. 1999. Detection of new Enterocytozoon genotypes in faecal samples of farm dogs and a cat. Parasite 6:189-193. [DOI] [PubMed] [Google Scholar]
- 37.Mathis, A., I. Tanner, R. Weber, and P. Deplazes. 1999. Genetic and phenotypic intraspecific variation in the microsporidian Encephalitozoon hellem. Int. J. Parasitol. 29:767-770. [DOI] [PubMed] [Google Scholar]
- 38.Moura, H., J. L. Da Silva, F. C. Sodre, P. Brasil, K. Wallmo, S. Wahlquist, S. Wallace, G. P. Croppo, and G. S. Visvesvara. 1996. Gram-chromotrope: a new technique that enhances detection of microsporidial spores in clinical samples. J. Eukaryot. Microbiol. 43:94S-95S. [DOI] [PubMed] [Google Scholar]
- 39.Muller, A., R. Bialek, A. Kamper, G. Fatkenheuer, B. Salzberger, and C. Franzen. 2001. Detection of microsporidia in travelers with diarrhea. J. Clin. Microbiol. 39:1630-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myers, R. M., L. H. Elleson, and K. Hayashi. 1997. Detection of DNA variation. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 41.Negm, A. Y. 2003. Human pathogenic protozoa in bivalves collected from local markets in Alexandria. J. Egypt Soc. Parasitol. 33:991-998. [PubMed] [Google Scholar]
- 42.Pulparampil, N., D. Graham, D. Phalen, and K. Snowden. 1998. Encephalitozoon hellem in two eclectus parrots (Eclectus roratus): identification from archival tissues. J. Eukaryot. Microbiol. 45:651-655. [DOI] [PubMed] [Google Scholar]
- 43.Rabodonirina, M., L. Cotte, S. Radenne, E. Besada, and C. Trepo. 2003. Microsporidiosis and transplantation: a retrospective study of 23 cases. J. Eukaryot. Microbiol. 50(Suppl.):583. [DOI] [PubMed] [Google Scholar]
- 44.Reetz, J., H. Rinder, A. Thomschke, H. Manke, M. Schwebs, and A. Bruderek. 2002. First detection of the microsporidium Enterocytozoon bieneusi in non-mammalian hosts (chickens). Int. J. Parasitol. 32:785-787. [DOI] [PubMed] [Google Scholar]
- 45.Reetz, J., M. Wiedemann, A. Aue, U. Wittstatt, A. Ochs, A. Thomschke, H. Manke, M. Schwebs, and H. Rinder. 2004. Disseminated lethal Encephalitozoon cuniculi (genotype III) infections in cotton-top tamarins (Oedipomidas oedipus)-a case report. Parasitol. Int. 53:29-34. [DOI] [PubMed] [Google Scholar]
- 46.Rinder, H., S. Katzwinkel-Wladarsch, A. Thomschke, and T. Loscher. 1998. Strain differentiation in microsporidia. Tokai J. Exp. Clin. Med. 23:433-437. [PubMed] [Google Scholar]
- 47.Rinder, H., A. Thomschke, B. Dengjel, R. Gothe, T. Loscher, and M. Zahler. 2000. Close genotypic relationship between Enterocytozoon bieneusi from humans and pigs and first detection in cattle. J. Parasitol. 86:185-188. [DOI] [PubMed] [Google Scholar]
- 48.Snowden, K., and K. Logan. 1999. Molecular identification of Encephalitozoon hellem in an ostrich. Avian Dis. 43:779-782. [PubMed] [Google Scholar]
- 49.Snowden, K. F., K. Logan, and D. N. Phalen. 2000. Isolation and characterization of an avian isolate of Encephalitozoon hellem. Parasitology 121:9-14. [DOI] [PubMed] [Google Scholar]
- 50.Sulaiman, I. M., R. Fayer, A. A. Lal, J. M. Trout, F. W. Schaefer III, and L. Xiao. 2003. Molecular characterization of microsporidia indicates that wild mammals harbor host-adapted Enterocytozoon spp. as well as human-pathogenic Enterocytozoon bieneusi. Appl. Environ. Microbiol. 69:4495-4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suter, C., A. Mathis, R. Hoop, and P. Deplazes. 1998. Encephalitozoon hellem infection in a yellow-streaked lory (Chalcopsitta scintillata) imported from Indonesia. Vet. Rec. 143:694-695. [PubMed] [Google Scholar]
- 52.Thurston-Enriquez, J. A., P. Watt, S. E. Dowd, R. Enriquez, I. L. Pepper, and C. P. Gerba. 2002. Detection of protozoan parasites and microsporidia in irrigation waters used for crop production. J. Food Prot. 65:378-382. [DOI] [PubMed] [Google Scholar]
- 53.Visvesvara, G. S., M. Belloso, H. Moura, A. J. Da Silva, I. N. Moura, G. J. Leitch, D. A. Schwartz, P. Chevez-Barrios, S. Wallace, N. J. Pieniazek, and J. D. Goosey. 1999. Isolation of Nosema algerae from the cornea of an immunocompetent patient. J. Eukaryot. Microbiol. 46:10S. [PubMed] [Google Scholar]
- 54.Visvesvara, G. S., G. J. Leitch, A. J. da Silva, G. P. Croppo, H. Moura, S. Wallace, S. B. Slemenda, D. A. Schwartz, D. Moss, R. T. Bryan, et al. 1994. Polyclonal and monoclonal antibody and PCR-amplified small-subunit rRNA identification of a microsporidian, Encephalitozoon hellem, isolated from an AIDS patient with disseminated infection. J. Clin. Microbiol. 32:2760-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Visvesvara, G. S., G. J. Leitch, H. Moura, S. Wallace, R. Weber, and R. T. Bryan. 1991. Culture, electron microscopy, and immunoblot studies on a microsporidian parasite isolated from the urine of a patient with AIDS. J. Protozool. 38:105S-111S. [PubMed] [Google Scholar]
- 56.Weber, R., and R. T. Bryan. 1994. Microsporidial infections in immunodeficient and immunocompetent patients. Clin. Infect. Dis. 19:517-521. [DOI] [PubMed] [Google Scholar]
- 57.Weber, R., R. T. Bryan, R. L. Owen, C. M. Wilcox, L. Gorelkin, and G. S. Visvesvara. 1992. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. N. Engl. J. Med. 326:161-166. [DOI] [PubMed] [Google Scholar]
- 58.Weber, R., R. T. Bryan, D. A. Schwartz, and R. L. Owen. 1994. Human microsporidial infections. Clin. Microbiol. Rev. 7:426-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weiss, L. M. 2003. Microsporidia 2003: IWOP-8. J. Eukaryot. Microbiol. 50(Suppl.):566-568. [DOI] [PubMed] [Google Scholar]
- 60.Xiao, L., L. Li, H. Moura, I. Sulaiman, A. A. Lal, S. Gatti, M. Scaglia, E. S. Didier, and G. S. Visvesvara. 2001. Genotyping Encephalitozoon hellem isolates by analysis of the polar tube protein gene. J. Clin. Microbiol. 39:2191-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiao, L., L. Li, G. S. Visvesvara, H. Moura, E. S. Didier, and A. A. Lal. 2001. Genotyping Encephalitozoon cuniculi by multilocus analyses of genes with repetitive sequences. J. Clin. Microbiol. 39:2248-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]