Abstract
Lactobacillus rhamnosus GG is an industrially significant probiotic strain with proven health benefits. In this study, the effect of glucose on L. rhamnosus GG survival was analyzed in simulated gastric juice at pH 2.0. It was found that the presence of 19.4 mM glucose resulted in up to 6-log10-enhanced survival following 90 min of exposure. Further work with dilute HCl confirmed that glucose was the sole component responsible. Comparative analysis with other Lactobacillus strains revealed that enhanced survival was apparent in all strains, but at different pH values. The presence of glucose at concentrations from 1 to 19.4 mM enhanced L. rhamnosus GG survival from 6.4 to 8 log10 CFU ml−1 in simulated gastric juice. The mechanisms behind the protective effect of glucose were investigated. Addition of N′,N′-dicyclohexylcarbodiimide to simulated gastric juice caused survival to collapse, which was indicative of a prominent role in inhibition of F0F1-ATPase. Further work with neomycin-resistant mutants that exhibited 38% to 48% of the F0F1-ATPase activity of the parent confirmed this, as the survival in the presence of glucose of these mutants decreased 3 × 106-fold compared with the survival of the wild type (which had a viability of 8.02 log10 CFU ml−1). L. rhamnosus GG survival in acidic conditions occurred only in the presence of sugars that it could metabolize efficiently. To confirm the involvement of glycolysis in the glucose effect, iodoacetic acid was used to inhibit glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity. The reduction in GAPDH activity caused survival to decrease by 8.30 log10 CFU ml−1 in the presence of glucose. The data indicate that glucose provides ATP to F0F1-ATPase via glycolysis, enabling proton exclusion and thereby enhancing survival during gastric transit.
Probiotics for human consumption, generally either lactobacilli or bifidobacteria, are of increasing interest due to the growing evidence of health benefits associated with their use, and they represent a significant growth area in the functional foods industry (1, 27, 49). Lactobacilli do not predominate among the intestinal microflora; however, they are isolated throughout the gastrointestinal tract (GIT) of healthy humans (22). It is desirable that probiotics have suitable general aspects (origin, identity, safety, and acid and bile resistance), technical aspects (growth properties in vitro and during processing), and functional and beneficial features (28). Lactobacilli fulfill these criteria (19), and there is sound evidence of clinical benefits (for a review see reference 43). For example, Lactobacillus rhamnosus GG has been found to be beneficial in the treatment of diarrhea (31) and atopic eczema (32).
Probiotics must survive in the acidic gastric environment if they are to reach the small intestine and colonize the host, thereby imparting their benefits. Lactobacillus species are considered intrinsically resistant to acid (51). Although there are differences between species and strains, organisms generally exhibit increased sensitivity at pH values below 3.0 (34, 44). Hence, acid tolerance is accepted as one of the desirable properties used to select potentially probiotic strains. As indicated above, the human-derived strain L. rhamnosus GG is a commercial probiotic strain with recognized health benefits, and it is also amenable to food processing (11, 38). The ability of L. rhamnosus GG to survive passage through the GIT has been demonstrated in both children and adults (26, 40, 45), and this strain is resistant to pH values as low as 2.5 for 4 h (33). In order to survive in this harsh environment, L. rhamnosus GG must prevail over host defense mechanisms, such as gastric activity and bile (50). Gastric transit studies of probiotics have been conducted using both simulated gastric juice and animal and human gastric juices (8, 9, 18, 25). Both of these approaches have limitations; the former does not accommodate the influence of dietary and nonacid constituents of gastric secretions on probiotic survival, and the latter is restricted by the availability of fresh material (8). In addition, the exploitation of rich media, such as acidified MRS medium, may provide protection to bacteria by providing energy and metabolic precursors. The use of food ingredients to enhance probiotic survival through the GIT has been extensively studied (8, 9, 18, 25, 50). However, few data are available to describe the effects of individual food components and their underlying mechanisms of action for enhancing the survival of lactobacilli (6, 7).
The acid tolerance of lactobacilli is attributed to the presence of a constant gradient between extracellular and cytoplasmic pH. When the internal pH reaches a threshold value, cellular functions are inhibited and the cells die (35). The F0F1-ATPase is a known mechanism that gram-positive organisms use for protection against acidic conditions (14). The F0F1-ATPase is a multiple-subunit enzyme consisting of a catalytic portion (F1) incorporating the α, β, γ, δ, and ɛ subunits for ATP hydrolysis and an integral membrane portion (F0) including the a, b, and c subunits, which function as a membranous channel for proton translocation (46). The role of the F0F1-ATPase in organisms devoid of a respiratory chain is to generate a proton motive force, via proton expulsion. As a consequence, it is thought that the F0F1-ATPase can increase the intracellular pH at a low extracellular pH. F0F1-ATPase is induced at low pH, and regulation appears to occur at the transcriptional level (23).
The increased survival of probiotic lactobacilli in acidic conditions in the presence of glucose has been reported previously (7). However, the mechanisms involved were not studied. In addition, it has been reported that lactic acid bacteria are capable of metabolizing glucose at low pH, albeit at lower rates (29, 54). The aims of this study were to evaluate the effect of glucose on L. rhamnosus GG survival in simulated gastric juice, to compare the protective effect of glucose on L. rhamnosus GG survival at low pH with that for other probiotic lactobacilli, and to elucidate the mechanisms responsible for the protective effect of glucose in acidic conditions.
MATERIALS AND METHODS
Bacterial strains.
The probiotic strains L. rhamnosus VTT E-97800 (= E800; VTT Biotechnology, Espoo, Finland), L. rhamnosus VTT E-94522 (= ATCC 53103 = GG; Valio Ltd., Finland), Lactobacillus salivarius VTT E-01878 (= UCC 500; University College, Cork, Ireland), and L. paracasei NFBC 338 (University College, Cork, Ireland) and Lactobacillus gasseri ATCC 33323 were obtained from University College Cork under a restricted materials transfer agreement. Harvested cells of these strains were stored as stock solutions in 50% (vol/vol) aqueous glycerol at −20°C.
Culture conditions.
Strains were subcultured (1%, vol/vol) in MRS (17) medium (Oxoid Ltd., Hampshire, United Kingdom) for ∼17 h at 37°C under anaerobic conditions. For enumeration of viable microorganisms in acid tolerance studies, samples were pour plated on MRS agar (Oxoid) in independent triplicate experiments. Cultures were serially diluted in maximum-recovery diluent (10% [wt/vol]; Oxoid), and the appropriate serial dilutions were prepared prior to pour plating on MRS agar.
Preparation of simulated gastric juice.
Simulated gastric juice was prepared as previously described (5), with modifications. Proteose peptone was omitted from the formulation as it may be a source of free amino acids, such as l-glutamate, which may have been used to extrude protons from the cell, thus potentially enhancing bacterial survival (13). Simulated gastric juice was formulated using glucose (3.5 g liter−1), NaCl (2.05 g liter−1), KH2PO4 (0.60 g liter−1), CaCl2 (0.11 g liter−1), and KCl (0.37 g liter−1), adjusted to pH 2.0 using 1 M HCl, and autoclaved at 121°C for 15 min. Porcine bile (0.05 g liter−1), lysozyme (0.1 g liter−1), and pepsin (13.3 mg liter−1) were added as stock solutions prior to analysis. Components were obtained from Sigma, AnalaR (BDH Chemicals Ltd., Poole, England), and Orthana (Orthana Kemisk Fabrik A/S, Kastrup, Denmark).
Comparative survival of probiotic lactobacilli in a simulated gastric environment.
Cultures of L. rhamnosus E800, L. rhamnosus GG, L. salivarius UCC 500, L. paracasei NFBC 338, and L. gasseri ATCC 33323 were grown overnight (16 h) in 25 ml MRS medium and subcultured by using 1% (vol/vol) inocula. The cultures were subsequently centrifuged at 7,000 × g at 4°C for 15 min, washed once in an equal volume of cold 0.25× Ringer's solution, and subsequently centrifuged (7,000 × g at 4°C for 15 min). Pellets were then resuspended in an equal volume of simulated gastric juice at 37°C and incubated for 90 min with constant stirring. Samples were taken at 0, 10, 30, 60, and 90 min, serially diluted in maximum-recovery diluent, plated on MRS medium, and incubated at 37°C for 72 h.
Effects of different components in simulated gastric juice on survival of L. rhamnosus GG and comparative survival of probiotic lactobacilli in the presence and absence of glucose in simulated gastric juice.
In order to analyze the effects of various components of simulated gastric juice on the survival of L. rhamnosus GG, components were systematically excluded from the gastric juice preparation, and viability was monitored as described above. L. rhamnosus GG survival was also assayed in dilute HCl (pH 2.0) with and without glucose, and viability was analyzed as described above. To study comparative survival of probiotic lactobacilli in the presence and absence of glucose in simulated gastric juice, the probiotic lactobacilli described above were assayed in a single-time experiment (45 min) in simulated gastric juice at pH 2.0. Cultures that did not show enhanced survival in the presence of glucose at pH 2.0 were assayed in simulated gastric juice at alternative pH values, depending on the strain studied.
Inactivation of the F0F1-ATPase of L. rhamnosus GG.
Four 25-ml cultures of L. rhamnosus GG (grown for 16 h) were prepared for studies of survival in simulated gastric juice, except that that cells were centrifuged, resuspended in 0.25× Ringer's solution, and incubated for 1 h at 37°C to deplete residual glucose. N′,N′-Dicyclohexylcarbodiimide (DCCD) (1.4 mM; Sigma), prepared as an ethanol stock containing 288.86 mg ml−1, was added to two of the cultures 15 min prior to harvesting via centrifugation. Cultures were then assayed for survival in simulated gastric juice, pH 2.0, either in the presence or in the absence of glucose, as described above.
Isolation of L. rhamnosus GG mutants with reduced F0F1-ATPase activity.
Isolation of spontaneous neomycin-resistant mutants of L. rhamnosus GG was performed as described by Yokota et al. (59), with some modifications. Bacteria were grown in 40 ml of MRS medium until the exponential phase was reached (optical density at 600 nm, 0.3). Cells were harvested by centrifugation (7,000 × g at 4°C for 15 min), concentrated in 2 ml of fresh MRS medium, and then spread onto MRS agar plates containing 300 μg ml−1 of neomycin sulfate (Sigma) and incubated anaerobically at 37°C for 72 h. Neomycin-resistant colonies were selected and inoculated into 5 ml of MRS medium containing 300 μg ml−1 neomycin sulfate. Forty isolates were selected on the basis of their growth characteristics under optimal growth conditions. Four neomycin-resistant mutants designated m5, m8, m14, and m18, whose growth profiles were most affected at pH 4.5, were selected for studies in simulated gastric juice containing glucose.
ATPase assay of L. rhamnosus GG mutants with reduced F0F1-ATPase activity.
The ATPase activity of permeabilized wild-type and mutant strains of L. rhamnosus GG was determined as previously described (3). Samples (5 ml) of fresh overnight cultures were centrifuged at 7,000 × g at 4°C, and cells from each sample were resuspended in 250 μl of 75 mM Tris-HCl buffer (pH 7.0) with 10 mM MgSO4. Toluene (25 μl) was added to each cell suspension prior to vigorous vortex mixing and incubation for 5 min at 37°C. Each cell suspension was then subjected to two cycles of freezing in ethanol at −80°C and thawing at 37°C. Permeabilized cells were then harvested by centrifugation at 15,000 × g. They were then resuspended in 200 μl of 75 mM Tris-HCl buffer (pH 7.0) with 10 mM MgSO4. The suspension was rapidly frozen and stored at −80°C.
A 25-μl sample of a permeabilized cell suspension was added to 1.0 ml of 50 mM Tris-maleate buffer (pH 6.0) with 10 mM MgSO4 at 37°C. The ATPase reaction was initiated by addition of 10 μl of 0.5 M ATP (pH 6.0) and was allowed to proceed at 37°C for 15 min. Samples (50 μl) were removed and assayed for inorganic phosphate liberated from cleavage of ATP by the Fiske-SubbaRow method (56). ATPase activities were expressed as micromoles of phosphate released from ATP per minute per mg of protein.
Growth of L. rhamnosus GG in metabolizable and nonmetabolizable carbohydrates.
Fresh overnight cultures of L. rhamnosus GG were inoculated (1% inocula) into MRS medium prepared from first principles using glucose, lactose, or fructose as a carbohydrate source. Growth was assessed by determining the optical density at 600 nm with a Genesys 5 Thermo Spectronic spectrophotometer (Milton Roy, Rochester, NY). The acidification of cultures was analyzed with a pH meter (model MP220; Mettler-Toledo, Griefensee, Switzerland) with a calibrated electrode (Mettler Toledo InLab 413).
Inactivation of L. rhamnosus GG GAPDH using iodoacetate and glycolysis analysis.
In order to reduce glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity, 10 μM iodoacetic acid (IAA) (Sigma) was added to overnight 25-ml cultures of L. rhamnosus GG 15 min prior to harvesting by centrifugation at 7,000 × g at 4°C. Cultures were subsequently washed in 0.25× Ringer's solution (4°C), centrifuged again (7,000 × g at 4°C), and assayed for survival in simulated gastric juice (pH 2.0) in the presence or absence of glucose as described above.
RESULTS
Comparative survival of probiotic lactobacilli in simulated gastric juice.
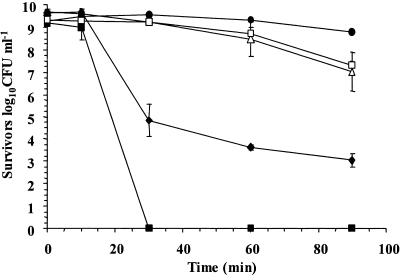
In order to evaluate the survival of lactobacilli in acidic conditions, we compared the survival of five Lactobacillus strains in simulated gastric juice, pH 2.0, for 90 min. L. rhamnosus GG had the highest survival rate over the 90 min of exposure to simulated gastric juice (pH 2.0), while the poorest survivor was Lactobacillus paracasei NFBC 338, whose concentration declined to undetectable levels after only 30 min of exposure (Fig. 1). While L. rhamnosus GG exhibited good survival in this system, a second L. rhamnosus strain, strain E800, was a poor survivor; the concentration of this strain declined by 6.62 log10 CFU ml−1 over 90 min. Overall, the data showed that the survival rates of Lactobacillus species differ and that differences are also apparent at the strain level.
FIG. 1.
Survival of L. rhamnosus GG (•), L. gasseri ATCC 33323 (□), L. salivarius UCC 500 (▵), L. rhamnosus E800 (♦), and L. paracasei NFBC 338 (▪) in simulated gastric juice, pH 2.0. The data are the means of triplicate experiments, and the error bars indicate standard deviations.
Effect of removal of components from simulated gastric juice on L. rhamnosus GG survival.
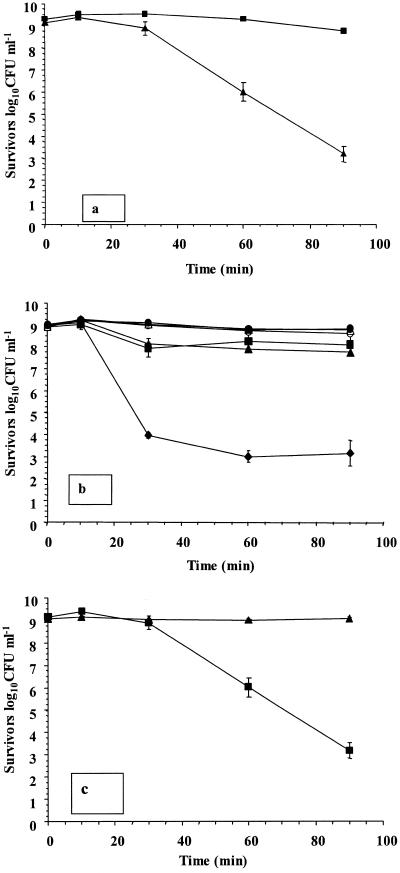
We then determined whether any individual component in simulated gastric juice was responsible for enhanced survival of L. rhamnosus GG cultures. The concentration of surviving L. rhamnosus GG was over 5.5 log10 CFU ml−1 lower in dilute acid (pH 2.0) than in simulated gastric juice (pH 2.0) after 90 min of exposure (Fig. 2a). Therefore, an analysis of individual components was conducted. It was found that the glucose component (19.4 mM) was responsible for the enhanced survival of L. rhamnosus GG in simulated gastric juice. The level of survival fell by approximately 5.6 log10 CFU ml−1 upon removal of this component (Fig. 2b). Small reductions in viability (0.97 to 1.15 log10 CFU ml−1) occurred in cultures devoid of lysozyme or CaCl2. In addition, L. rhamnosus GG survived in dilute HCl, pH 2.0, when glucose was included (Fig. 2c), confirming that the presence of glucose alone in acidic conditions was responsible for the protective effect observed. In addition, microscopic analysis indicated that the chain length or morphology of cells did not change during the exposure period, either in the presence or in the absence of glucose (results not shown).
FIG. 2.
(a) Survival of L. rhamnosus GG in simulated gastric juice containing glucose, pH 2.0 (▪), and dilute HCl, pH 2.0 (▴); (b) survival of L. rhamnosus GG in simulated gastric juice, pH 2.0, without lysozyme (▴), KCl (•), glucose (♦), CaCl2 (▪), pepsin (▵), or KH2PO4 (○); (c) survival of L. rhamnosus GG in dilute HCl, pH 2.0, in the presence (▴) or absence (▪) of 19.4 mM glucose. The data are the means of triplicate experiments, and the error bars indicate standard deviations.
Comparative analysis of the effect of glucose on survival of probiotic lactobacilli.
In order to analyze whether glucose enhances survival of other probiotic or intestinal lactobacilli in a simulated gastric environment, stationary-phase cultures (approximately 109 CFU ml−1) were assayed for survival in a single-time analysis following exposure for 45 min (Table 1). The results showed that the greatest survival effect attributable to glucose occurred in L. rhamnosus GG cultures, while L. gasseri ATCC 33323 did not require the presence of glucose at pH 2.0 for optimal survival. The results therefore indicated that L. gasseri ATCC 33323 was the most intrinsically acid-resistant strain studied, as it had the best survival in the absence of glucose at pH 2.0 (7.63 log10 CFU ml−1). However, when the pH of simulated gastric juice was reduced to 1.75, an enhanced survival effect in L. gasseri ATCC 33323 cultures grown in the presence of glucose was clearly observed (4.80 log10 CFU ml−1), although the concentration was approximately 2.7 log10 CFU ml−1 lower than the concentration of L. rhamnosus GG. Similarly, when the pH was increased to 2.25, an enhanced protective effect of glucose on L. paracasei NFBC 338 was observed (5.10 log10 CFU ml−1).
TABLE 1.
Survival of cultures of probiotic lactobacilli exposed to simulated gastric juice in the presence of 19.4 mM glucose for 45 mina
| Strain | pH | Glucose | Concn (log10 CFU ml−1) at:
|
|
|---|---|---|---|---|
| Zero time | 45 min | |||
| L. rhamnosus GG | 2.0 | Yes | 9.03 (0.08)b | 8.84 (0.24) |
| L. rhamnosus GG | 2.0 | No | 9.09 (0.10) | 1.31 (0.38) |
| L. rhamnosus E800 | 2.0 | Yes | 8.96 (0.09) | 4.39 (1.17) |
| L. rhamnosus E800 | 2.0 | No | 9.02 (0.01) | 1.71 (1.22) |
| L. salivarius UCC 500 | 2.0 | Yes | 9.38 (0.04) | 5.35 (0.77) |
| L. salivarius UCC 500 | 2.0 | No | 9.38 (0.07) | 3.29 (0.32) |
| L. paracasei NFBC 338 | 2.0 | Yes | 8.83 (0.05) | 3.52 (0.74) |
| L. paracasei NFBC 338 | 2.0 | No | 8.90 (0.09) | 1.91 (0.31) |
| L. paracasei NFBC 338 | 2.25 | Yes | 9.05 (0.03) | 7.93 (0.45) |
| L. paracasei NFBC 338 | 2.25 | No | 9.14 (0.12) | 2.81 (1.22) |
| L. gasseri ATCC 33323 | 2.0 | Yes | 8.84 (0.12) | 7.71 (0.21) |
| L. gasseri ATCC 33323 | 2.0 | No | 9.09 (0.07) | 7.63 (0.33) |
| L. gasseri ATCC 33323 | 1.75 | Yes | 8.91 (0.04) | 6.58 (0.37) |
| L. gasseri ATCC 33323 | 1.75 | No | 8.94 (0.13) | 1.77 (0.58) |
The data are the means for triplicate experiments.
The values in parentheses are the standard errors.
We compared the levels of survival of L. rhamnosus GG in the presence of decreasing concentrations of glucose and found that even at glucose concentrations as low as 1.0 mM, the protective effect was apparent (4.03 log10 CFU ml−1 better survival compared to the survival in the absence of glucose) (Table 2). Only small increases in survival occurred with higher concentrations of glucose (5 and 19.4 mM) (Table 2).
TABLE 2.
Survival of cultures of L. rhamnosus GG exposed to simulated gastric juice, pH 2.0, in the presence of glucose for 45 mina
| Strain | pH | Glucose concn (mM) | Concn (log10 CFU ml−1) at:
|
|
|---|---|---|---|---|
| Zero time | 45 min | |||
| L. rhamnosus GG | 2.0 | 0 | 8.94 (0.07)b | 2.39 (0.16) |
| L. rhamnosus GG | 2.0 | 1 | 9.00 (0.08) | 6.42 (0.40) |
| L. rhamnosus GG | 2.0 | 5 | 8.97 (0.12) | 6.80 (0.40) |
| L. rhamnosus GG | 2.0 | 19.4 | 9.03 (0.02) | 8.15 (0.46) |
The data are the means for triplicate experiments.
The values in parentheses are the standard errors.
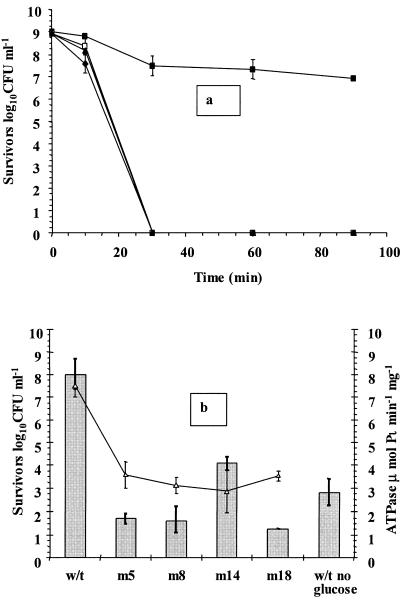
Addition of DCCD leads to the loss of the protective effect of glucose in L. rhamnosus GG cultures.
In order to evaluate the significance of the role played by the membrane-bound F0F1-ATPase complex in probiotic Lactobacillus survival in the presence of glucose, we added the inhibitor DCCD to two cultures prior to analysis, subjected the cultures to simulated gastric juice, pH 2.0, in the presence and absence of glucose, and compared the levels of survival of these cultures with those of controls. The addition of DCCD abolished the protective effect of glucose in simulated gastric juice containing glucose, so that no viable cells were detected within 30 min (Fig. 3a). A 2-log10 CFU ml−1 decline occurred in control cultures containing glucose, probably as a result of the starvation step prior to analysis, which may have reduced intracellular glucose and ATP supplies. The toxicity of 1.4 mM DCCD to stationary-phase L. rhamnosus GG cells was also studied at pH 7.00, and cells were found to be insensitive to DCCD at this pH (results not shown).
FIG. 3.
(a) Survival of stationary-phase L. rhamnosus GG in simulated gastric juice, pH 2.0, containing glucose (▪), glucose and DCCD (□), no glucose (♦), or no glucose and DCCD (▴). (b) Survival of stationary-phase parent (w/t) and neomycin-resistant L. rhamnosus GG (m5, m8, m14, and m18) cultures in simulated gastric juice containing glucose, pH 2.0, following 45 min of exposure (bars) and ATPase activity of permeabilized cells (▵). The data are the means of triplicate experiments, and the error bars indicate standard deviations.
In order to fully evaluate the role of the F0F1-ATPase in L. rhamnosus GG, spontaneous mutants were created using neomycin sulfate. Similar to mutants in other studies (23, 24, 52), these mutants had slower growth at pH 4.5 (results not shown). In addition, the ATPase activities of mutant cells were lower than those of the parent strain, and the four mutant strains selected for further study had 52 to 62% less F0F1-ATPase activity than the parent strain, which corresponded to activities of 2.9 to 3.6 μmol Pi min−1 mg−1 (Fig. 3b). These mutants also had lower growth rates at pH 6.5 than the parent strain (0.214 to 0.216 h−1 for mutants and 0.237 h−1 for the parent strain).
Survival of these mutants in simulated gastric juice in the presence of glucose was also evaluated following exposure for 45 min (Fig. 3b). The data revealed that the levels of survival of cultures ranged from 1.28 to 4.08 log10 CFU ml−1. Interestingly, the wild-type strain survived better in the absence of glucose than some mutants, which may indicate that the surviving cultures require fully functioning F0F1-ATPase activity. Cultures with reduced F0F1-ATPase activity had lower growth rates, and their inability to utilize the glucose at low pH may also have contributed to their reduced viability.
Enhanced survival of L. rhamnosus GG occurs only in the presence of metabolizable sugars.
In order to determine if there was a link between glycolysis and the enhanced survival in the presence of glucose, we established the relationship between the survival of L. rhamnosus GG in the presence of metabolizable and nonmetabolizable sugars. Growth over 16 h was found to occur in MRS medium containing glucose and fructose, while low levels of growth occurred in MRS medium containing lactose (Table 3). In addition, growth was also confirmed by the decrease in pH of the cultures containing glucose and fructose, while there were only small decreases in cultures growing in lactose (Table 3). Further analysis showed that glucose and fructose enhanced survival of L. rhamnosus GG in simulated gastric juice at pH 2.0, while lactose and sucrose, which L. rhamnosus GG could not metabolize, did not enhance survival (Table 3).
TABLE 3.
Growth of L. rhamnosus GG in MRS medium containing different sugars (indicated by OD600 and pH) and survival of L. rhamnosus GG grown in MRS medium containing glucose and exposed to simulated gastric juice, pH 2.0, in the presence of different sugars for 45 mina
| Carbohydrate | OD600b | pH of 16-h culturec | Concn (log10 CFU ml−1) at:
|
|
|---|---|---|---|---|
| Zero timed | 45 min | |||
| Glucose | 2.18 (0.03)e | 3.68 (0.13) | 9.15 (0.03) | 8.91 (0.26) |
| Fructose | 1.808 (0.01) | 3.54 (0.02) | 9.06 (0.08) | 8.86 (0.09) |
| Lactose | 0.599 (0.07) | 5.84 (0.01) | 9.07 (0.04) | 0.99 (0.74) |
| Sucrose | 0.764 (0.08) | 5.53 (0.01) | 9.16 (0.03) | 0.93 (0.63) |
The data are the means for triplicate experiments.
The optical density at 600 nm (OD600) was recorded following 16 h of growth at 37°C in the different sugars.
The medium pH was 6.2 ± 0.2 at the start of growth.
Numbers of viable cells.
The values in parentheses are the standard errors.
The glycolytic inhibitor iodoacetic acid eliminates the glucose effect.
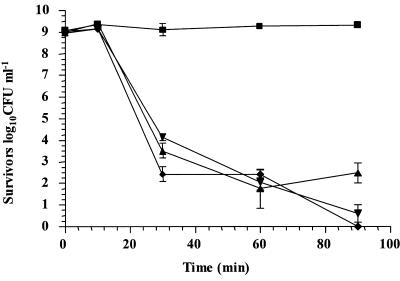
In order to further study the ability of the glycolytic system to provide ATP to cultures of L. rhamnosus GG, we used iodoacetic acid, which has previously been used to specifically inhibit GAPDH, a key enzyme in the glycolytic pathway (21). The results showed that addition of IAA to cultures prior to analysis reduced viability by 8.30 log10 CFU ml−1 in simulated gastric juice, pH 2.0, with glucose (Fig. 4). Interestingly, the rate of decline of cultures containing IAA was similar to that of L. rhamnosus GG in simulated gastric juice without glucose (Fig. 2b). Therefore, the data imply that glucose protected L. rhamnosus GG by glycolysis for provision of ATP for homeostasis. We also determined that the concentration of IAA used (10 μM) was not toxic to cells at neutral pH, as determined by viable cell counts, and that this concentration was sufficient to reduce the growth rate of L. rhamnosus GG (results not shown). In addition, we observed that L. rhamnosus GG grew only slightly slower in the presence of IAA (the growth rates were 0.24 h−1 and 0.233 h−1 in the absence and in the presence of IAA, respectively).
FIG. 4.
Survival of stationary-phase L. rhamnosus GG cultures in simulated gastric juice, pH 2.0, containing glucose (▪), glucose and IAA (▾), no glucose (▴), or no glucose and IAA (♦).
DISCUSSION
Lactobacilli of intestinal origin are considered intrinsically resistant to acid environments and are often employed in fermented foods as probiotics. In order to increase survival of probiotics during gastric transit, components such as milk (9), milk proteins (8), cheese and yogurt (25), reconstituted skim milk with gum acacia (18), and cereal extracts have been used (7). Buffering capacities, carbohydrate and protein constituents, and encapsulation are some of the technologies used previously for increased probiotic survival in acidic environments. However, the precise mechanisms which enhanced survival were not studied. In this study we found that the survival of Lactobacillus cultures varied among species and that the commercially significant organism L. rhamnosus GG had the best survival properties in acidic conditions among the strains tested. Different survival rates of Lactobacillus species have been observed in previous studies (7, 8, 20, 44). In addition, variation in acid survival among species has also been observed in propionibacteria (30) and bifidobacteria (39). Some variation at the strain level also occurred between L. rhamnosus GG and L. rhamnosus E800, and such variation has also been observed between two Lactobacillus brevis stains (44). Nitrate entering the stomach from saliva can also have bactericidal effects on lactobacilli (57), but this effect was not assessed in our study, which, similar to other studies, focused on the effect of acid alone (7, 8).
It was observed that the enhanced survival of L. rhamnosus GG was not due to its intrinsic properties per se but to the presence of glucose in simulated gastric juice. The removal of glucose from simulated gastric juice caused a dramatic loss of viability that was up to 4 log10 CFU ml−1 greater than that observed by Charalampopoulos et al. (7), who previously determined that glucose can increase the survival of lactobacilli in acid environments over 4 h of exposure. Under acidic conditions, the intracellular pH was higher in cells in a medium containing glucose at lower extracellular pH values, indicating that glucose plays a significant role in homeostasis (48). Small reductions in viability occurred among cultures devoid of lysozyme and CaCl2 (Fig. 2b), which may indicate a need for either component for optimal survival. Indeed, glucose-energized Streptococcus mutans cells could maintain a higher intracellular pH in the presence of 25 mM K+, which was used by a K+-ATPase to maintain pH homeostasis (15). However, the survival effect outlined in this study does not appear to be a K+-ATPase-mediated process, as the effect was observed in dilute HCl in the presence of glucose (Fig. 2c), although dilute HCl may have been less lethal to L. rhamnosus GG than simulated gastric juice (compare Fig. 2b and c). Therefore, we postulate that the enhanced survival observed resulted from proton extrusion by the F0F1-ATPase alone.
We compared the effects of glucose on the survival of different strains, which showed different results. L. gasseri ATCC 33323 had the best intrinsic properties in simulated gastric juice in the absence of glucose, and some strains, particularly L. rhamnosus GG, utilized glucose to survive better. Glucose has previously been shown to have different protective effects for different Lactobacillus species (7). Charalampopoulos et al. (7) showed that although Lactobacillus plantarum and Lactobacillus acidophilus experienced a protective effect from up to 8.33 g liter−1 glucose, Lactobacillus reuteri did not over 4 h of exposure. However, with the adjustment of the pH of simulated gastric juice in our study, a protective effect did occur for L. gasseri ATCC 33323 and L. paracasei NFBC 338. It is therefore not unreasonable to assume that the beneficial effect of glucose occurs for each Lactobacillus at a critical pH value. The survival of L. rhamnosus GG in simulated gastric juice in the presence of glucose also appeared to be dependent on glucose concentrations as low as 1 mM. This observation has been previously seen in lactobacilli (7).
We linked the importance of F0F1-ATPase to the survival effect observed in simulated gastric juice in the presence of glucose. The F0F1-ATPase was upregulated as a result of acid stress in lactobacilli (37). The presence of DCCD inhibits F0F1-ATPase by covalent modification of the Glu 54 residue of the c subunit, preventing proton translocation and thereby causing cell death at low pH (12, 16). Greater cell death occurred in cultures containing DCCD and glucose than in cultures without glucose (Table 1), probably as a result of the inability of ATPase to pump out protons, and thus viability was reduced. In addition, we starved cells in this study prior to analysis, further reducing the intracellular reserves necessary for maintenance, which may explain the total loss of viable cells in the simulated gastric juice sample without glucose (Fig. 3a). DCCD was not toxic to cells at a higher pH, which is in agreement with previous results (16), and therefore, the viability losses were solely attributable to reductions in F0F1-ATPase activity. A previous study of ATP turnover showed that the presence of DCCD led to stable retention of ATP levels, while the proton gradient collapsed, linking the association of ATPase activity with ATP supplies (10).
Spontaneous neomycin-resistant L. rhamnosus GG mutants with lower ATPase activity were unable to exploit glucose to survive, unlike the parent strains. They had 38% to 48% of the activity found in the parent strain, which is in accordance with other studies (23, 24, 52, 58). ATPase mutants have been reported to have slower growth rates (12, 36). However, the growth rates of L. rhamnosus GG mutants in MRS medium (pH 6.5) were only approximately 10% lower than that of the parent strain, so a lower growth rate is unlikely to be a major factor contributing to the lower survival rate observed. Neomycin-resistant mutants with no ATPase activity were not isolated, suggesting that this enzyme mechanism may be necessary for growth of lactobacilli. Mutants resistant to neomycin tend to have a mutation in the γ-subunit which is thought to prevent F1-ATPase assembly, and a defective proton pathway is formed (47). The F0F1-ATPase has been found to be an important complex in the survival of bifidobacteria in acidic environments and is highly conserved among eubacteria (39, 55). In addition, higher activity has been observed in Lactobacillus casei than in Actinomyces viscosus (4), which may have resulted in greater potential for survival of the Lactobacillus strain. In cultures lacking a respiratory chain, the function of the F0F1-ATPase is to provide a mechanism for pH homeostasis in acidic conditions (14). To fulfill this function, sufficient ATP reserves must be generated.
There have been a number of energy-generating mechanisms described that link survival in low-pH environments with ATP generation (53). In addition, Shabala et al. (48) also noted that increased bacterial survival is directly proportional to glucose availability in the media. It was postulated that the increased glucose availability met the high energy demands of maintaining pH homeostasis (48). We further linked the relationship between the survival of L. rhamnosus GG in the presence of glucose and its ability to utilize sugars. L. rhamnosus GG is unable to ferment sucrose and lactose (26), while fructose has been used as a supplement for growth in milk (42). In our study, it was observed that inclusion of carbohydrates which could be utilized by L. rhamnosus GG resulted in enhanced survival, while the survival effect was lost in cultures containing nonmetabolizable sugars, thereby establishing a relationship between glycolysis and enhanced survival in acidic conditions.
Intracellular ATP concentrations have been reported to increase in the presence of glucose in stationary-phase lactic acid bacteria (2). The effect of iodoacetate on the GAPDH activity of Lactococcus lactis has been described (21). The mechanism of inactivation consists of covalent fixation of IAA at the active site cysteine. This inhibits formation of an enzyme-NAD+ complex and the transfer between cysteine and NAD+ which occurs during glyceraldehyde-3-phosphate dehydrogenation (41). It has been established that the IAA concentration that provokes partial inhibition of GAPDH activity should not affect other catabolic enzymes (21). Therefore, the effect of IAA can be considered specific for GAPDH at the concentrations used. A 50% decrease in ATP concentrations was observed in the presence of IAA, and there was also a 94% loss of GAPDH activity (21). Indeed, IAA has been reported to reduce ATP concentrations in cells in other studies (2, 10). Based on the assumption that addition of IAA prior to analysis impairs glycolysis and reduces ATP concentrations, we analyzed its effect on the survival of L. rhamnosus GG in simulated gastric juice in the presence of glucose. IAA caused the glucose effect to decrease dramatically, and this observation establishes a link between glycolysis, ATP generation, and the activity of F0F1-ATPase in this phenomenon.
F0F1-ATPase requires ATP for expulsion of H+ from the cell, thereby maintaining pH homeostasis and cell viability. The accumulation of ATP is as a result of energy-generating factories, such as the glycolytic system. In conclusion, we found that lactobacilli could sequester glucose to survive in a simulated gastric environment. We also found that the inhibition of glycolysis affected the ability of L. rhamnosus GG to survive in simulated gastric juice in the presence of 19.4 mM glucose. Glucose in acid conditions can therefore enhance probiotic survival by providing the ATP pool required, permitting optimal H+ extrusion by F0F1-ATPase. Such a mechanism can provide more effective delivery of viable probiotic lactobacilli to the human GIT.
Acknowledgments
B. Corcoran is in receipt of a Teagasc Walsh Fellowship. This work was funded by the Irish Government under National Development Plan 2000-2006, by the European Research and Development Fund, by Science Foundation Ireland, and by EU project QLK1-CT-2000-30042.
REFERENCES
- 1.Alvarez-Olmos, M. I., and R. A. Oberhelman. 2001. Probiotic agents and infectious diseases: a modern perspective on a traditional therapy. Clin. Infect. Dis. 32:1567-1575. [DOI] [PubMed] [Google Scholar]
- 2.Bakker, E. P., and F. M. Harold. 1980. Energy coupling to potassium transport in Streptococcus faecalis. J. Biol. Chem. 255:433-440. [PubMed] [Google Scholar]
- 3.Belli, W. A., and R. E. Marquis. 1991. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl. Environ. Microbiol. 57:1134-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender, G. R., and R. E. Marquis. 1987. Membrane ATPases and acid tolerance of Actinomyces viscosus and Lactobacillus casei. Appl. Environ. Microbiol. 53:2124-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beumer, R. R., J. de Vries, and F. M. Rombouts. 1992. Campylobacter jejuni non-culturable coccoid cells. Int. J. Food Microbiol. 15:153-163. [DOI] [PubMed] [Google Scholar]
- 6.Champomier Vergès, M.-C., M. Zuñiga, F. Morel-Deville, G. Peréz-Martínez, M. Zagorec, and S. D. Ehrlich. 1999. Relationships between arginine degradation, pH and survival in Lactobacillus sakei. FEMS Microbiol. Lett. 180:297-304. [DOI] [PubMed] [Google Scholar]
- 7.Charalampopoulos, D., S. S. Pandiella, and C. Webb. 2003. Evaluation of the effect of malt, wheat and barley extracts on the viability of potentially probiotic lactic acid bacteria under acidic conditions. Int. J. Food Microbiol. 82:133-141. [DOI] [PubMed] [Google Scholar]
- 8.Charteris, W. P., P. M. Kelly, L. Morelli, and J. K. Collins. 1998. Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J. Appl. Microbiol. 84:759-768. [DOI] [PubMed] [Google Scholar]
- 9.Conway, P. L., S. L. Gorbach, and B. R. Goldin. 1987. Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J. Dairy Sci. 70:1-12. [DOI] [PubMed] [Google Scholar]
- 10.Cook, G. M., and J. B. Russell. 1994. Energy-spilling reactions of Streptococcus bovis and resistance of its membrane to proton conductance. Appl. Environ. Microbiol. 60:1942-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corcoran, B. M., R. P. Ross, G. F. Fitzgerald, and C. Stanton. 2004. Comparative survival of probiotic lactobacilli spray-dried in the presence of prebiotic substances. J. Appl. Microbiol. 96:1024-1039. [DOI] [PubMed] [Google Scholar]
- 12.Cotter, P. D., C. G. Gahan, and C. Hill. 2000. Analysis of the role of the Listeria monocytogenes F0F1-ATPase operon in the acid tolerance response. Int. J. Food Microbiol. 60:137-146. [DOI] [PubMed] [Google Scholar]
- 13.Cotter, P. D., C. G. Gahan, and C. Hill. 2001. A glutamate decarboxylase system protects Listeria monocytogenes in gastric fluid. Mol. Microbiol. 40:465-475. [DOI] [PubMed] [Google Scholar]
- 14.Cotter, P. D., and C. Hill. 2003. Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 67:429-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dashper, S. G., and E. C. Reynolds. 1992. pH regulation by Streptococcus mutans. J. Dent. Res. 71:1159-1165. [DOI] [PubMed] [Google Scholar]
- 16.Datta, A. R., and M. M. Benjamin. 1997. Factors controlling acid tolerance of Listeria monocytogenes: effects of nisin and other ionophores. Appl. Environ. Microbiol. 63:4123-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.deMan, J. C., M. Rogosa, and M. E. Sharpe. 1960. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 23:130-135. [Google Scholar]
- 18.Desmond, C., R. P. Ross, E. O'Callaghan, G. Fitzgerald, and C. Stanton. 2002. Improved survival of Lactobacillus paracasei NFBC 338 in spray-dried powders containing gum acacia. J. Appl. Microbiol. 93:1003-1011. [DOI] [PubMed] [Google Scholar]
- 19.Dunne, C., L. O'Mahony, L. Murphy, G. Thornton, D. Morrissey, S. O'Halloran, M. Feeney, S. Flynn, G. Fitzgerald, C. Daly, B. Kiely, G. C. O'Sullivan, F. Shanahan, and J. K. Collins. 2001. In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am. J. Clin. Nutr. 73:386S-392S. [DOI] [PubMed] [Google Scholar]
- 20.Ehrmann, M. A., P. Kurzak, J. Bauer, and R. F. Vogel. 2002. Characterization of lactobacilli towards their use as probiotic adjuncts in poultry. J. Appl. Microbiol. 92:966-975. [DOI] [PubMed] [Google Scholar]
- 21.Even, S., C. Garrigues, P. Loubiere, N. D. Lindley, and M. Cocaign-Bousquet. 1999. Pyruvate metabolism in Lactococcus lactis is dependent upon glyceraldehyde-3-phosphate dehydrogenase activity. Metab. Eng. 1:198-205. [DOI] [PubMed] [Google Scholar]
- 22.Finegold, S. M., V. L. Sutter, P. T. Sugihara, H. A. Elder, S. M. Lehmann, and R. L. Phillips. 1977. Fecal microbial flora in Seventh Day Adventist populations and control subjects. Am. J. Clin. Nutr. 30:1781-1792. [DOI] [PubMed] [Google Scholar]
- 23.Fortier, L. C., R. Tourdot-Maréchal, C. Diviès, B. H. Lee, and J. Guzzo. 2003. Induction of Oenococcus oeni H+-ATPase activity and mRNA transcription under acidic conditions. FEMS Microbiol. Lett. 222:165-169. [DOI] [PubMed] [Google Scholar]
- 24.Galland, D., R. Tourdot-Maréchal, M. Abraham, K. S. Chu, and J. Guzzo. 2003. Absence of malolactic activity is a characteristic of H+-ATPase-deficient mutants of the lactic acid bacterium Oenococcus oeni. Appl. Environ. Microbiol. 69:1973-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardiner, G. E., R. P. Ross, J. M. Wallace, F. P. Scanlan, P. P. Jagers, G. F. Fitzgerald, J. K. Collins, and C. Stanton. 1999. Influence of a probiotic adjunct culture of Enterococcus faecium on the quality of cheddar cheese. J. Agric. Food Chem. 47:4907-4916. [DOI] [PubMed] [Google Scholar]
- 26.Goldin, B. R., S. L. Gorbach, M. Saxelin, S. Barakat, L. Gualtieri, and S. Salminen. 1992. Survival of Lactobacillus species (strain GG) in human gastrointestinal tract. Dig. Dis. Sci. 37:121-128. [DOI] [PubMed] [Google Scholar]
- 27.Guarner, F., and J.-R. Malagelada. 2003. Gut flora in health and disease. Lancet 360:512-519. [DOI] [PubMed] [Google Scholar]
- 28.Holzapfel, W. H., and U. Schillinger. 2002. Introduction to pre- and probiotics. Food Res. Int. 35:109-116. [Google Scholar]
- 29.Hong, S. I., Y. J. Kim, and Y. R. Pyun. 1999. Acid tolerance of Lactobacillus plantarum from kimchi. Lebensm.-Wiss. Technol. 32:142-148. [Google Scholar]
- 30.Huang, Y., and M. C. Adams. 2004. In vitro assessment of the upper gastrointestinal tolerance of potential probiotic dairy propionibacteria. Int. J. Food Microbiol. 91:253-260. [DOI] [PubMed] [Google Scholar]
- 31.Isolauri, E., M. Juntunen, T. Rautanen, P. Sillanaukee, and T. Koivula. 1991. A human Lactobacillus strain (Lactobacillus casei sp. strain GG) promotes recovery from acute diarrhea in children. Pediatrics 88:90-97. [PubMed] [Google Scholar]
- 32.Isolauri, E., S. Salminen, and T. Mattila-Sandholm. 1999. New functional foods in the treatment of food allergy. Ann. Med. 31:299-302. [DOI] [PubMed] [Google Scholar]
- 33.Jacobsen, C. N., V. Rosenfeldt Nielsen, A. E. Hayford, P. L. Moller, K. F. Michaelsen, A. Paerregaard, B. Sandstrom, M. Tvede, and M. Jakobsen. 1999. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl. Environ. Microbiol. 65:4949-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin, L. Z., Y. W. Ho, N. Abdullah, and S. Jalaludin. 1998. Acid and bile tolerance of Lactobacillus isolated from chicken intestine. Lett. Appl. Microbiol. 27:183-185. [DOI] [PubMed] [Google Scholar]
- 35.Kashket, E. R. 1987. Bioenergetics of lactic acid bacteria: cytoplasmic pH and osmotolerance. FEMS Microbiol. Rev. 46:233-244. [Google Scholar]
- 36.Koebmann, B. J., D. Nilsson, O. P. Kuipers, and P. R. Jensen. 2000. The membrane-bound H+-ATPase complex is essential for growth of Lactococcus lactis. J. Bacteriol. 182:4738-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kullen, M. J., and T. R. Klaenhammer. 1999. Identification of the pH-inducible, proton-translocating F1F0-ATPase (atpBEFHAGDC) operon of Lactobacillus acidophilus by differential display: gene structure, cloning and characterization. Mol. Microbiol. 33:1152-1161. [DOI] [PubMed] [Google Scholar]
- 38.Marteau, P. R., M. de Vrese, C. J. Cellier, and J. Schrezenmeir. 2001. Protection from gastrointestinal diseases with the use of probiotics. Am. J. Clin. Nutr. 73:430S-436S. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto, M., H. Ohishi, and Y. Benno. 2004. H+-ATPase activity in Bifidobacterium with special reference to acid tolerance. Int. J. Food Microbiol. 93:109-113. [DOI] [PubMed] [Google Scholar]
- 40.Millar, M. R., C. Bacon, S. L. Smith, V. Walker, and M. A. Hall. 1993. Enteral feeding of premature infants with Lactobacillus GG. Arch. Dis. Child. 69:483-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagradova, N. K., E. V. Schmalhausen, P. A. Levashov, R. A. Asryants, and V. I. Muronetz. 1996. d-Glyceraldehyde-3-phosphate dehydrogenase. Properties of the enzyme modified at arginine residues. Appl. Biochem. Biotechnol. 61:47-56. [DOI] [PubMed] [Google Scholar]
- 42.Østlie, H. M., M. H. Helland, and J. A. Narvhus. 2003. Growth and metabolism of selected strains of probiotic bacteria in milk. Int. J. Food Microbiol. 87:17-27. [DOI] [PubMed] [Google Scholar]
- 43.Reid, G., J. Jass, M. T. Sebulsky, and J. K. McCormick. 2003. Potential uses of probiotics in clinical practice. Clin. Microbiol. Rev. 16:658-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ronka, E., E. Malinen, M. Saarela, M. Rinta-Koski, J. Aarnikunnas, and A. Palva. 2003. Probiotic and milk technological properties of Lactobacillus brevis. Int. J. Food Microbiol. 83:63-74. [DOI] [PubMed] [Google Scholar]
- 45.Saxelin, M., T. Pessi, and S. Salminen. 1995. Fecal recovery following oral administration of Lactobacillus strain GG (ATCC 53103) in gelatine capsules to healthy volunteers. Int. J. Food Microbiol. 25:199-203. [DOI] [PubMed] [Google Scholar]
- 46.Sebald, W., P. Friedl, H. U. Schairer, and J. Hoppe. 1982. Structure and genetics of the H+-conducting F0 portion of the ATP synthase. Ann. N. Y. Acad. Sci. 402:28-44. [DOI] [PubMed] [Google Scholar]
- 47.Sekine, H., T. Shimada, C. Hayashi, A. Ishiguro, F. Tomita, and A. Yokota. 2001. H+-ATPase defect in Corynebacterium glutamicum abolishes glutamic acid production with enhancement of glucose consumption rate. Appl. Microbiol. Biotechnol. 57:534-540. [DOI] [PubMed] [Google Scholar]
- 48.Shabala, L., B. Budde, T. Ross, H. Siegumfeldt, and T. McMeekin. 2002. Responses of Listeria monocytogenes to acid stress and glucose availability monitored by measurements of intracellular pH and viable counts. Int. J. Food Microbiol. 75:89-97. [DOI] [PubMed] [Google Scholar]
- 49.Shanahan, F. 2002. Probiotics and inflammatory bowel disease: from fads and fantasy to facts and future. Br. J. Nutr. 88(Suppl. 1):S5-S9. [DOI] [PubMed] [Google Scholar]
- 50.Stadler, M., and H. Viernstein. 2003. Optimization of a formulation containing viable lactic acid bacteria. Int. J. Pharm. 256:117-122. [DOI] [PubMed] [Google Scholar]
- 51.Tannock, G. W. 2004. A special fondness for lactobacilli. Appl. Environ. Microbiol. 70:3189-3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tourdot-Maréchal, R., L. C. Fortier, J. Guzzo, B. Lee, and C. Diviès. 1999. Acid sensitivity of neomycin-resistant mutants of Oenococcus oeni: a relationship between reduction of ATPase activity and lack of malolactic activity. FEMS Microbiol. Lett. 178:319-326. [DOI] [PubMed] [Google Scholar]
- 53.van de Guchte, M., P. Serror, C. Chervaux, T. Smokvina, S. D. Ehrlich, and E. Maguin. 2002. Stress responses in lactic acid bacteria. Antonie Leeuwenhoek 82:187-216. [PubMed] [Google Scholar]
- 54.Venkatesh, K. V., M. R. Okos, and P. C. Wankat. 1993. Kinetic model of growth and lactic acid production from lactose by Lactobacillus bulgaricus. Process Biochem. 28:231-241. [Google Scholar]
- 55.Ventura, M., C. Canchaya, D. van Sinderen, G. F. Fitzgerald, and R. Zink. 2004. Bifidobacterium lactis DSM 10140: identification of the atp (atpBEFHAGDC) operon and analysis of its genetic structure, characteristics, and phylogeny. Appl. Environ. Microbiol. 70:3110-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wiseman, N., and V. J. Pileggi. 1972. Determination of inorganic phosphorous, p. 723-727. In R. J. Henry, D. C. Cannon, and J. W. Winkleman (ed.), Clinical chemistry: principles and techniques. Harper & Row, Publishers, Inc., New York, N.Y.
- 57.Xu, J., X. Xu, and W. Verstraete. 2001. The bactericidal effect and chemical reactions of acidified nitrite under conditions simulating the stomach. J. Appl. Microbiol. 90:523-529. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto, N., Y. Masujima, and T. Takano. 1996. Reduction of membrane-bound ATPase activity on a Lactobacillus helveticus strain with slower growth at low pH. FEMS Microbiol. Lett. 138:179-184. [Google Scholar]
- 59.Yokota, A., S. Amachi, S. Ishii, and S. Tomita. 1995. Acid sensitivity of membrane bound ATPase activity in Lactobacillus helveticus strain with slower growth at low pH. FEMS Microbiol. Lett. 138:2004-2007. [Google Scholar]