Abstract
Ileal lesions in 36.4% of patients with Crohn's disease are colonized by pathogenic adherent-invasive Escherichia coli. The aim of this study was to determine the in vitro inhibitory effects of the probiotic strain, Lactobacillus casei DN-114 001, on adhesion to and invasion of human intestinal epithelial cells by adherent-invasive E. coli isolated from Crohn's disease patients. The experiments were performed with undifferentiated Intestine-407 cells and with undifferentiated or differentiated Caco-2 intestinal epithelial cells. Bacterial adhesion to and invasion of intestinal epithelial cells were assessed by counting CFU. The inhibitory effects of L. casei were determined after coincubation with adherent-invasive E. coli or after preincubation of intestinal cells with L. casei prior to infection with adherent-invasive E. coli. Inhibitory effects of L. casei on adherent-invasive E. coli adhesion to differentiated and undifferentiated intestinal epithelial cells reached 75% to 84% in coincubation and 43% to 62% in preincubation experiments, according to the cell lines used. Addition of L. casei culture supernatant to the incubation medium increased L. casei adhesion to intestinal epithelial cells and enhanced the inhibitory effects of L. casei. The inhibitory effects on E. coli invasion paralleled those on adhesion. This effect was not due to a bactericidal effect on adherent-invasive E. coli or to a cytotoxic effect on epithelial intestinal cells. As Lactobacillus casei DN-114 001 strongly inhibits interaction of adherent-invasive E. coli with intestinal epithelial cells, this finding suggests that the probiotic strain could be of therapeutic value in Crohn's disease.
Crohn's disease (CD) is a chronic inflammatory bowel disease (IBD) for which the etiology is still unknown, but several factors, including genetic, environmental, immunological, and other endogenous host factors, have been incriminated (40). Among the environmental triggers, luminal bacteria seem to play a substantial role. Indeed, the onset of inflammation in IBD may be associated with an imbalance in the intestinal microflora, with a relative predominance of aggressive bacteria and an insufficient amount of protective bacteria (46). Moreover, the efficacy of antibiotic therapy suggests a role of bacterial flora in CD. However, antibiotic treatments are often associated with gastrointestinal side effects and bacterial resistance, which may contribute to treatment failure (14, 21, 41, 43, 48).
In early and chronic ileal lesions of CD, an abnormal predominance of Escherichia coli has been observed (between 50 and 100% of the total number of aerobes and anaerobes). Most of these strains are able to adhere to and invade intestinal epithelial cells and to replicate within macrophages (9, 17, 19). These E. coli strains belong to a pathogenic group of E. coli designated AIEC (for adherent-invasive E. coli) (9). In neoterminal ileal specimens, AIEC strains were found in 36.4% of CD patients (16). Treatments aimed at eradicating these pathogenic strains and replacing them by nonpathogenic bacteria such as probiotic strains may be beneficial for the course of CD and may provide an innovative approach to treatment.
Probiotics are living microorganisms that upon ingestion in sufficient numbers exert benefits on human health. By modulating enteric flora, probiotic strains are effective in the prevention and treatment of antibiotic-associated, rotavirus, Clostridium difficile-associated, or traveler's diarrhea (for a review, see reference 47). The efficacy of several probiotics for IBD has been investigated in clinical trials. E. coli Nissle 1917, Saccharomyces boulardii, and a formula consisting of species of Bifidobacterium, Lactobacillus, and Streptococcus salivarius subsp. Thermophilus (VSL #3) have been reported as being as effective as standard treatment in preventing relapse in ulcerative colitis and chronic pouchitis (24, 42, 51). Probiotics are used in these pathologies basically to restore the unbalanced indigenous microflora, to inhibit the adverse effects of enteric pathogens, and to counteract the inflammatory process (28, 45).
Lactobacillus casei DN-114 001 is a probiotic strain that survives intestinal transit (35) and exerts beneficial effects in vivo. It is able to modify the digestive microflora and enhance the immune system during its transit in the digestive tract (23, 39). It was shown to reduce the incidence and duration of diarrhea in children (37, 38). Moreover, a recent study has provided evidence that this probiotic interacts with human intestinal mucosa and can markedly reduced the mucosal release of tumor necrosis factor alpha and interleukin-8 in active Crohn's disease (5, 6).
The aim of the present study was to investigate whether L. casei DN-114 001 could inhibit the ability of pathogenic adherent-invasive-E. coli strains isolated from patients with Crohn's disease to adhere to and invade intestinal epithelial cells in vitro.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
L. casei DN-114 001 was provided by Danone Vitapole (Paris, France). L. casei DN-114 001 was grown in De Man, Rogosa, and Sharpe (MRS) broth (Difco, Becton Dickinson, Meylan, France) at 37°C for 18 h. The culture was centrifuged (10,000 × g for 5 min at 4°C), and bacteria were suspended in cell culture medium. The final suspension was adjusted to obtain the appropriate concentration. The number of CFU was determined by plating serial 10-fold dilutions from bacterial suspensions on MRS agar plates. Plates were incubated at 37°C in a CO2 atmosphere for 48 h.
Seven AIEC strains were assessed: the AIEC reference strain LF82 (9) and strains LF9, LF15, LF31, LF65, LF110, and LF134 (16). All these strains isolated from patients with Crohn's disease were characterized by using the adhesion and invasion assays described below. All strains were highly sensitive to gentamicin. E. coli LF32 was used as a positive control for cytotoxicity assay, since this strain produces α-hemolysin (17). E. coli strain K-12 C600 was used as a negative control. All E. coli strains were grown either in Luria-Bertani broth without shaking or on Mueller-Hinton agar plates (Institut Pasteur Production, Marnes-la-Coquette, France) overnight at 37°C.
Intestinal cell lines and cell cultures.
The Intestine-407 cells (ATCC CCL6; Flow Laboratories, Inc., McLean, VA) derived from human embryonic jejunum and ileum were used as an intestinal model for undifferentiated intestinal epithelial cells mimicking the cells found in the crypts of the intestinal villi. They were cultured for 20 h and in an atmosphere containing 5% CO2 at 37°C in Eagle minimum essential medium (Eagle MEM; BioWhittaker-Cambrex, Emerainville, France) supplemented with 10% (vol/vol) fetal bovine serum (BioWhittaker-Cambrex, Emerainville, France), 1% (vol/vol) nonessential amino acids (BioWhittaker), 1% (vol/vol) l-glutamine (Gibco BRL-Life Technologies, Cergy-Pontoise, France), 200 U of penicillin, 50 mg of streptomycin, 0.25 mg/liter of amphotericin B (Gibco BRL-Life Technologies, Cergy-Pontoise, France), and 1% (vol/vol) MEM vitamin solution X-100 (BioWhittaker). Caco-2 cells established from human colonic adenocarcinoma were kindly provided by Alain Zweibaum (INSERM U178, Villejuif, France). These cells were used as undifferentiated cells to mimic cells of the crypts and as differentiated cells to mimic mature enterocytes of the small intestine (30). Undifferentiated and differentiated Caco-2 cells were grown for 2 days and 15 days, respectively. Cells were cultured in Dulbecco's modified Eagle medium (DMEM) with 4.5 g/liter of glucose (BioWhittaker) supplemented with 20% (vol/vol) fetal bovine serum (BioWhittaker), 1% (vol/vol) nonessential amino acids (BioWhittaker), 1% (vol/vol) l-glutamine (Gibco BRL-Life Technologies, Cergy-Pontoise, France), 200 U of penicillin, 50 mg of streptomycin, 0.25 mg/liter of amphotericin B (Gibco BRL-Life Technologies, Cergy-Pontoise, France), and 1% (vol/vol) MEM vitamin solution X-100 (BioWhittaker). The cells were grown at 37°C in 5% CO2.
Adhesion and invasion assays.
Intestine-407 cells were seeded in 24-well tissue culture plates (Polylabo, Strasbourg, France) at 4 × 105 cells per well and grown for 20 h. Caco-2 cells were seeded in 24-well tissue culture plates (Polylabo, Strasbourg, France) at 2 × 105 cells per well and grown for 2 days for undifferentiated cells and 15 days for differentiated cells. The culture medium was changed every 2 days. The cells were washed twice with phosphate-buffered saline (BioWhittaker).
To study the adhesion of L. casei DN-114 001, each cell line was infected in 1 ml of the cell culture medium supplemented with heat-inactivated (30 min; 56°C) fetal bovine serum, at a multiplicity of infection (MOI) of either 10, 100, or 500 bacteria per epithelial cell. After a 1- to 6-h incubation period at 37°C with 10% CO2, the infected cells were washed three times with phosphate-buffered saline. To determine the total number of cell-associated bacteria, the cells were lysed with 1% (vol/vol) Triton X-100 (Sigma) in deionized water. This concentration of Triton X-100 did not affect bacterial viability for at least 30 min (data not shown). Samples were diluted and plated onto MRS agar plates to determine the number of CFU recovered from the lysed cells. To study the effect of spent L. casei culture supernatant (SN) on the adhesion of the strain, 10% (vol/vol) of its spent culture SN or neutralized spent culture SN were added to the cell culture medium. The spent culture supernatant of L. casei DN-114 001 was centrifuged and sterilized by filtration through a sterile 0.22-μm-pore-size filter unit (Millipore Molsheim, France). To check for potential interference of pH reduction linked to organic acid production, the spent culture supernatant was also adjusted to a neutral value (pH 7.0) using 4 M NaOH.
AIEC adhesion was measured utilizing the same protocol. MOIs of 10 and 100 were used. To determine the number of CFU recovered from the lysed cells, samples were diluted and plated onto Mueller-Hinton agar plates. For invasion assays, fresh cell culture medium containing 100 μg/ml of gentamicin was added after the infection period to kill extracellular bacteria. After incubation for an additional hour, cultured cells were treated as described above. Each assay was performed three times with successive passages of intestinal cells.
Adhesion and invasion inhibition assays.
Two different procedures were used to assess exclusion of AIEC strain by L. casei DN-114 001 and competition between the two strains. Exclusion was assessed by performing preinfection experiments in which cultured intestinal epithelial cells were first incubated with L. casei DN-114 001 (MOI, 500) alone or in the presence of 10% of its spent culture supernatant for 6 h at 37°C. AIEC strain LF82 (MOI, 100) was added and incubation was continued for a further 3 h. Competition was assessed by performing coinfection experiments in which L. casei DN-114 001 (MOI, 500) bacteria alone or in the presence of 10% of its spent culture supernatant (or neutralized spent culture supernatant), and each of the AIEC strains tested were added to the cultured cells at an MOI of 10 for 6 h. The numbers of strains adhering to or invading the intestinal cells were determined as described above. For each assay, a minimum of three experiments was performed with successive passage of intestinal cells. To evaluate the number of adherent or intracellular bacteria per intestinal epithelial cells, two additional wells were prepared when the cells were seeded. At the end of the culture period, the cells were trypsinized and enumerated microscopically.
Epithelial cell viability: lactate dehydrogenase (LDH) measurement.
At the same time as each study of L. casei DN-114 001 adherence, a duplicate 24-well plate of cultured epithelial cells was inoculated with bacteria and assayed as described above. At the end of the incubation period, supernatants of the infected cells containing released LDH were collected, centrifuged at 2,500 × g for 3 min at 4°C, and assayed for lactate dehydrogenase activity. Enzymatic activity was determined in the supernatants by using NADH as the substrate. Release of LDH was expressed as units per liter of supernatant. The percentage of cytotoxicity was calculated as follows: [(experimental release − spontaneous release)/(total release − spontaneous release)] × 100, where spontaneous release is the amount of LDH activity in supernatants of cells incubated in medium alone and total release is the LDH activity measured in cell lysates. E. coli LF32 producing an α-hemolysin was used as a positive control (17). E. coli strain K-12 C600 was used as a negative control.
Statistical analysis.
The data were analyzed by Student's t test. P values of ≤0.05 were considered to be statistically significant.
RESULTS
Ability of L. casei DN-114 001 to adhere to intestinal epithelial cells.
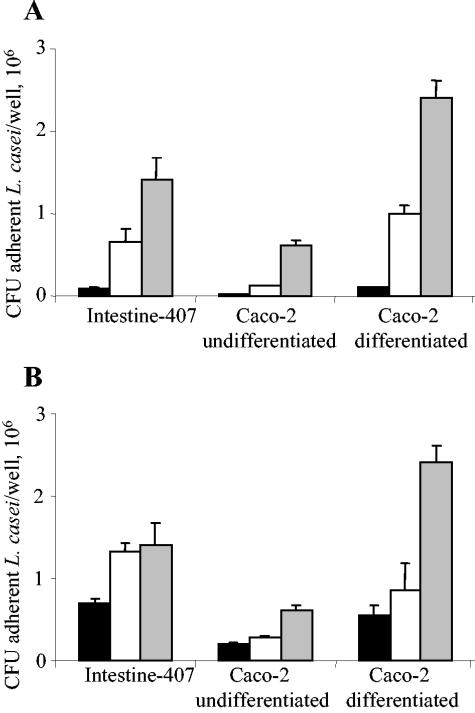
The ability of L. casei DN-114 001 to adhere to intestinal epithelial cells was determined using undifferentiated Intestine-407 and Caco-2 cells and differentiated Caco-2 cells. The adhesion of L. casei DN-114 001 increased with the MOI as shown in Fig. 1A. It also increased with the incubation period (Fig. 1B). The observed levels of adhesion varied according to the cell lines tested. With Intestine-407 cells, adhesion was maximal after 3 h of incubation, reaching an adhesion level of 3 bacteria/cell. With undifferentiated and differentiated Caco-2 cells, marked increases in adhesion levels were observed between 3 and 6 h of incubation. After 6 h of incubation, the adhesion levels were 2.7 and 3.9 bacteria/cell with undifferentiated and differentiated Caco-2 cells, respectively. Thus, L. casei DN-114 001 exhibited a dose-dependent and incubation time-dependent ability to adhere to undifferentiated and differentiated intestinal epithelial cells.
FIG. 1.
Adhesion of L. casei DN-114 001 to intestinal epithelial cells according to multiplicity of infection (A) or to time of incubation (B). Adhesion of L. casei DN-114 001 was tested with undifferentiated Intestine-407 or Caco-2 cells cultured for 2 days and with differentiated Caco-2 cells cultured for 15 days. (A) Cultured cells were incubated at an MOI of 10 (black bars), 100 (white bars), and 500 (grey bars) for 6 h. (B) Cultured cells were incubated at an MOI of 500 for 1 h (black bars), 3 h (white bars), and 6 h (grey bars). Adhesion levels are expressed as the number of CFU per well. Data are given as means ± the standard error of the mean (SEM) of at least three separate experiments.
Increased ability of L. casei DN-114 001 to adhere to intestinal epithelial cells in the presence of its spent culture supernatant.
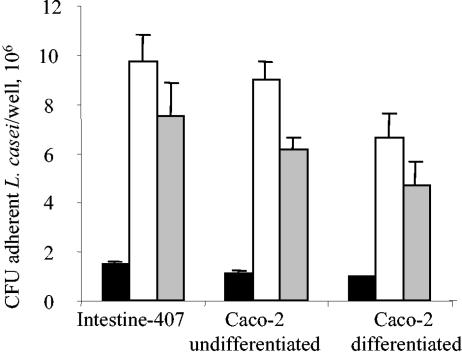
Since Lactobacilli can produce secreted compounds able to interact with their adhesive abilities (10, 22), the effects of putative secreted products on ability to adhere were tested. L. casei DN-114 001 adhesion assays were performed at an MOI of 500 for 6 h in the presence of 10% of L. casei DN-114 001 spent culture supernatant. The addition of 10% of L. casei DN-114 001 spent culture supernatant to the incubation medium induced 6.8-, 7.7-, and 7.1-fold increases in levels of adhesion of L. casei DN-114 001 to Intestine-407, undifferentiated Caco-2, and differentiated Caco-2 cells, respectively (Fig. 2). Since the observed effect could be related to a drop in pH due to acid lactic production, we performed similar experiments in the presence of 10% spent culture supernatant adjusted to a neutral pH value. It continued to induce a marked increase in L. casei DN-114 001 adhesion levels, indicating that an acidic pH was not the main factor involved in the increased ability of L. casei DN-114 001 to adhere to undifferentiated and differentiated intestinal epithelial cells.
FIG. 2.
Increased adhesion of L. casei DN-114 001 in the presence of its spent culture supernatant. Cultured cells were incubated with L. casei DN-114 001 at an MOI of 500 for 6 h in cell culture medium alone (black bars), or supplemented with 10% (vol/vol) of its spent culture supernatant (white bars) and with 10% of neutralized supernatant (grey bars). Adhesion levels were determined as described in the legend to Fig. 1.
Inhibitory effect of L. casei DN 114 001 on AIEC LF82 adhesion and invasion in preincubation experiments.
The probiotic activity of L. casei DN-114 001 in terms of antiadhesive and anti-invasive effects on adherent-invasive E. coli colonization of the gut was determined in vitro using undifferentiated and differentiated intestinal epithelial cells.
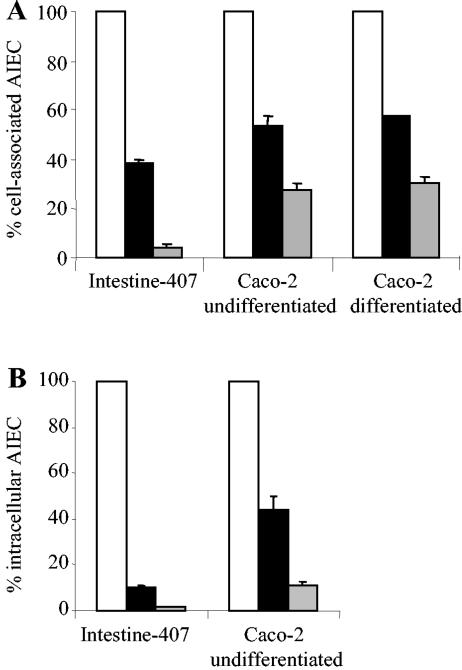
Preincubation of cultured intestinal epithelial cells was performed with L. casei DN-114 001 prior to infection with AIEC LF82. As shown in Fig. 3A, L. casei DN-114 001 significantly (P < 0.05) inhibited the ability of LF82 to adhere to undifferentiated Intestine-407 cells (62%) and to undifferentiated and differentiated Caco-2 cells (47% and 43%, respectively). The inhibitory effects on LF82 adhesion were significantly (P < 0.01) increased when the preincubation of L. casei DN-114 001 was performed in the presence of 10% of its spent culture supernatant. Under such conditions, the LF82 adhesion level to Intestine-407 cells was reduced by 96%, and we observed percentages of inhibition of 73% and 70% on LF82 adhesion to undifferentiated and differentiated Caco-2 cells, respectively.
FIG. 3.
Inhibitory effects of L. casei DN-114 001 on the abilities of AIEC LF82 to adhere to and to invade intestinal epithelial cells in preincubation experiments. Adhesion (A) and invasion (B) of AIEC LF82 with intestinal epithelial cells preincubated with L. casei DN-114 001 alone (black bars) or supplemented with 10% (vol/vol) of its spent culture supernatant (grey bars), compared with adhesion and invasion levels of AIEC LF82 to untreated epithelial cells (white bars), taken as 100%. Preincubation of cultured cells was performed for 6 h with L. casei DN-114 001 at an MOI of 500. Infection with AIEC LF82 was performed for 3 h with an MOI of 100. Invasion was determined after gentamicin treatment for an additional hour. Results are expressed as cell-associated bacteria (adherent plus intracellular bacteria) or intracellular bacteria relative to those obtained for strain LF82 with untreated cells. Each value is the mean ± SEM of three to four separate experiments.
The inhibitory effect of L. casei DN-114 001 on LF82 invasion was only examined with undifferentiated intestinal epithelial cells (Intestine-407 and Caco-2 cells), since low levels of intracellular LF82 are observed with differentiated intestinal cells (9). The inhibitory effects of L. casei DN-114 001 on LF82 invasion were slightly higher than those obtained on LF82 adhesion (Fig. 3B). In preincubation experiments of intestinal epithelial cells with L. casei DN-114 001 alone, inhibitory effects on LF82 invasion of 90% with Intestine-407 and 56% with Caco-2 cells were observed. When preincubation with L. casei DN-114 001 was performed in the presence of 10% of its spent culture supernatant, inhibition of LF82 invasion was 98.7% with Intestine-407 and 89% with Caco-2 cells.
Inhibitory effect of L. casei DN 114 001 on AIEC adhesion and invasion in coincubation experiments.
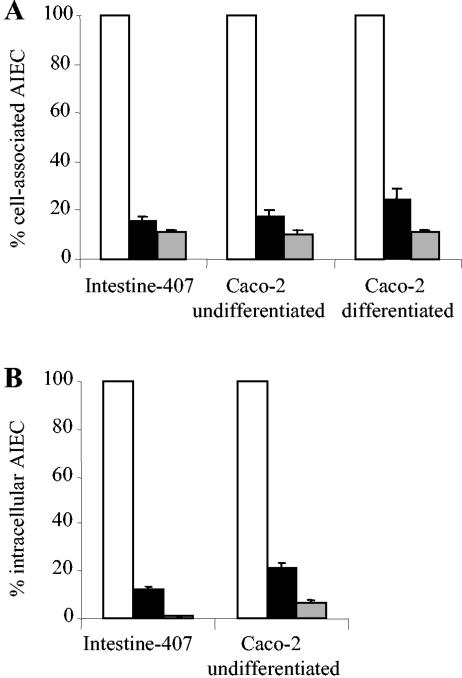
Adhesion and invasion levels of AIEC strains with respect to intestinal epithelial cells were determined by coincubation experiments where AIEC and L. casei DN-114 001 were added together with intestinal epithelial cells. In coincubation with L. casei DN-114 001, very marked decreases in AIEC LF82 adhesion levels were observed (Fig. 4A) with differentiated and undifferentiated cells. The inhibitory effect on LF82 adhesion was highly significant (P < 0.01) and percentage of inhibition was 82% and 84% with undifferentiated Caco-2 and Intestine-407 cells, respectively. It was 75% with differentiated Caco-2 cells. The inhibitory effect was even more pronounced when coincubations of AIEC LF82 and L. casei DN-114 001 were performed in the presence of 10% of L. casei DN-114 001 spent culture supernatant. Under these conditions, shown above to increase the ability of L. casei DN-114 001 to adhere to intestinal epithelial cells, the percentage of inhibition of LF82 adhesion was 89, 90, and 89% with Intestine-407, undifferentiated Caco-2, and differentiated Caco-2 cells, respectively. When coincubation of AIEC LF82 and L. casei DN-114 001 was performed in the presence of 10% of neutralized L. casei spent culture supernatant, similar inhibitory effects on AIEC LF82 adhesion to Intestine-407 cells were observed (Table 1).
FIG. 4.
Adhesion (A) and invasion (B) abilities of AIEC LF82 with respect to intestinal epithelial cells in coincubation experiments with L. casei DN-114 001 alone (black bars) or supplemented with 10% (vol/vol) of L. casei DN-114 001 spent culture supernatant (grey bars), compared with monoinfection experiments with LF82 alone (white bars). A multiplicity of infection of 500 was used for L. casei DN-114 001 and an MOI of 10 was used for AIEC LF82. Cell-associated bacteria were quantified after a 6-h incubation period. Invasion was determined after gentamicin treatment for an additional hour. Results are expressed as the percentage of cell-associated bacteria (adherent plus intracellular bacteria) or intracellular bacteria relative to those obtained in monoinfection with strain LF82, taken as 100%. Each value is the mean ± SEM of three to five separate experiments.
TABLE 1.
Inhibition of adhesion and invasion of various AIEC strains by probiotic L. casei DN-114 001c
| AIEC strain | Coincubation of L. casei DN-114 001 with:
|
|||
|---|---|---|---|---|
| 10% L. casei spent culture SN
|
10% L. casei neutralized spent culture SN
|
|||
| % Inhibition of cell associationa | % Inhibition of cell invasionb | % Inhibition of cell associationa | % Inhibition of cell invasionb | |
| LF 82 | 89.1 ± 2.8 | 99.7 ± 0.2 | 84.9 ± 4.1 | 99.4 ± 0.2 |
| LF 9 | 87.1 ± 2.4 | 97.6 ± 0.7 | 80.3 ± 5.1 | 97.4 ± 0.7 |
| LF 15 | 87.4 ± 2.8 | 98.0 ± 1.0 | 81.4 ± 3.6 | 97.8 ± 1.0 |
| LF 31 | 90.0 ± 3.3 | 98.9 ± 0.6 | 86.9 ± 2.9 | 98.5 ± 0.6 |
| LF 65 | 87.2 ± 3.4 | 98.9 ± 0.7 | 78.6 ± 3.9 | 98.6 ± 0.7 |
| LF 110 | 89.1 ± 2.4 | 99.0 ± 0.6 | 86.0 ± 2.7 | 98.4 ± 0.8 |
| LF 134 | 83.4 ± 2.7 | 96.1 ± 1.4 | 76.3 ± 4.0 | 93.0 ± 2.6 |
Inhibition (%) = [(cell-associated bacteria alone − cell-associated bacteria in coincubation experiments with L. casei DN-114 001)/(cell-associated bacteria alone)] × 100.
Inhibition (%) = [(intracellular bacteria alone − intracellular bacteria in coincubation experiments with L. casei DN-114 001)/(intracellular bacteria alone)] × 100.
Coincubation experiments were performed with Intestine-407 cells for 6 h at a multiplicity of infection of 500 for the probiotic strain L. casei DN-114 001 and of 10 for AIEC strains. Results are expressed as a percent inhibition of cell-associated bacteria or intracellular bacteria relative to those obtained in monoinfections with AIEC strains (taken as 100%). Each value is the mean ± SEM of three separate experiments.
The inhibitory effect of L. casei DN-114 001 on LF82 invasion paralleled inhibition of adhesion (Fig. 4B). In coincubation with L. casei DN-114 001, the number of intracellular LF82 bacteria significantly (P < 0.001) decreased. The percentage of inhibition of LF82 invasion was similar to that observed with LF82 adhesion, 88 and 79% with Intestine-407 and Caco-2 cells, respectively. Similar to results obtained on LF82 adhesion, the addition of 10% of L. casei DN-114 001 spent culture supernatant in the incubation medium induced a more pronounced inhibitory effect on LF82 invasion, 99 and 93% with Intestine-407 and Caco-2 cells, respectively. When the L. casei culture supernatant was neutralized, similar inhibitory effects on AIEC LF82 invasion of Intestine-407 cells were observed (Table 1).
Inhibitory effects of L. casei DN-114 001 on bacterial adhesion and invasion of other AIEC strains were investigated with Intestine-407 cultured cells (Table 1). Strong inhibitory effects on AIEC adhesion and invasion were observed for all the AIEC strains tested. Adhesion inhibition levels ranged from 83 to 90%, and invasion inhibition levels ranged from 96 to 99%. These inhibitory effects continued to be observed when the pH of L. casei DN-114 001 spent culture supernatant was adjusted to a neutral value. Slight decreases were observed, but adhesion inhibition levels still ranged from 76 to 86%, and invasion inhibition levels ranged from 93 to 98%.
The inhibitory effect of L. casei DN 114 001 on AIEC LF82 adhesion and invasion is not related to cell cytotoxicity or to antibacterial activity.
Since the decreased ability of AIEC LF82 to adhere to and invade intestinal epithelial cells may be related to cultured cell death induced by L. casei and/or its spent culture supernatant, we determined the amounts of the cytoplasmic enzyme LDH released when the integrity of the cytoplasmic membrane of eukaryotic cells was breached (Table 2). As a positive control inducing LDH release, we used E. coli strain LF32 producing an α-hemolysin (17). Infection of the different cell models with E. coli LF32 for 6 h induced marked release of LDH (19.3 to 22.7%). In contrast, LDH release by intestinal epithelial cells incubated for 6 h with an MOI of 500 of L. casei DN-114 001 alone or in the presence of 10% of its spent culture supernatant was very low (2.0 to 5.7), and values were similar to those observed when incubations were performed with the nonpathogenic strain E. coli K-12 C600. This indicates that L. casei DN-114 001 alone or in association with its spent culture supernatant did not induce any cell cytotoxicity even at an MOI of 500 and after 6 h of incubation with intestinal epithelial cells.
TABLE 2.
Absence of LDH release by intestinal epithelial cells incubated with L. casei DN-114 001 alone and in the presence of its spent culture SN
| Strain | % LDH releasea
|
||
|---|---|---|---|
| Intestine-407 | Caco-2
|
||
| Undifferentiated | Differentiated | ||
| E. coli LF 32 | 22.7 ± 0.2 | 19.3 ± 0.7 | 20.2 ± 2.0 |
| E. coli K12-C600 | 2.6 ± 0.9 | 5.0 ± 0.4 | 6.2 ± 0.2 |
| L. casei DN-114 001 | 2.0 ± 0.6 | 5.7 ± 0.7 | 2.3 ± 0.1 |
| L. casei DN-114 001 + 10% SN | 3.1 ± 0.9 | 5.2 ± 0.4 | 4.2 ± 0.9 |
LDH release was determined in supernatants using NADH as the substrate. It was measured after 6 h of incubation with a multiplicity of infection of 500 for L. casei DN-114 001 and of 100 for E. coli strains. Results are expressed as percent cytotoxycity, calculated as (experimental release − spontaneous LDH release)/(total LDH release − spontaneous release) × 100. E. coli LF32 producing an α-hemolysin was used as a positive control (17). E. coli strain K-12 C600 was used as a negative control.
Since inhibition of LF82 adhesion and invasion may be related to bactericidal effects or inhibition of bacterial growth induced by L. casei DN-114 001, LF82 bacterial growth was analyzed by incubation with DMEM with L. casei DN-114 001 alone and in the presence of its spent culture supernatant (Table 3). Coincubation for 3 h with L. casei DN-114 001 alone or with 10% of its spent culture supernatant did not induce any significant (P > 0.05) decrease in LF82 bacterial growth. After 6 h of coincubation with L. casei DN-114 001, a 26% decrease in LF82 replication was observed. This decrease reached 38% when coincubation of AIEC LF82 was performed with L. casei DN-114 001 in the presence of 10% of the spent supernatant. When the pH of the L. casei DN-114 001 supernatant was adjusted to a neutral value (pH 7.0), the decrease in LF82 bacterial growth was similar to that observed with L. casei alone. Compared to the inhibitory effects of L. casei on AIEC LF82 adhesion and invasion (89 to 98% of inhibition), the influence of L. casei on LF82 bacterial growth could not fully explain the resulting inhibitory effects of L. casei DN-114 001 on the abilities of strain LF82 to adhere to and invade cultured intestinal epithelial cells.
TABLE 3.
Activity of L. casei DN-114 001 alone or in the presence of 10% of its spent culture supernatant on AIEC LF82 bacterial growtha
| Bacterial growth in CFU (107)/ml (% of growth inhibition/DMEM)
| ||
|---|---|---|
| Growth medium | Time of incubation
|
|
| 3 h | 6 h | |
| DMEM | 2.9 ± 0.2 | 43.5 ± 5.2 |
| DMEM + L. casei DN-114 001 | 3.0 ± 0.4 (0) | 32.2 ± 3.3 (26) |
| DMEM + L. casei DN-114 001 + 10% SN | 2.7 ± 0.4 (5) | 26.7 ± 2.1 (38) |
| DMEM + L. casei DN-114 001 + 10% SN (pH = 7) | 3.0 ± 0.3 (0) | 31.8 ± 2.1 (27) |
Control bacterial growth of LF82 was determined in cell culture medium (DMEM). Bacterial growth of LF82 was also determined in coincubation with L. casei DN-114 001 alone or supplemented with 10% of L. casei DN-114 001 spent culture supernatant (10% SN) or of neutralized supernatant (10% SN, pH 7.0). Results are expressed as (CFU) after 3 h or 6 h incubation. Each value is the mean ± SEM of five separate experiments.
DISCUSSION
The onset of inflammation in IBD may be associated with an imbalance in the intestinal microflora, with a relative predominance of aggressive bacteria and an insufficient amount of protective species. Since early and chronic ileal lesions of patients with Crohn's disease are abnormally colonized by AIEC strains (16), the eradication of these pathogenic bacteria and their replacement by probiotic bacteria may provide a new option for the treatment of CD and for the maintenance of remission.
To provide clinical benefits, adhesion of probiotic bacteria to the intestinal mucosa is an interesting trait for antagonistic activity against pathogens. We showed in the present study that the probiotic strain L. casei DN-114 001 exhibited a dose- and incubation time-dependent ability to adhere to undifferentiated Intestine-407 and Caco-2 cells and to differentiated Caco-2 cells. This result was expected, since several studies showed that various Lactobacillus strains adhere to cultured intestinal epithelial cells (18, 20, 22, 29, 44, 50). Their ability to adhere is independent of bacterial species, but is strain specific (10, 49), and is increased in the presence of spent culture supernatant (3, 10, 12, 44, 50). A markedly increased ability of L. casei DN-114 001 to adhere to intestinal epithelial cells was also observed in the presence of 10% of its spent culture supernatant. This indicates that a secreted factor enhancing bacterial adhesion or the low pH due to acid lactic production may interfere with the adhesion of Lactobacilli to intestinal epithelial cells (3, 10, 12, 15, 22, 25). However, this study shows that low pH is not required for adhesion of L. casei DN-114 001 strain to adhere. Besides, Greene and Klaenhammer (22) previously reported that lowering the pH of fresh MRS cannot account for the increased adherence of Lactobacilli to Caco-2 cells following the addition of the spent culture supernatant.
An important function of probiotic bacteria is to provide protection of the host gastrointestinal tract from invading pathogens. In this study, we showed that preincubation of intestinal epithelial cells with L. casei DN-114 001 prior to infection with pathogenic AIEC strain LF82 resulted in a strong decrease in LF82 adhesion (43 to 62%) and invasion (56 to 90%). In coincubation experiments, where L. casei DN-114 001 and AIEC strain LF82 were added together with the intestinal epithelial cells, higher inhibitory effects on AIEC adhesion (75 to 84%) and invasion (79 to 88%) were observed. The inhibitory effects of L. casei DN-114 001 on both LF82 adhesion and invasion are not surprising, since this result is consistent with previous observations showing that the adhesion step is crucial for AIEC to invade epithelial cells (7). Several reports have confirmed the ability of probiotic Lactobacilli to inhibit the ability of pathogenic bacteria to adhere to and to invade intestinal epithelial cells (4, 10, 20, 26, 27). The inhibition levels observed with L. casei DN-114 001 are similar to or even higher than those reported for Lactobacilli on the adhesion of various pathogens (4, 11, 18, 20, 49). We noticed that L. casei DN-114 001 exerts stronger inhibitory effects on AIEC invasion than those reported with various Lactobacilli on invasive enteropathogens (11, 13, 20). Concerning its inhibitory role on AIEC adhesion and invasion, L. casei DN-114 001 is as efficient as E. coli Nissle (8).
The inhibitory activity of L. casei DN-114 001 on LF82 adhesion and invasion was enhanced when 10% of spent L. casei DN-114 001 culture supernatant was added to coincubation and preincubation media. This finding continued to be observed when the acidic pH of the supernatant was neutralized. The increased inhibitory effects may be related to the increased adhesion of L. casei DN-114 001 that we observed in the presence of its spent culture supernatant. Such increased adhesion could induce greater competition between probiotic and pathogens for attachment sites by specific blockage or steric hindrance. This could also be due to the production of antimicrobial substances active against AIEC. Several studies have reported the ability of Lactobacilli to secrete antimicrobial compounds such as bacteriocins (1, 2, 31, 36, 52, 53). Lactic acid produced by all Lactobacillus strains was also shown to inhibit bacterial growth (32-34). Under the experimental conditions of this study, even though the secretion by L. casei DN-114 001 of an antibacterial substance active on AIEC LF82 bacterial growth was observed, this substance alone could not explain the high inhibitory effects of L. casei DN-114 001 on the abilities of strain LF82 to adhere to and to invade cultured intestinal epithelial cells. Thus, the high inhibitory effects of L. casei on AIEC adhesion to and invasion of intestinal epithelial cells would result from several cumulative factors such as the adhesion of L. casei to the intestinal epithelial cells and the secretion by L. casei of compounds, which induce a high increase in L. casei adhesion and a moderate decrease in AIEC bacterial growth.
In conclusion, probiotic strain L. casei DN-114 001 exerts strong inhibitory effects on both AIEC adhesion to and invasion of intestinal epithelial cells. The present in vitro study indicates that this probiotic may be efficient for preventive and curative probiotic therapy, since inhibitory effects were observed when intestinal cells were preincubated with L. casei DN-114 001 and when the probiotic was used in coincubation experiments. Thus, the use of this probiotic could be of great interest, especially in maintaining remission in a subset of CD patients harboring pathogenic AIEC colonizing early and chronic ileal lesions.
REFERENCES
- 1.Allison, G. E., C. Fremaux, and T. R. Klaenhammer. 1994. Expansion of bacteriocin activity and host range upon complementation of two peptides encoded within the lactacin F operon. J. Bacteriol. 176:2235-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Axelsson, L., A. Holck, S. E. Birkeland, T. Aukrust, and H. Blom. 1993. Cloning and nucleotide sequence of a gene from Lactobacillus sake Lb706 necessary for sakacin A production and immunity. Appl. Environ. Microbiol. 59:2868-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernet, M. F., D. Brassart, J. R. Neeser, and A. L. Servin. 1994. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut 35:483-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernet-Camard, M. F., V. Lievin, D. Brassart, J. R. Neeser, A. L. Servin, and S. Hudault. 1997. The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance(s) active in vitro and in vivo. Appl. Environ. Microbiol. 63:2747-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borruel, N., M. Carol, F. Casellas, M. Antolin, F. de Lara, E. Espin, J. Naval, F. Guarner, and J. R. Malagelada. 2002. Increased mucosal tumour necrosis factor alpha production in Crohn's disease can be downregulated ex vivo by probiotic bacteria. Gut 51:659-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borruel, N., F. Casellas, M. Antolin, M. Llopis, M. Carol, E. Espiin, J. Naval, F. Guarner, and J. R. Malagelada. 2003. Effects of nonpathogenic bacteria on cytokine secretion by human intestinal mucosa. Am. J. Gastroenterol. 98:865-870. [DOI] [PubMed] [Google Scholar]
- 7.Boudeau, J., N. Barnich, and A. Darfeuille-Michaud. 2001. Type 1 pili-mediated adherence of Escherichia coli strain LF82 isolated from Crohn's disease is involved in bacterial invasion of intestinal epithelial cells. Mol. Microbiol. 39:1272-1284. [DOI] [PubMed] [Google Scholar]
- 8.Boudeau, J., A. L. Glasser, S. Julien, J. F. Colombel, and A. Darfeuille-Michaud. 2003. Inhibitory effect of probiotic Escherichia coli strain Nissle 1917 on adhesion to and invasion of intestinal epithelial cells by adherent-invasive E. coli strains isolated from patients with Crohn's disease. Aliment. Pharmacol. Ther. 18:45-56. [DOI] [PubMed] [Google Scholar]
- 9.Boudeau, J., A. L. Glasser, E. Masseret, B. Joly, and A. Darfeuille-Michaud. 1999. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect. Immun. 67:4499-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chauviere, G., M. H. Coconnier, S. Kerneis, A. Darfeuille-Michaud, B. Joly, and A. L. Servin. 1992. Competitive exclusion of diarrheagenic Escherichia coli (ETEC) from human enterocyte-like Caco-2 cells by heat-killed Lactobacillus. FEMS Microbiol. Lett. 70:213-217. [DOI] [PubMed] [Google Scholar]
- 11.Coconnier, M. H., M. F. Bernet, S. Kerneis, G. Chauviere, J. Fourniat, and A. L. Servin. 1993. Inhibition of adhesion of enteroinvasive pathogens to human intestinal Caco-2 cells by Lactobacillus acidophilus strain LB decreases bacterial invasion. FEMS Microbiol. Lett. 110:299-305. [DOI] [PubMed] [Google Scholar]
- 12.Coconnier, M. H., T. R. Klaenhammer, S. Kerneis, M. F. Bernet, and A. L. Servin. 1992. Protein-mediated adhesion of Lactobacillus acidophilus BG2FO4 on human enterocyte and mucus-secreting cell lines in culture. Appl. Environ. Microbiol. 58:2034-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coconnier, M. H., V. Lievin, M. Lorrot, and A. L. Servin. 2000. Antagonistic activity of Lactobacillus acidophilus LB against intracellular Salmonella enterica serovar Typhimurium infecting human enterocyte-like Caco-2/TC-7 cells. Appl. Environ. Microbiol. 66:1152-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colombel, J. F., M. Lemann, M. Cassagnou, Y. Bouhnik, B. Duclos, J. L. Dupas, B. Notteghem, J. Y. Mary, et al. 1999. A controlled trial comparing ciprofloxacin with mesalazine for the treatment of active Crohn's disease. Am. J. Gastroenterol. 94:674-678. [DOI] [PubMed] [Google Scholar]
- 15.Conway, P. L., and S. Kjelleberg. 1989. Protein-mediated adhesion of Lactobacillus fermentum strain 737 to mouse stomach squamous epithelium. J. Gen. Microbiol. 135:1175-1186. [DOI] [PubMed] [Google Scholar]
- 16.Darfeuille-Michaud, A., J. Boudeau, P. Bulois, C. Neut, A. L. Glasser, N. Barnich, M. A. Bringer, A. Swidsinski, L. Beaugerie, and J. F. Colombel. 2004. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology 127:412-421. [DOI] [PubMed] [Google Scholar]
- 17.Darfeuille-Michaud, A., C. Neut, N. Barnich, E. Lederman, P. Di Martino, P. Desreumaux, L. Gambiez, B. Joly, A. Cortot, and J. F. Colombel. 1998. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology 115:1405-1413. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez, M. F., S. Boris, and C. Barbes. 2003. Probiotic properties of human lactobacilli strains to be used in the gastrointestinal tract. J. Appl. Microbiol. 94:449-455. [DOI] [PubMed] [Google Scholar]
- 19.Glasser, A. L., J. Boudeau, N. Barnich, M. H. Perruchot, J. F. Colombel, and A. Darfeuille-Michaud. 2001. Adherent invasive Escherichia coli strains from patients with Crohn's disease survive and replicate within macrophages without inducing host cell death. Infect. Immun. 69:5529-5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gopal, P. K., J. Prasad, J. Smart, and H. S. Gill. 2001. In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against an enterotoxigenic Escherichia coli. Int. J. Food Microbiol. 67:207-216. [DOI] [PubMed] [Google Scholar]
- 21.Greenbloom, S. L., A. H. Steinhart, and G. R. Greenberg. 1998. Combination ciprofloxacin and metronidazole for active Crohn's disease. Can. J. Gastroenterol. 12:53-56. [DOI] [PubMed] [Google Scholar]
- 22.Greene, J. D., and T. R. Klaenhammer. 1994. Factors involved in adherence of lactobacilli to human Caco-2 cells. Appl. Environ. Microbiol. 60:4487-4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerin-Danan, C., C. Chabanet, C. Pedone, F. Popot, P. Vaissade, C. Bouley, O. Szylit, and C. Andrieux. 1998. Milk fermented with yogurt cultures and Lactobacillus casei compared with yogurt and gelled milk: influence on intestinal microflora in healthy infants. Am. J. Clin. Nutr. 67:111-117. [DOI] [PubMed] [Google Scholar]
- 24.Guslandi, M., G. Mezzi, M. Sorghi, and P. A. Testoni. 2000. Saccharomyces boulardii in maintenance treatment of Crohn's disease. Dig. Dis. Sci. 45:1462-1464. [DOI] [PubMed] [Google Scholar]
- 25.Henriksson, A., R. Szewzyk, and P. L. Conway. 1991. Characteristics of the adhesive determinants of Lactobacillus fermentum 104. Appl. Environ. Microbiol. 57:499-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirano, J., T. Yoshida, T. Sugiyama, N. Koide, I. Mori, and T. Yokochi. 2003. The effect of Lactobacillus rhamnosus on enterohemorrhagic Escherichia coli infection of human intestinal cells in vitro. Microbiol. Immunol. 47:405-409. [DOI] [PubMed] [Google Scholar]
- 27.Hudault, S., V. Lievin, M. F. Bernet-Camard, and A. L. Servin. 1997. Antagonistic activity exerted in vitro and in vivo by Lactobacillus casei (strain GG) against Salmonella typhimurium C5 infection. Appl. Environ. Microbiol. 63:513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isolauri, E., P. V. Kirjavainen, and S. Salminen. 2002. Probiotics: a role in the treatment of intestinal infection and inflammation? Gut 50(Suppl. 3):III54-III59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobsen, C. N., V. Rosenfeldt Nielsen, A. E. Hayford, P. L. Moller, K. F. Michaelsen, A. Paerregaard, B. Sandstrom, M. Tvede, and M. Jakobsen. 1999. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl. Environ. Microbiol. 65:4949-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerneis, S., S. S. Bilge, V. Fourel, G. Chauviere, M. H. Coconnier, and A. L. Servin. 1991. Use of purified F1845 fimbrial adhesin to study localization and expression of receptors for diffusely adhering Escherichia coli during enterocytic differentiation of human colon carcinoma cell lines HT-29 and Caco-2 in culture. Infect. Immun. 59:4013-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsen, A. G., F. K. Vogensen, and J. Josephsen. 1993. Antimicrobial activity of lactic acid bacteria isolated from sour doughs: purification and characterization of bavaricin A, a bacteriocin produced by Lactobacillus bavaricus MI401. J. Appl. Bacteriol. 75:113-122. [DOI] [PubMed] [Google Scholar]
- 32.Lehto, E. M., and S. J. Salminen. 1997. Inhibition of Salmonella typhimurium adhesion to Caco-2 cell cultures by Lactobacillus strain GG spent culture supernate: only a pH effect? FEMS Immunol. Med. Microbiol. 18:125-132. [DOI] [PubMed] [Google Scholar]
- 33.Midolo, P. D., J. R. Lambert, R. Hull, F. Luo, and M. L. Grayson. 1995. In vitro inhibition of Helicobacter pylori NCTC 11637 by organic acids and lactic acid bacteria. J. Appl. Bacteriol. 79:475-479. [DOI] [PubMed] [Google Scholar]
- 34.Ogawa, M., K. Shimizu, K. Nomoto, R. Tanaka, T. Hamabata, S. Yamasaki, T. Takeda, and Y. Takeda. 2001. Inhibition of in vitro growth of Shiga toxin-producing Escherichia coli O157:H7 by probiotic Lactobacillus strains due to production of lactic acid. Int. J. Food Microbiol. 68:135-140. [DOI] [PubMed] [Google Scholar]
- 35.Oozeer, R., N. Goupil-Feuillerat, C. A. Alpert, M. van de Guchte, J. Anba, J. Mengaud, and G. Corthier. 2002. Lactobacillus casei is able to survive and initiate protein synthesis during its transit in the digestive tract of human flora-associated mice. Appl. Environ. Microbiol. 68:3570-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parente, E., and A. Ricciardi. 1999. Production, recovery and purification of bacteriocins from lactic acid bacteria. Appl. Microbiol. Biotechnol. 52:628-638. [DOI] [PubMed] [Google Scholar]
- 37.Pedone, C. A., C. C. Arnaud, E. R. Postaire, C. F. Bouley, and P. Reinert. 2000. Multicentric study of the effect of milk fermented by Lactobacillus casei on the incidence of diarrhoea. Int. J. Clin. Pract. 54:568-571. [PubMed] [Google Scholar]
- 38.Pedone, C. A., A. O. Bernabeu, E. R. Postaire, C. F. Bouley, and P. Reinert. 1999. The effect of supplementation with milk fermented by Lactobacillus casei (strain DN-114 001) on acute diarrhoea in children attending day care centres. Int. J. Clin. Pract. 53:179-184. [PubMed] [Google Scholar]
- 39.Perdigon, G., E. Vintini, S. Alvarez, M. Medina, and M. Medici. 1999. Study of the possible mechanisms involved in the mucosal immune system activation by lactic acid bacteria. J. Dairy Sci. 82:1108-1114. [DOI] [PubMed] [Google Scholar]
- 40.Podolsky, D. K. 2002. Inflammatory bowel disease. N. Engl. J. Med. 347:417-429. [DOI] [PubMed] [Google Scholar]
- 41.Prantera, C., F. Zannoni, M. L. Scribano, E. Berto, A. Andreoli, A. Kohn, and C. Luzi. 1996. An antibiotic regimen for the treatment of active Crohn's disease: a randomized, controlled clinical trial of metronidazole plus ciprofloxacin. Am. J. Gastroenterol. 91:328-332. [PubMed] [Google Scholar]
- 42.Rembacken, B. J., A. M. Snelling, P. M. Hawkey, D. M. Chalmers, and A. T. Axon. 1999. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet 354:635-639. [DOI] [PubMed] [Google Scholar]
- 43.Rutgeerts, P., M. Hiele, K. Geboes, M. Peeters, F. Penninckx, R. Aerts, and R. Kerremans. 1995. Controlled trial of metronidazole treatment for prevention of Crohn's recurrence after ileal resection. Gastroenterology 108:1617-1621. [DOI] [PubMed] [Google Scholar]
- 44.Sarem, F., L. O. Sarem-Damerdji, and J. P. Nicolas. 1996. Comparison of the adherence of three Lactobacillus strains to Caco-2 and Int-407 human intestinal cell lines. Lett. Appl. Microbiol. 22:439-442. [DOI] [PubMed] [Google Scholar]
- 45.Shanahan, F. 2000. Probiotics and inflammatory bowel disease: is there a scientific rationale? Inflamm. Bowel Dis. 6:107-115. [DOI] [PubMed] [Google Scholar]
- 46.Shanahan, F. 2001. Probiotics in inflamatory bowel disease. Gut 48:609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sullivan, A., and C. E. Nord. 2002. Probiotics in human infections. J. Antimicrob Chemother. 50:625-627. [DOI] [PubMed] [Google Scholar]
- 48.Sutherland, L., J. Singleton, J. Sessions, S. Hanauer, E. Krawitt, G. Rankin, R. Summers, H. Mekhjian, N. Greenberger, M. Kelly, et al. 1991. Double blind, placebo controlled trial of metronidazole in Crohn's disease. Gut 32:1071-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Todoriki, K., T. Mukai, S. Sato, and T. Toba. 2001. Inhibition of adhesion of food-borne pathogens to Caco-2 cells by Lactobacillus strains. J. Appl. Microbiol. 91:154-159. [DOI] [PubMed] [Google Scholar]
- 50.Tuomola, E. M., and S. J. Salminen. 1998. Adhesion of some probiotic and dairy Lactobacillus strains to Caco-2 cell cultures. Int. J. Food Microbiol. 41:45-51. [DOI] [PubMed] [Google Scholar]
- 51.Venturi, A., P. Gionchetti, F. Rizzello, R. Johansson, E. Zucconi, P. Brigidi, D. Matteuzzi, and M. Campieri. 1999. Impact on the composition of the faecal flora by a new probiotic preparation: preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment. Pharmacol. Ther. 13:1103-1108. [DOI] [PubMed] [Google Scholar]
- 52.Yamato, M., K. Ozaki, and F. Ota. 2003. Partial purification and characterization of the bacteriocin produced by Lactobacillus acidophilus YIT 0154. Microbiol. Res. 158:169-172. [DOI] [PubMed] [Google Scholar]
- 53.Zhu, W. M., W. Liu, and D. Q. Wu. 2000. Isolation and characterization of a new bacteriocin from Lactobacillus gasseri KT7. J. Appl. Microbiol. 88:877-886. [DOI] [PubMed] [Google Scholar]