Abstract
Nitrate and phosphate concentrations higher than those found in the natural environment slowed down growth of two strains of non-bloom-forming, phycoerythrin-rich Synechococcus spp. isolated from mesotrophic subalpine lakes. The results make clear why isolation of these picocyanobacteria in standard cultivation media was difficult. At low concentrations, closely related strains exhibited distinct growth characteristics with respect to these two nutrients, possibly explaining differences in their seasonal appearance in the natural environment.
At low levels of nutrient loading, small red-pigmented phycoerythrin (PE)-rich cyanobacteria of the Synechococcus type dominate the autotrophic picoplankton in freshwater ecosystems (10, 12, 16, 21). These non-bloom-forming Synechococcus spp. (19) belong to the same phylogenetic clade as marine Synechococcus spp. and Prochlorococcus spp. (7, 8, 20). Despite their ubiquity, they were discovered in the late 1970s only, when epifluorescence microscopy and flow cytometry were introduced as counting techniques (see references in reference 18) and the first isolates from marine and freshwater ecosystems became available (6, 23). The difficulty in cultivating red-pigmented freshwater Synechococcus spp. contrasts with the easy accessibility of blue-green, phycocyanin-rich species, particularly those of the closely related genus Cyanobium (8, 22; for strain histories, see reference 14).
Recently, Becker et al. (3) reported that PE-rich picocyanobacteria from a biofilm of tiles deposited for 6 weeks in Lake Constance formed small colonies on agar plates amended with a mineral medium lacking nitrate, BG110, although none of them was capable of nitrogen fixation. A similar inoculum spread on plates with nitrate (BG11) produced blue-green colonies only. The mineral medium BG11 was introduced by Stanier et al. (17) for the cultivation of coccoid cyanobacteria. In its original description, it is particularly rich in nitrate (1.5 g/liter NaNO3, equivalent to 247 mg/liter NO3-N) and exhibits an N:P molar ratio of 100:1, which is far above the molar ratio of these elements in biomass (Redfield ratio, 16:1). Nevertheless, phosphate also is plentiful in BG11 compared to the environmental concentrations often limiting the growth of photoautotrophs in meso- and oligotrophic lakes.
Cyanobacteria have developed highly efficient uptake and retention mechanisms for three nutrient anions: bicarbonate, nitrate, and phosphate (1, 11, 15). The apparent competitive disadvantage of PE-rich Synechococcus spp. on a cultivation medium rich in nitrate prompted us to examine the effect of high nutrient concentrations on the growth of two strains, Synechococcus rubescens strain SAG 3.81 and Synechococcus sp. strain BO 8807, isolated from Lake Zürich and Lake Constance, respectively.
Growth inhibition by phosphate and nitrate.
The two red-pigmented Synechococcus strains BO 8807 and SAG 3.81 of subalpine cluster I (8) were raised in 96-well microtiter plates. Before growth experiments were conducted, aliquots of batch cultures were diluted into fresh growth medium (BG11 amended with sterile solutions of sodium bicarbonate [0.168 g/liter] and vitamin B12 [0.02 mg/liter]) and maintained at least 20 h under continuous light (10 ± 1 μmol quanta m−2 s−1) at 19 ± 1°C, the conditions used during growth experiments. After this preadaptation, cells were collected by centrifugation (10 min; 10,000 × g), washed, and resuspended in this medium lacking nitrate and phosphate. Sterile dilutions of NaNO3 and K2HPO4 were added, in a volume of 10 μl each, to the 96 wells of flat-bottom microtiter plates (Greiner). Finally, the resuspended cells were added in a volume of 130 μl. The microtiter plates were taped at the long sides and then placed on a microtiter plate vortexer (Applied Quality Services, United Kingdom) that was set to 2 min of vigorous shaking per h to counteract sedimentation of cells.
The 96-well format allowed not only the application of a wide range of nutrient loadings and N:P ratios but also monitoring of growth with a microplate reader (ELx808; Bio-Tek, Germany), a convenient and nonintrusive detection method. Growth was followed by measuring the optical density at 750 nm (OD750). At this wavelength, the pigments of cyanobacteria exhibit negligible absorbance and, hence, turbidity can be used as a measure for biomass.
At low initial nutrient concentrations (0.3 mg/liter K2HPO4 and 15 mg/liter NaNO3), biomass did not increase significantly. Both concentrations were higher than those measured in Lake Constance, which exhibited a total P concentration of 40 μg/liter and 4.3 mg/liter NO3 in 1988, when a maximum of 7.7 × 105 cells/ml of autotrophic picoplankton was reported (9). The initial cell density of (2 to 4) × 107 cells/ml in our experiments may have already exceeded the carrying capacity. Also, at an initial NaNO3 concentration of 9.9 g/liter, the highest nitrate concentration used, biomass of both strains did not increase during the duration of the experiments (10 to 14 days in different series). At intermediate concentrations, growth strongly depended on the initial phosphate and nitrate concentrations (Fig. 1). The total biomass formation was limited by nitrate if the molar N:P ratio was ≤33 and was limited by phosphate at higher N:P ratios, a factor two higher than that expected from the Redfield ratio. For both strains, high initial nitrate and phosphate concentrations delayed growth. The initial phosphate concentration used in BG11 (0.03 g/liter K2HPO4) inhibited growth of both strains even at low nitrate concentrations (Fig. 1c and f). In combination with the high nitrate concentration of BG11 (1.5 g/liter; Fig. 1c and f), both strains did not reach stationary phase within 2 weeks. Similar growth characteristics were reported earlier for two other strains of the subalpine cluster I (13).
FIG. 1.
Growth of Synechococcus sp. strain BO 8807 (a to c) and Synechococcus rubescens strain SAG (d to f) in microtiter plates. Initial concentrations of K2HPO4 (mg/liter) were 3.0 in a and d; 9.0 in b and e; and 30 in c and f. The initial NaNO3 concentrations (g/liter) are indicated in panel c. Growth was recorded as absorbance at 750 nm. The initial concentrations of chlorophyll a (μg/ml in a methanol extract) varied between 0.23 and 0.31 in different experimental series. Continuous lines represent logistic fits of growth. Note that the scale of the y axis differs for the different initial phosphate concentrations.
In batch cultures, growth rates are not constant because the concentration of the limiting nutrient decreases during growth. Growth limited by a vanishing resource is described by logistic growth models. For the logistic fit of the growth measured at OD750, we applied the equation
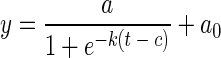 |
(1) |
The sigmoid shape of the growth curve is characterized by four parameters: the upper asymptote, a, represented by the absorbance reached in the stationary phase, a rate parameter, k, describing the rate at which growth initially accelerates, and a time constant, c, that describes the time elapsing between the beginning of the growth experiment and the point of maximum increase in biomass (turning point). To fit the data of our experiments, an offset in the absorbance (a0), which remained constant throughout the experiment, was added. In Fig. 1, the fits of individual growth curves are depicted as continuous lines. The model fitted growth of the two PE-rich Synechococcus strains well except at high nutrient concentrations at which growth was inhibited and cultures did not reach stationary phase before termination of the experiments.
From the parameters we calculated the biomass-specific initial growth rate at t = 0 (see Appendix). These calculations showed that k is an excellent approximation of the initial, biomass-specific growth rate. Hence, we used k as an approximation for the maximum growth rate at the indicated nutrient concentration.
For both Synechococcus strains, we conducted two series of growth experiments, both with an internal replicate for every concentration used. To calculate the growth parameters a, a0, c, and k for multiple experiments, the OD750 values were imported into the program Origin (version 7.5; OriginLab Corp.). A script for data management and analysis was written in LabTalk, a C-based scripting language provided by this program. The script was structured in four subroutines: first, the extinction values (triplicates) were averaged and the intrinsic absorption of each well filled with 150 μl H2O was subtracted. Next, time series were constructed with the net extinction values. Third, the time series were fitted assuming a logistic growth function (equation 1). Finally, the growth parameters of experiments with identical initial nutrient concentrations were averaged, and standard deviations were calculated (the peripheral wells of the 96-well plates were not considered). The results (Fig. 2) show that carrying capacity (a) was similar for both strains. Growth rate constants k and time constants c (not shown) were almost identical in multiple experiments conducted with the same starting material (internal replicates) but differed between experimental series. This variation is reflected in the error bars of the growth rate constant (Fig. 2). However, this variation did not obscure the effects of nutrient concentrations on growth outlined above. The generation time of both strains was about six times longer for BG11 than that in media with lower, optimal nutrient concentrations.
FIG. 2.
Carrying capacity a and growth rate constant k of Synechococcus strains BO 8807 (a) and SAG 3.81 (b) in media containing different initial concentrations of nitrate and phosphate. The figure is divided into six sections, each representing a series of experiments for which the initial K2HPO4 concentration is as indicated below the section. For each K2HPO4 concentration, six initial NaNO3 concentrations were used as indicated in panel a. The growth parameters a (hatched bars) and k (▾) were derived from logistic fits of individual growth curves (see Fig. 1) and represented as an average with standard deviation for two to eight growth experiments. Note that in some experiments, the increase in biomass was too small to determine growth rate constants.
With these results in mind, we prepared agarose plates with BG11 (plus vitamin B12) and four versions of the bicarbonate-enriched medium, one with original concentrations of nutrients and three in which phosphate was reduced to 30% and nitrate to either 10%, 3%, or 1%. These plates were inoculated with serial dilutions of the two red-pigmented Synechococcus strains used in this study and a blue-green isolate, strain BO 8806, which is closely related to Cyanobium gracile PCC 6307 (8). The blue-green strain was able to form colonies with high plating efficiency (>90%) on all variants of the medium (in Table 1, only colony numbers of a 104-fold dilution of the preadapted cultures are shown). In contrast, dilutions of the red-pigmented strains failed to form colonies in full-strength BG11 with or without bicarbonate. However, both strains grew with only slightly lower plating efficiency on all plates with lowered nutrient concentrations (Table 1). Apparently, on the surfaces of agarose plates, the inhibitory effect of high nutrient concentrations on the growth of the red-pigmented strains was enhanced by evaporation, resulting in a very strong, negative isolation bias. The results are in full agreement with the observations of Becker et al. (3) reporting a selective suppression of colony formation of red-pigmented picoplankton by BG11.
TABLE 1.
Number of colonies of Synechococcus sp. strains BO 8806 and BO 8807 and Synechococcus rubescens strain SAG 3.81 on agarose plates amended with variations of the mineral medium BG11
| Growth medium | No. of colonies of:
|
||
|---|---|---|---|
| BO 8806a | BO 8807b | SAG 3.81b | |
| BG11c | 213 | 0 | 0 |
| BG11+Bd | 195 | 0 | 0 |
| P30, N10e | 252 | 30 | 199 |
| P30, N3f | 197 | 23 | 181 |
| P30, N1g | 187 | 43 | 208 |
Colonies per plate counted after 4 to 6 weeks.
Colonies per plate counted after 12 weeks.
Original BG11 (see reference 17), vitamin B12 added.
As described in footnote c but with bicarbonate added.
As described in footnote d but with phosphate lowered to 30% and nitrate lowered to 10% of the original.
As described in footnote e but with nitrate lowered to 3% of the original.
As described in footnote f but with nitrate lowered to 1% of the original.
Clonal effects of nitrate and phosphate.
Figures 1 and 2 indicate that nitrate and phosphate affected growth of the two Synechococcus strains differently. Increasing nitrate concentrations delayed growth of strain BO 8807 (Fig. 1a to c and 3a) even at low initial phosphate levels, while growth of strain SAG 3.81 was affected by high nitrate only. On the other hand, growth of strain SAG. 3.81 was delayed and inhibited by increasing phosphate (Fig. 1d to f and 3b), while strain BO 8807 not only needed higher initial phosphate concentrations to obtain highest growth rates but also tolerated high phosphate concentrations for cultivation, provided nitrate was low. Thus, in strain BO 8807 the tolerance towards phosphate seemed to be paired with a high sensitivity towards nitrate, while in strain SAG 3.81 tolerance of nitrate is paired with high sensitivity towards phosphate. Contour plots summarize the combined effect of nitrate and phosphate on carrying capacity and growth rate constants of the two strains (Fig. 3).
FIG. 3.
Contour plots depicting the growth parameters a and k of Synechococcus strains BO 8807 (a, b) and SAG (c, d) as a function of the initial concentrations of nitrate and phosphate. Contour plots were constructed from the growth parameters shown in Fig. 2, placed at the positions indicated by black dots. The lines indicate constant values of a or k, respectively, that were calculated using the weighted distance algorithm provided by the program Statistica with a stiffness of 0.2. Note that the logarithmic scales in a and c (carrying capacity a) deviate from those of b and d (growth rate constant k).
The observation that strain BO 8807 tolerates high concentrations of phosphate but is highly sensitive towards nitrate, while strain SAG 3.81 can tolerate high nitrate if phosphate is low, indicates that uptake and assimilation of these two anions is endogenously limited by a common resource. Thus, the question remains why non-bloom-forming Synechococcus spp. cannot cope with high nutrient levels or what is the limiting endogenous resource. Most studies of the regulation of N, P, and C assimilation were conducted with blue-green freshwater cyanobacteria growing well in presence of high concentrations of nitrate and phosphate. Presumably, all these strains are capable of efficiently limiting uptake and assimilation of N (via NtrA [11]) and P (via the P-regulon [4]) when high concentrations (at low light intensity) threaten to impair photosynthetic assimilation of CO2. Such a control may be missing not only in the oceanic Synechococcus sp. strain WH 8103 (5) but also in non-bloom-forming freshwater Synechococcus spp., with the reported effects with cultivation in the presence of high nutrient levels. However, at ambient nutrient levels, the particular growth characteristics of the two strains may be of relevance. In summer picoplankton, when phosphate reaches a minimum in the euphotic zone of Lake Constance (9), genotypes closely related to that of strain SAG 3.81 are abundant, while the genotype of isolate BO 8807 reaches a relative and absolute minimum (2; S. Becker, P. Richl, P. Boger, and A. Ernst, unpublished data). As growth of this genotype is more constrained by low levels of phosphate, this may reflect its particular growth characteristics.
Acknowledgments
This work was partly financed by the Commission of the European Community through RTD project MIRACLE (contract: EVK3-CT-2002-00087).
The comments of L. Stal during preparation of the manuscript were greatly appreciated.
APPENDIX
The growth rate of a logistic model can be calculated from the equation
 |
From this equation, the initial biomass-specific growth rate (IGR) at t = 0 and y = y0 can be calculated as
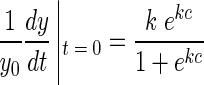 |
For c ≫ k, the biomass-specific IGR approaches the value of the growth rate constant k, and the biomass-specific growth rate at the turning point is half that of the initial growth rate. In all our experiments, k provided an excellent approximation of IGR.
Footnotes
This is publication 3506 NIOO-KNAW of the Netherlands Institute of Ecology.
REFERENCES
- 1.Badger, M. R., and G. D. Price. 2003. CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. J. Exp. Bot. 54:609-622. [DOI] [PubMed] [Google Scholar]
- 2.Becker, S., M. Fahrbach, P. Böger, and A. Ernst. 2002. Quantitative tracing, by Taq nuclease assays, of a Synechococcus ecotype in a highly diversified natural population. Appl. Environ. Microbiol. 68:4486-4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker, S., A. K. Singh, C. Postius, P. Böger, and A. Ernst. 2004. Genetic diversity and distribution of periphytic Synechococcus spp. in biofilms and picoplankton of Lake Constance. FEMS Microbiol. Ecol. 49:181-190. [DOI] [PubMed] [Google Scholar]
- 4.Bhaya, D., D. Vaulot, P. Amin, A. W. Takahashi, and A. R. Grossman. 2000. Isolation of regulated genes of the cyanobacterium Synechocystis sp. strain PCC 6803 by differential display. J. Bacteriol. 182:5692-5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bird, C., and M. Wyman. 2003. Nitrate/nitrite assimilation of the marine picoplanktonic cyanobacterium Synechococcus sp. strain WH 8103: effect of nitrogen source and availability on gene expression. Appl. Environ. Microbiol. 69:7009-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, T.-P. 1980. Zwei neue Synechococcus-Arten aus dem Zurichsee. Schweiz. Z. Hydrobiol. 42:247-254. [Google Scholar]
- 7.Crosbie, N. D., M. Pöckl, and T. Weisse. 2003. Dispersal and phylogenetic diversity of nonmarine picocyanobacteria, inferred from 16S rRNA gene and cpcBA-intergenic spacer sequence analysis. Appl. Environ. Microbiol. 69:5716-5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernst, A., S. Becker, U. I. A. Wollenzien, and C. Postius. 2003. Ecosystem-dependent adaptive radiations of picocyanobacteria inferred from 16S rRNA and ITS-1 sequence analysis. Microbiology (United Kingdom) 149:217-228. [DOI] [PubMed] [Google Scholar]
- 9.Gaedke, U., and T. Weisse. 1998. Seasonal and interannual variability of picocyanobacteria in Lake Constance (1987-1997). Arch. Hydrobiol. Spec. Issues Adv. Limnol. 53:143-158. [Google Scholar]
- 10.Hawley, G. R. W., and B. A. Whitton. 1991. Seasonal changes in chlorophyll-containing picoplankton populations of ten lakes in Northern England. Int. Rev. Gesamten Hydrobiol. 76:545-554. [Google Scholar]
- 11.Herrero, A., A. M. Muro-Pastor, and E. Flores. 2001. Nitrogen control in cyanobacteria. J. Bacteriol. 183:411-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pick, F. R. 1991. The abundance and composition of freshwater picocyanobacteria in relation to light penetration. Limnol. Oceanogr. 36:1457-1462. [Google Scholar]
- 13.Postius, C., U. Kenter, A. Wacker, A. Ernst, and P. Böger. 1998. Light causes selection among two phycoerythrin-rich Synechococcus isolates from Lake Constance. FEMS Microbiol. Ecol. 25:171-178. [Google Scholar]
- 14.Rippka, R., J. Deruelles, J. B. Waterbeury, M. Herdman, and R. Y. Stanier. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1-61. [Google Scholar]
- 15.Ritchie, R. J., D. A. Trautman, and A. W. D. Larkum. 2001. Phosphate uptake in the cyanobacterium Synechococcus R-2 PCC 7942. Plant Cell Physiol. 38:1232-1241. [Google Scholar]
- 16.Søndergaard, M. 1991. Phototrophic picoplankton in temperate lakes: seasonal abundance and importance along a trophic gradient. Int. Rev. Gesamten Hydrobiol. 76:505-522. [Google Scholar]
- 17.Stanier, R. Y., R. Kunisawa, M. Mandel, and G. Cohen-Baziere. 1971. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 35:171-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stockner, J. G. 1988. Phototrophic picoplankton: an overview from marine and freshwater ecosystems. Limnol. Oceanogr. 33:765-775. [Google Scholar]
- 19.Stockner, J. G., C. Callieri, and C. Cronberg. 2000. Picoplankton and other non-bloom-forming cyanobacteria in lakes, p. 195-231. In B. A Whitton and M. Potts (ed.), The ecology of cyanobacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 20.Urbach, E., D. J. Scanlan, D. L. Distel, J. B. Waterbury, and S. W. Chisholm. 1998. Rapid diversification of marine picoplankton with dissimilar light harvesting structures inferred from sequences of Prochlorococcus and Synechococcus (cyanobacteria). J. Mol. Evol. 46:188-201. [DOI] [PubMed] [Google Scholar]
- 21.Vörös, L., C. Callieri, K. V. Balogh, and R. Bertoni. 1998. Freshwater picocyanobacteria along trophic gradient and light quality range. Hydrobiologia 369/370:117-125. [Google Scholar]
- 22.Waterbury, J. B., and R. Rippka. 1989. Subsection I, order Chroococcales Wettstein 1924, emend. Rippka et al., 1979, p. 1728-1746. In S. T. Williams, M. E. Sharpe and J. G. Holt (ed.), Bergey's manual of systematic bacteriology. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 23.Waterbury, J. B., S. W. Watson, R. R. Guillard, and L. E. Brand. 1979. Widespread occurrence of a unicellular, marine planktonic cyanobacterium. Nature 277:293-294.16069038 [Google Scholar]





