Abstract
Populations of the causal agent of wheat tan spot, Pyrenophora tritici-repentis, that are collected from fields frequently treated with reduced fungicide concentrations have reduced sensitivity to strobilurin fungicides and azole fungicides (C14-demethylase inhibitors). Energy-dependent efflux transporter activity can be induced under field conditions and after in vitro application of sublethal amounts of fungicides. Efflux transporters can mediate cross-resistance to a number of fungicides that belong to different chemical classes and have different modes of action. Resistant isolates can grow on substrata amended with fungicides and can infect plants treated with fungicides at levels above recommended field concentrations. We identified the hydroxyflavone derivative 2-(4-ethoxy-phenyl)-chromen-4-one as a potent inhibitor of energy-dependent fungicide efflux transporters in P. tritici-repentis. Application of this compound in combination with fungicides shifted fungicide-resistant P. tritici-repentis isolates back to normal sensitivity levels and prevented infection of wheat leaves. These results highlight the role of energy-dependent efflux transporters in fungicide resistance and could enable a novel disease management strategy based on the inhibition of fungicide efflux to be developed.
The ascomycete fungus Pyrenophora tritici-repentis (Died.) Drechs. [anamorph, Drechslera tritici-repentis (Died.) Shoemaker] is the causal agent of tan spot of wheat (11), and it may cause up to 50% yield loss in wheat grown in Europe, North America, and Australia (30). P. tritici-repentis was of minor importance in Germany until the mid-1990s, but now it is one of the most prominent wheat pathogens (23). The sudden occurrence of severe epidemics was caused by changes in agricultural practice, including early sowing of winter wheat, minimum tillage, and increasing cereal proportions in crop rotations (44). Fungicide treatment and appropriate agricultural practices are the most efficient ways to control this pathogen (6). Several fungicides against P. tritici-repentis are available commercially (http://www.bvl.bund.de/; http://www.ext.nodak.edu/extpubs/plantsci/pests/pp1249w.htm). Most of the active substances are either azole (C14-demethylase inhibitors [DMIs]) or strobilurin-like fungicides (quinone “outside” inhibitors). Frequent application of fungicides with similar modes of action increases the risk that fungicide resistance will emerge and could eventually lead to the loss of an entire fungicide class. Several examples of fungicide resistance in various pathogens have been attributed to inadequate fungicide management (9).
Qualitative and quantitative fungicide resistance has been identified in pathogen populations. In contrast to qualitative resistance resulting from mutations in genes encoding fungicide targets (18, 21), quantitative resistance is polygenic (3) and may affect the activity of several fungicides with different modes of action. Reduction of intracellular fungicide concentrations by enzymatic degradation of antifungal compounds and secretion of fungicides by plasma membrane-localized efflux transporters have been reported (10). Furthermore, utilization of alternative metabolic pathways also may increase quantitative fungicide resistance (24).
Members of the ATP-binding cassette (ABC) transporter family contribute to fungicide resistance in different fungi (10). Several of these proteins can actively transport a wide range of substrates across the plasma membrane (32). Broad, overlapping substrate specificity of efflux pumps may explain the cross-resistance to compounds belonging to the same and different fungicide classes (9, 10).
Both DMIs, which interfere with fungal sterol biosynthesis, and strobilurins, which bind to cytochrome b and inhibit mitochondrial ATP synthesis, are highly successful classes of fungicides for many different crops. The appearance of pathogen strains not affected by fungicides is a cause of concern and needs to be prevented by antiresistance management strategies. To develop such management strategies, the resistance mechanisms in fungal populations need to be better understood (3).
In this study we analyzed the sensitivity of field isolates of the tan spot fungus, which differed in fungicide history, to commercially available DMI and strobilurin fungicides in order to determine whether plasma membrane transporters contribute to quantitative fungicide resistance. We tested the hypothesis that the chemical inhibition of efflux transport enhances fungicide activity and allows efficient chemical control of insensitive isolates. Our findings add to the understanding of mechanisms of fungicide resistance and suggest novel approaches for fungicide resistance and disease control management.
MATERIALS AND METHODS
Isolation and cultivation of P. tritici-repentis.
P. tritici-repentis-infected wheat leaves were collected from conventionally fungicide-treated fields and from nontreated control fields at different locations in Saxonia-Anhalt (Aschersleben, Bad Lauchstädt, Beelitz, Bernburg, Dohndorf, Greifenhagen, Halberstadt, Hohenthurm, Jeggeleben, Magdeburg, Quenstedt, Rödgen, Salzwedel, Schwenda, Seyda, Walbeck, Weißenfels, Wittenberg, Zeppernick) and Schleswig-Holstein (Pölitz), Germany, in June 1999 and June 2000. Leaf material was air dried at ∼25°C for 7 days and stored at room temperature at 40 to 50% relative humidity.
Fungicide sensitivity was assayed with spore suspensions obtained directly from stored leaves. Conidiation was induced by incubating dry leaf samples in a humid chamber (room temperature, 100% relative humidity) for 1 to 3 days until sporulation occurred. To cultivate the fungus in vitro, conidia were transferred to biomalt agar plates (30 g · liter−1 biomalt, 20 g · liter−1 agar), potato dextrose agar (Merck, Darmstadt, Germany), or V8 agar plates (200 ml V8 juice [Bio-Gemüsesaft, Grünland GmbH, Hamburg, Germany], 3 g · liter−1 CaCO3, 30 g · liter−1 agar). Liquid cultures were grown in 20 g · liter−1 biomalt. Fungicides were adjusted to the concentrations required (see below).
Fungicide resistance development.
The fungicides used in this study were the strobilurins Discus (active ingredient, kresoxim-methyl; BASF AG, Ludwigshafen, Germany), Amistar (active ingredient, azoxystrobin; Syngenta Agro GmbH, Frankfurt/Main, Germany), and Acanto (active ingredient, picoxystrobin, Syngenta Agro GmbH, Frankfurt/Main, Germany) and the DMI fungicide Opus (active ingredient, epoxyconazole; BASF AG, Ludwigshafen, Germany).
A set of matched strains (i.e., strains with different fungicide sensitivities derived from the same P. tritici-repentis isolate) were obtained by sequential transfers of agar plugs from a colony edge to biomalt agar containing increasing concentrations (1, 2.5, 5, 10, and 50 mg · liter−1 active ingredient) of the strobilurin fungicide kresoxim-methyl, azoxystrobin, or picoxystrobin or the DMI epoxyconazole.
The cross-resistance of P. tritici-repentis strains that were adapted to different strobilurin (kresoxim-methyl, azoxystrobin, or picoxystrobin) or DMI (epoxyconazole) concentrations was assessed on biomalt agar plates containing 50 mg · liter−1 azoxystrobin or epoxyconazole. The corresponding nonadapted isolate served as the control.
For plant inoculation experiments, fungicide-adapted and nonadapted mycelia from liquid cultures were fragmented in an AKA KM8 blender (type 03259; Suhl, Germany) at level III for 90 s.
Quantitative fungicide sensitivity assays.
Fungicide sensitivity was determined by determining 50% effective doses (ED50s) and ED90s by spore germination assays. At least 200 spores were incubated with kresoxim-methyl (Discus; 0, 0.3, 0.38, 0.50, 0.75, 1.5, 3.0, and 6.0 g · l−1 active ingredient) or epoxyconazole (Opus; 0, 0.04, 0.05, 0.08, 0.09, 0.13, 0.19, 0.38, and 0.75 g · liter−1 active ingredient) at room temperature in water for 24 h. Since DMIs may not directly affect spore germination because endogenous sterols are not limiting, spores were counted as germinated only when the germ tube length was at least twice the spore length. Analyses of confidence intervals were performed to evaluate fungicide effects. The regression line between the logit-transformed fungicide efficiency and the transformed fungicide concentrations was calculated by linear regression with Microsoft Excel 2002 (Microsoft Corp., Redmond, WA) (27).
Fungicide assays on leaf segments.
In planta fungicide performance assays were carried out by using leaf segments (2 to 3 cm) excised from 9- to 11-day-old fungicide-treated wheat (Triticum aestivum cv. Ritmo) plants. Fungicides were sprayed 1 h before excision of segments. Control leaf segments were obtained from plants sprayed with water. The leaf segments were placed onto water agar containing 35 mg · liter−1 benzimidazole and 5 g · liter−1 agar, inoculated with a suspension of mycelial fragments from a 2- to 3-week-old liquid culture, and kept in plant growth chambers (Percival Scientific AR-75HIL; Percival Scientific, Inc., Perry, IA) at 100% relative humidity at 21°C during a 12-h light period and at 18°C during a 12-h dark period until symptoms occurred.
Microscopy.
Microscopy was performed with a Nikon Eclipse 600 epifluorescence microscope or a modular confocal laser scanning microscope (system C1; Nikon GmbH, Düsseldorf, Germany). A UV-2A filter block (EX 340-380, DM 400, BA 420) and a 4′,6′-diamidino-2-phenylindole (DAPI) filter block (EX 340-380, DM 400, BA 435-485), both obtained from Nikon, were used. Digital images were taken with a CCD-1300 camera (VDS Vosskühler GmbH, Osnabrück, Germany) or a Basler A113C camera (Basler, Inc., Exton, PA) and then archived and processed with the software package Lucia 4.61 (Nikon GmbH, Düsseldorf, Germany).
Visualization of efflux transporter activity.
Fluorescence microscopy was used to assess efflux transporter activity in hyphal plasma membranes. In vitro-grown fungicide-adapted and nonadapted mycelia of P. tritici-repentis were incubated in the dark with aqueous solutions of two fluorescent transporter substrates, the imidazole dye Hoechst 33342 (20 μg · ml−1; MoBiTec, Göttingen, Germany) and ethidium bromide (5 μg · ml−1; Roche, Mannheim, Germany), for 30 min. Under these conditions the fluorescent dyes did not induce transporter activity. After three washes (30 s each) in phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 8.5 mM Na/K phosphate, pH 7.2), samples were transferred to 0.8% (wt/vol) NaCl and evaluated microscopically.
Inhibition of membrane transporter activity.
Hyphae were incubated with the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP) (50 μM; Sigma-Aldrich, Seelze, Germany) (29) for 20 min or with the specific ATPase inhibitor oligomycin (10 μg · ml−1; A.G. Scientific, Inc., San Diego, CA) (8) for 1 h and then stained with Hoechst 33342 or ethidium bromide. Hyphal washing in phosphate-buffered saline and fluorescence microscopy were performed as described above.
Natural substances from plants and some analogs of these compounds can inhibit efflux transporter activity (14). We tested flavone-based inhibitors (14) for the ability to prevent Hoechst 33342 and ethidium bromide transport across the plasma membrane of P. tritici-repentis isolates adapted to 1, 2, 5, and 10 mg · liter−1 of different fungicides. Substances identified as inhibitors in microscopic fluorescence assays also were tested in vitro (hyphal growth rate assays) and in planta (leaf segment assay), alone and in combination with fungicides. Kresoxim-methyl (only used in leaf segment assays), azoxystrobin, picoxystrobin, and epoxyconazole were tested with three strains of the fungus adapted to each fungicide group. Matched nonadapted isolates served as controls.
RESULTS
Sensitivities of P. tritici-repentis populations to kresoxim-methyl and epoxyconazole.
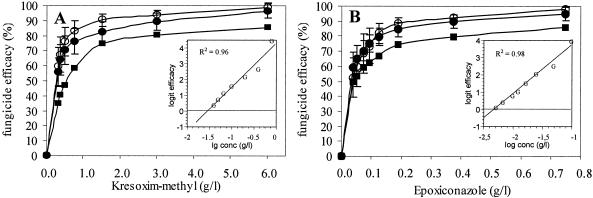
The sensitivities of P. tritici-repentis populations from fungicide-treated and nontreated control fields were determined by spore germination assays (Fig. 1). The dose-effect curves for nontreated populations collected from different locations in Saxonia-Anhalt were not statistically different. Therefore, values were combined, and they reflected normal sensitivity. “Normal sensitivity,” as defined by the European and Mediterranean Plant Protection Organization (EPPO standard for the efficacy evaluation of plant protection products [http://www.eppo.org./Standards/Gl213.html]), means that baseline sensitivity values cannot be provided, as DMI and probably strobilurin fungicides have been applied extensively. ED50s were determined from logit transformations for populations from nontreated fields, and they were 31 ± 7.6 mg · liter−1 and 5.3 ± 1.2 mg · liter−1 for kresoxim-methyl and epoxyconazole, respectively; the corresponding ED90s were 210 ± 72 mg · liter−1and 29 ± 9.3 mg · liter−1, respectively. Spores from fungicide-treated populations from fields in Saxonia-Anhalt germinated at slightly higher rates in the presence of kresoxim-methyl and epoxyconazole than spores from nontreated fields germinated (Fig. 1), but the differences were not statistically significant (P = 0.05). Fungal populations also were collected in Schleswig-Holstein from fields that had been continuously cultured in wheat for 10 years with reduced tillage. These fields were treated four or five times per year with a fungicide mixture containing ∼90% sterol biosynthesis inhibitor or strobilurin. Fungicides were applied at reduced concentrations that corresponded to 50 to 70% of the recommended concentrations. Populations collected from these fields had ED90s of 500 ± 200 mg · liter−1 and 94 ± 41 mg · liter−1 for kresoxim-methyl and epoxyconazole, respectively. These values indicated that the fungicide resistance was significantly higher (P = 0.05) than that found in populations collected from fields that had not been treated with fungicides.
FIG. 1.
Sensitivity of spore germination in P. tritici-repentis populations from conventionally fungicide-treated (•) and nontreated control fields (○) to kresoxim-methyl (A) and epoxyconazole (B). For comparison, populations from fields intensively treated with reduced fungicide doses (▪) were also tested. The insets show the regression lines for logit-transformed normal sensitivity. The error bars indicate standard deviations.
In vitro adaptation experiments.
Isolates adapted to 1 mg · liter−1 azoxystrobin could grow on biomalt agar plates containing a 50-fold-higher concentration of this fungicide. Isolates adapted to 5 or 10 mg · liter−1 of strobilurin had slightly higher growth rates than isolates adapted to 1 mg · liter−1. Nonadapted isolates could not grow on plates containing 50 mg · liter−1 azoxystrobin. Similar results were obtained with plates containing other strobilurin fungicides and with plates containing epoxyconazole (data not shown). Adaptation usually was not limited by the fungal capacity to cope with the fungicide, but rather was limited by the solubility of the fungicide in aqueous media (data not shown). Several successive transfers to fungicide-free growth media did not significantly reduce fungicide resistance. Isolates that were successfully adapted to fungicides did not revert to complete fungicide sensitivity even after eight transfers.
Strains that had previously been adapted to either a strobilurin fungicide or to epoxyconazole could grow on 50 mg · liter−1 azoxystrobin. Similarly, strobilurin- and DMI-adapted strains could grow on plates containing 50 mg · liter−1 epoxyconazole (data not shown). The nonadapted matched control did not grow in either case.
Water-treated leaf segments were infected by both azoxystrobin-adapted and nonadapted isolates (Fig. 2), but fungicide-treated leaf segments were infected only by fungicide-adapted strains. Similar results were obtained with epoxyconazole-adapted isolates (data not shown). In the absence of fungicides, fungicide-adapted strains sporulated less when they were grown on agar plates, perhaps indicating some reduction in fitness, but the symptom development and sporulation rates of fungicide-adapted and nonadapted control strains on leaves were not statistically different (P = 0.05) (data not shown).
FIG. 2.
Infection assay with fungal isolates adapted to 2.5 mg · liter−1 azoxystrobin (+) and nonadapted isolates (−) on leaf segments treated with different strobilurin or epoxyconazole concentrations. The following fungicide concentrations were used: azoxystrobin, 840, 250, and 125 mg · liter−1; kresoxim-methyl, 750, 500, and 300 mg · liter−1; picoxystrobin, 2,500, 1,250, and 1,000 mg · liter−1; and epoxyconazole, 750, 375, and 126 mg · liter−1. Water-treated leaves were used to check the virulence of adapted and nonadapted isolates in the absence of fungicides.
Membrane transporter activity in fungicide-resistant isolates.
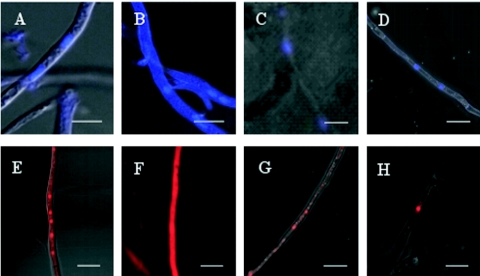
The spatial distribution of membrane transporter activity in fungicide-treated P. tritici-repentis was different in nonadapted (Fig. 3A and E) and adapted isolates, which had substantial fluorescence of the plasma membrane-cell wall area, in addition to some nuclear fluorescence (Fig. 3B and F). Thus, efflux transporters might increase fungicide resistance. One of the candidate transporter inhibitory compounds, 2-(4-ethoxy-phenyl)-chromen-4-one (NG5-13a), completely eliminated plasma membrane-associated fluorescence (Fig. 3C and G) and was tested in hyphal growth and leaf segment assays (see below). The disappearance of plasma membrane-cell wall-associated fluorescence immediately after addition of the protonophore CCCP at a concentration of 50 μM shows that efflux transport activity is proton gradient dependent (data not shown). Elimination of membrane-associated fluorescence by adding 10 μg · ml−1 oligomycin indicates that membrane efflux transport is energy dependent (Fig. 3D and H).
FIG. 3.
Confocal fluorescence microscopy of Hoechst 33342-stained hyphae (A to D) and ethidium bromide-stained hyphae (E to H) of P. tritici-repentis. In contrast to nonadapted hyphae, which show nuclear fluorescence (A and E), hyphae adapted to 2.5 mg · liter−1 azoxystrobin show strong fluorescence of the plasma membrane-cell wall area (B and F). Fluorescence of the plasma membrane-cell wall area disappeared after addition of 4 μg · ml−1 of the inhibitor NG5-13a, leaving only fluorescing nuclei in inhibitor-treated hyphae (C and G). Likewise, addition of 10 μg · ml−1 of the ATP synthase inhibitor oligomycin eliminated fluorescence of the plasma membrane-cell wall area (D and H). Bars = 10 μm.
To test whether efflux transporter-mediated fluorescence patterns reflect fungicide treatment in the field, isolates from nontreated and conventionally fungicide-treated wheat fields also were tested. Mycelia that developed from leaf samples collected from nontreated fields showed only nuclear fluorescence, but isolates from fungicide-treated leaves also fluoresced at the plasma membrane-cell wall after treatment with ethidium bromide or Hoechst 33342, indicating induction of efflux transporter activity.
Effect of an inhibitor of efflux transporter activity on growth of fungicide-adapted isolates.
The 4′-hydroxyflavone derivative NG5-13a (14) was a potent inhibitor of efflux transporter activity in P. tritici-repentis (Fig. 3C and G) and rendered fungicide-resistant isolates sensitive in growth assays on agar plates (Table 1). In the absence of fungicides, NG5-13a did not significantly alter the growth of either nonadapted or azoxystrobin-adapted isolates (Table 1). However, in the absence of fungicides this inhibitor reduced the growth rates of epoxyconazole-adapted isolates by 26%. Relative to the controls (i.e., the nonadapted isolates), the growth rates of azoxystrobin-adapted strains decreased by 95, 93, and 91% on sublethal concentrations (2.5 mg · liter−1) of azoxystrobin, picoxystrobin, and epoxyconazole, respectively, in the presence of 2 mg · liter−1 2-(4-ethoxy-phenyl)-chromen-4-one. The corresponding levels of growth rate inhibition for epoxyconazole-adapted isolates were 96, 88, and 42%. The levels of growth rate inhibition in the presence of the less soluble fungicide kresoxim-methyl and 2-(4-ethoxy-phenyl)-chromen-4-one were 88 and 80% for azoxystrobin- and epoxyconazole-adapted isolates. These data demonstrate that there was enhancement of fungicide activity by NG5-13a in fungicide-resistant isolates (Table 1). Other compounds, e.g., NG3-53b6 or NG3-55-16 (14) with inhibitory activity towards ABC transporters of the pathogenic bacterium Staphylococcus aureus, had no effect on the efflux transporter activity of P. tritici-repentis (data not shown). Growth rate measurements in the presence of sublethal fungicide concentrations are not used to assess the fungicide sensitivity of nonadapted isolates. These assays take 24 h, which is long enough to induce efflux transporter activity and fungicide resistance, so the sensitivity of the nonadapted isolates would be underestimated.
TABLE 1.
Enhancement of fungicide efficacy by the membrane efflux transport inhibitor 2-(4-ethoxy-phenyl)-chromen-4-one
| Fungicide applieda | Strain adaptationb | Inhibitorc | Growth rate (μm/24 h)d |
|---|---|---|---|
| None (control) | Nonadapted | − | 540 ± 23 |
| + | 500 ± 20 | ||
| Epoxiconazole | − | 160 ± 8.2 | |
| + | 120 ± 5.8 | ||
| Azoxystrobine | − | 440 ± 18 | |
| + | 320 ± 16 | ||
| Kresoxim-methyl | Epoxiconazolee | − | 210 ± 12 |
| + | 67 ± 4.0 | ||
| Azoxystrobine | − | 130 ± 7.3 | |
| + | 110 ± 9.4 | ||
| Azoxystrobin | Epoxiconazolee | − | 190 ± 12 |
| + | 29 ± 2.6 | ||
| Azoxystrobine | − | 350 ± 15 | |
| + | 26 ± 2.1 | ||
| Picoxystrobin | Epoxiconazolee | − | 190 ± 12 |
| + | 40 ± 5.3 | ||
| Azoxystrobine | − | 170 ± 12 | |
| + | 62 ± 5.8 | ||
| Epoxyconazole | Epoxiconazolee | − | 160 ± 6.6 |
| + | 50 ± 2.9 | ||
| Azoxystrobine | − | 310 ± 13 | |
| + | 200 ± 12 |
The concentration of each fungicide was 2.5 mg · liter−1.
Isolates were adapted to 1 mg · liter−1 epoxyconazole or azoxystrobin.
The inhibitor concentration was 2 mg · liter−1.
Mean growth rates of nonadapted and adapted isolates of P. tritici-repentis were measured on biomalt agar in the presence or absence of fungicides and/or inhibitor 24 h after inoculation. The results are based on three replicate experiments, and the values are means ± standard errors.
There were statistically different rates (P ≤ 0.05) of hyphal growth in the presence and in the absence of the inhibitor for each strain and fungicide tested under these conditions.
In wheat leaf segment assays (Fig. 4) nonadapted isolates were controlled by all concentrations of the strobilurin azoxystrobin tested, but adapted isolates were not controlled even by the highest fungicide concentration used, 840 mg · liter−1. This concentration is well above the recommended field concentration for this compound (625 mg · liter−1) in the commercial product Amistar. When the 4′-hydroxyflavone inhibitor NG5-13a was added at a concentration of 30 mg · liter−1, there was complete control of disease symptom development, even at the lowest fungicide concentration tested (125 mg · liter−1) (Fig. 4). Comparable results were obtained with the second strobilurin tested (kresoxim-methyl) and with the DMI fungicide epoxyconazole (data not shown). Application of only 2-(4-ethoxy-phenyl)-chromen-4-one to plants at a concentration that blocks fungal efflux transporter activity (30 mg · liter−1) did not inhibit symptom development (Fig. 4) or cause any other visible alteration to the wheat leaves.
FIG. 4.
Infection assay with fungal isolates adapted to 2.5 mg · liter−1 azoxystrobin and nonadapted isolates in the presence (+) or absence (−) of the efflux transport inhibitor 2-(4-ethoxy-phenyl)-chromen-4-one (30 mg · liter−1) on leaf segments treated with different azoxystrobin concentrations (840, 330, 250, 200, and 125 mg · liter−1). Water-treated leaves were used to check the effect of the inhibitor in the absence of fungicides and the virulence of adapted and nonadapted isolates.
DISCUSSION
Fungicides have been used intensively in cereal production in Europe since the mid-1960s (17). A major force driving the emergence of antibiotic resistance in populations of pathogenic microorganisms is the volume of drug use (1), and increasing rates of fungicide resistance have been reported for both animal pathogens and plant-pathogenic fungi (37). It is now clear that efflux transporters are involved in fungicide resistance (4, 15, 26, 37, 46).
Genome-wide expression profiling of >5,000 open reading frames of Candida albicans indicated that the expression of 301 genes was significantly altered in response to treatment with the DMI fungicide fluconazole (5). This group of up-regulated genes included the ABC transporter gene CDR2 and the major facilitator superfamily transporter (MFS) gene MDR1, both of which can affect fungicide resistance (5, 25, 43). In other fungi that cause invasive mycoses, including Aspergillus species and Cryptococcus neoformans, efflux transporters contribute to quantitative fungicide resistance (33, 37).
To reduce the risk of transporter-based fungicide resistance, it is important to monitor fungicide sensitivity and to develop antiresistance strategies. Chemical inhibitors that block transporter activity may be important in managing and reducing fungicide resistance problems. In this study we showed that a derivative of naturally occurring 4′-hydroxyflavones, 2-(4-ethoxy-phenyl)-chromen-4-one, blocks the energy-dependent efflux transporter activity of the wheat pathogen P. tritici-repentis. This inhibitor, when it is combined with fungicides, renders fungicide-resistant strains susceptible to the fungicide and could enable chemical control of resistant strains under field conditions. A complex set of flavonoids, including 4′-hydroxyflavone, has been detected in Sophora species, which belong to the Fabaceae (31). Although the role of the individual compounds is not yet clear, some of the compounds could inhibit membrane transporter activity and enhance the effect of antimicrobial secondary metabolites. Several of these metabolites have been identified as phytoalexins in the Fabaceae. Hydrophobic flavonoids can occur on leaf surfaces at significant levels (40), and 2-(4-ethoxy-phenyl)-chromen-4-one, which is hydrophobic, is expected to associate with the plant cuticle after application.
Based on the fungicide sensitivity of strains from conventionally treated field populations of P. tritici-repentis, both the DMI fungicide epoxyconazole (Opus) and the strobilurin kresoxim-methyl (Discus) remained efficacious. However, a population regularly exposed to reduced fungicide doses was less sensitive to these fungicides than a population that was not exposed to these fungicides at all (Fig. 1). That the fungicide application regimen affects the sensitivity of a population has been shown for the powdery mildews of barley and cucurbits, the wheat eyespot fungus Pseudocercosporella herpotrichoides, and the apple scab fungus Venturia inaequalis (2, 7, 19, 22).
The ability to adapt to fungicide stress is not sufficient to permit conclusions about the resistance mechanism to be drawn. All P. tritici-repentis isolates tested, once adapted to either DMI or strobilurin fungicides, exhibited cross-resistance and could either grow on agar media with the fungicide or infect leaves treated with the fungicide (Fig. 2). Since stepwise adaptation to fungicide stress is required and since the adapted isolates are cross resistant to classes of fungicides to which they have not been exposed (Fig. 2), it is unlikely that the fungicide resistance observed results from mutations in the fungicide target genes. In vitro adaptation experiments were successful with all isolates tested when the initial fungicide concentration was <1 mg · liter−1. Even lower fungicide concentrations can be found in field margins and areas exposed to spray drift (34). In this context the reduction in the fungicide concentration in spray applications that is recommended, based on ecological and economic arguments (39), could increase the proportion of resistant fungal strains in the population. When adapted to fungicide concentrations as low as 2.5 mg · liter−1, P. tritici-repentis isolates could no longer be controlled chemically in our experiments.
There was a fitness penalty in fungicide-adapted P. tritici-repentis in growth rate assays on agar lacking fungicides, and this penalty occurred preferentially for strobilurin-adapted isolates (Table 1). The fitness penalty observed on agar plates amended with fungicides may have been due to the energy demand of hyphal efflux transporters. However, reduced fitness of adapted strains was not observed in infection assays, as the severities of the disease symptoms caused by fungicide-adapted and nonadapted matched isolates of P. tritici-repentis on nontreated wheat leaves (Fig. 2) and the sporulation rates on necrotic tissue were not significantly different. Since the fitness penalty did not occur in infection assays and since samples collected from fungicide-treated fields had hyphal efflux transporter activity, this resistance mechanism may be important under commercial field conditions.
ABC transporters and MFS multidrug transporters are thought to contribute to fungicide resistance in plant-pathogenic fungi (9, 10, 36). In the causal agent of Septoria leaf blotch of wheat, Mycosphaerella graminicola, the role of five different ABC transporters in fungicide resistance has been studied in detail (46). Complementation of Saccharomyces cerevisiae mutants by the transporter genes MgAtr1 to MgAtr5 showed that each transporter provided protection against several chemically unrelated compounds. M. graminicola mutants in which individual transporter genes were deleted or disrupted lacked a clear phenotype, which probably was due to distinct but overlapping substrate specificities of the transporters (46). In the citrus green mold Penicillium digitatum, disruption of the ABC transporter gene PMR1 demonstrated that this transporter was an important determinant of DMI resistance (26), and in the gray mold Botrytis cinerea the ABC transporter BcatrD synergized the MFS transporter Bcmfs1 to mediate DMI resistance (15). However, data demonstrating the role of transporters in fungicide resistance in plant pathogens under field conditions have not been presented yet.
The ability to transport the fluorescent dyes Hoechst 33342 and ethidium bromide in an ATP-dependent manner is taken as proof of efflux transporter activity in fungi (4). Fluorescence staining patterns in fungicide-adapted and nonadapted P. tritici-repentis isolates indicate that efflux transporters are induced by fungicide treatment under laboratory and field conditions. Results obtained with Hoechst 33342 are of special interest, because this dye exhibits structural similarity with commercially available imidazole fungicides. Treatment of adapted isolates with the protonophore CCCP, which interferes with generation of a proton gradient (28), or with oligomycin, which specifically inhibits mitochondrial ATP synthase (45), resulted in an immediate and complete loss of plasma membrane-associated fluorescence. These results are consistent with the hypothesis that plasma membrane-associated fluorescence in P. tritici-repentis results from energy-dependent efflux transporter activity. As neither Hoechst 33342 nor ethidium bromide is a specific substrate of ABC or MFS transporters, the experiments that have been described are not sufficient to determine whether transporters of one or both classes contribute to fungicide resistance. Addition of the transporter inhibitor 2-(4-ethoxy-phenyl)-chromen-4-one (14) also eliminated plasma membrane-associated fluorescence (Fig. 3), and addition of this compound to fungicides (Fig. 4) allowed chemical control of fungicide-resistant isolates of the wheat tan spot fungus. More importantly, this inhibitor is neither fungitoxic nor phytotoxic. These results show clearly that efflux transporters contribute to fungicide resistance in P. tritici-repentis.
Identification of chemical inhibitors that are active against a broad range of transporters is important because there are several ABC and MSF transporters that are possibly involved in fungicide efflux in fungal genomes. In the genome of the yeast S. cerevisiae there are 29 putative transporters (38), in the wheat pathogen M. graminicola there are five putative transporters (35), and in Botryotinia fuckeliana >14 ABC transporter genes (36) have been identified. Common to all of these transporters are the broad substrate spectra and the inducibility of gene expression by specific patterns of synthetic and natural fungicidal compounds (26, 33, 46).
Novel compounds that inhibit these export pumps could be used as synergists in combination with fungicides to control a plant disease(s). Wang et al. (42) used fluorescent P-glycoprotein substrates to screen for and evaluate potential ABC transporter inhibitors. The first-generation transporter inhibitor verapamil and subsequently developed compounds, such as cyclosporine A (41), have been used in cancer and antifungal therapies to overcome multidrug resistance phenotypes. Hayashi et al. (16) demonstrated that the calmodulin antagonist chlorpromazine and the immunosuppressor tacrolimus, both of which reduce multidrug resistance in tumor cells, can increase or alter the activity of a DMI fungicide in the gray mold fungus B. cinerea. Inhibitors of fungal efflux transporters not only may enhance fungicide activity but also may increase the efficiency of phytoalexins, thereby reinforcing natural plant defense mechanisms. Zwiers et al. (46) have shown that MgAtr5 deletion mutants of M. graminicola are more sensitive to the putative wheat defense compound resorcinol and to the grape phytoalexin resveratrol. Similarly, mutants of Gibberella pulicaris that lack the ABC transporter gene Gpabc1 also are sensitive to the potato phytoalexin rishitin and are no longer virulent on potato (13).
Resistance to antimicrobial compounds is a serious clinical and agricultural problem today (9, 20). At the beginning of the 20th century, Ehrlich (12) recommended “to hit hard and hit early” and to combine drugs with different modes of action in order to overcome or delay the acquisition of resistance. These recommendations are still valid in antiresistance management strategies for chemical plant protection (9). To counteract transporter-based resistance, it is necessary to develop strategies directed against the transporters involved. Such strategies could involve efflux transporter inhibitors that, if used in combination with existing fungicides, could lengthen their useful life span and reduce the levels needed for effective disease control.
Acknowledgments
We thank the Ministry of Agriculture and the Ministry of Education of Saxonia-Anhalt for financial support (grants FKZ 3205A0020H and FKZ 32852A0080T [EFRE221800100004/50]).
We thank Frank Stermitz, Colorado State University, Fort Collins, for donating the efflux transporter inhibitors and for critically reading the manuscript; Maarten de Waard, University of Wageningen, The Netherlands, and Stefan G. R. Wirsel, Martin Luther University, Halle, for helpful comments on the manuscript; Roswitha Ende for technical assistance; H. Hartleb, G. Hartmann, H. Herold, and C. Wolf, State Institute for Agriculture and Horticulture Saxonia-Anhalt, for help collecting field samples; K. Nettesheim and H.-J. Pischeli, Nikon GmbH, Düsseldorf, Germany, for help with confocal microscopy; and K. Warnstorff, Martin Luther University, Halle, for help with statistical analysis.
REFERENCES
- 1.Austin, D. J., K. G. Kristinsson, and R. M. Anderson. 1999. The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proc. Natl. Acad. Sci. USA 96:1152-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bierman, S. M., B. D. L. Fitt, F. van den Bosch, G. L. Bateman, J. F. Jenkyn, and S. J. Welham. 2002. Changes in populations of the eyespot fungi Tapesia yallundae and T. acuformis under different fungicide regimes in successive crops of winter wheat, 1984-2000. Plant Pathol. 51:191-201. [Google Scholar]
- 3.Brent, K. J., and D. W. Hollomon. 1998. Fungicide resistance: the assessment of risk. FRAC monograph II. Global Crop Protection Federation, Brussels, Belgium.
- 4.Calabrese, D., J. Bille, and D. Sanglard. 2000. A novel multidrug efflux transporter gene of the major facilitator superfamily from Candida albicans (FLU1) conferring resistance to fluconazole. Microbiology 146:2743-2754. [DOI] [PubMed] [Google Scholar]
- 5.Cowen, L. E., A. Nantel, M. S. Whiteway, D. Y. Thomas, D. C. Tessier, L. M. Kohn, and J. B. Anderson. 2002. Population genomics of drug resistance in Candida albicans. Proc. Natl. Acad. Sci. USA 99:9284-9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuiffetti, L. M., and R. P. Tuori. 1999. Advances in the characterization of the Pyrenophora tritici-repentis-wheat interaction. Phytopathology 89:444-449. [DOI] [PubMed] [Google Scholar]
- 7.Damgaard, C., and B. J. Nielsen. 1999. The effect of fungal density on fungicide dose-response curves in barley powdery mildew (Erysiphe graminis f. sp. hordei). Plant Pathol. 48:402-407. [Google Scholar]
- 8.Decottignies, A., A. M. Grant, J. W. Nichols, H. de Wet, D. B. McIntosh, and A. Goffeau. 1998. ATPase and multidrug transport activities of the overexpressed yeast ABC Yor1p. J. Biol. Chem. 273:12612-12622. [DOI] [PubMed] [Google Scholar]
- 9.Deising, H. B., S. Reimann, A. Peil, and W. E. Weber. 2002. Disease management of rusts and powdery mildews, p. 243-269. In F. Kempken (ed.), The mycota, vol. XI. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 10.del Sorbo, G., H.-J. Schoonbeek, and M. A. de Waard. 2000. Fungal transporters involved in efflux of natural toxic compounds and fungicides. Fungal Genet. Biol. 30:1-15. [DOI] [PubMed] [Google Scholar]
- 11.Drechsler, C. 1923. Some graminicolous species of Helminthosporium: I. J. Agric. Res. 24:641-740. [Google Scholar]
- 12.Ehrlich, P. 1913. Chemotherapeutics: scientific principles, methods, and results. Lancet ii:445-451. [Google Scholar]
- 13.Fleissner, A., C. Sopalla, and K. M. Weltring. 2002. An ATP-binding cassette multidrug-resistance transporter is necessary for tolerance of Gibberella pulicaris to phytoalexins and virulence on potato tubers. Mol. Plant-Microbe Interact. 15:102-108. [DOI] [PubMed] [Google Scholar]
- 14.Guz, N. R., F. R. Stermitz, J. B. Johnson, T. D. Beeson, S. Willen, J. Hsiang, and K. Lew. 2001. Flavolignan and flavone inhibitors of a Staphylococcus aureus multidrug resistance pump: structure-activity relationships. J. Med. Chem. 44:261-268. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi, K., H. J. Schoonbeek, and M. A. de Waard. 2002. Bcmfs1, a novel major facilitator superfamily transporter from Botrytis cinerea, provides tolerance towards the natural toxic compounds camptothecin and cercosporin and towards fungicides. Appl. Environ. Microbiol. 68:4996-5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi, K., H.-J. Schoonbeek, and M. A. de Waard. 2003. Modulators of membrane drug transporters potentiate the activity of the DMI fungicide oxpoconazole against Botrytis cinerea. Pest Manag. Sci. 59:294-302. [DOI] [PubMed] [Google Scholar]
- 17.Hewitt, H. G. 1998. Fungicides in crop protection. CAB International, Oxon, United Kingdom.
- 18.Ishii, H., B. A. Fraaije, T. Sugiyama, K. Noguchi, T. Takeda, T. Amano, and D. W. Hollomon. 2001. Occurrence and molecular characterization of strobilurin resistance in cucumber powdery mildew and downy mildew. Phytopathology 91:1166-1171. [DOI] [PubMed] [Google Scholar]
- 19.Kunz, S., H. Deising, and K. Mendgen. 1997. Acquisition of resistance to sterol demethylation inhibitors by populations of Venturia inaequalis. Phytopathology 87:1272-1278. [DOI] [PubMed] [Google Scholar]
- 20.Levin, B. R., M. Lipsitch, and S. Bonhoeffer. 1999. Population biology, evolution, and infectious disease: convergence and synthesis. Science 283:806-809. [DOI] [PubMed] [Google Scholar]
- 21.Ma, Z., M. A. Yoshimura, and T. J. Michailides. 2003. Identification and characterization of benzimidazole resistance in Monilinia fructicola from stone fruit orchards in California. Appl. Environ. Microbiol. 69:7145-7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGrath, M. T. 1996. Increased resistance to triadimefon and to benomyl in Sphaerotheca fuliginea populations following fungicide usage over one season. Plant Dis. 80:633-639. [Google Scholar]
- 23.Mielke, H. 1999. Zur DTR-Weizenblattdürre-Anfälligkeit inländischer Weizensorten und mögliche Bekämpfung des Erregers. Nachrbl. Dtsch. Pflanzenschutzd. (Berlin) 51:91-94. [Google Scholar]
- 24.Miguez, M., C. Reeve, P. M. E. Wood, and D. W. Hollomon. 2004. Alternative oxidase reduces the sensitivity of Mycospherella graminicola to QoI fungicides. Pest Manag. Sci. 60:3-7. [DOI] [PubMed] [Google Scholar]
- 25.Morschhäuser, J. 2002. The genetic basis of fluconazole resistance development in Candida albicans. Biochim. Biophys. Acta 1587:240-248. [DOI] [PubMed] [Google Scholar]
- 26.Nakaune, R., K. Adachi, O. Nawata, M. Tomiyama, K. Akutsu, and T. Hibi. 1998. A novel ATP-binding cassette transporter involved in multidrug resistance in the phytopathogenic fungus Penicillium digitatum. Appl. Environ. Microbiol. 64:3983-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neter, J., W. Wassermann, and M. H. Kutner. 1983. Applied linear regression models. Richard D. Irwin, Inc., Homewood, IL.
- 28.Peric, M., B. Bozdogan, M. R. Jacobs, and P. C. Appelbaum. 2003. Effects of an efflux mechanism and ribosomal mutations on macrolide susceptibility of Haemophilus influenzae clinical isolates. Antimicrob. Agents Chemother. 47:1017-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prudencio, C., F. Sansonetty, M. J. Sousa, M. Corte-Real, and C. Leao. 2000. Rapid detection of efflux pumps and their relation with drug resistance in yeast cells. Cytometry 39:26-35. [DOI] [PubMed] [Google Scholar]
- 30.Rees, R. G., and G. J. Platz. 1983. Effects of yellow spot on wheat: comparison of epidemics at different stages of crop development. Aust. J. Agric. Res. 34:39-46. [Google Scholar]
- 31.Ruiz, E., C. Donoso, F. González, J. Becerra, C. Marticorena, and M. Silva. 1999. Phenetic relationships between Juan Fernandez and continental Chilean species of Sophora (Fabaceae) based on flavonoid patterns. Bol. Soc. Chil. Quim. 44:351-356. [Google Scholar]
- 32.Saurin, W., M. Hofnung, and E. Dassa. 1999. Getting in or out: early segregation between importers and exporters in the evolution of ATP-binding cassette (ABC) transporters. J. Mol. Evol. 48:22-41. [DOI] [PubMed] [Google Scholar]
- 33.Semighini, C. P., M. Marins, M. H. Goldman, and G. H. Goldman. 2002. Quantitative analysis of the relative transcript levels of ABC transporter Atr genes in Aspergillus nidulans by real-time reverse transcription-PCR assay. Appl. Environ. Microbiol. 68:1351-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaw, M. W. 2000. Models of the effects of dose heterogeneity and escape on selection pressure for pesticide resistance. Phytopathology 90:333-339. [DOI] [PubMed] [Google Scholar]
- 35.Stergiopoulos, I., M. M. Gielkens, S. D. Goodall, K. Venema, and M. A. de Waard. 2002. Molecular cloning and characterization of three new ATP-binding cassette transporter genes from the wheat pathogen Mycosphaerella graminicola. Gene 289:141-149. [DOI] [PubMed] [Google Scholar]
- 36.Stergiopoulos, I., L.-H. Zwiers, and M. A. de Waard. 2002. Secretion of natural and synthetic toxic compounds from filamentous fungi by membrane transporters of the ATP-binding cassette and major facilitator superfamily. Eur. J. Plant Pathol. 108:719-734. [Google Scholar]
- 37.St. Georgiev, V. 2000. Membrane transporters and antifungal drug resistance. Curr. Drug Targets 1:261-284. [DOI] [PubMed] [Google Scholar]
- 38.Taglicht, D., and S. Michaelis. 1998. Saccharomyces cerevisiae ABC proteins and their relevance to human health and disease. Methods Enzymol. 292:130-162. [DOI] [PubMed] [Google Scholar]
- 39.Urech, P. A. 1999. The agrichemical industry: its contribution to crop protection and environmental policy. Plant Pathol. 48:689-692. [Google Scholar]
- 40.Valkama, E., J.-P. Salminen, J. Koricheva, and K. Pihlaja. 2004. Changes in leaf trichomes and epicuticular flavonoids during leaf development in three birch taxa. Ann. Bot. (London) 94:233-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Zuylen, L., K. Nooter, A. Sparreboom, and J. Verweij. 2000. Development of multidrug-resistance convertors: sense or nonsense? Investig. New Drugs 18:205-220. [DOI] [PubMed] [Google Scholar]
- 42.Wang, E. J., C. N. Casciano, R. P. Clement, and W. W. Johnson. 2001. Active transport of fluorescent P-glycoprotein substrates: evaluation as markers and interaction with inhibitors. Biochem. Biophys. Res. Commun. 289:580-585. [DOI] [PubMed] [Google Scholar]
- 43.White, T. C., S. Holleman, F. Dy, L. F. Mirels, and D. A. Stevens. 2002. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob. Agents Chemother. 46:1704-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, W., and W. F. Pfender. 1992. Effect of residue management on wetness duration and ascocarp production by Pyrenophora tritici-repentis in wheat residue. Phytopathology 82:1434-1439. [Google Scholar]
- 45.Zheng, J., and V. D. Ramirez. 2000. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br. J. Pharmacol. 130:1115-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zwiers, L. H., I. Stergiopoulos, M. M. Gielkens, S. D. Goodall, and M. A. de Waard. 2003. ABC transporters of the wheat pathogen Mycosphaerella graminicola function as protectants against biotic and xenobiotic toxic compounds. Mol. Genet. Genom. 269:499-507. [DOI] [PubMed] [Google Scholar]