Abstract
Ergot alkaloids are mycotoxins that interact with several monoamine receptors, negatively affecting cardiovascular, nervous, reproductive, and immune systems of exposed humans and animals. Aspergillus fumigatus, a common airborne fungus and opportunistic human pathogen, can produce ergot alkaloids in broth culture. The objectives of this study were to determine if A. fumigatus accumulates ergot alkaloids in a respirable form in or on its conidia, to quantify ergot alkaloids associated with conidia produced on several different substrates, and to measure relevant physical properties of the conidia. We found at least four ergot alkaloids, fumigaclavine C, festuclavine, fumigaclavine A, and fumigaclavine B (in order of abundance), associated with conidia of A. fumigatus. Under environmentally relevant conditions, the total mass of ergot alkaloids often constituted >1% of the mass of the conidium. Ergot alkaloids were extracted from conidia produced on all media tested, and the greatest quantities were observed when the fungus was cultured on latex paint or cultured maize seedlings. The values for physical properties of conidia likely to affect their respirability (i.e., diameter, mass, and specific gravity) were significantly lower for A. fumigatus than for Aspergillus nidulans, Aspergillus niger, and Stachybotrys chartarum. The demonstration of relatively high concentrations of ergot alkaloids associated with conidia of A. fumigatus presents opportunities for investigations of potential contributions of the toxins to adverse health effects associated with the fungus and to aspects of the biology of the fungus that contribute to its success.
The ergot alkaloids are a complex family of indole-derived alkaloids that have a long history of association with human suffering. The contamination of rye and other grain crops with alkaloid-rich sclerotia of the ergot fungus Claviceps purpurea was responsible for gangrenous and convulsive forms of ergotism known as St. Anthony's fire or holy fire (19). Other ergot alkaloid-producing fungi, such as the closely related Neotyphodium spp. endophytes of forage grasses, negatively affect agriculture by reducing animal productivity and health (2, 22). The ability of different ergot alkaloids to act as partial agonists or antagonists of various serotonin, dopamine, and α-adrenaline receptors results in effects on nervous, circulatory, reproductive, and immune systems, leading to high or low blood pressure, muscle contractions, reduced fertility, disturbances in sleep-wake cycles, lowered immune responses, and, at high doses, hallucinations and gangrene of the extremities (14, 16, 22, 25, 31).
Screening analyses of other fungi for ergot alkaloids have identified several distantly related fungi as potential sources (13, 17, 29). Among these fungi, the best characterized is Aspergillus fumigatus. This fungus was first noted to produce the ergot alkaloid festuclavine and two novel derivatives of festuclavine, fumigaclavine A and fumigaclavine B, in semidefined broth culture (29). Later, Cole et al. (3) described an additional festuclavine derivative, fumigaclavine C, from broth cultures of A. fumigatus originally isolated from moldy silage.
A. fumigatus is associated with several human health issues. It is the most common airborne fungal pathogen of humans (6, 18). It can cause invasive aspergillosis in immunocompromised individuals, and the resulting mortality rate is >50%. In immunocompetent individuals, this fungus can colonize preexisting cavities in the lungs or sinuses without penetrating into tissues, a condition known as aspergilloma or fungus ball (6, 11, 18). A. fumigatus also is associated with air quality issues in indoor environments and near composting facilities (11, 12, 27). The presence of conidia of A. fumigatus and of several other fungi in such environments has been loosely associated with respiratory allergic symptoms and miscellaneous other ailments, but causal associations have not been demonstrated.
Most mycotoxin-producing fungi produce their toxins in the substrate in which they grow, and ingestion of the contaminated substrate is required for intoxication. Although less frequently documented, the presence of mycotoxins in or on conidia of the producing fungus provides the potential for delivery of mycotoxins via the alternate and less voluntary route of inhalation. For example, the black mold fungus Stachybotrys chartarum contains trichothecenes associated with its spores (28).
Our objectives in this study were to determine if conidia of A. fumigatus contain ergot alkaloids in a respirable form, to quantify ergot alkaloids associated with conidia produced on several environmentally relevant substrates, and to measure physical properties of conidia likely to affect their respirability. Detection and quantification of ergot alkaloids associated with conidia are prerequisites for investigations of potential contributions of the alkaloids to adverse human health effects or to other aspects of the biology of the fungus.
MATERIALS AND METHODS
Fungi and culture conditions.
A. fumigatus isolate WVU1943 (= FGSC A1141) was cultured from the lungs of a deceased parakeet in Charleston, West Virginia, during 1977. Isolate WVU2026 (= FGSC A1142) was obtained by passive sampling of the air (by opening a petri dish containing potato dextrose agar for 20 min under ambient conditions) in a house in Monongalia County, West Virginia, during 2003. All viable cultures were manipulated in a class II biosafety cabinet. Isolates were maintained on potato dextrose agar (PDA) (20 g/liter dehydrated mashed potatoes [Idaho Spuds; Pillsbury, Minneapolis, MN], 20 g/liter glucose, 15 g/liter agar [Bacto agar; Difco, Detroit, MI]) at room temperature or at 37°C, and most ergot alkaloid analyses were conducted with cultures grown on this medium. Additional alkaloid analyses were conducted with A. fumigatus isolates cultured on the following substrates: 1.5% (wt/vol) water agar plates (prepared with Difco Bacto agar); Pittsburgh Paint (Pittsburgh, PA) interior latex base 88-110 (low luster semigloss latex 100% acrylic white and pastel mixing base) that had been applied with a brush in two thin layers on the inside of lids of petri dishes and allowed to dry for 2 h prior to inoculation; maize (Zea mays DeKalb hybrid DK626RR; DeKalb, DeKalb, IL) seedlings 4 days after germination of surface-sterilized kernels on water agar; and water-rinsed and autoclaved pistachio (Pistacia vera; Blue Diamond, Sacramento, CA) shells. Painted and inoculated petri dish lids were placed over 1.5% water agar plates to promote high humidity for growth of the fungus. Pistachio shells were incubated in petri dishes containing 8 ml of sterile distilled water to promote high humidity. Cultures were inoculated by lightly dusting the medium with conidia that had adhered to a petri dish lid formerly covering a mature culture. In mock inoculations, an average of 1.2 × 107 conidia per petri dish were deposited by this inoculation method.
Large numbers of conidia, free of culture medium and hyphae, were collected from PDA cultures by tapping a culture dish so that conidia accumulated on the inside of the lid. In this way, alkaloids could be extracted exclusively from conidia. Alternatively, conidia were made airborne from a petri dish lid by agitating the lid by hand. Airborne conidia were collected on a 0.2-μm SFCA filter (Nalge Nunc International, Rochester, NY) or a 0.45-μm nylon filter (Millipore, Bedford, MA) under a vacuum and extracted by washing the filter with 800 μl of 80% methanol. In other experiments, conidia were collected from water agar and paint cultures by very briefly washing small portions of the sporulating cultures with 800 μl of 80% methanol. Conidia were harvested from corn or pistachio tissue by suspending pieces of the colonized and spore-bearing tissue in 1 ml of 80% methanol. Microscopic examination of conidial suspensions prepared from any of these alternate substrates revealed a lack of hyphae. However, with the exception of the conidia collected from PDA cultures, the extraction solvent was briefly in contact with hyphae and the colonized substrate or medium.
S. chartarum and Aspergillus nidulans were isolated from contaminated building materials; Aspergillus niger was isolated by passive air sampling (as described above for A. fumigatus). Conidia of these fungi and of A. fumigatus collected for measurement of their physical properties were harvested with 80% methanol from cultures grown on PDA. The number of conidia per ml of suspension was determined by counting dilutions with a hemacytometer (Hausser Scientific, Horsham, PA). Conidia (a defined number) were collected by filtration though tared 0.22-μm nylon filters (Costar, Corning, NY) and were retained for mass determination with a Mettler-Toledo (Columbus, OH) AG104 balance after drying in a Labconco (Kansas City, MO) Centrivap concentrator. Conidial diameters were determined microscopically with the aid of an ocular micrometer.
High-performance liquid chromatography (HPLC), chemical manipulations, and mass spectrometry.
Aliquots of conidia were suspended in 80% methanol, transferred to 1.5-ml microcentrifuge tubes, and rotated end over end at 40 rpm for 1 h on a Glas-Col (Terre Haute, IN) mini-rotator. The number of conidia in an extraction tube was determined by counting dilutions with a hemacytometer. Conidia from PDA-based cultures were separated from the alkaloid extract by centrifugal filtration and weighed as described above. For conidia derived from cultures on alternate substrates, the conidial mass was calculated by multiplying the number of conidia counted in the sample by the value previously calculated for the mass of an individual conidium. These calculations were based on the assumption that the mass of a conidium produced on these alternate substrates was the same as the mass of a conidium produced on PDA.
Methanol extracts of conidia (with no further preparation beyond removal of conidia by filtration) were analyzed by HPLC by using conditions described previously (23), except for changes in the equipment used. The HPLC apparatus consisted of a model 600 pump controller with an in-line degasser, a model 717plus autosampler, a model 2487 absorbance detector or a model 996 photodiode array detector (all from Waters Corp., Milford, MA), and a Rainin (Woburn, MA) Fl2 fluorescence detector set at excitation and emission wavelengths of 272 nm and 372 nm, respectively. Alkaloid quantities were determined based on a comparison of peak areas to a standard curve derived from four dilutions each of three parallel samples of the highly similar ergot alkaloid agroclavine (Sigma, St. Louis, MO), and the values were adjusted for the relative molecular weights of analytes and the standard.
For PDA-based cultures, several dilutions of each A. fumigatus spore extract were analyzed, and the concentration of each alkaloid was determined by linear regression analysis. Analyses of PDA-based cultures (see Table 2) were conducted in triplicate on two separate occasions. Three additional preliminary analyses yielded similar results. Extracts from A. fumigatus-colonized plant material, water agar, or paint-based cultures (three to six cultures per medium per isolate), which were less dense and contained fewer conidia than PDA-based cultures, were analyzed without dilution.
TABLE 2.
Ergot alkaloid contents associated with A. fumigatus conidia
| Isolate | Medium | Concn (mg/g [dry wt] of conidia) ofa:
|
||||
|---|---|---|---|---|---|---|
| Festuclavine | Fumigaclavine A | Fumigaclavine B | Fumigaclavine C | Total | ||
| WVU1943 | PDA | 0.61 ± 0.16 CD | 0.39 ± 0.09 B | 0.04 ± 0.01 ABC | 9.9 ± 3.0 AB | 11 ± 3.3 AB |
| WVU2026 | PDA | 1.5 ± 0.30 BC | 0.32 ± 0.09 B | 0.04 ± 0.01 BC | 3.8 ± 1.1 CD | 5.7 ± 1.5 BC |
| WVU1943 | Paint | 9.5 ± 2.2 A | 1.5 ± 0.50 A | 0.33 ± 0.16 A | 16 ± 3.7 A | 27 ± 6.5 A |
| WVU2026 | Paint | 5.1 ± 1.3 AB | 1.6 ± 0.62 A | 0.18 ± 0.04 AB | 8.1 ± 2.6 AB | 15 ± 2.6 A |
| WVU1943 | Water agar | 0.97 ± 0.44 CD | 0.14 ± 0.04 B | 0.01 ± 0.003 C | 1.2 ± 0.32 D | 2.3 ± 0.74 C |
| WVU2026 | Water agar | 0.71 ± 0.08 CD | 0.16 ± 0.03 B | 0.02 ± 0.001 C | 1.4 ± 0.22 CD | 2.3 ± 0.26 C |
| WVU1943 | Maize | 0.31 ± 0.05 D | 1.2 ± 0.18 A | 0.05 ± 0.01 ABC | 12 ± 1.1 A | 14 ± 1.1 A |
| WVU1943 | Pistachio | 0.57 ± 0.20 D | 0.33 ± 0.05 B | 0.02 ± 0.004 C | 4.0 ± 0.53 BC | 4.9 ± 0.72 BC |
The values are means ± standard errors. For values followed by the same letter within a column the means are not significantly different (P < 0.05) as determined by the Turkey-Kramer honestly significant differences test.
Agroclavine was validated as a standard for the A. fumigatus clavines by comparing experimentally measured concentrations of it with calculated concentrations of four separate preparations of dihydroergotamine and dihydroergocristine (Sigma), which have the same ergoline chromophore as the A. fumigatus ergot alkaloids. The concentrations of dihydroergotamine measured based on fluorescence relative to an agroclavine standard curve were 1.0 ± 0.04 (mean ± standard error) times the calculated values, whereas the measured concentrations of dihydroergocristine solutions were 0.98 ± 0.05 times the calculated values.
The relationships among compounds represented in different peaks were investigated by deacetylation of alkaloid fractions with 1 M NaOH at room temperature for 15 h (29). Base-treated samples were neutralized with an equinormal solution of acetic acid and analyzed by HPLC as described above. The masses of untreated and base-treated compounds were determined with a Finnegan mass spectrometer (model SSQ7000) at the WVU Mass Spectrometry Center. Isolated peaks collected after HPLC were concentrated 10-fold under a vacuum, introduced into the ion source with an infusion pump, and ionized in positive electrospray ionization mode.
Statistical analyses.
Data were analyzed by analysis of variance, and means were compared by the Tukey-Kramer honestly significant difference test in the JMP software package (SAS Institute, Cary, NC). Sets of values having unequal variances were log transformed prior to analysis.
RESULTS
Identification and quantification of ergot alkaloids associated with conidia of A. fumigatus.
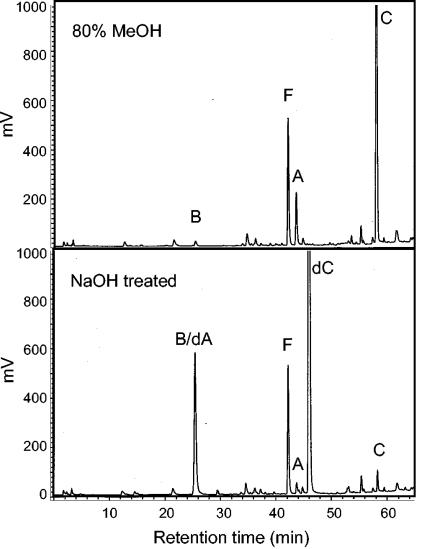
Several compounds from simple 80% methanolic extracts of conidia from PDA-cultured A. fumigatus produced large fluorescent peaks when they were analyzed by HPLC with excitation and emission wavelengths of 272 nm and 372 nm, respectively (Fig. 1). Electrospray ionization-mass spectrometry of the compound in peak C (Fig. 1), which had the greatest area and was the most nonpolar of the alkaloids analyzed, revealed an ion at m/z 367 whose mass corresponded to the mass of [fumigaclavine C + H]+, which had previously been described from broth cultures of A. fumigatus (3). Treatment of this compound with 1 M NaOH resulted in a more polar compound (Fig. 1, peak dC) that yielded an ion at m/z 325 whose mass corresponded to the mass of [deacetylated fumigaclavine C + H]+.
FIG. 1.
Chromatography of ergot alkaloids in an untreated 80% methanolic extract and an NaOH-treated methanolic extract of conidia of A. fumigatus WVU1943 cultured on PDA. Detection was by fluorescence with excitation and emission wavelengths of 272 nm and 372 nm, respectively. B, fumigaclavine B; F, festuclavine; A, fumigaclavine A; C, fumigaclavine C; dA, deactylfumigaclavine A; dC, deactylfumigaclavine C.
Similarly, the compound represented by peak A (Fig. 1) yielded an ion at m/z 299 in its native form, which was consistent with the proposed identity as fumigaclavine A, and it was converted after treatment with NaOH to a more polar compound (Fig. 1, peak dA) with an ion at m/z 257, which was consistent with [deacetylated fumigaclavine A + H]+. Peak B, which cochromatographed with experimentally deacetylated fumigaclavine A (Fig. 1), is proposed to be fumigaclavine B, which is identical to deacetylated fumigaclavine A (3, 29). Small quantities of fumigaclavine B also were detected in untreated extracts of A. fumigatus conidia (Fig. 1, peak B). The compound in peak F (Fig. 1) yielded an ion at m/z 241 when electrospray ionization-mass spectrometry was used and was not affected by NaOH treatment. These characteristics are consistent with identification of this compound as festuclavine, which was characterized previously from culture extracts of A. fumigatus (29).
The absorbance spectra for these compounds (data not shown) and their relative retention times on C18 silica were consistent with their proposed identities. Analyses of the same conidial extracts with an excitation wavelength of 310 nm and an emission wavelength of 410 nm did not result in detection of any of the previously characterized lysergyl-derived ergot alkaloids (data not shown). Conidia collected on the inner lid of a petri dish and conidia aerosolized and collected on filters under a vacuum yielded the same spectrum of ergot alkaloids.
To relate the quantities of ergot alkaloids to the spore mass and to investigate the buoyancy of A. fumigatus conidia, physical properties of conidia were measured (Table 1). The conidia of A. fumigatus were significantly smaller, lighter, and less dense than those of some other common Aspergillus species and the black mold fungus S. chartarum (Table 1). (Although the ranges of diameters for conidia of Aspergillus species are well established [26], we determined means and report them here so that our calculations of density were based on the volume and mass of the same collections of conidia.) Cultures of A. fumigatus on PDA sporulated abundantly; a 2-month-old, 85-mm culture contained 5.8 × 1010 conidia.
TABLE 1.
Physical properties of conidia from selected fungia
| Fungus | Mass (pg) | Diam (μm) | Vol (μm3) | Specific gravity |
|---|---|---|---|---|
| Aspergillus fumigatus | 2.9 ± 0.3 A | 2.8 ± 0.08 A | 12 ± 0.9 A | 0.24 |
| Aspergillus nidulans | 8.8 ± 1.8 B | 3.5 ± 0.06 B | 22 ± 1.3 B | 0.40 |
| Aspergillus niger | 14 ± 0.6 B | 4.0 ± 0.05 C | 33 ± 1.2 C | 0.42 |
| Stachybotrys chartarumb | 140 ± 39 C | 4.9 ± 0.11, 9.4 ± 0.21 D | 170 ± 11 D | 0.82 |
The values are means ± standard errors. For values followed by different letters within a column the means are significantly different (P < 0.05) as determined by the Tukey-Kramer honestly significant difference test.
The spores are ellipsoid and were measured in two dimensions. The smaller diameter was used for diameter comparisons. The volume was estimated by using the formula for a cylinder.
Each of the four defined ergot alkaloids was quantified in extracts of defined numbers of conidia with defined conidial masses (Table 2). Ergot alkaloids accounted for 1.1% of the mass of conidia of A. fumigatus isolate WVU1943 grown on PDA, whereas conidia of isolate WVU2026 appeared to contain slightly lower quantities of ergot alkaloids, although the difference was not statistically significant. Both isolates accumulated the same array of ergot alkaloids when they were cultured on latex-based paint (Table 2). Isolate WVU2026 contained significantly more total ergot alkaloids when it was cultured on paint than when it was cultured on PDA; isolate WVU1943 also contained abundant total ergot alkaloids when it was cultured on paint, but the difference compared to the culture on PDA was not statistically significant. Colonies on paint were relatively small and consisted mainly of conidiophores and conidia along with very little vegetative hyphae. Cultures on water agar (also mostly conidiophores and conidia) still accumulated ergot alkaloids despite the limited exogenous nutrients in this medium. However, the concentrations of ergot alkaloids extracted from the water agar-derived conidia were generally lower than the concentrations extracted from conidia produced on the other substrates. The same set of ergot alkaloids also was detected in or on conidia of cultures grown on cultured maize seedlings (on which the fungus colonized mainly the residual scutellum and kernel tissue), where they accumulated to relatively high levels, and on sterilized pistachio shells (on which the fungus grew poorly), where the concentrations of ergot alkaloids were relatively low (Table 2).
The yield of ergot alkaloids recovered after a more harsh extraction procedure was assessed in a separate experiment. Breaking of ∼63% of the conidia by bead beating followed by a 1-h extraction in 80% methanol resulted in a 38% increase in the total yield of ergot alkaloids compared to the ergot alkaloids extracted from unbeaten conidia.
DISCUSSION
Our results demonstrate that high levels of certain ergot alkaloids are associated with conidia of A. fumigatus. The alkaloids are present in or on conidia produced by cultures grown on a variety of substrates. Moreover, the conidia of A. fumigatus are smaller, lighter, and less dense than those of closely related species. These physical properties may promote the aerosolization and buoyancy of the conidia, which serve as vehicles for the alkaloids.
Whether the ergot alkaloids are on the surface of the conidia, contained within the conidia, or in both locations cannot be answered definitively with the available data. The majority of the ergot alkaloids were easily extracted from intact conidia, which suggests a surface location. A bead-beating treatment that physically disrupted the spore wall increased the amount of alkaloid extracted by 38%. However, in addition to cell breakage, this treatment also more vigorously extracted any surface compounds. The data from this more disruptive extraction also indicate that the values in Table 2 represent minimum values for conidium-associated alkaloids. The question of the location of the ergot alkaloids relative to the spore surface may not be significant from a health perspective. In immunocompetent individuals, inhaled conidia are likely to be killed and lysed by macrophages (18), releasing any mycotoxins contained in them. Conidia that are not killed and lysed present a threat of infection and probably additional toxin production. A role for ergot alkaloids in invasive aspergillosis has not been investigated. Such studies would be facilitated by comparison of wild-type isolates with the ergot alkaloid-deficient mutants described in the accompanying paper (4). In the absence of infection, conidia may serve as vehicles for exposure to ergot alkaloids.
The issue of health risks posed by inhalation of mycotoxin-containing conidia is complex and is affected by several factors, including the physical nature of the conidia with respect to their potential for dispersal and inhalation and the production of mycotoxins on environmentally relevant substrates. The conidia of A. fumigatus have properties that appear to facilitate their dispersal and inhalation. The 2.8-μm diameter of conidia is small enough for them to penetrate deep into the alveoli of the lungs (18). The low specific gravity of the conidia (0.24) probably promotes efficient air dispersal. For comparison, the conidia of the trichothecene producer S. chartarum, which have generated considerable health concerns (10, 28), have a mass that is 48 times greater and a specific gravity that is four times greater than those of conidia of A. fumigatus.
Ergot alkaloids were detected on all media tested, including environmentally relevant substrates such as latex paint, two different plant materials, and water agar (representative of moist, nutritionally poor environments). The reported concentrations of ergot alkaloids associated with conidia produced on substrates other than PDA were based on the number of conidia extracted and a value for conidial mass determined from conidia produced on PDA. Thus, these values were based on the assumption that conidia produced on any of the alternate substrates have the same mass as conidia produced on PDA. We contend that this is a reasonable assumption. However, if the mass of each conidium were in fact greater on a natural substrate than on PDA, then the calculated values for the amount of ergot alkaloid per conidium would be lower. Conversely, if the conidial mass were lower on a natural substrate than on PDA, then the alkaloid concentration would be greater than that shown in Table 2.
A more complicated factor in determination of the health risks associated with conidial mycotoxins is the issue of whether the toxins are encountered in quantities sufficient for them to exert their effects. There are at least three components to this issue, including (i) the concentration of mycotoxin in each conidium, (ii) the number of conidia encountered, and (iii) the toxicity of the mycotoxins.
Concentrations of ergot alkaloids that exceed 1% of the mass of the A. fumigatus conidia are relatively high for fungal natural products. There are few data available for direct comparison, because most mycotoxin data are expressed as mass of toxin per unit volume of culture. Data for respirable trichothecenes from S. chartarum (28) revealed a mean of 17 ng of trichothecenes per mg of dust aerosolized from S. chartarum cultured on rice. Assuming that conidia, which constituted 85% of the particles in the aerosolized dust (28), contributed 85% of the mass of the dust sample, then 0.002% of the conidium mass was trichothecenes. Using a different isolate of the same fungal species and measuring only the most abundant trichothecene, Nikulin et al. (21) reported 0.1 pg of satratoxin H per conidium. Based on a mass of 140 pg per conidium (Table 1), satratoxin H accounted for 0.0007% of the conidial mass. Another source of alkaloid data based on fungal mass is the poisonous mushroom literature. For example, in basidiocarps of Amanita muscaria ibotenic acid accounts for 0.45% of the dry weight and muscimol accounts for 0.036% (30) (assuming that 12.5 mg [fresh weight] of basidiocarp yields 1 mg [dry weight] [9]). Amatoxins have been found to account for 0.1% to 0.7% of the dry weight of Amanita phalloides basidiocarps (9). In a more relevant example, ergot sclerotia of field-grown C. purpurea contain 1% to 2% ergot alkaloids by mass (5, 20).
The number of A. fumigatus conidia available in the air depends on the substrate and the environment in which the fungus is growing. Under favorable conditions the fungus can sporulate prolifically. A typical culture of A. fumigatus on PDA can yield ∼109 conidia per cm2 of culture surface area. The number of viable A. fumigatus conidia per m3 of air ranges from 0 to 200 CFU in clean environments (27) to 107 CFU near composting facilities (12) to 1011 CFU near moldy hay or other stored organic materials (27). If the intake rate was 0.63 m3 of air per h (32), the conidial ergot alkaloid content was 1% by mass, and there was no further ergot alkaloid production after inhalation of the fungus, the ergot alkaloid dose would range from 3.7 pg per h (at 200 CFU/m3) to 180 ng per h (at 107 CFU per m3) to 1.8 mg per h (at 1011 CFU per m3). An interesting point of reference is that an ingested dose of the illicit and highly active ergot alkaloid lysergic acid diethylamide (LSD) can range from hundreds of nanograms to hundreds of micrograms (1), but based on U.S. Drug Enforcement Agency data the dose is frequently in the range from 20 to 80 μg (http://www.nida.nih.gov/Infofax/lsd.html). To inhale a comparable mass of A. fumigatus ergot alkaloids in 1 h would require exposure to 107 to 1010 CFU per m3. Such high concentrations of conidia are encountered only rarely. A more practical issue to consider is whether there are potential health effects of a less remarkable nature (e.g., effects on depression, blood pressure, or sleep-wake cycles) that are associated with chronic daily doses of ergot alkaloids in the nanogram to microgram range. This issue has not been investigated.
The toxicity of the particular ergot alkaloids associated with A. fumigatus conidia has not been studied extensively, but the available data suggest that these mycotoxins have considerable biological activity. Similar to other ergot alkaloids that have been studied, festuclavine interacts with receptors for serotonin, dopamine, and α-adrenaline (7, 14, 24, 25). Festuclavine and synthetic derivatives of festuclavine also are cytostatic in in vitro assays with several bacteria and mouse lymphoma cell lines (7, 8). Moreover, festuclavine is unique among the naturally occurring ergot alkaloids in that it is directly mutagenic in the Ames test (15). In the only published animal study of fumigaclavine C, ingestion of relatively crude preparations of this ergot alkaloid greatly reduced feed intake by treated calves and caused hemorrhagic enteritis of the small and large intestines, as well as patchy interstitial thickening of alveolar walls of the animals (3).
Demonstration of the presence of ergot alkaloids at relatively high concentrations in or on conidia of A. fumigatus does not ipso facto indicate that the toxins play a role in pathogenesis, other health effects, or any specific aspect of the biology of the fungus. However, it does raise interesting questions for further research. Studies with ergot alkaloid-deficient mutants, such as the dmaW knockout strain described in the accompanying paper (4), should be useful for assessing the contribution of ergot alkaloids to virulence to animals or potential contributions of the alkaloids to the ecological success of A. fumigatus. Since minimization of the mass of a conidium appears to have been selected for in this fungus, the presence of alkaloids in quantities that exceed 1% of the mass of the conidium should have been selected against unless they provided some advantage to the fungus.
Acknowledgments
This work was supported by USDA-NRI grant 2001-35319-10930 and Hatch funds.
We thank Robert Smith of the WVU Mass Spectrometry Center for assistance with mass spectrometry, Barton Baker and Jim Kotcon for helpful discussions, and Brian Lewis and Jessica Cipoletti for technical assistance.
Footnotes
This paper is published with the approval of the Director of the West Virginia Agricultural and Forestry Experiment Station as Scientific Article no. 2895.
REFERENCES
- 1.Baudot, P., and J. C. Andre. 1985. Identification and quantitative determination of LSD by fluorescence: new data. Bull. Narc. 37:79-94. [PubMed] [Google Scholar]
- 2.Clay, K., and C. Schardl. 2002. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am. Nat. 160:S99-S127. [DOI] [PubMed] [Google Scholar]
- 3.Cole, R. J., J. W. Kirksey, J. W. Dorner, D. M. Wilson, J. C. Johnson, Jr., A. N. Johnson, D. M. Bedell, J. P. Springer, K. K. Chexal, J. C. Clardy, and R. H. Cox. 1977. Mycotoxins produced by Aspergillus fumigatus species isolated from molded silage. J. Agric. Food Chem. 25:826-830. [DOI] [PubMed] [Google Scholar]
- 4.Coyle, C. M., and D. G. Panaccione. 2005. An ergot alkaloid biosynthesis gene and clustered hypothetical genes from Aspergillus fumigatus. Appl. Environ. Microbiol. 71:3112-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cvak, L. 1999. Industrial production of ergot alkaloids, p. 373-409. In V. Křen and L. Cvak (ed.), Ergot: the genus Claviceps. Harwood Academic Publishers, Amsterdam, The Netherlands.
- 6.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-805. [DOI] [PubMed] [Google Scholar]
- 7.Eich, E., and H. Pertz. 1999. Antimicrobial and antitumor effects of ergot alkaloids and their derivatives, p. 411-440. In V. Křen and L. Cvak (ed.), Ergot: the genus Claviceps. Harwood Academic Publishers, Amsterdam, The Netherlands.
- 8.Eich, E., C. Becker, and W. E. G. Müller. 1984. Clavines—new antibiotics with cytostatic activity. Biochem. Pharmacol. 33:523-526. [DOI] [PubMed] [Google Scholar]
- 9.Enjalbert, F., C. Gallion, F. Jehl, H. Monteil, and H. Faulstich. 1993. Amatoxins and phallotoxins in Amanita species: high-performance liquid chromatography determination. Mycologia 85:579-584. [DOI] [PubMed] [Google Scholar]
- 10.Etzel, R. A., E. Montana, W. G. Sorenson, G. J. Kullman, T. M. Allan, and D. G. Dearborn. 1998. Acute pulmonary hemorrhage in infants associated with exposure to Stachybotrys atra and other fungi. Arch. Pediatr. Adolesc. Med. 152:757-762. [DOI] [PubMed] [Google Scholar]
- 11.Fischer, G., and W. Dott. 2003. Relevance of airborne fungi and their secondary metabolites for environmental, occupational and indoor hygiene. Arch. Microbiol. 179:75-82. [DOI] [PubMed] [Google Scholar]
- 12.Fischer, G., T. Muller, R. Schwalbe, R. Ostrawski, and W. Dott. 2000. Species-specific profiles of mycotoxins produced in cultures and associated with conidia of airborne fungi derived from biowaste. Int. J. Hyg. Environ. Health 203:105-116. [DOI] [PubMed] [Google Scholar]
- 13.Flieger, M., M. Wurst, and R. Shelby. 1997. Ergot alkaloids—sources, structures, and analytical methods. Folia Microbiol. 42:3-30. [DOI] [PubMed] [Google Scholar]
- 14.Fuxe, K., B. B. Fredholm, L. F. Agnati, S.-O. Ögren, B. J. Everitt, G. Jonsson, and J.-Ä. Gustafsson. 1978. Interactions of ergot drugs with central monoamine systems. Pharmacology 16:99-134. [DOI] [PubMed] [Google Scholar]
- 15.Glatt, H., H. Pertz, R. Kasper, and E. Eich. 1992. Clavine alkaloids and derivatives as mutagens detected in the AMES test. Anti-Cancer Drugs 3:609-614. [DOI] [PubMed] [Google Scholar]
- 16.Gröger, D., and H. G. Floss. 1998. Biochemistry of ergot alkaloids—achievements and challenges. Alkaloids 50:171-218. [Google Scholar]
- 17.Kozlovsky, A. G. 1999. Producers of ergot alkaloids out of Claviceps genus, p. 479-499. In V. Křen and L. Cvak (ed.), Ergot: the genus Claviceps. Harwood Academic Publishers, Amsterdam, The Netherlands.
- 18.Latgé, J.-P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matossian, M. K. 1989. Poisons of the past: molds, epidemics, and history. Yale University Press, New Haven, CT.
- 20.Németh, E. 1999. Parasitic production of ergot alkaloids, p. 303-319. In V. Křen and L. Cvak (ed.), Ergot: the genus Claviceps. Harwood Academic Publishers, Amsterdam, The Netherlands.
- 21.Nikulin, M., K. Reijula, B. B. Jarvis, and E. L. Hintikka. 1996. Experimental lung mycotoxicosis in mice induced by Stachybotrys atra. Int. J. Exp. Pathol. 77:213-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panaccione, D. G., and C. L. Schardl. 2003. Molecular genetics of ergot alkaloid biosynthesis, p. 399-424. In J. F. White, Jr., C. W. Bacon, N. L. Hywel-Jones, and J. W. Spatafora (ed.), The clavicipitalean fungi: evolutionary biology, chemistry, biocontrol, and cultural impacts. Marcel-Dekker, New York, N.Y.
- 23.Panaccione, D. G., B. A. Tapper, G. A. Lane, E. Davies, and K. Fraser. 2003. Biochemical outcome of blocking the ergot alkaloid pathway of a grass endophyte. J. Agric. Food Chem. 51:6429-6437. [DOI] [PubMed] [Google Scholar]
- 24.Pertz, H. 1996. Naturally occurring clavines: antagonism/partial agonism at 5-HT2A receptors and antagonism at α1-adrenoceptors in blood vessels. Planta Med. 62:387-392. [DOI] [PubMed] [Google Scholar]
- 25.Pertz, H., and E. Eich. 1999. Ergot alkaloids and their derivatives as ligands for serotoninergic, dopaminergic, and adrenergic receptors, p. 411-440. In V. Křen and L. Cvak (ed.), Ergot: the genus Claviceps. Harwood Academic Publishers, Amsterdam, The Netherlands.
- 26.Raper, K. B., and D. I. Fennell. 1965. The genus Aspergillus. Williams & Wilkins, Balitmore, MD.
- 27.Solomon, W. R., and H. A. Burge. 1984. Allergens and pathogens in indoor air quality, p. 173-191. In P. J. Walsh, C. S. Dudney, and E. D. Copenhaver (ed.), Indoor air quality. CRC Press, Boca Raton, FL.
- 28.Sorenson, W. G., D. G. Fraser, B. B. Jarvis, J. Simpson, and V. A. Robinson. 1987. Trichothecene mycotoxins in aerosolized conidia of Stachybotrys atra. Appl. Environ. Microbiol. 53:1370-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spilsbury, J. F., and S. Wilkinson. 1961. The isolation of festuclavine and two new clavine alkaloids from Aspergillus fumigatus Fres. J. Chem. Soc. 5:2085-2091. [Google Scholar]
- 30.Tsunoda, K., N. Inoue, Y. Aoyagi, and T. Sugahara. 1993. Changes in concentration of ibotenic acid and muscimol in the fruit body of Amanita muscaria during the reproduction stage. J. Food Hyg. Soc. Jpn. 34:18-24. [Google Scholar]
- 31.Tudzynski, P., T. Correia, and U. Keller. 2001. Biotechnology and genetics of ergot alkaloids. Appl. Microbiol. Biotechnol. 57:593-605. [DOI] [PubMed] [Google Scholar]
- 32.U.S. Environmental Protection Agency. 1997. Volume I. General factors. Exposure factors handbook. EPA Office of Research and Development Publication EPA/600/P-95/002Fa-c. U.S. Environmental Protection Agency, Washington, DC.