Abstract
Chlorine dioxide (ClO2) inactivation experiments were conducted with adenovirus type 40 (AD40) and feline calicivirus (FCV). Experiments were carried out in buffered, disinfectant demand-free water under high- and low-pH and -temperature conditions. Ct values (the concentration of ClO2 multiplied by contact time with the virus) were calculated directly from bench-scale experiments and from application of the efficiency factor Hom (EFH) model. AD40 Ct ranges for 4-log inactivation (Ct99.99%) at 5°C were >0.77 to <1.53 mg/liter × min and >0.80 to <1.59 mg/liter × min for pH 6 and 8, respectively. For 15°C AD40 experiments, >0.49 to <0.74 mg/liter × min and <0.12 mg/liter × min Ct99.99% ranges were observed for pH 6 and 8, respectively. FCV Ct99.99% ranges for 5°C experiments were >20.20 to <30.30 mg/liter × min and >0.68 mg/liter × min for pH 6 and 8, respectively. For 15°C FCV experiments, Ct99.99% ranges were >4.20 to <6.72 and <0.18 mg/liter × min for pH 6 and 8, respectively. Viral inactivation was higher at pH 8 than at pH 6 and at 15°C than at 5°C. Comparison of Ct values and inactivation curves demonstrated that the EFH model described bench-scale experiment data very well. Observed bench-scale Ct99.99% ranges and EFH model Ct99.99% values demonstrated that FCV is more resistant to ClO2 than AD40 for the conditions studied. U.S. Environmental Protection Agency guidance manual Ct99.99% values are higher than Ct99.99% values calculated from bench-scale experiments and from EFH model application.
According to the U.S. Environmental Protection Agency (EPA) National Primary Drinking Water Standards, enteric viruses must be removed or inactivated by 4 logs (99.99%) from source water by filtration and disinfection or by a combination of these technologies (48). Viral pathogens can bypass conventional filtration processes due to their small size, making disinfection an important treatment barrier between drinking water consumers and viral gastroenteritis. While chlorine is the most common disinfectant in the United States for drinking water and wastewater treatment, alternative disinfectants are needed to reduce highly chlorine resistant pathogens, such as Cryptosporidium parvum. UV light disinfection is effective at reducing Cryptosporidium oocysts (6, 34), and it is therefore an attractive alternative disinfectant to chlorine for drinking water treatment. Recent evidence, however, has shown that UV light is ineffective at reducing enteric adenovirus at doses commonly applied by water treatment systems (45). Furthermore, UV light water disinfection leaves no residual for protection of potable water after it leaves the treatment plant. To effectively reduce viruses by 99.99% and to maintain a disinfectant residual in the distribution system, a secondary disinfectant is needed.
ClO2 is an alternative to chlorine as a primary disinfectant or can serve as a secondary disinfectant for UV treatment systems. Advantages of ClO2 disinfection include (i) oxidation of iron and manganese (reduces discoloration of finished water), (ii) no trihalomethane formation, (iii) no reaction with ammonia, (iv) less affected by the pH conditions typical of drinking water than is chlorine, (v) a relatively persistent residual, and (vi) reduction of tastes and odors caused by organic and sulfurous compounds (1, 5). Disadvantages of ClO2 disinfection include (i) formation of organic halides, (ii) formation of chlorite and chlorate, and (iii) production of taste and odors at concentrations of >0.5 mg/liter (5). However, this strong oxidant is a useful and attractive alternative to chlorine for the advantages listed above and its reported increased ability to reduce pathogenic microorganisms in water. Previous studies have reported that ClO2 effectively inactivates several viruses in water and sewage (14, 21, 39, 43), but limited information is available concerning the reduction of caliciviruses and adenoviruses in drinking water.
The EPA, mandated by the Safe Drinking Water Act, published the Drinking Water Contaminant Candidate List (CCL) in 1998 (12). This list includes chemical and microbial contaminants that are known or anticipated to occur in public water systems. These contaminants are under regulatory consideration, since little to no information regarding health, drinking, wastewater treatment, or analytical methodology is currently available. Enteric viruses and caliciviruses are included in the CCL and were investigated in this study.
Members of the human calicivirus genus, noroviruses (NVs), are a principal cause of nonbacterial acute gastroenteritis (11, 28) and have been identified as etiological agents of waterborne outbreaks (20, 29, 31, 32). Caliciviruses range in diameter from 27 to 40 nm and have a single-stranded RNA genome and an icosahedral capsid structure. Commonly reported symptoms include diarrhea and vomiting. Previous outbreaks caused by NV-contaminated ice and cooked shellfish have suggested that these viruses are capable of withstanding harsh environmental conditions (16). Their ability to withstand current drinking water disinfection practices is largely unknown, since there are no known animal or mammalian cell culture systems that determine NV infectivity. Due to these difficulties, two alternative studies, a human feeding study and a PCR-based study, were carried out previously (30, 41). However, conflicting results between these studies made conclusions regarding NV chlorine resistance difficult. More recently, an NV surrogate, feline calicivirus (FCV), has been used as a surrogate for NV inactivation in several disinfection studies (9, 36, 42, 44, 45). Since FCV has genome organization (7, 26) and capsid architecture (38) similar to those of NVs and can be easily grown in cell culture, it is an appropriate surrogate for NV. Chlorine inactivation experiments carried out with FCV resulted in conclusions similar to those reported in the NV PCR-based study. Results from these two studies suggest that chlorine is effective at reducing these caliciviruses by 4 logs at commonly applied chlorine concentrations (44).
Like NVs, the enteric adenoviruses, enteric adenovirus 40 (AD40) and AD41, are also important causes of self-limiting, acute gastroenteritis, especially in children <4 years of age (23). Ranging from 70 to 90 nm in size, these viruses are considerably larger than noroviruses, and their capsid structure is complex. The adenovirus icosahedron contains 240 hexons, 12 pentons, and 12 fibers that extend from each penton base; its genome consists of linear, double-stranded DNA. Enteric adenoviruses are shed in high numbers in the feces (2), are typically shed in the feces for long periods, and infection can be caused by low numbers of viral particles (16, 23). Enteric adenoviruses have greater environmental stability than other enteric viruses (10), so their presence in sewage and surface water makes them likely contaminants in public water supplies (24, 25). Moreover, enteric adenoviruses and noroviruses were identified as two of the etiological agents causing acute gastroenteritis in a waterborne outbreak in Finland (31), and waterborne outbreaks of pharyngoconjunctivitis from swimming have been reported for nonenteric adenoviruses (13, 37). Enteric adenoviruses are susceptible to chlorine (44) but are very resistant to UV light (45).
Based on previous viral disinfection studies, the EPA published the Guidance Manual for Compliance with the Filtration and Disinfection Requirements for Public Water Sources (46). Ct values, which are the disinfectant concentration (C) multiplied by the contact time (t) between the disinfectant and microorganism, for 2- to 4-log viral inactivation by ClO2 and other water disinfectants at different pH and temperature conditions are listed in the manual. Ct values for viral inactivation are based on experiments conducted with hepatitis A virus (HAV). The guidance manual's Ct values (in milligrams per liter, multiplied by the number of minutes) direct public water utilities to ensure that disinfection practices meet regulatory microbial log inactivation requirements. However, ClO2 Ct values may not be adequate for caliciviruses and adenoviruses whose susceptibility to this disinfectant is largely unknown.
The objectives of this study were to (i) compare viral inactivation by ClO2 for AD40 and FCV in water under high- and low-pH (pH 8 and 6) and -temperature (15°C and 5°C) conditions, (ii) use a previously described disinfection model to determine Ct values for each virus and experimental condition, and (iii) compare predicted Ct values to the EPA guidance manual Ct values and disinfection practices commonly applied in the United States.
MATERIALS AND METHODS
Virus propagation and assay.
AD40 (strain Dugan), FCV (strain F9), primary liver carcinoma cell line (PLC/PRF/5), and Crandell Reese feline kidney cell lines were obtained from the American Type Culture Collection (Rockville, MD). AD40 and FCV stocks were propagated, enumerated, concentrated, and purified to reduce disinfection demand in the same manner described by Thurston-Enriquez et al. (45). All viral stocks were stored at 4°C until use. Determination of viral titer before and after chlorine disinfection was accomplished by assaying 5- or 10-fold dilutions in quadruplicate in 24-well tissue culture trays with the appropriate cells in suspension (44).
ClO2 production and measurement.
ClO2 was generated using the iodometric method (4). ClO2 concentrations of the stock solution and in buffered, disinfectant demand-free (BDF) water throughout disinfection experiments were measured according to Hach (Loveland, CO) DPD method 10126 with a Hach DR2000 spectrophotometer.
Experimental protocol.
Glassware and BDF water were prepared. The experimental protocol was carried out according to protocols used by Thurston-Enriquez et al. (45). Briefly, BDF water was kept at a constant temperature (5°C or 15°C) in a refrigerated water bath. ClO2 was added at a volume necessary to achieve an initial disinfectant dose close to 0.50 mg/liter or 1.0 mg/liter. Chlorine dioxide doses applied in this study's disinfection experiments ranged from 0.47 to 1.01 (Table 1). Four experimental reaction beakers were analyzed for every experimental condition. The first beaker, containing only BDF water and ClO2, was measured at 15 s to determine the initial disinfectant dose (at 15 s) in the absence of disinfectant demand from the viral stock. The second and third reaction beakers were inoculated with one of the studied viruses, FCV or AD40, at concentrations that would allow detection of at least 2 logs of viral inactivation. The second and third beakers were also inoculated with ClO2 and immediately stirred. The second beaker was sampled to determine disinfectant concentration at the beginning (15 s) and end of each disinfection experiment. These measurements were necessary to determine disinfectant demand of each viral preparation and disinfectant decay during each experiment. To determine viral inactivation, 2-ml samples were taken from the third beaker at predetermined times throughout the reaction. These 2-ml samples were immediately inoculated into collection tubes containing 20 μl of sterile 10% sodium thiosulfate solution to quench any residual disinfectant activity. The fourth reaction beaker, or control beaker, contained only virus and BDF water and was considered to be representative of viral concentrations per milliliter of BDF water in beakers two and three. This control beaker was necessary to (i) determine the initial virus concentration for every experiment and (ii) evaluate whether virus inactivation occurred under the tested BDF water, pH, and temperature conditions (in the absence of disinfectants). Viral samples were kept on ice during the experiment and then stored at 4°C until assay.
TABLE 1.
Summary of BDF water conditions and EFH model coefficients for AD40 and FCV chorine dioxide disinfection experiments
| Virus | BDF water conditions
|
No. of replicates | k′ (min−1) | ka | na | ma | ||
|---|---|---|---|---|---|---|---|---|
| ClO2b (mg/liter) | °C | pH | ||||||
| AD40 | 0.51 | 5 | 6 | 2 | 0.03 | 8.32 | 0.62 | 0.57 |
| AD40 | 0.53 | 5 | 8 | 2 | 0.04 | 362.0 | 6.01 | 0.60 |
| AD40 | 0.49 | 15 | 6 | 2 | 0.10 | 5.61 | 0.01 | 0.80 |
| AD40c | 0.47 | 15 | 8 | 2 | 0.14 | 44.87 | 0.01 | 1.10 |
| FCV | 1.01 | 5 | 6 | 5 | 0.03 | 1.59 | 0.01 | 0.52 |
| FCV | 0.90 | 5 | 8 | 4 | 0.03 | 8.58 | 0.01 | 0.40 |
| FCV | 0.84 | 15 | 6 | 4 | 0.05 | 2.20 | 0.01 | 0.67 |
| FCVd | 0.72 | 15 | 8 | 3 | 0.07 | 167.01 | 0.01 | 2.17 |
EFH model parameters k, n, and m are dimensionless.
Average ClO2 concentration applied in replicate experiments.
Virus not detected by cell culture assays after 15 s of contact time and ≥4.21-log inactivation.
Virus not detected by cell culture assays after 15 s of contact time and ≥4.15-log inactivation.
Kinetic modeling and Ct values.
Chlorine decay constants (k′) for each experiment were calculated using the Solver function in Microsoft Excel 2000 (Microsoft Corp.) to regress the first-order kinetic equation (equation 1) using the least-squares method.
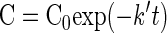 |
(1) |
where C and C0 are the ClO2 residual (in milligrams per liter) at time t (in minutes) and time 0.25 min (the closest possible measurement to time zero), respectively; k′ is the first-order disinfectant decay rate constant (per minute) (19). Disinfectant decay (k′) values are listed in Table 1 for each set of disinfection experiments.
Efficiency factor Hom (EFH) model parameters (Table 1) and Ct values (Table 2) were calculated by applying the EFH model (equation 2) to data obtained from bench-scale disinfection experiments (19). The EFH model is an analytical approximation of the incomplete gamma Hom (IGH) model. These models are considered to adequately describe the kinetics of disinfection experiments that do not follow Chick-Watson relationships and are subject to disinfectant decay (19). Unlike the IGH model, the EFH model enables researchers to describe disinfection kinetics by using mathematical functions available in commonly used computer packages such as Microsoft Excel (Microsoft Corp.) (19). IGH and EFH models have been employed to describe disinfection kinetics in previous studies (17, 18, 33) and have been used to predict enteric adenovirus type 40 and feline calicivirus inactivation by chlorine (44).
TABLE 2.
AD40 and FCV Ct99.99% ranges observed from bench-scale inactivation experiments, Ct99.99% values calculated by fitting the EFH model to bench-scale data, and EPA guidance manual Ct99.99% values
| BDF water conditions
|
Ct99.99% (mg/liter × min)
|
|||||
|---|---|---|---|---|---|---|
| °C | pH | AD40 observed ranges | AD40 EFH modela | FCV observed ranges | FCV EFH modela | EPA guidance manual |
| 5 | 6 | >0.77 to <1.53 | 1.28 | >20.20 to <30.30 | 20.85 | 33.5 |
| 5 | 8 | >0.80 to <1.59 | 0.67 | >0.68 (3.60 log)b | 1.08 | 33.5 |
| 15 | 6 | >0.49 to <0.74 | 0.92 | >4.20 to <6.72 | 7.13 | 16.8 |
| 15 | 8 | <0.12c | 0.11 | <0.18d | 0.19 | 16.8 |
Ct values calculated using k′ = 0.0001 (conditions of negligible disinfectant decay).
Duration of experiment was not long enough to achieve 99.99% inactivation. Ct value corresponds to 3.60-log inactivation by 45 s.
Value is ≥4.21-log inactivation by 15 s.
Value is ≥4.15-log inactivation by 15 s.
Viral most-probable-number values for each experiment, grouped by virus type, pH, and temperature conditions, were fit into the EFH model (equation 2)
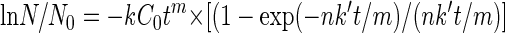 |
(2) |
where t is exposure time (min), k is the viral inactivation rate constant (dimensionless), n is the coefficient of dilution (dimensionless), k′ is the first-order disinfectant decay rate constant (per minute), and m is the constant for the inactivation rate law which describes deviation from ideal Chick-Watson kinetics (dimensionless) (13). ln N/N0 is the natural log of the survival ratio (the number of viruses remaining at time t divided by the initial viral concentration). Microsoft Excel Solver (Microsoft Excel 2000; Microsoft Corp.) was used to minimize the sum of squares of the difference between the observed and ln N/N0 value for viral disinfection experiments performed with the same virus and conditions, to determine EFH model coefficients for each viral and set of conditions (Table 1). Using GraphPad Prism version 4.00 for Windows (San Diego, CA), average viral concentrations versus time were charted for observed viral inactivation and for values fitted by the EFH model (Fig. 1 and 2).
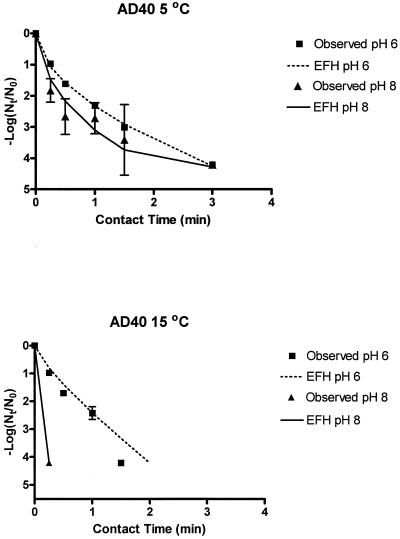
FIG. 1.
Observed and EFH model AD40 ClO2 inactivation curves (ClO2 doses ranged from 0.47 to 0.53 mg/liter in buffered, demand-free water).
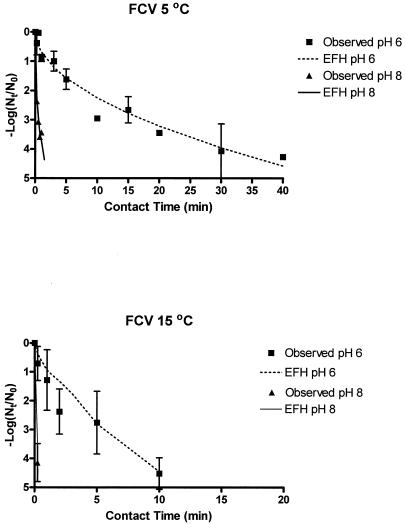
FIG. 2.
Observed and EFH model FCV ClO2 inactivation curves (ClO2 doses ranged from 0.72 to 1.01 mg/liter in buffered, demand-free water).
A Ct value is determined by multiplying the disinfectant concentration (C) in milligrams per liter by the time (t) in minutes when a specific log inactivation (2, 3, or 4 log; 99%, 99.9%, or 99.99%) occurred. Ct values (Ct99.0%, Ct99.9%, and Ct99.99%) were used to assess viral sensitivity to ClO2 and evaluate the ability of the EFH model to fit data obtained from bench-scale viral inactivation experiments (Table 2). EFH model Ct99.99% values were determined through application of EFH model parameters (Table 1). A value of 0.0001 for k′ (conditions of negligible disinfectant decay) was used for EFH Ct values. This value was chosen to produce baseline Ct values and because k′ varied between experiments. The average ClO2 dose applied in replicate experiments was also used to determine EFH model Ct values. Ct value ranges were calculated by multiplying the average ClO2 concentration applied for each set of replicate experiments by the closest time points to 4-log viral inactivation. For example, AD40 experiments conducted at pH 8 and 5°C had an average log inactivation of 3.41 and 4.21 at 1.5 min and 3 min, respectively. The average ClO2 dose for this set of experiments was 0.53 mg/liter. The range, therefore, is >0.80 to <1.59 mg/liter × min.
Statistical analysis.
Average microbial concentrations and standard deviations were calculated and graphed using GraphPad Prism, version 4.00, for Windows (San Diego, CA). F tests were carried out using Microsoft Excel 2000 (Microsoft Corp.) to determine whether differences in viral inactivation were significant (P < 0.05) between different viruses and pH and temperature conditions.
RESULTS
ClO2 disinfection experiments were carried out in at least in duplicate for AD40 and FCV under high- and low-pH and -temperature conditions in BDF water. The EFH model was used to model disinfectant inactivation kinetics for every experimental condition applied to each virus. Table 1 lists BDF water conditions applied to viral disinfection experiments and parameter estimates for EFH model analysis.
Table 2 compares Ct99.99% ranges observed from bench-scale inactivation experiments, Ct99.99% values calculated by fitting the EFH model to bench-scale data, and EPA guidance manual Ct99.99% values. The EFH model fit the observed bench-scale data well, producing Ct99.99% values close to or within the range of observed Ct99.99% values. AD40 and FCV EFH Ct99.99% values were lower than Ct99.99% values recommended in the EPA guidance manual for viral inactivation in water.
When observed Ct99.99% ranges and EFH Ct99.99% values were compared, it was noted that FCV is more resistant to ClO2 than FCV for most of the conditions studied. Differences in viral sensitivities under pH 8 and 15°C conditions, however, are unclear, since AD40 and FCV were completely inactivated by the first sample collection taken at 15 s. For all other tested conditions, FCV appears to be much more resistant to ClO2 than AD40.
The Ct99.99% ranges calculated for viral inactivation are difficult to compare when 4-log inactivation was not observed. At pH 8 and 5°C, the FCV Ct99.99% range is >0.68, since only 3.60-log inactivation was observed at the last time point sampled. For this set of experiments, 3.60 log was inactivated within 45 s. For FCV inactivation at pH 6 and 5°C, however, an average of only 0.90 logs was inactivated by 1 min. Thus, FCV appears to be more resistant at pH 6 than at pH 8. This difference is better reflected by comparing EFH model Ct99.99% values where the difference in inactivation rates is obvious between FCV inactivation at pH 6 (20.85 mg/liter × min) and pH 8 (1.08 mg/liter ×min).
Observed bench-scale viral inactivation curves and EFH model inactivation curves for all tested water conditions are shown in Fig. 1 and 2. Data points listed as observed in the charts are average viral concentrations from replicate bench-scale experiments. Fitting bench-scale viral inactivation data into the EFH model generated curves listed as EFH. Significant differences (P < 0.05) in viral inactivation rates for high- and low-temperature and -pH conditions were observed. For AD40 experiments conducted at 5°C, inactivation rates are not significant, starting at the 1-min time point (Fig. 1). However, the rate of AD40 inactivation was higher under pH 8 conditions than under pH 6 conditions for the first 30 s. Tailing of the curves was similar, resulting in insignificant inactivation from the 1-min to the 3-min time point. Viral inactivation rates were higher for experiments carried out at pH 8 than at pH 6 (AD40, 15°C; FCV, 5 and 15°C) and 15°C than at 5°C.
DISCUSSION
To our knowledge, this is the first report of ClO2 inactivation of enteric adenovirus and generation of Ct values for ClO2 inactivation of AD40 and FCV in water at high- and low-pH and -temperature conditions. This information is important not only for evaluation of ClO2 as a secondary disinfectant for water systems employing UV light but also for systems utilizing ClO2 as a primary disinfectant. Ct99.99% values, calculated based on bench-scale experiments and those predicted by the EFH model for AD40 and FCV, suggest that EPA guidance manual Ct values (46) are sufficient for reducing these viruses in treated water under the temperature and pH conditions tested by this study. Considering that the range in ClO2 dosage employed by the United States water industry is 0.07 to 2.0 mg/liter (47) and that the average contact time is 237 min (derived from water treatment plants employing chlorination) (49), the studied viruses would be inactivated by at least 4 logs for the majority of water conditions studied. However, using the average contact time (237 min), the range in Ct values would be from 16.59 to 474 mg/liter × min for water systems in the United States. The Ct99.99% value for FCV in BDF water at pH 6 and 5°C, however, was within this range (Ct99.99% = 20.85 mg/liter × min).
Viral inactivation kinetics varies between different viral types, disinfectants, and water disinfection conditions. EFH model Ct99.99% values for ClO2 inactivation experiments at 5°C were 1.28 and 0.67 mg/liter × min for AD40 and 20.85 and 1.08 mg/liter × min for FCV for BDF water at pH 6 and 8, respectively. Thus, Ct99.99% values for AD40 and FCV were 1.9 and 19.3 times higher at pH 6 than at pH 8, respectively. Previous studies have also shown that the potency of ClO2 is increased at higher pH levels. IT has been reported that poliovirus type 1 is inactivated by ClO2 4.6 times faster at pH 9 than at pH 7 (8). In related studies, poliovirus (3), coliphage f2 (35), and Norwalk (norovirus) virus, poliovirus, and coliphage MS-2 (41) were more rapidly inactivated by ClO2 at pH 10 than at pH 6.
The rate of microbial inactivation generally increases by a factor of 2 or 3 as temperature increases by 10°C (22). Results generated by Cronier et al. (8) demonstrate that poliovirus type 1 was more rapidly inactivated in pH 7 BDF water at 15°C than at 5°C. Inactivation curves illustrated that poliovirus was roughly 3.5 times more resistant to ClO2 at 5°C than at 15°C (8). Similar to AD40 and FCV inactivation by chlorine (44), the disinfection efficiency of ClO2 increased at higher experimental temperatures. At pH 6 and 5°C, the Ct99.99% value was 1.39 and 2.9 times higher than pH 6 and 15°C for AD40 and FCV, respectively.
ClO2 Ct values for AD40 and FCV are higher than those reported for chlorine at pH 6 and 8 (44). This is a contradiction of earlier reports that demonstrated increased viral inactivation by ClO2 at high pH levels compared to chlorine (27, 39). For example, Ct99.99% values for rotavirus inactivation by chlorine and ClO2 at pH 10 and 5°C revealed that the Ct99.99% was 0.14 mg/liter × min (0.1 mg/liter chlorine dose for 1.4 min) for chlorine and ≤0.13 mg/liter × min (0.5 mg/liter ClO2 dose for 15 s or less) for ClO2 (39). Other studies, however, have reported that ClO2 and chlorine inactivation were similar for poliovirus (8) and coxsackievirus (40, 47). Similar to the results observed in the current study, Shin and colleagues (41) reported that ClO2 did not reduce poliovirus type 1, coliphage MS-2, and Norwalk virus as rapidly as free chlorine. Harakeh et al. (21) observed varying susceptibilities of viruses to different disinfectants. Coliphage f2 was more resistant to chlorine but less resistant to ClO2 than enteroviruses (coxsackievirus, echovirus, and poliovirus). Harakeh et al. (21) demonstrated that viral inactivation could differ for one virus challenged by different disinfectants or under different disinfection conditions. In the current study, FCV was more resistant to chlorine dioxide than to chlorine. For chlorine disinfection in water, however, our group observed that AD40 was more resistant than FCV. These results support early recommendations regarding the cautious use of indicator viruses as models for disinfectant efficacy (21). Proper evaluation of disinfectant efficacy should include representative enteric viruses known or thought to occur in source water and under various conditions typical of source water.
Very few studies have been conducted on enteric viral ClO2 inactivation in water. In comparison to a few of these earlier studies, AD40 inactivation appears to be comparable to other viruses. For example, AD40 Ct99.0% values fall within the Ct99.0% ranges for poliovirus (0.2 to 0.67 mg/liter × min) and rotavirus (0.2 to 0.3 mg/liter × min) (15, 43). In the current study, the EFH Ct99.0% value calculated for AD40 at pH 6 and 5°C was 0.38 mg/liter × min. For HAV inactivation in water (pH 6), however, it appears that HAV (Ct99.99% = 16.75 mg/liter × min) is much more resistant than AD40 (Ct99.99% = 0.83 mg/liter × min) but less resistant than FCV (Ct99.99% = 20.85 mg/liter × min) (46).
EFH Ct values provided a means for comparison of complicated data, taking into consideration replicate experiments that varied in viral concentration, disinfectant dose, disinfectant demand, and viral inactivation kinetics under different water conditions. Overall, the EFH model fit bench-scale inactivation data well for all tested conditions. EFH Ct values were within or slightly higher than Ct ranges derived from bench-scale experiments. When considering that replicate experiments varied in viral inactivation, ClO2 dose, and disinfectant decay, EFH curves modeled bench-scale inactivation data very well. The use of this model to accurately predict Ct values out of the range of bench-scale experiments, however, needs to be evaluated.
All disinfection reactions were carried out in BDF water that was inoculated with purified (removal of cell debris) and dispersed (chloroform extraction) AD40 and FCV virus stocks. The controlled disinfection reactions described in this paper provide baseline information necessary for understanding ClO2 efficacy against CCL viral pathogens in treated water under high- and low-pH and -temperature conditions. Studies of chlorine inactivation of aggregated FCV, however, reported Ct99% values 31.0 times higher than those observed for dispersed FCV virus particles (44). Moreover, Ct values for chlorine inactivation of AD40 and FCV in groundwater were higher than those calculated for experiments conducted with BDF water (44). Further studies are needed to determine whether EPA guidance manual ClO2 Ct values are adequate for reducing viruses in an aggregated state, associated with particulate matter, and in natural waters.
Acknowledgments
This work was supported by the American Water Works Association Research Foundation (project 442).
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
REFERENCES
- 1.Aieta, E. M., and J. D. Berg. 1986. A review of chlorine dioxide in drinking water treatment. J. Am. Water Works Assoc. June:62-72. [Google Scholar]
- 2.Albert, M. J. 1986. Enteric adenoviruses. Arch. Virol. 88:1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez, M. E., and R. T. O'Brien. 1982. Mechanisms of inactivation of poliovirus by chlorine dioxide and iodine. Appl. Environ. Microbiol. 44:1064-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Public Health Association. 1998. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Washington, D.C.
- 5.American Water Works Association. 1995. Water treatment: principles and practices of water supply operations, 2nd ed. American Water Works Association, Denver, Colo.
- 6.Clancy, J. 2000. UV rises to the Cryptosporidium challenge. Water21 10:14-16. [Google Scholar]
- 7.Clarke, I. N., and P. R. Lambden. 2000. Organization and expression of Calicivirus genes. J. Infect. Dis. 181:S309-S316. [DOI] [PubMed] [Google Scholar]
- 8.Cronier, S., P. V. Scarpino, and M. L. Zink. 1978. Chlorine dioxide destruction of viruses and bacteria in water, p. 651-658. .In R. L. Jolly, H. Gorchev, and D. M. Hamilton (ed.), Water chlorination: environmental impacts and health effects, vol. 2. Ann Arbor Science Publishers, Ann Arbor, Mich. [Google Scholar]
- 9.Doultree, J. C., J. D. Druce, C. J. Birch, D. S. Bowden, and J. A. Marshall. 1999. Inactivation of feline calicivirus, a Norwalk virus surrogate. J. Hosp. Infect. 41:51-57. [DOI] [PubMed] [Google Scholar]
- 10.Enriquez, C. E., C. J. Hurst, and C. P. Gerba. 1995. Survival of the enteric adenovirus-40 and adenovirus-41 in tap, sea, and waste-water. Water Res. 29:2548-2553. [Google Scholar]
- 11.Fankhauser, R. L., J. S. Noel, S. S. Monroe, T. Ando, and R. I. Glass. 1998. Molecular epidemiology of “Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J. Infect. Dis. 178:1571-1578. [DOI] [PubMed] [Google Scholar]
- 12.Federal Register. 1998. Announcement of the drinking water contaminant candidate list. Fed. Regist. 63:10273-10287. [Google Scholar]
- 13.Foy, H. M., M. K. Cooney, and J. B. Hatlen. 1968. Adenovirus type 3 epidemic associated with intermittent chlorination of a swimming pool. Arch. Environ. Health 17:795-802. [DOI] [PubMed] [Google Scholar]
- 14.Fujioka, R. S., M. A. Dow, and B. S. Yoneyama. 1986. Comparative disinfection of indicator bacteria and poliovirus by chlorine dioxide. Water Sci. Technol. 18:125-132. [Google Scholar]
- 15.Gerba, C. P. 2000. Disinfection, p. 543-556. In R. M. Maier, I. L. Pepper, and C. P. Gerba (ed.), Environmental microbiology. Academic Press, San Diego, Calif..
- 16.Glass, R. I., J. Noel, T. Ando, R. Fankhauser, G. Belliot, A. Mounts, U. D. Parashar, J. S. Bresee, and S. S. Monroe. 2000. The epidemiology of enteric caliciviruses from humans: a reassessment using new diagnostics. J. Infect. Dis. 181:S254-S261. [DOI] [PubMed] [Google Scholar]
- 17.Gyurek, L. L., and G. R. Finch. 1998. Modeling water treatment chemical disinfection kinetics. J. Environ. Eng. 124:783-793. [Google Scholar]
- 18.Gyurek, L. L., L. Hanbin, M. Belosevic, and G. B. Finch. 1999. Ozone inactivation kinetics of Cryptosporidium in phosphate buffer. J. Environ. Eng. 125:913-924. [Google Scholar]
- 19.Haas, C. N., and J. Joffe. 1994. Disinfection under dynamic conditions: modification of Hom's model for decay. Environ. Sci. Technol. 28:1367-1369. [DOI] [PubMed] [Google Scholar]
- 20.Hafliger, D., P. Hubner, and J. Luthy. 2000. Outbreak of viral gastroenteritis due to sewage-contaminated drinking water. Int. J. Food Microbiol. 54:123-126. [DOI] [PubMed] [Google Scholar]
- 21.Harakeh, S. 1987. The behavior of viruses on disinfection by chlorine dioxide and other disinfectants in effluent. FEMS Microbiol. Lett. 44:335-341. [Google Scholar]
- 22.Hoff, J. C. 1986. Inactivation of microbial agents by chemical disinfectants. EPA 600-S2-86-067. Office of Water, U.S. Environmental Protection Agency, Washington, D.C.
- 23.Horowitz, M. S. 1996. Adenoviruses, p. 2149-2171. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields Virology, 3rd ed., vol. 1. Lippincott-Raven, Philadelphia, Pa. [Google Scholar]
- 24.Hurst, C. J., K. A. McClellan, and W. H. Benton. 1988. Comparison of cytopathogenicity, immunofluorescence and in situ DNA hybridization as methods for the detection of adenoviruses. Water Res. 22:1547-1552. [Google Scholar]
- 25.Irving, L. G., and P. A. Smith. 1981. One-year survey of enteroviruses, adenoviruses, and reoviruses isolated from effluent at an activated-sludge purification plant. Appl. Environ. Microbiol. 41:51-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang, X., M. Wang, K. Wang, and M. K. Estes. 1993. Sequence and genomic organization of Norwalk virus. Virology 195:51-61. [DOI] [PubMed] [Google Scholar]
- 27.Junli, H., L. Wang, R. Nenqu, L. Li, S. Fun, and Y. Guanle. 1997. Disinfection effect of chlorine dioxide on viruses, algae, and animal planktons in water. Water Res. 31:455-460. [Google Scholar]
- 28.Kapikian, A. Z., M. K. Estes, and R. M. Chanock. 1996. Norwalk group of viruses, p. 783-810. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields Virology, 3rd ed., vol. 1. Lippincott-Raven, Philadelphia, Pa. [Google Scholar]
- 29.Kaplan, J. E., R. A. Goodman, L. B. Schonberger, E. C. Lippy, and G. W. Gary. 1982. Gastroenteritis due to Norwalk virus: an outbreak associated with a municipal water system. J. Infect. Dis. 146:190-197. [DOI] [PubMed] [Google Scholar]
- 30.Keswick, B. H., T. K. Satterwhite, P. C. Johnson, H. L. DuPont, S. L. Secor, J. A. Bitsura, G. W. Gary, and J. C. Hoff. 1985. Inactivation of Norwalk virus in drinking water by chlorine. Appl. Environ. Microbiol. 50:261-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kukkula, M., P. Arstila, M. L. Klossner, L. Maunula, C. H. V. Bonsdorff, and P. Jaatinen. 1997. Waterborne outbreak of viral gastroenteritis. Scand. J. Infect. Dis. 29:415-418. [DOI] [PubMed] [Google Scholar]
- 32.Kukkula, M., L. Maunula, E. Silvennoinen, and C.-H. v. Bonsdorff. 1999. Outbreak of viral gastroenteritis due to drinking water contaminated by Norwalk-like viruses. J. Infect. Dis. 180:1771-1776. [DOI] [PubMed] [Google Scholar]
- 33.Li, H., L. L. Gyurek, G. B. Finch, D. W. Smith, and M. Belosevic. 2001. Effect of temperature on ozone inactivation of Cryptosporidium parvum in oxidant demand-free phosphate buffer. J. Environ. Eng. 127:456-467. [Google Scholar]
- 34.Morita, S., A. Namikoshi, T. Hirata, K. Oguma, H. Katayama, and e. al. 2002. Efficacy of UV irradiation in inactivating Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 68:5387-5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moss, C. I., and V. P. Olivieri. 1985. Disinfecting capabilities of oxychlorine compounds. Appl. Environ. Microbiol. 50:1162-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nuanualsuwan, S., and D. O. Cliver. 2003. Capsid functions of inactivated human picornaviruses and feline calicivirus. Appl. Environ. Microbiol. 69:350-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papapetropoulou, M., and A. C. Vantarakis. 1998. Detection of adenovirus outbreak at a municipal swimming pool by nested PCR amplification. J. Infect. 36:101-103. [DOI] [PubMed] [Google Scholar]
- 38.Prasad, B. V. V., M. E. Hardy, and M. K. Estes. 2000. Structural studies of recombinant Norwalk capsids. J. Infect. Dis. 181:S317-S321. [DOI] [PubMed] [Google Scholar]
- 39.Rizet, M., N. Dumoutier, and D. Bellahcen. 1986. Techniques for the elimination of viral particles. Aqua 6:343-348. [Google Scholar]
- 40.Scarpino, P. V., F. A. Brigano, S. Cronier, and M. L. Zink. 1979. Effects of particulates on disinfection of enteroviruses in water by chlorine dioxide. EPA 600-2-79-054. U.S. Environmental Protection Agency, Washington, D.C.
- 41.Shin, G. A., D. Battigelli, and M. D. Sobsey. 1998. Reduction of norwalk virus, poliovirus 1, and coliphage MS2 by free chlorine, chlorine dioxide, and ozone disinfection of water. Proceedings of the Water Quality Technology Conference. American Water Works Association, Denver, CO.
- 42.Slomka, M. J., and H. Appleton. 1998. Feline calicivirus as a model system for heat inactivation studies of small round structured viruses in shellfish. Epidemiol. Infect. 121:401-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sobsey, M. D. 1989. Inactivation of health-related microorganisms in water by disinfection processes. Water Sci. Tech. 21:171-195. [Google Scholar]
- 44.Thurston-Enriquez, J. A., C. N. Haas, J. G. Jacangelo, and C. P. Gerba. 2003. Inactivation of feline calicivirus and adenovirus type 40 by UV radiation. Appl. Environ. Microbiol. 69:577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thurston-Enriquez, J. A., C. N. Haas, J. G. Jacangelo, and C. P. Gerba. 2003. Chlorine inactivation of adenovirus type 40 and feline calicivirus. Appl. Environ. Microbiol. 69:3979-3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.U.S. Environmental Protection Agency. 1989. Guidance manual for compliance with the filtration and disinfection requirements for public water systems using surface water sources. Office of Water, U.S. Environmental Protection Agency, Washington, D.C.
- 47.U.S. Environmental Protection Agency. 1999. EPA guidance manual: alternative disinfectants and oxidants. EPA 815-R-99-014. Office of Water, U.S. Environmental Protection Agency, Washington, D.C.
- 48.U.S. Environmental Protection Agency. 2001. National primary drinking water standards. EPA 816-F-01-007. Office of Water, U.S. Environmental Protection Agency, Washington, D.C.
- 49.White, G. C. 1999. Handbook of chlorination and alternative disinfectants, 4th ed. John Wiley and Sons, Inc., New York, N.Y.