Abstract
Anaerobic starvation conditions are frequent in industrial fermentation and can affect the performance of the cells. In this study, the anaerobic carbon or nitrogen starvation response of Saccharomyces cerevisiae was investigated for cells grown in anaerobic carbon or nitrogen-limited chemostat cultures at a dilution rate of 0.1 h−1 at pH 3.25 or 5. Lactic or benzoic acid was present in the growth medium at different concentrations, resulting in 16 different growth conditions. At steady state, cells were harvested and then starved for either carbon or nitrogen for 24 h under anaerobic conditions. We measured fermentative capacity, glucose uptake capacity, intracellular ATP content, and reserve carbohydrates and found that the carbon, but not the nitrogen, starvation response was dependent upon the previous growth conditions. All cells subjected to nitrogen starvation retained a large portion of their initial fermentative capacity, independently of previous growth conditions. However, nitrogen-limited cells that were starved for carbon lost almost all their fermentative capacity, while carbon-limited cells managed to preserve a larger portion of their fermentative capacity during carbon starvation. There was a positive correlation between the amount of glycogen before carbon starvation and the fermentative capacity and ATP content of the cells after carbon starvation. Fermentative capacity and glucose uptake capacity were not correlated under any of the conditions tested. Thus, the successful adaptation to sudden carbon starvation requires energy and, under anaerobic conditions, fermentable endogenous resources. In an industrial setting, carbon starvation in anaerobic fermentations should be avoided to maintain a productive yeast population.
Microorganisms in nature frequently encounter nutrient excess, nutrient starvation, and rapid shifts between these two extremes. Studies of starvation responses of Saccharomyces cerevisiae, both a model and a commercially important yeast, are limited and focused primarily on the starvation response of aerobically starved cells (8, 9, 12, 14, 17, 21, 22). Anaerobic starvation conditions (25, 29) are also important for this yeast during the production of bread, alcoholic beverages, and fuel alcohol (16).
Studies of the starvation response of aerobically grown cells have shown that the growth history prior to starvation is an important variable (20, 22). Cells in stationary phase are generally more tolerant to osmotic stress or heat stress than are cells in the logarithmic-growth phase (3, 32). In aerobic studies of starvation for a single nutrient in an otherwise complete medium, carbon starvation is better tolerated than nitrogen starvation (9, 21). The lack of tolerance for nitrogen starvation could result from degradation of the glucose uptake system in cells starved for nitrogen in the presence of a fermentable carbon source (4). However, even in the presence of a nonfermentable carbon source, e.g., ethanol instead of glucose, there was a greater decrease in fermentative capacity during nitrogen starvation than during carbon starvation (21).
Storage carbohydrate accumulation also affects starvation tolerance. Trehalose and glycogen accumulate in response to nutrient limitation (5) and may be found in large amounts during starvation for nitrogen in the presence of a carbon source (14). In chemostats, storage carbohydrates accumulate in carbon-limited cultures at low dilution rates and low glycolytic flux (11). However, if the glycolytic flux is increased by imposing nitrogen limitation or by the addition of weak organic acids, then the storage carbohydrate content of the cells will diminish (11).
Trehalose is a stress protectant of proteins and cell membranes (1, 10, 27, 33) and is important for the quality and viability of baker's yeast (23, 26). The inability to produce trehalose and glycogen before a starvation period results in lower viability (26), and cells whose intracellular trehalose content was increased by the addition of extracellular trehalose had a higher starvation tolerance (23).
The starvation response of cells grown under anaerobic conditions is different from the response of aerobically grown cells (29). Anaerobically grown logarithmic-phase cells subjected to a sudden carbon starvation period have a reduction in fermentative capacity and a rapid drop in ATP immediately after harvest that lasts through the starvation period. Cells from the same growth conditions that are starved for nitrogen also had severely reduced fermentative capacity but maintained their initial ATP levels. The lack of fermentative capacity following carbon starvation could not be explained by the absence of glucose transport capacity. We previously suggested that during carbon starvation the cells rapidly lost their energy reserves and were not able to adapt successfully to starvation conditions (29).
Industrial fermentation based on wheat hydrolysate (for example) frequently becomes nitrogen limited (24). Due to both a low and variable homogeneity of large-scale fermenters, there may even be times when cells encounter a starvation situation. For example, nitrogen limitation-starvation can result in so-called stuck fermentation (2, 24). Furthermore, the logistics of the propagation procedure may involve starvation conditions, e.g., when cells are stored in a NaCl solution before being inoculated in the subsequent step. Lactic acid is also a common stress factor under industrial conditions, due to contamination of the fermentation by lactic acid bacteria (18). Weak organic acids are believed to enter the cell by crossing the membrane in their undissociated form. The proportion of dissociated and undissociated acid depends on the pH and the pKa of the acid.
Our objective in this study was to evaluate the response to a sudden anaerobic starvation with respect to fermentative capacity in cells cultivated under carbon or nitrogen limitation and with or without lactic acid. Nutrient limitation and weak organic acids affect the rate of fermentation, which in turn affects endogenous energy reserves. Understanding the consequences of the anaerobic starvation response is needed to develop and implement an optimized ethanol production process and to maintain a productive yeast population.
MATERIALS AND METHODS
Yeast strain and medium.
An industrial strain provided by Jästbolaget AB (Järfälla, Sweden) was used for these experiments. The cells were cultured in a defined medium (30), with a few alterations, containing the following: KH2PO4, 3 g; MgSO4 · 7H2O, 0.5 g; EDTA, 15 mg; ZnSO4 · 7H2O, 4.5 mg; CoCl2 · 6H2O, 0.3 mg; MnCl2 · 4H2O, 1 mg; CuSO4 · 5H2O, 0.3 mg; CaCl2 · 2H2O, 4.5 mg; FeSO4 · 7H2O, 3 mg; Na2MoO4 · 2H2O, 0.4 mg; H3BO3, 1 mg; KI, 0.1 mg; biotin, 0.05 mg; calcium pantothenate, 1 mg; nicotinic acid, 1 mg; inositol, 25 mg; thiamine HCl, 1 mg; pyridoxine HCl, 1 mg; and p-aminobenzoic acid, 0.2 mg. Addition of ergosterol to a final concentration of 10 mg/liter was made from a stock solution of ergosterol (10 g/liter) in Tween 80 (420 g/liter) in ethanol. The alterations were that Polypropylene-2000 (Fluka, Steinheim, Switzerland) was used as an antifoaming agent and was added before sterilization to a final concentration of 0.1 ml/liter. The glucose concentration was 50 g/liter unless otherwise specified. The concentrations of (NH4)2SO4 were 5 g/liter in the carbon-limited medium and 0.3 g/liter in the nitrogen-limited medium. Benzoic acid was included in this study because it affects glycolytic flux more strongly than does lactic acid (11, 19). Aqueous solutions of lactic acid (British Drug House, Poole, United Kingdom) or sodium benzoate (Merck, Darmstadt, Germany) were filter sterilized (0.22-μm-pore-size diameter filter) and added to the medium after autoclaving.
Cultivation setup.
A chemostat culture with a working volume of 1.5 liter and a constant dilution rate of 0.1 h−1 was prepared in a 2 l Braun Biostat A fermentor (Braun, Melsungen, Germany). The temperature was kept constant at 30°C, and the stirring rate was 500 rpm. The pH was kept at either 3.25 or 5.0 by the automatic addition of 1 or 2 M NaOH. Anaerobic conditions were maintained with a continuous N2 gas flow through the fermentor at a rate of 500 ml/min. The gas flow was controlled by a mass flow controller (Bronkhorst High-Tech B.V., Ruurlo, The Netherlands).
Batch cultures were used to start the chemostat. Cells were cultivated on a yeast extract-peptone-glucose plate for 48 h at 30°C and then in a 300-ml shake flask containing 100 ml of complete medium with 1% glucose for 24 h at 30°C and 200 rpm. The fermentor contained 2 liter of complete medium and was inoculated with 2 ml of the preculture. The feed of fresh substrate was started shortly after entry into stationary-phase, following glucose depletion, as indicated by the CO2 production rate.
Steady-state criteria.
Constant CO2 production after at least three residence periods was used as the criterion for a steady-state fermentation. Exhaust gas was monitored continuously with a gas analyzer (Brüel & Kjær, Nærum, Denmark). The different steady-state conditions were achieved following successive changes in the substrate reservoir. First, the lactic acid concentration was increased from 0 to 90 and finally to 280 mM at pH 5. Then, the pH was reduced to 3.25 and the lactic acid level was reduced successively from 280 to 90 and 0 mM. Finally, the pH was reset to 5, and 5 mM benzoic acid was added. The first seven steady states were run under carbon-limiting conditions and the following seven were run under nitrogen-limiting conditions.
Starvation protocol.
The starvation media were identical to the growth medium, except that carbon starvation was performed in the absence of glucose and nitrogen starvation was performed in the absence of ammonium sulfate. Samples of 20 to 125 ml were taken in duplicate for each starvation condition at steady state from the same fermentor. The volume of the sample depended on the dry weight (wt/vol) of the fermentor culture at the particular growth condition. The cells were centrifuged at 4,000 × g for 5 min at 25°C and washed once in the starvation medium to be studied (either carbon or nitrogen starvation medium). Cells were resuspended in 100 ml of starvation medium, resulting in an initial dry weight concentration at the beginning of starvation of approximately 1 g (wt/vol) per liter. The subsequent 24-h anaerobic incubation was performed with anaerobic 100-ml shake flasks (28).
Measurement of fermentative capacity.
Fermentative capacity was measured as the ethanol production rate before and after starvation in a medium identical to the growth medium but without a nitrogen source (ergosterol-Tween 80) and with a glucose concentration of 10 g/liter. The nitrogen source was omitted to avoid de novo protein synthesis during the test. For unstarved cells, 5 to 25 ml of duplicate samples, depending on dry weight, were sampled and harvested by centrifugation at 4,000 × g for 5 min at 25°C. After being washed once in the fermentative capacity test medium, the cells were resuspended in 50 ml test medium in 250-ml shake flasks, resulting in a final cell density of approximately 0.5 g (dry weight)/liter. The cells were incubated at 30°C on a shaker at 200 rpm, and glucose was added to a final concentration of 10 g/liter. Extracellular samples (1 ml) were taken every 10 min by centrifugation for 1 min at 16,000 × g. The supernatant was collected and frozen in liquid N2 and stored at −20°C. Ethanol was analyzed enzymatically with kits from Boehringer-Mannheim GmbH (Mannheim, Germany). To measure the fermentative capacity of the starved cells, 25 ml of the starvation culture was sampled, and the cells were washed and resuspended as indicated above. The ethanol production capacity of starved cells was normalized to the dry weight of the culture at the onset of starvation.
Glucose uptake capacity experiments.
Glucose uptake capacity was measured as previously described (31) with minor modifications. Approximately 25 ml of the starved culture was centrifuged for 5 min at 4,000 × g and washed twice with growth medium lacking both a carbon and a nitrogen source. The pellet was resuspended in 4 ml of medium lacking the carbon and nitrogen source and kept on ice until measurement. Immediately before measurement, the cells were transferred to a 30°C water bath for 4 min and flushed with N2. From the flushed-cell suspension, 50 μl was transferred and mixed with 12.5 μl solution containing 100 mM potassium phosphate buffer (pH 6.5) and 14C-labeled glucose to a final concentration of 50 mM. The activity was 60 μCi/ml. The mixture was incubated for 5 s, and glucose uptake was quenched by transferring 50 μl of the mixture to 10 ml of ice-cold quench buffer containing 100 mM potassium phosphate buffer (pH 6.5) and 500 mM unlabeled glucose maintained at or below −5°C in a salt-ice bath. The cells were collected by filtration on glass fiber filters and washed twice with 2 × 10 ml of quench buffer. Radioactivity was determined with a Beckman (Fullerton, California) liquid scintillator counter (LS6000LL). Five determinations were made for each starvation culture.
Protein determination.
Duplicates of 150 μl each were mixed with an equal volume of 2 M NaOH, frozen in liquid N2, and stored at −20°C. The proteins were hydrolyzed by being boiled for 15 min and centrifuged at 16,000 × g at 4°C for 15 min. Total protein in the supernatant was measured as described by Lowry (15) with bovine serum albumin as the standard.
Determination of intracellular ATP.
Duplicate 1-ml samples were taken, and ATP was extracted by the addition of 1.2 ml of 0.51 M trichloroacetic acid (6). The analysis was carried out with the CLSII ATP bioluminiscence assay kit (Boehringer-Mannheim GmbH) on a Pico-Lite luminometer (Packard Instruments, Downers Grove, Illinois).
Determination of trehalose and glycogen.
Duplicate samples of 35 to 50 mg (dry weight) of unstarved cells or 10 to 50 mg (dry weight) of starved cells were taken. The cells were centrifuged for 5 min at 4,000 × g at 4°C and washed twice in cold 0.9% NaCl. The pellet was frozen in liquid N2, kept at −20°C, and analyzed as previously described (29). The content of storage carbohydrates was normalized to the cell dry weight of the same culture.
Determination of dry weight.
Samples (2 × 5 or 2 × 10 ml) were centrifuged in dried, tared conical glass tubes for 5 min at 2,300 × g and washed twice with deionized water. Pellets were dried in the tubes for 24 h at 110°C, stored in a desiccator until cooled to room temperature, and then weighed.
RESULTS
Fermentative capacity of carbon-limited cells.
The fermentative capacity of unstarved cells grown under carbon-limiting conditions was unaffected by the presence of lactic acid or benzoic acid during growth (Table 1). After carbon starvation, the decrease in fermentative capacity of cells grown at pH 5 was greater in response to 5 mM benzoic acid than to 280 mM lactic acid. Nitrogen starvation of carbon-limited cells at pH 5 decreased the fermentative capacity to between 4.8 and 7.2 mmol/g/h, irrespective of weak acid addition (Table 1). Cells grown at pH 3.25 had a fermentative capacity of 8.1 mmol/g/h after carbon starvation, but in the presence of 90 or 280 mM of lactic acid during growth the carbon starvation tolerance was affected and the fermentative capacity was reduced by >70% (Table 1). For nitrogen-starved cells, the fermentative capacity of 5.8 mmol/g/h when no lactic acid was present during growth was reduced further by 35 and 20% at lactic acid concentrations of 90 or 280 mM, respectively (Table 1).
TABLE 1.
Fermentative capacity before and after carbon or nitrogen starvationd with performance of a statistical analysis using Student's two-sample t test (P = 0.01)e
| Growth condition (pH) | Acid present | Fermentative capacity (mmol/g/h)
|
||
|---|---|---|---|---|
| Unstarved | C starved | N starved | ||
| Carbon limited (5) | 0a | 12 ± 0.2(A)(D) | 9.8 ± 0.5(B)(D) | 6.4 ± 0.7(C) |
| L90b | 13 ± 2.6(A)(D) | 9.4 ± 0.2(B)(D) | 5.0 ± 0.6(C) | |
| L280b | 9.9 ± 0.4(A)(D) | 7.3 ± 0.4(B)(D) | 4.8 ± 0.4(C) | |
| B5c | 14 ± 0.4(A)(D) | 5.3 ± 0.5(B)(D) | 7.2 ± 0.4(C) | |
| Carbon limited (3.25) | 0a | 8.6 ± 1.8(A)(E) | 8.1 ± 0(B)(E) | 5.8 ± 0(C) |
| L90b | 8.9 ± 1.6(A)(E) | 1.5 ± 0.5(B)(E) | 3.7 ± 0(C) | |
| L280b | 9.0 ± 0.2(A)(E) | 1.9 ± 0(B)(E) | 4.6 ± 0.2(C) | |
| Nitrogen limited (5) | 0a | 8.0 ± 0.2(A)(F) | 0.3 ± 0.1 | 3.2 ± 0.2(C) |
| L90b | 11 ± 1.1(A)(F) | 0.1 ± 0.1 | 5.8 ± 0.6(C) | |
| L280b | 10 ± 4.3(A)(F) | 0.0 ± 0.0 | 5.6 ± 0.2(C) | |
| B5c | 13 ± 0.4(A)(F) | 1.4 ± 0.2 | 5.8 ± 0(C) | |
| Nitrogen limited (3.25) | 0a | 9.1 ± 0.2(A)(G) | 0.3 ± 0 | 6.4 ± 0.2(C)(G) |
| L90b | 13 ± 0.9(A)(G) | 0.4 ± 0.2 | 7.6 ± 0.2(C)(G) | |
| L280b | 7.1 ± 0.2(A)(G) | 0.3 ± 0 | 4.7 ± 0.2(C)(G) | |
No weak acid present.
Lactic acid present at a concentration of 90 or 280 mM.
Benzoic acid present at a concentration of 5 mM.
Standard deviations were calculated from two separate starvation cultures.
Student's two-sample t test (P > 0.01; df = 4, 5, or 6) was used for a statistical analysis of the data. The influence of growth condition on fermentative capacity of unstarved, carbon-starved, or nitrogen-starved cells was analyzed. The respective group of values obtained for cells grown under carbon limitation at pH 5 irrespective of weak acid addition that were unstarved (A), carbon-starved (B), or nitrogen-starved (C) cells was used as a reference group when data groups from other growth conditions in the same column were compared. The influence of carbon or nitrogen starvation on fermentative capacity of cells grown at the same growth conditions was also analyzed. The respective group of data obtained for unstarved cells under the four growth conditions—carbon limitation at pH 5 (D), carbon limitation at pH 3.25 (E), nitrogen limitation at pH 5 (F), or nitrogen limitation at pH 3.25 (G), irrespective of weak acid concentration—was used as the reference group. The reference group was compared with each of the two groups of data for carbon- or nitrogen-starved cells grown under the same growth conditions in the same row.
Fermentative capacity of nitrogen-limited cells.
The fermentative capacity of unstarved cells grown under nitrogen-limiting conditions at either pH 5 or 3.25 was similar to that of cells grown under carbon limitation irrespective of acid concentration (Table 1). Nitrogen-limited cells were much more sensitive to carbon starvation than were cells grown under carbon limitation. The fermentative capacity after carbon starvation was close to zero for all nitrogen-limited cells except for cells cultured in the presence of benzoic acid, where a fermentative capacity of 1.4 mmol/g/h was recorded after carbon starvation (Table 1). Nitrogen starvation resulted in similar fermentative capacities for both nitrogen- and carbon-limited cells (Table 1).
Glucose uptake capacity of starved cells.
In general, cells grown at pH 5, irrespective of carbon or nitrogen limitation during growth, had similar glucose uptake capacities after starvation when carbon- and nitrogen-starved cells were compared (Table 2). At pH 3.25, the presence of lactic acid during growth under carbon- and nitrogen-limiting conditions reduced glucose uptake capacity after carbon starvation. However, most nitrogen-limited cells subjected to carbon starvation (resulting in a fermentative capacity close to zero) retained substantial glucose uptake capacity (Table 2).
TABLE 2.
Glucose uptake capacity at a glucose concentration of 50 mM (9 g/liter) after carbon or nitrogen starvation of cells grown in a chemostat,e with a statistical analysis using Student's two-sample t test performedf
| Growth condition (pH) | Acid present | Glucose uptake capacity (μmol/g protein/min)
|
|
|---|---|---|---|
| C starved | N starved | ||
| Carbon limited (5) | 0a | NDd | NDd |
| L90b | 180 ± 4 | 200 ± 3 | |
| L280b | 120 ± 20 | 99 ± 12 | |
| B5c | 140 ± 4 | 98 ± 28 | |
| Carbon limited (3.25) | 0a | 96 ± 31 | 130 ± 0 |
| L90b | 20 ± 2 | 95 ± 28 | |
| L280b | 25 ± 2 | 92 ± 15 | |
| Nitrogen limited (5) | 0a | 120 ± 46 | 150 ± 58 |
| L90b | 290 ± 112 | 200 ± 13 | |
| L280b | 99 ± 64 | 150 ± 3 | |
| B5c | 340 ± 60 | 210 ± 71 | |
| Nitrogen limited (3.25) | 0a | 170 ± 92 | 190 ± 12 |
| L90b | 77 ± 4 | 170 ± 7 | |
| L280b | 18 ± 6 | 88 ± 8 | |
No weak acid present.
Lactic acid present at a concentration of 90 or 280 mM.
Benzoic acid present at a concentration of 5 mM.
ND, not determined.
Standard deviations were calculated from two separate starvation conditions.
Student's two-sample t test (P > 0.01; df = 4 or 5) was used for a statistical analysis of the data. The group of values obtained from cells grown under carbon limitation at pH 5 and starved for carbon or nitrogen, respectively, was used as a reference when comparing with similarly starved cells grown at carbon limitation at pH 3.25, nitrogen limitation at pH 5, or at nitrogen limitation at pH 3.25, respectively. The P value was never < 0.01, indicating that there were no statistically significant differences between the groups. A comparison between carbon- and nitrogen-starved cells grown under the same growth conditions was also performed and always resulted in a P value of >0.01.
Storage carbohydrates before and after starvation.
Under all growth conditions tested, the trehalose content of unstarved cells was below the detection level. Glycogen accumulations were dependent upon growth conditions (Table 3). Carbon limitation generally resulted in the accumulation of more glycogen than did nitrogen limitation. Decreasing the pH also reduced the amount of glycogen accumulated (Table 3). Addition of lactic acid decreased glycogen accumulation in a concentration-dependent manner. The presence of benzoic acid also reduced glycogen accumulation (Table 3). The glycogen levels before starvation were 1.6 to 13.1% during growth under carbon limitation and 0.5 to 3.6% during growth under nitrogen limitation. A statistical analysis (Student's t test) indicated that the higher level for carbon-limited cells was statistically significant (P = 0.007). Most or all of the accumulated glycogen was consumed during the subsequent carbon starvation period. During nitrogen starvation, however, the response was the opposite, i.e., glycogen and trehalose were synthesized. The extent of storage carbohydrate accumulated during nitrogen starvation in carbon-limited cells was inversely correlated with the original amount, i.e., the lower the glycogen content before starvation, the greater the amount of glycogen synthesized during the subsequent starvation period (Table 3).
TABLE 3.
Intracellular levels of glycogen and trehalose in unstarved, carbon-starved, or nitrogen-starved cells,f with a statistical analysis using Student's two-sample t testg
| Growth condition (pH) | Acid present | Glycogen (%)
|
Trehalosed (%) | ||
|---|---|---|---|---|---|
| Unstarved | C starved | N starved | |||
| Carbon limited (5) | 0a | 13 ± 0.2(A)(D) | 1.3 ± 0.3(B) | 21 ± 0.3(C)(D) | 0.6 ± 0.2 |
| L90b | 13 ± 1.3(A)(D) | 1.5 ± 0.3(B) | 19 ± 1.8(C)(D) | 2.8 ± 0.5 | |
| L280b | 8.3 ± 0.2(A)(D) | 1.6 ± 0.0(B) | 16 ± 1.6(C)(D) | 3.2 ± 0.5 | |
| B5c | 1.6 ± 0.1 | 0.1 ± 0.0 | 15 ± 0.8 | 3.5 ± 0.0 | |
| Carbon limited (3.25) | 0a | 8.9 ± 0.1(A)(E) | 1.9 ± 0.0(B)(E) | 17 ± 0.8(C) | 3.2 ± 0.9 |
| L90b | 4.5 ± 0.2(A)(E) | 0.6 ± 0.0(B)(E) | 19 ± 0.1(C) | 4.6 ± 0.9 | |
| L280b | 2.9 ± 0.0(A)(E) | 0.9 ± 0.1(B)(E) | 18 ± 0.0(C) | 4.5 ± 0.9 | |
| Nitrogen limited (5) | 0a | 3.6 ± 0.2(F) | 0.9 ± 0.2(B)(F) | 10 ± 0.2 | 3.9 ± 0.6 |
| L90b | 1.3 ± 0.1(F) | NDe(B)(F) | 8.7 ± 0.0 | 2.8 ± 0.1 | |
| L280b | 1.2 ± 0.2(F) | NDe(B)(F) | 10 ± 1.2 | 6.1 ± 4.3 | |
| B5c | 1.7 ± 0.1 | 0.2 ± 0.0 | 12 ± 0.4 | 3.7 ± 0.0 | |
| Nitrogen limited (3.25) | 0a | 1.8 ± 0.1(G) | NDe(G) | 10 ± 0.6 | 3.5 ± 0.6 |
| L90b | 1.2 ± 0.0(G) | NDe(G) | 7.3 ± 1.7 | 1.7 ± 0.1 | |
| L280b | 0.5 ± 0.3(G) | NDe(G) | 9.6 ± 1.0 | 3.3 ± 0.2 | |
No weak acid present.
Lactic acid present at a concentration of 90 or 280 mM.
Benzoic acid present at a concentration of 5 mM.
The concentration of trehalose was below the estimated limit for determination for unstarved cells and for carbon-starved cells; thus, only data for N-starved cells are shown.
The concentration was below the estimated limit for determination.
Deviations are given as standard deviation calculated from two separate starvation cultures.
Student's two-sample t test (P > 0.01; df = 4) was used for a statistical analysis of the data on glycogen. Data for cells grown in presence of benzoic acid were excluded. The influence of growth conditions on glycogen content of unstarved, carbon-starved, or nitrogen-starved cells was analyzed. The respective group of values obtained from cells grown under carbon limitation at pH 5 irrespective of lactic acid concentration, i.e., unstarved (A), starved for carbon (B), or nitrogen (C), respectively, was used as reference when comparing with data groups from other growth conditions in the same column. The influence of carbon or nitrogen starvation on glycogen content of cells grown at the same growth conditions was also analyzed. The respective group of data obtained for unstarved cells under the four growth conditions—carbon limitation at pH 5 (D), at carbon limitation at pH 3.25 (E), at nitrogen limitation at pH 5 (F), or nitrogen limitation at pH 3.25 (G), irrespective of weak acid concentration—was used as the reference group. The reference group was compared with each of the two groups of data for carbon-or nitrogen-starved cells grown under the same growth conditions in the same row.
Correlation between glycogen content, fermentative capacity, and ATP level.
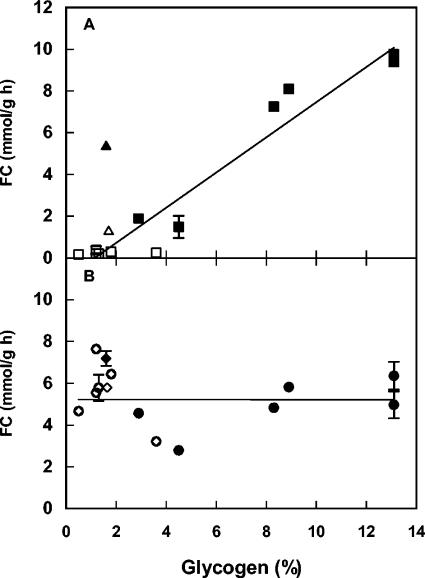
In most cases, there was a positive correlation between the amount of storage carbohydrates before starvation and fermentative capacity after carbon starvation (Fig. 1A). The exception was cells grown in the presence of benzoic acid, which had a much higher fermentative capacity after carbon starvation than predicted from their initial glycogen content (Fig. 1A). Omission of benzoic acid samples and application of a linear regression results in a correlation factor (R2) of 0.94. There was no correlation in nitrogen-starved cells between fermentative capacity and the amount of storage carbohydrates before starvation, i.e., linear regression gave a correlation coefficient (R2) of <0.001 (Fig. 1B).
FIG. 1.
Glycogen content before starvation (percentage of dry weight) versus fermentative capacity (FC, millimoles per gram per hour) after carbon starvation (A) and nitrogen starvation (B). Growth and starvation conditions are indicated by the following symbols: ▪, carbon-limited growth followed by carbon starvation; □, nitrogen-limited growth followed by carbon starvation; •, carbon-limited growth followed by nitrogen starvation; ○, nitrogen-limited growth followed by nitrogen starvation; ▴, carbon-limited growth in the presence of 5 mM benzoic acid followed by carbon starvation; ▵, nitrogen-limited growth in the presence of 5 mM benzoic acid followed by carbon starvation; ⧫, carbon-limited growth in the presence of 5 mM benzoic acid, followed by nitrogen starvation; ◊, nitrogen-limited growth in the presence of 5 mM benzoic acid, followed by nitrogen starvation. Error bars indicate standard deviation when >10% of the value of the point. The error bars for glycogen content indicate standard deviation, calculated from two separately analyzed samples originating from the same fermenter cultivation. The error bars for fermentative capacity indicate standard deviation from two separately starved cultures originating from the same fermenter cultivation. The lines are linear regressions (samples with benzoic acid were omitted) with R2 = 0.94 (A) and R2 = 0.001 (B), respectively.
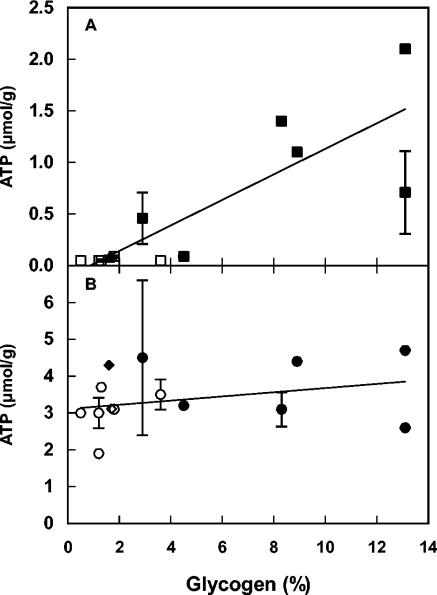
Access to an intracellular energy source, e.g., glycogen, could result in cells that can maintain a higher energy status in the form of ATP throughout the carbon starvation period. There was a correlation (R2 = 0.69) between the amount of glycogen at the onset of carbon starvation and ATP content after the starvation period (Fig. 2A). Carbon-limited cells had ATP levels after carbon starvation of between 0.1 and 2.1 μmol/g (dry weight), while the corresponding values for nitrogen-limited cells were ≤0.1 μmol/g (Fig. 2A). Nitrogen starvation did not result in a drastic or systematic reduction in ATP content, and glycogen accumulation and ATP content after nitrogen starvation were not correlated (R2 = 0.10) (Fig. 2B).
FIG. 2.
Glycogen content (percentage of dry weight) versus ATP level (in micromoles per gram [dry weight]) after carbon starvation (A) and nitrogen starvation (B). Growth and starvation conditions are indicated by the following symbols: ▪, carbon-limited growth followed by carbon starvation; □, nitrogen-limited growth followed by carbon starvation; •, carbon-limited growth followed by nitrogen starvation; ○, nitrogen-limited growth followed by nitrogen starvation; ▴, carbon-limited in the presence of 5 mM benzoic acid followed by carbon starvation; ▵, nitrogen-limited growth in the presence of 5 mM benzoic acid followed by carbon starvation; ⧫, carbon-limited growth in the presence of 5 mM benzoic acid, followed by nitrogen starvation; ◊, nitrogen-limited growth in the presence of 5 mM benzoic acid, followed by nitrogen starvation. Error bars indicate standard deviation when >10% of the value of the point. The error bars for glycogen content indicate standard deviation, calculated from two separately analyzed samples originating from the same fermenter cultivation. The error bars for ATP levels indicate standard deviation from the level measured in two separately starved cultures originating from the same fermenter cultivation. The lines are linear regressions (samples with benzoic acid were omitted) with R2 = 0.69 (A) and R2 = 0.10 (B), respectively.
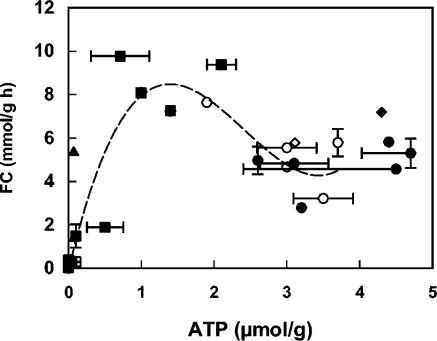
Fermentative capacity following starvation probably reflects the ATP content of the cells (Fig. 3). However, there was no simple positive or negative correlation between the two. Instead, at low ATP concentrations, e.g., during carbon starvation, fermentative capacity was positively correlated with ATP concentrations. At high ATP levels, e.g., during nitrogen starvation, the correlation disappeared, perhaps because the ATP concentration was no longer important as a limiting factor. Carbon-starved cells exposed to benzoic acid contained <0.1 μmol ATP/g (dry weight), yet these cells still retained substantial fermentative capacity (Fig. 3).
FIG. 3.
ATP levels (micromoles per gram[dry weight] of cells) versus fermentative capacity (FC; in millimoles per gram per hour) after carbon and nitrogen starvation. Growth and starvation conditions are indicated by the following symbols: ▪, carbon-limited growth followed by carbon starvation; □, nitrogen-limited growth followed by carbon starvation; •, carbon-limited growth followed by nitrogen starvation; ○, nitrogen-limited growth followed by nitrogen starvation; ▴, carbon-limited growth in the presence of 5 mM benzoic acid followed by carbon starvation; ▵, nitrogen-limited growth in the presence of 5 mM benzoic acid followed by carbon starvation; ⧫, carbon-limited growth in the presence of 5 mM benzoic acid, followed by nitrogen starvation; ◊, nitrogen-limited growth in the presence of 5 mM benzoic acid, followed by nitrogen starvation. Error bars indicate standard deviation when >10% of the mean value of the point. The error bars for the ATP levels indicate standard deviation from the level, measured in two separately starved cultures originating from the same fermenter cultivation. The error bars for fermentative capacity indicate standard deviation from two separately starved cultures originating from the same fermenter cultivation. The trend line shown is a polynomial of the third order in the interval between 0 to 4 μmol/g of ATP. The correlation factor (R2) was 0.75.
Assuming intracellular volumes of 1.6 ± 0.4 and 0.9 ± 0.4 ml/g (dry weight) (estimated at a 95% confidence interval) for aerobically grown carbon- and nitrogen-starved cells, respectively (E. Albers, personal communication), then ATP has an activating effect at an intracellular concentration up to 0.5 to 1.0 mM and an increasingly inhibitory effect up to an intracellular concentration of ∼4 mM.
DISCUSSION
In this study, we showed that the ability to withstand a sudden carbon starvation situation depends on the previous growth conditions. Carbon-limited anaerobic chemostat cultures performed better under this stress than did their nitrogen-limited counterparts. The relatively poor performance of the nitrogen-limited cells during a subsequent carbon starvation period probably is due to the low level of storage carbohydrates accumulated under these growth conditions. In carbon-limited cells, there was a positive correlation between the initial amount of storage carbohydrates before starvation and fermentative capacity after the carbon starvation period (Fig. 1A). No such correlation was observed for cells subjected to nitrogen starvation (Fig. 1B), and nitrogen starvation tolerance was independent of the previous growth conditions.
The almost complete lack of fermentative capacity in response to externally added glucose by nitrogen-limited cells subjected to carbon starvation cannot be explained simply by reductions in glucose uptake capacity. Glucose uptake capacity after starvation was very similar for both carbon and nitrogen starvation at each respective previous growth condition, except for growth at a pH of 3.25 with high concentrations of lactic acid. Under the latter conditions, both carbon- and nitrogen- limited cells had less glucose uptake capacity following carbon starvation than they did following nitrogen starvation. However, glucose uptake capacity remained in these cells. Thus, lack of glucose uptake capacity does not explain the effect of carbon starvation on cells previously grown under nitrogen limitation.
One hypothesis to explain the inability of anaerobic cells to cope with a sudden carbon starvation could be a rapid depletion of ATP (29). For example, cells growing exponentially in anaerobic batch cultures subjected to carbon starvation were rapidly depleted of their ATP and also lost fermentative capacity (29). Consistent with these earlier results, we found that a high-glycogen content preserved fermentative capacity during carbon starvation and that it also prevented the cells from becoming ATP depleted. Thus, successful adaptation to starvation requires energy; in the absence of an extracellular energy source(s), endogenous energy reserves are required to provide the cells with energy in the form of ATP. Even though the cells were supplied with an external energy source, e.g., glucose (during the fermentation capacity test), after carbon starvation they could not catabolize it. We interpret these results to mean that a minimum amount of ATP is needed internally to initiate glucose degradation. This general effect was demonstrated previously by testing the effect of different ATP concentrations on the glycolytic flux of permeabilized cells of S. cerevisiae (13). The intracellular ATP is probably required for the phosphorylation of glucose and fructose-6-phosphate by hexokinase and phosphofructokinase, respectively, both of which act in glycolysis before ATP production begins (13).
The starvation tolerance/response for anaerobic conditions is different from that for cells growing under aerobic conditions. In aerobically grown cells, nitrogen starvation usually provokes a more drastic reduction in catabolic capacity than does carbon starvation (9, 12, 21). In addition, a high level of storage carbohydrates is not required to preserve fermentative capacity during carbon starvation under aerobic conditions (21). These differences can, at least in part, be explained by differences in the range of substrates that can be utilized for energy generation under aerobic and anaerobic conditions. Aerobically, lipids, proteins, amino acids, etc., are all potential substrates, but anaerobically, energy generation is limited to the fermentation of carbohydrates. Access to energy will be important for successful adaptation to starvation conditions since this adaptation requires protein synthesis, the most energy-expensive process in the cell.
Cells grown in the presence of benzoic acid are more tolerant of carbon starvation than expected, given the relatively low-storage-carbohydrate content of the cells prior to starvation (Fig. 1A). Thus, exposure to one kind of stress, e.g., a weak acid stress, may induce increased tolerance to another type of stress, e.g., starvation. The fact that one kind of mild stress might improve the ability to withstand a different more severe type of stress is well known (7). To conclude, successful adaptation to sudden carbon starvation requires access to intracellular energy in the form of ATP; under anaerobic conditions, this requires fermentable endogenous energy resources such as glycogen. The practical implications of these results are that aeration during propagation is not only advantageous for obtaining a high cell yield but also for making the cells more tolerant of carbon starvation. As large, nonaerated fermenters are often not homogenous, it is crucially important to avoid zones of carbon source depletion in which cell performance will be reduced.
Acknowledgments
This work was financially supported by the Swedish Research Council (dnr 285-2000-656).
REFERENCES
- 1.Attfield, P. V. 1997. Stress tolerance: the key to effective strains of industrial baker's yeast. Nat. Biotechnol. 15:1351-1357. [DOI] [PubMed] [Google Scholar]
- 2.Bisson, L. F. 1999. Stuck and sluggish fermentations. Am. J. Enol. Vitic. 50:107-119. [Google Scholar]
- 3.Blomberg, A., C. Larsson, and L. Gustafsson. 1988. Microcalorimetric monitoring of growth of Saccharomyces cerevisiae: osmotolerance in relation to physiological state. J. Bacteriol. 170:4562-4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busturia, A., and R. Lagunas. 1986. Catabolite inactivation of the glucose transport system in Saccharomyces cerevisiae. J. Gen. Microbiol. 132:379-385. [DOI] [PubMed] [Google Scholar]
- 5.de Winde, J. H., J. M. Thevelein, and J. Winderickx. 1997. From feast to famine: adaption to nutrient depletion, p. 7-52. In S. Hohmann and W. H. Mager (ed.), Yeast stress responses. R. G. Landes Company, Austin, Tex.
- 6.Gustafsson, L. 1979. The ATP pool in relation to the production of glycerol and heat during growth of the halotolerant yeast Debaromyces hansenii. Arch. Microbiol. 120:15-23. [Google Scholar]
- 7.Hohmann, S. 2002. Osmotic stress signaling and osmoadaptation in yeast. Microbiol. Mol. Biol. Rev. 66:300-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jona, G., M. Choder, and O. Gileadi. 2000. Glucose starvation induces a drastic reduction in the rates of both transcription and degradation of mRNA in yeast. Biochim. Biophys. Acta 1491:37-48. [DOI] [PubMed] [Google Scholar]
- 9.Jorgensen, H., L. Olsson, B. Ronnow, and E. Palmqvist. 2002. Fed-batch cultivation of baker's yeast followed by nitrogen or carbon starvation: effects on fermentative capacity and content of trehalose and glycogen. Appl. Microbiol. Biotechnol. 59:310-317. [DOI] [PubMed] [Google Scholar]
- 10.Kim, J., P. Alizadeh, T. Harding, A. Hefner-Gravink, and D. J. Klionsky. 1996. Disruption of the yeast ATH1 gene confers better survival after dehydration, freezing, and ethanol shock: potential commercial applications. Appl. Environ. Microbiol. 62:1563-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsson, C., A. Nilsson, A. Blomberg, and L. Gustafsson. 1997. Glycolytic flux is conditionally correlated with ATP concentration in Saccharomyces cerevisiae: a chemostat study under carbon- or nitrogen-limiting conditions. J. Bacteriol. 179:7243-7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsson, C., A. Nilsson, and L. Gustafsson. 1994. Catabolic capacity of carbon- or nitrogen-starved cultures of Saccharomyces cerevisiae, p. 232-237. In E. Gnaiger, F. N. Gellerich, and M. Wyss (ed.), What is controlling life?, vol. 3. Innsbruck University Press, Innsbruck, Austria. [Google Scholar]
- 13.Larsson, C., I.-L. Påhlman, and L. Gustafsson. 2000. The importance of ATP as a regulator of glycolytic flux in Saccharomyces cerevisiae. Yeast 16:797-809. [DOI] [PubMed] [Google Scholar]
- 14.Lillie, S., and J. R. Pringle. 1980. Reserve carbohydrate metabolism in Saccharomyces cerevisiae: responses to nutrient limitation. J. Bacteriol. 143:1384-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 16.Lyons, T. P. 2003. Ethanol around the world: rapid growth in policies, technology and production, p. 1-9. .In K. A. Jaques, T. P. Lyons, and D. R. Kelsall (ed.), The alcohol textbook, 4th ed. Nottingham University Press, Nottingham, United Kingdom.
- 17.Martinez-Pastor, M. T., and F. Estruch. 1996. Sudden depletion of carbon source blocks translation, but not transcription, in the yeast Saccharomyces cerevisiae. FEBS Lett. 390:319-322. [DOI] [PubMed] [Google Scholar]
- 18.Narendranath, N. V. 2003. Bacterial contamination and control in ethanol production, p. 287-298. In K. A. Jaques, T. P. Lyons, and D. R. Kelsall (ed.), The alcohol textbook, 4th ed. Nottingham University Press, Nottingham, United Kingdom.
- 19.Narendranath, N. V., K. C. Thomas, and W. M. Ingledew. 2001. Effects of acetic and lactic acid on the growth of Saccharomyces cerevisiae in a minimal medium. J. Ind. Microbiol. Biotechnol. 26:171-177. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson, A., Christer Larsson, and Lena Gustafsson. 1995. Catabolic capacity of Saccharomyces cerevisiae in relation to the physiological state and maintenance requirement. Thermochim. Acta 250:233-245. [Google Scholar]
- 21.Nilsson, A., I.-L. Påhlman, P. A. Jovall, A. Blomberg, C. Larsson, and L. Gustafsson. 2001. The catabolic capacity is preserved to a higher extent during carbon compared to nitrogen starvation. Yeast 18:1371-1381. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson, A., J. Norbeck, R. Oelz, A. Blomberg, and L. Gustafsson. 2001. Fermentative capacity after cold storage of baker's yeast is dependent on the initial physiological state but not correlated to the levels of glycolytic enzymes. Int. J. Food Microbiol. 71:111-124. [DOI] [PubMed] [Google Scholar]
- 23.Plourde-Owobi, L., S. Durner, G. Goma, and J. Francois. 2000. Trehalose reserve in Saccharomyces cerevisiae: phenomenon of transport, accumulation and role in cell viability. Int. J. Food Microbiol. 55:33-40. [DOI] [PubMed] [Google Scholar]
- 24.Russel, I. 2003. Understanding yeast fundamentals, p. 85-119. In K. A. Jaques, T. P. Lyons, and D. R. Kelsall (ed.), The alcohol textbook, 4th ed. Nottingham University Press, Nottingham, United Kingdom.
- 25.Schulze, U., G. Liden, J. Nielsen, and J. Villadsen. 1996. Physiological effects of nitrogen starvation in an anaerobic batch culture of Saccharomyces cerevisiae. Microbiology 142:2299-2310. [DOI] [PubMed] [Google Scholar]
- 26.Silljé, H. H. W., J. W. G. Paalman, E. G. ter Schure, S. Q. B. Olsthoorn, A. J. Verkleij, J. Boonstra, and C. T. Verrips. 1999. Function of trehalose and glycogen in cell cycle progression and cell viability in Saccharomyces cerevisiae. J. Bacteriol. 181:396-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singer, M. A., and Susan Lindquist. 1998. Thermotolerance in Saccharomyces cerevisiae: the yin and yang of trehalose. Trends Biotechnol. 16:460-468. [DOI] [PubMed] [Google Scholar]
- 28.Taherzadeh, M. J., R. Eklund, L. Gustafsson, C. Niklasson, and G. Lidén. 1997. Characterization and fermentation of dilute-acid hydrolyzates from wood. Ind. Eng. Chem. Res. 36:4659-4665. [Google Scholar]
- 29.Thomsson, E., C. Larsson, E. Albers, A. Nilsson, C. J. Franzén, and L. Gustafsson. 2003. Carbon starvation can induce energy deprivation and loss of fermentative capacity in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 69:3251-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verduyn, C., E. Postma, W. A. Scheffers, and J. P. Van Dijken. 1992. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 8:501-517. [DOI] [PubMed] [Google Scholar]
- 31.Walsh, M. C., H. Smits, M. Scholte, and K. van Dam. 1994. Affinity of glucose transport in Saccharomyces cerevisiae is modulated during growth on glucose. J. Bacteriol. 176:953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werner-Washburne, M., E. Braun, G. Johnston, and R. Singer. 1993. Stationary phase in Saccharomyces cerevisiae. Microbiol. Rev. 57:384-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiemken, A. 1990. Trehalose in yeast, stress protectant rather than reserve carbohydrate. Antonie Leeuwenhoek 58:209-217. [DOI] [PubMed] [Google Scholar]