Abstract
Several regulators of methionine biosynthesis have been reported in Escherichia coli, which might represent barriers to the production of excess l-methionine (Met). In order to examine the effects of these factors on Met biosynthesis and metabolism, deletion mutations of the methionine repressor (metJ) and threonine biosynthetic (thrBC) genes were introduced into the W3110 wild-type strain of E. coli. Mutations of the metK gene encoding S-adenosylmethionine synthetase, which is involved in Met metabolism, were detected in 12 norleucine-resistant mutants. Three of the mutations in the metK structural gene were then introduced into metJ and thrBC double-mutant strains; one of the resultant strains was found to accumulate 0.13 g/liter Met. Mutations of the metA gene encoding homoserine succinyltransferase were detected in α-methylmethionine-resistant mutants, and these mutations were found to encode feedback-resistant enzymes in a 14C-labeled homoserine assay. Three metA mutations were introduced, using expression plasmids, into an E. coli strain that was shown to accumulate 0.24 g/liter Met. Combining mutations that affect the deregulation of Met biosynthesis and metabolism is therefore an effective approach for the production of Met-excreting strains.
l-Methionine (Met) is a sulfur-containing amino acid that is essential in mammals and is used both as a food additive and a medication (15). Met has a central role in the metabolism of sulfur-containing substances and is also involved in methyl group transfer via its derivative S-adenosylmethionine (SAM), which is an intermediate in the polyamine biosynthetic pathway (7).
Industrial Met is produced mainly from dl-methionine, which is widely used as a feed additive. This compound is chemically synthesized through the generation of N-acetyl-dl-methionine by the acetylation of dl-methionine, followed by enzymatic selective deacetylation of the N-acetyl-l-methionine. Industrial-scale microbial fermentation has not yet been developed for the production of Met. This is partly due to the complexity of the Met biosynthetic pathway and the strong metabolic regulation that results from its essential cellular functions.
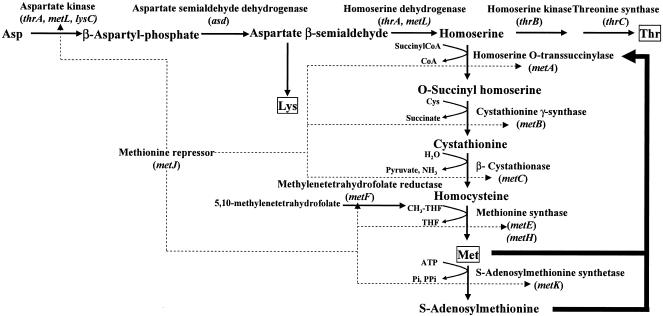
Escherichia coli is one of the most important microorganisms used in the manufacture of amino acids (8). Met biosynthesis and metabolism have been well studied in this bacterium, and several regulatory factors have been identified (7). At the transcriptional level, the methionine repressor inhibits the Met biosynthetic genes metA, metBJ, metC, metE, and metF (Fig. 1). This activity is mediated by the MetJ repressor protein and its corepressor, SAM, which also acts as a methyl donor and a substrate for polyamine biosynthesis.
FIG. 1.
Biosynthesis and regulation of methionine in E. coli. The boldface arrows indicate feedback inhibition, and the dotted arrows indicate repression. CoA, coenzyme A.
Homoserine succinyltransferase, which is the first enzyme in the Met biosynthetic pathway from homoserine, is encoded by the metA gene. This enzyme is inhibited by the combined activity of Met and SAM; the latter compound is synthesized from Met and ATP by S-adenosylmethionine synthetase (MetK), which is encoded by the metK gene. Mutations in metK lead to elevated levels of Met biosynthetic enzymes and defective feedback inhibition. It is well known that analogue resistance is effective in suppressing metabolic control (18). Selection for resistance to Met analogues, such as α-methylmethionine (α-MM) and norleucine, has therefore been suggested to lead to mutants with desensitized MetJ, homoserine succinyltransferase (MetA), and MetK (1). However, no nucleotide substitutions or corresponding amino acid exchanges have been identified so far in the metA and metK genes.
In this study, we attempted to deregulate the controls of Met biosynthesis and metabolism in the W3110 wild-type strain of E. coli. metK mutations were identified in norleucine-resistant mutants, and desensitization of the metA gene to Met and SAM inhibition was obtained through α-MM resistance.
MATERIALS AND METHODS
Strains, plasmids, media, and cultivation.
The W3110 strain of E. coli was used as the parent strain, and the JM109 strain (22) was used for plasmid preparation. Plasmids pUC18, pHSG298, pHSG398, pSTV28 (Takara Shuzo, Kyoto, Japan), and pMW118 (Nippon Gene, Toyama, Japan) were used for plasmid construction and gene amplification. Details of these strains and plasmids are summarized in Table 1. Cultivation for fermentative Met excretion was performed for 24 h (for W3110) or 48 h (for Thr-auxotrophic mutants) in a 500-ml Sakaguchi flask with a working volume of 20 ml at 37°C in MS medium containing (per liter of distilled water) 40 g glucose, 1 g MgSO4 · 7H2O, 16 g (NH4)2SO4, 1 g KH2PO4, 2 g Bacto yeast extract, 0.01 g MnSO4 · 4H2O, 0.01 g FeSO4 · 7H2O, 0.5 g Thr (for Thr-auxotrophic mutants), and 30 g CaCO3 (added after it was sterilized separately) (13). Growth was monitored by measuring the optical density at 600 nm. The Met concentration in the culture medium was evaluated by the analytical method for physiological fluids using an L-8500 amino acid analyzer (Hitachi, Tokyo, Japan). For mutant isolation, E. coli was grown at 37°C in a minimal medium based on the medium described by Davis and Mingioli (4), containing (per liter) 2 g glucose, 7 g K2HPO4, 3 g KH2PO4, 0.5 g Na2 citrate · 2H2O, 0.1 g MgSO4 · 7H2O, and 5 g (NH4)2SO4. Luria-Bertani (LB) medium was used for all other manipulations.
TABLE 1.
List of bacterial strains and plasmids
| Strain or plasmid | Genotype or gene | Source or reference |
|---|---|---|
| Strains | ||
| W3110 | Wild type | Our collection |
| WΔthrBC | W3110 thrBC | This study |
| WΔthrBCΔmetJ | W3110 thrBC metJ | This study |
| WΔthrBCΔmetJmetK2 | W3110 thrBC metJ metK2 | This study |
| WΔthrBCΔmetJmetK24 | W3110 thrBC metJ metK24 | This study |
| WΔthrBCΔmetJmetK32 | W3110 thrBC metJ metK32 | This study |
| Plasmids | ||
| pMW118 | Plasmid vector, Ampr | Nippon Gene |
| pMWPthrmetA | Wild-type metA gene under the control of Pthr | This study |
| pMWPthrmetA4 | metA4 gene under the control of Pthr | This study |
| pMWPthrmetA5 | metA5 gene under the control of Pthr | This study |
| pMWPthrmetA9 | metA9 gene under the control of Pthr | This study |
| pMWPthrmetA4+9 | metA gene with combined mutation of metA4 and metA9 under the control of Pthr | This study |
| pMWPthrmetA5+9 | metA gene with combined mutation of metA4 and metA9 under the control of Pthr | This study |
| pMWPthrmetA4+5+9 | metA gene with combined mutation of metA4, metA5, and metA9 under the control of Pthr | This study |
Chemicals.
dl-[U-14C]homoserine (50 mCi/mmol) was synthesized by Muromachi Chemical Industry (Tokyo, Japan). O-Succinylhomoserine and pyridoxal phosphate were purchased from Sigma-Aldrich (St. Louis, MO).
Gene cloning and DNA manipulations.
General DNA manipulation procedures were performed as described previously (19). Sequencing was carried out using the dideoxy chain termination method with a Taq DyeDeoxy terminator cycle sequencing kit and a 377 DNA sequencer (Applied Biosystems, Foster City, CA). Genomic DNA was extracted using a genomic DNA purification kit (Advanced Genetic Technologies, Gaithersburg, MD). Gene replacement was performed using a temperature-sensitive origin of replication plasmid, pMAN997 (13), which was derived from plasmid pMAN031 (14). The plasmids for recombination were transformed and the integrants were selected at a nonpermissive temperature (42°C) in the presence of an antibiotic (ampicillin). The second recombination was performed at a permissive temperature (30°C) without ampicillin. The generation of recombinants was confirmed by the length of amplified PCR fragments.
Threonine-auxotrophic and metJ-deficient strains.
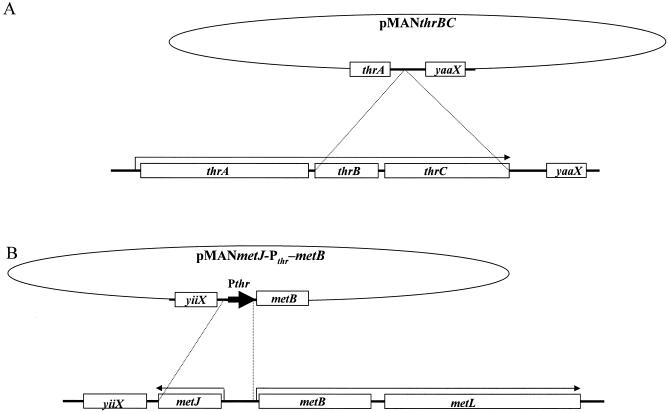
To construct an l-threonine (Thr)-auxotrophic strain (Fig. 2A), the thrB region inside the thrABC operon was amplified by PCR with primers 5′-GGGAATTCTGGCAGGAGGAACTGGCGCA-3′ (EcoRI site underlined) and 5′-GGGTCGACGCTCATATTGGCACTGGAAG-3′ (SalI site underlined) and digested using EcoRI and SalI. The thrC region was amplified by PCR with primers 5′-GGGTCGACATCAGTAAAATCTATTCATT-3′ (SalI site underlined) and 5′-GGAAGCTTGCCCGAGGGAAAGATCTGTA-3′ (HindIII site underlined) and treated with SalI and HindIII. The amplified fragments were mixed, ligated into plasmid pMAN997, and digested using EcoRI and HindIII. The resultant plasmid, pMANthrBC, was used to obtain the thrBC-deficient mutant from W3110 by homologous recombination. The Thr-auxotrophic strain selected was designated WΔthrBC.
FIG. 2.
Schematic representation of recombination strategies. (A) Construction of thrBC disrupted strain. The lower part depicts the structure of plasmid pMANthrBC. The upper part depicts the gene organization of the wild-type thr operon (thrABC) on the chromosome. The dotted lines show the points of homologous recombination. (B) Construction of the strain with metJ disruption and thr operon promoter (Pthr) insertion into the metBL operon. The lower part depicts the structure of plasmid pMANmetJ-Pthr-metB. The upper part depicts the gene organization of the wild-type metJ and metBL operon on chromosome. Pthr is indicated by a boldface arrow. The dotted lines show the points of recombination.
A 1-kb fragment containing the region of the metB gene was then amplified using primers 5′-GGGCATGCCCAGGGAACTTCATCACATG-3′ (SphI site underlined) and 5′-GGGAATTCTCATGGTTGCGGCGTGAGAG-3′ (EcoRI site underlined) and digested using SphI and EcoRI. The metJ gene region was amplified using primers 5′-GGAAGCTTGCGTGAGATGGGGATTAACC-3′ (HindIII site underlined) and 5′-GGGAATTCTACTGCTAGCTGCTCTTGCG-3′ (EcoRI site underlined) and restricted using HindIII and EcoRI. Both of the 75-bp strands of the thr operon promoter (Pthr) region, 5′-GGAAGCTTAAAATTTTATTGACTTAGGTCACTAAATACTTTAACCAATATAGGCATAGCGCACAGACGCATGCCC-3′ (HindIII and SphI sites underlined, respectively), were synthesized, annealed, and digested using HindIII and SphI.
Three fragments and plasmid pHSG298 digested with EcoRI were ligated. Plasmid pHSGmetJ-Pthr-metB, into which the three fragments were inserted, was then selected and sequenced for confirmation. The metJ-Pthr-metB fragments that were excised from pHSGmetJ-Pthr-metB were inserted into pMAN997, and the resultant plasmid was designated pMANmetJ-Pthr-metB. Using this plasmid, a metJ-deficient strain and a strain with the promoter replaced were derived from WΔthrBC and W3110 and designated WΔthrBCΔmetJ and WΔmetJ, respectively (Fig. 2B).
Isolation of metK mutants.
W3110 cells were cultivated for 24 h in LB medium. The cells from 1 ml of culture were collected by centrifugation, washed twice in 0.9% NaCl, inoculated into Davis-Mingioli minimal medium plates containing 100 μg/ml of norleucine, and incubated for 5 days. Colonies were then isolated, and 12 norleucine-resistant strains were confirmed again to be resistant to 100 μg/ml of norleucine on minimal medium plates.
The chromosomal DNA was extracted, and the metK gene was amplified from these 12 strains using primers 5′-GGAAGCTTAAGCAGAGATGCAGAGTGCG-3′ and 5′-GGAAGCTTGGTGCGGTATAAGAGGCCAC-3′ (HindIII sites underlined). Mutations in the metK structural gene were identified by DNA sequencing using the following six internal sequencing primers: 5′-CAACAGTTTGAGCTAACC-3′, 5′-GCGGTTTTTTTGCCGGATGC-3′, 5′-TCGGCTACGCAACTAATG-3′, 5′-GAGAATGCACCGCCACCG-3′, 5′-TGGCGCGTCACGGTGGCG-3′ and 5′-GCACGTCGGTTTCATTAG-3′. To introduce the mutation into the host strains, fragments amplified from the metK mutants were introduced into plasmid pSTV28 at the HindIII site, transferred to pMAN997, and then subjected to homologous recombination.
Isolation of metA mutants and construction of metA expression plasmids.
W3110 cells were cultivated for 24 h in LB medium. The cells from 1 ml of culture were collected by centrifugation, washed twice in 0.9% NaCl, inoculated into 5 ml of Davis-Mingioli minimal medium containing 1 g/liter α-MM, and cultured for 3 days. The culture was then diluted and spread on minimal medium plates containing 1 g/liter α-MM. Grown colonies were subsequently isolated, and they were confirmed to be resistant to 1 g/liter of α-MM on minimal medium plates again.
The metA fragment was amplified using primers 5′-GGGCATGCTGTAGTGAGGTAATCAGGTT-3′ (SphI site underlined) and 5′-GGGTCGACTTAATCCAGCGTTGGATTCA-3′ (SalI site underlined) and the W3110 genome and cloned into plasmid pHSG398 at the SphI and SalI sites. Sequencing of wild and mutated metA genes was performed using the internal primers 5′-TGTCGCTGGGCGGTACA-3′ and 5′-AGAGAGTTTTTCGGTGCG-3′. The wild and mutated metA fragments were digested with SphI and SalI, the Pthr fragments were digested with HindIII and SphI, and plasmid pMW118 treated with HindIII and SalI was mixed and ligated. The resultant plasmid, which contained the metA gene under the control of Pthr, was selected and used to produce the metA expression plasmid (designated pMWPthrmetA for wild-type metA). In order to combine the metA mutations, 5′-phosphorylated primers containing the metA mutation points, 5′-CCAGACGCACAAGAAGTTGTC-3′ for metA9 and 5′-TAGATCGTATAGCGTGTCTCTGGTAGAC-3′ for the metA4 mutation plus the metA5 mutation, were used for site-directed mutagenesis.
Enzyme assays.
For the enzyme assays, cells were suspended in 3 ml of 50 mM potassium phosphate buffer (pH 7.5) containing 1 mM dithiothreitol. The suspension was subjected to cell disruption treatment using sonication. The sonicated suspension was then centrifuged at 18,000 × g for 30 min, and the supernatant was desalted in a Sephadex G-50 column (Amersham-Pharmacia Biotech, Tokyo, Japan) to obtain the crude enzyme extract. To measure the MetA activity, 5 μl of the crude enzyme extract was added to a reaction mixture (final volume, 50 μl) containing 0.1 M potassium phosphate (pH 7.5), 1 mM succinyl coenzyme A (Sigma-Aldrich), 0.2 mM l-homoserine, and 2 nM dl-[U-14C]homoserine. The mixture was incubated at 30°C for 10 min. Subsequently, 1 ml of the reaction mixture was spotted onto a cellulose plate (Merck, Whitehouse Station, NJ) and developed with a mixed solvent containing acetone, butanol, water, and diethylamine at a ratio of 10:10:5:2. After the plate was air dried, autoradiography was performed using a BAS2000 image analyzer (Fuji Photo Film, Tokyo, Japan). The conversion of [14C]homoserine to [14C]O-succinylhomoserine was used to calculate MetA activity. To measure the cystathionine γ-synthase (MetB) activity, 100 μl of the crude enzyme extract was added to a reaction mixture (final volume, 1 ml) containing 0.2 M Tris-HCl (pH 8.0), 5 mM O-succinylhomoserine (Sigma-Aldrich), and 0.25 mM pyridoxal phosphate (Sigma-Aldrich). The mixture was incubated at 37°C for 20 min and then cooled with ice. The amount of pyridoxal phosphate-dependent reduction of O-succinylhomoserine was used to calculate the MetB activity.
RESULTS
Disruption of the metJ and thrBC genes.
As Thr is synthesized from homoserine, which is a common substrate for Met biosynthesis (Fig. 1), a Thr-auxotrophic strain was expected to be effective in inducing Met excretion. In addition, the growth of such a strain can be controlled by the addition of Thr to the medium. We therefore produced a Thr-auxotrophic strain by deleting the thrBC genes of W3110 through homologous recombination (Fig. 2A). Auxotrophy was confirmed through growth on minimal medium, and the resultant disruption mutant was designated WΔthrBC.
The metJ gene encodes an aporepressor that mediates the suppression of the Met biosynthetic genes metA, metB, metC, metE, metF, metH, and metL (Fig. 1). We attempted to disrupt the metJ repressor and the adjacent metBL promoter region with Pthr simultaneously using the temperature-sensitive origin of replication plasmid (Fig. 2B). We obtained a metJ-deficient mutant from WΔthrBC and mutants WΔthrBCΔmetJ and WΔmetJ from W3110. To confirm derepression of the Met biosynthetic genes, we measured MetA and MetB activities. The level of MetA activity in the presence of Met in minimal medium was significantly higher in the WΔmetJ strain (126 mmol/min/mg protein) than in the W3110 strain (0.3 mmol/min/mg protein). Furthermore, the MetB activity of WΔmetJ was 1,300 mmol/min/mg protein, compared with 140 mmol/min/mg protein in W3110. These results clearly demonstrated that there was successful derepression of the Met biosynthetic genes.
Isolation of MetK mutations.
MetK catalyzes the formation of SAM from Met and ATP, and it has been suggested that metK is an essential gene (21). Resistance to the Met analogues norleucine and ethionine was reported to lead to metK mutations, as mapped using P1 transduction (1). Using norleucine to isolate metK mutants, we obtained 12 resistant strains from W3110. The metK region of each strain was amplified using PCR, and the nucleotide sequences were determined. Mutations in the structural metK gene were observed in 3 of the 12 norleucine-resistant strains; these mutations were designated metK2, metK24, and metK32. The first two strains both had a nucleotide substitution that led to an amino acid substitution (metK2 and metK24), whereas the third strain had a nucleotide deletion that caused a frameshift in the translated polypeptide (metK32) (Table 2). These mutations were introduced into the WΔthrBCΔmetJ strain using the plasmid pMAN997 vector, and the resultant strains containing metK mutations were desig-nated WΔthrBCΔmetJmetK2, WΔthrBCΔmetJmetK24, and WΔthrBCΔmetJmetK32. In order to test the effects of these mutations on Met excretion, the wild-type metA expression plasmid pMWPthrmetA was constructed (as described above). This plasmid was introduced into W3110, WΔthrBC, WΔthrBCΔmetJ, WΔthrBCΔmetJmetK2, WΔthrBCΔmetJmetK24, and WΔthrBCΔmetJmetK32, and the Met excretion levels were determined by cultivating the strains in MS medium (Table 3). The results showed that the metJ deletion had a significant effect on Met excretion in Thr-auxotrophic mutants. Furthermore, of the metK mutations, the metK24 mutation clearly had the greatest impact on Met production.
TABLE 2.
metK mutations found in norleucine-resistant strains
| Mutation | Change in nucleotide | Change in amino acid(s) |
|---|---|---|
| metK2 | 907A → C | 303Ile → Leu |
| metK24 | 554T → A | 185Val → Glu |
| metK32 | 1132C deletion | 378Arg-Asp-Ala-Ala-Gly-Leu-Lys-stop → 378Ala-Met-Leu-Pro-Val-stop |
TABLE 3.
Met excretion levels of wild-type strains with the metA gene introduceda
| Strain | Plasmid | Optical density at 600 nm | Excreted Met concn (g/liter) |
|---|---|---|---|
| W3110 | pMW118 | 21.3 ± 1.7 | NDb |
| W3110 | pMWPthrmetA | 23.1 ± 1.0 | ND |
| WΔthrBC | pMWPthrmetA | 9.5 ± 0.8 | 0.008 ± 0.001 |
| WΔthrBCΔmetJ | pMWPthrmetA | 7.9 ± 0.1 | 0.022 ± 0.004 |
| WΔthrBCΔmetJmetK2 | pMWPthrmetA | 6.9 ± 0.2 | 0.014 ± 0.002 |
| WΔthrBCΔmetJmetK24 | pMWPthrmetA | 6.9 ± 0.6 | 0.141 ± 0.004 |
| WΔthrBCΔmetJmetK32 | pMWPthrmetA | 6.5 ± 0.1 | 0.023 ± 0.002 |
Each value is the mean ± standard deviation of the mean for two replicate cultures.
ND, not detected.
Desensitization of MetA.
MetA is the first unique enzyme in the Met biosynthetic pathway, which catalyzes the succinylation of homoserine. Its activity is reported to be inhibited by the combined effects of Met and SAM (11, 20). In Salmonella enterica serovar Typhimurium, α-MM-resistant mutants are known to have feedback-resistant mutations in the metA gene (10). However, the nucleotide sequence information that is involved in these mutations remains unknown. Here, we obtained six spontaneous α-MM-resistant mutants from W3110 in independent experiments. The nucleotide sequences of the mutants were determined using PCR-amplified metA gene fragments. No mutations were detected in one strain; point mutations were observed in three strains, in which the mutant genes were designated metA4, metA5, and metA9; and an IS2 transposition (6) with a duplication of five nucleotides (886ATCTC) was found at the same position in the remaining two strains, in which the mutant genes were designated metA7 and metA8. As a consequence of the mutations in metA7 and metA8, the amino acid sequence from 298Pro onward was altered to 298Arg-Leu-Ala-Pro-stop (Table 4).
TABLE 4.
metA mutations in α-MM-resistant strains
| Mutation | Change in nucleotide | Change in amino acid |
|---|---|---|
| metA4 | 887T → G | 296Ile → Ser |
| metA5 | 893C → T | 298Pro → Leu |
| metA9 | 79C → T | 27Arg → Cys |
| metA7 | IS insertion | 298Pro-Tyr-Asp-Leu-Arg-His-Met-Asn-Pro-Thr-Leu-Asp-stop → 298Arg-Leu-Ala-Pro-stop |
| metA8 | IS insertion | 298Pro-Tyr-Asp-Leu-Arg-His-Met-Asn-Pro-Thr-Leu-Asp-stop → 298Arg-Leu-Ala-Pro-stop |
Crude extracts from strains containing metA4, metA5, and metA9 were prepared, and their MetA activities were measured using chemically synthesized dl-[14C]homoserine as a substrate (Table 5). The MetA activity in these extracts showed significant desensitization against α-MM, Met, and SAM, although the specific activities were reduced by approximately one-quarter. No MetA activity was detected in the metA7 extract as a result of the amino acid modification caused by the IS2 insertion. In both metA4 and metA5 extracts, the inhibition caused by SAM alone and that caused by SAM and Met together were reduced to the level reported previously by Lawrence (10).
TABLE 5.
Inhibition to desensitized MetA derived from α-MM-resistant mutants
| Inhibitor | MetA activity (mmol/min/mg protein)
|
||||
|---|---|---|---|---|---|
| W3110 | metA9 | metA4 | metA5 | metA7 | |
| None | 22.3 (100)b | 5.0 (100) | 4.5 (100) | 4.5 (100) | NDa |
| 0.1 mM α-MM | 18.6 (83) | 4.9 (99) | 4.1 (93) | 4.6 (102) | ND |
| 1 mM α-MM | 7.0 (31) | 2.7 (54) | 4.6 (103) | 4.8 (107) | ND |
| 0.1 mM Met | 14.3 (64) | 2.5 (51) | 4.5 (101) | 4.2 (94) | ND |
| 1 mM Met | 0.8 (4) | 2.2 (44) | 4.0 (89) | 4.0 (88) | ND |
| 0.1 mM SAM | 17.0 (76) | 1.1 (22) | 4.6 (103) | 3.6 (79) | ND |
| 1 mM SAM | 3.0 (13) | 0.5 (10) | 2.6 (58) | 3.3 (72) | ND |
| 0.1 mM Met + SAM | 0.0 (0) | 0.9 (19) | 5.6 (125) | 2.8 (61) | ND |
ND, not detected.
Numbers in parentheses are relative activities (%).
Met excretion caused by the introduction of desensitized metA genes.
In order to examine the effects of metA gene mutations that were feedback resistant to Met and SAM inhibition, we constructed expression plasmids carrying the metA4, metA5, and metA9 mutations as follows: pMWPthrmetA4, pMWPthrmetA5, and pMWPthrmetA9 harbored single mutations; pMWPthrmetA4+9 and pMWPthrmetA5+9 harbored double mutations; and pMWPthrmetA4+5+9 harbored all three mutations. These plasmids were introduced into host strain WΔthrBCΔmetJmetK32, which showed the greatest effect on Met excretion, and cultivated in MS medium.
As shown in Table 6, the metA4, metA5, and metA9 single mutations all had notable effects on Met excretion. Furthermore, combinations of these mutations (metA4 plus metA9, metA5 plus metA9, and metA4 plus metA5 plus metA9) increased the amount of Met excretion. These findings indicate that inhibition of MetA is critical for Met biosynthesis. All of the mutations investigated during our research had significant effects on Met excretion levels.
TABLE 6.
Met excretion by desentisized metA gene-containing strainsa
| Strain | Plasmid | Optical density at 600 nm | Excreted Met concn (g/liter) |
|---|---|---|---|
| WΔthrBCΔmetJmetK32 | pMWPthrmetA | 6.5 ± 0.4 | 0.023 ± 0.002 |
| WΔthrBCΔmetJmetK32 | pMWPthrmetA4 | 7.4 ± 0.3 | 0.158 ± 0.008 |
| WΔthrBCΔmetJmetK32 | pMWPthrmetA5 | 6.9 ± 0.3 | 0.108 ± 0.007 |
| WΔthrBCΔmetJmetK32 | pMWPthrmetA9 | 7.3 ± 0.2 | 0.131 ± 0.006 |
| WΔthrBCΔmetJmetK32 | pMWPthrmetA4+9 | 8.1 ± 0.2 | 0.206 ± 0.021 |
| WΔthrBCΔmetJmetK32 | pMWPthrmetA5+9 | 8.6 ± 0.7 | 0.207 ± 0.017 |
| WΔthrBCΔmetJmetK32 | pMWPthrmetA4+5+9 | 9.5 ± 0.3 | 0.236 ± 0.007 |
Each value is the mean ± standard deviation of the mean for two replicate cultures.
DISCUSSION
Many regulators of methionine biosynthesis and metabolism have been reported in E. coli (7). In order to determine the effects of factors predicted to be important for Met production by fermentation, we deregulated several of the known controls in the Met biosynthetic and metabolic pathways of E. coli. Introduction of deletion mutations of metJ and thrBC into the W3110 strain had a significant effect on the amount of Met excreted into the medium. In addition, three mutations of the metK structural gene, which were obtained from norleucine-resistant mutants, also significantly increased Met excretion levels when they were introduced into the metJ and thrBC double-mutant strain.
Mutations of the metA structural gene that were isolated from α-MM-resistant mutants were found to encode feedback-resistant enzymes using a 14C-labeled homoserine assay. A strain containing three metA mutations, which were introduced using expression plasmids, was shown to accumulate 0.24 g/liter Met in the medium. The metA9 mutation showed only a slight effect on MetA inhibition (Table 5) and little effect on Met excretion (Table 6) compared with the metA4 and metA5 mutations. However, the combination of the metA9 mutation with metA4 or metA5 significantly affected Met excretion. The metA9 mutation exhibited significant effects with high Met concentrations (compare 1 mM Met and 1 mM Met + 1 mM SAM in Table 5). The influence of the metA9 mutation might therefore appear only in combination with other mutations. The 27Arg residue that was replaced in the protein encoded by the metA9 mutant gene was located in a region near the amino terminus; this residue is well conserved across the bacteria. Similarly, the 298Pro residue that was replaced in the protein encoded by the metA5 gene, which was located close to the carboxy terminus of the protein, is also conserved across many species. By contrast, the 296Ile residue that was replaced in the protein encoded by the metA4 gene, which was located in an area near the carboxy terminus, is conserved only in closely related bacteria, such as Salmonella (accession no. P37413), Shigella (Q7UBA4), and Yersinia (Q8ZAR4). Combinations of these amino- and carboxy-terminal mutations had additive effects on Met excretion (Table 6), which suggests that both regions are important for the feedback inhibition of MetA.
The two other mutations detected in the metA structural gene were both insertions at the same position, which caused a frameshift in the carboxy-terminal region after 298Pro. Interestingly, some bacteria, such as Bacillus subtilis (accession no. P54167) and Lactobacillus plantarum (Q88UF5), lack this region. Although we were unable to measure the activity of the frameshifted enzyme (probably due to a lower specific activity compared with the enzymes with point mutations), these findings suggest that the carboxy-terminal region of MetA is essential for its regulation.
Thr auxotrophy is an important trait for Met excretion in E. coli. LB medium was enough for basal growth of WΔthrBC and its derivatives. However, addition of 0.5 g/liter of Thr to MS medium was appropriate for Met excretion. We could not detect the effects of wild-type metA amplification (Table 3) and metJ deletion (data not shown) on W3110. A Thr auxotroph might be useful for restricting the biomass of E. coli and for blocking off the branching pathway for the formation of by-products. For the latter reason, an l-lysine (Lys) auxotroph could also be effective in promoting Met excretion.
The metK gene has been proposed to be an essential gene (21), and we were unable to achieve complete metK deletion in this study. However, we did obtain norleucine-resistant MetK mutants. When the wild-type metA gene was amplified, the metK24 mutant was found to be most effective for Met production in a thrBC metJ background (Table 3). However, when the feedback-resistant MetA was amplified in the same background, the metK32 mutation resulted in the greatest effects on Met excretion (Table 6 and data not shown). These findings imply that the extent of the attenuation of MetK activity might vary depending on the genetic background of the strain. Clarification of the relationship between various levels of MetK activity and the amount of Met excretion is very important and will be the subject of further investigation.
Nakamori and colleagues (17) reported a MetJ mutant with replacement of 54Ser by Asn, which accumulated Met in the culture medium. This mutation had an effect similar to the effect of MetJ disruption. Chattopadhyay and coworkers (2, 3) also described enhanced methionine production in Thr analogue- and 5-bromouracil-resistant mutants. However, from the point of view of industrialization, the amounts of Met excretion reported in these studies were far from sufficient.
Many factors must be considered in order to increase the accumulation of Met. The most notable feature in the biosynthesis of this amino acid is the incorporation of sulfur as l-cysteine (Cys); this step is catalyzed by cystathionine γ-synthetase, which is encoded by metB. Furthermore, C1 transfer is involved in the last step of the pathway, which is catalyzed by methionine synthase (encoded by metE and metH). These steps are both potentially rate limiting in the large-scale production of Met. The C1 unit provided as methyl-tetrahydrofolate is derived from l-glycine (Gly), and both Cys and Gly are synthesized from l-serine (Ser). Therefore, the balanced synthesis of Cys and Gly from Ser, as well as the synthesis of Ser and oxaloacetate from 3-phosphoglycerate, is essential for the coordinated biosynthesis of Met. Cys production by modified serine acetyltransferase has been reported previously (16). Another factor that might be effective in promoting Met excretion is the Met exporter, and recent studies have revealed the importance of efflux carriers for amino acid excretion (5, 9, 12, 23). A Met exporter might be functional when excess Met is toxic inside the cell. This may explain why there were no negative effects on growth due to Met overproduction under the deregulated conditions for Met biosynthesis in this study (Table 6). Met biosynthesis seems to be regulated tightly, possibly because Met is valuable for cells due to the requirement for energy, including sulfur reduction. We were able to observe Met excretion into the medium only when energy was in excess due to growth restriction by auxotrophy. Therefore, a combination of deregulation of Met biosynthesis, restricted growth, and Met export activity is necessary for excretion of large amounts of Met.
In conclusion, in this study, we deregulated some of the controls of Met biosynthesis and metabolism in the W3110 wild-type strain of E. coli. Using a combination of gene disruption and amplification, we produced strains that could excrete increased amounts of Met into the growth medium. Further studies are necessary to identify additional factors that are essential for the realization of large-scale Met production by fermentation using E. coli.
Acknowledgments
We thank H. Kojima and K. Hashiguchi for helpful advice. We are also grateful to T. Inamura for amino acid measurements and to S. Murata for technical assistance.
REFERENCES
- 1.Chattopadhyay, M. K., A. K. Ghosh, and S. Sengupta. 1991. Control of methionine biosynthesis in Escherichia coli K-12: a closer study with analogue-resistant mutants. J. Gen. Microbiol. 137:685-691. [DOI] [PubMed] [Google Scholar]
- 2.Chattapadhyay, M. K., A. K. Ghosh, S. Sengupta, D. Sengupta, and S. Sengupta. 1995. Threonine analogue resistant mutants of Escherichia coli K-12. Biotechnol. Lett. 17:567-570. [Google Scholar]
- 3.Chattapadhyay, M. K., D. Sengupta, and S. Sengupta. 1995. Fermentative production of methionine by 5-bromouracil resistant mutants of Escherichia coli K-12. Med. Sci. Res. 23:775. [Google Scholar]
- 4.Davis, B. D., and E. S. Mingioli. 1950. Mutants of Escherichia coli requiring methionine or vitamin B12. J. Bacteriol. 60:17-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franke, I., A. Resch, T. Dassler, T. Maier, and A. Bock. 2003. YfiK from Escherichia coli promotes export of O-acetylserine and cysteine. J. Bacteriol. 185:1161-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosal, D., H. Sommer, and H. Saedler. 1979. Nucleotide sequence of the transposable DNA-element IS2. Nucleic Acids Res. 6:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greene, R. C. 1996. Biosynthesis of methionine, p. 542-560. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology Press, Washington, D.C.
- 8.Ikeda, M. 2003. Amino acid production processes. Adv. Biochem. Eng. Biotechnol. 79:1-35. [DOI] [PubMed] [Google Scholar]
- 9.Kruse, D., R. Kramer, L. Eggeling, M. Rieping, W. Pfefferle, J. H. Tchieu, Y. J. Chung, M. H. Saier, and A. Burkovski. 2002. Influence of threonine exporters on threonine production in Escherichia coli. Appl. Microbiol. Biotechnol. 59:205-210. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence, D. A. 1972. Regulation of the methionine feedback sensitive enzyme in mutants of Salmonella typhimurium. J. Bacteriol. 109:8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee, L. W., J. M. Ravel, and W. Shive. 1966. Multimetabolite control of a biosynthetic pathway by sequential metabolites. J. Biol. Chem. 241:5479-5480. [PubMed] [Google Scholar]
- 12.Livshits, V. A., N. P. Zakataeva, V. V. Aleshin, and M. V. Vitushkina. 2003. Identification and characterization of the new gene rhtA involved in threonine and homoserine efflux in Escherichia coli. Res. Microbiol. 154:123-135. [DOI] [PubMed] [Google Scholar]
- 13.Matsui, H., H. Kawasaki, M. Shimaoka, and O. Kurahashi. 2001. Investigation of various genotype characteristics for inosine accumulation in Escherichia coli W3110. Biosci. Biotechnol. Biochem. 65:570-578. [DOI] [PubMed] [Google Scholar]
- 14.Matsuyama, S., and S. Mizushima. 1985. Construction and characterization of a deletion mutant lacking micF, a proposed regulatory gene for OmpF synthesis in Escherichia coli. J. Bacteriol. 162:1196-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller, U., and S. Huebner. 2003. Economic aspects of amino acid production. Adv. Biochem. Eng. Biotechnol. 79:137-170. [DOI] [PubMed] [Google Scholar]
- 16.Nakamori, S., S. Kobayashi, C. Kobayashi, and H. Takagi. 1998. Overproduction of l-cysteine and l-cystine by Escherichia coli strains with a genetically altered serine acetyltransferase. Appl. Environ. Microbiol. 64:1607-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamori, S., S. Kobayashi, T. Nishimura, and H. Takagi. 1999. Mechanism of l-methionine overproduction by Escherichia coli: the replacement of Ser-54 by Asn in the MetJ protein causes the derepression of l-methionine biosynthetic enzymes. Appl. Microbiol. Biotechnol. 52:179-185. [DOI] [PubMed] [Google Scholar]
- 18.Pfefferle, W., B. Möckel, B. Bathe, and A. Marx. 2003. Biotechnical manufacture of lysine. Adv. Biochem. Eng. Biotechnol. 79:59-112. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Savin, M. A., M. Flavin, and C. Slaughter. 1972. Regulation of homocysteine biosynthesis in Salmonella typhimurium. J. Bacteriol. 111:547-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei, Y., and E. B. Newman. 2002. Studies on the role of the metK gene product of Escherichia coli K-12. Mol. Microbiol. 43:1651-1656. [DOI] [PubMed] [Google Scholar]
- 22.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequence of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 23.Zakataeva, N. P., V. V. Aleshin, I. L. Tokmakova, P. V. Troshin, and V. A. Livshits. 1999. The novel transmembrane Escherichia coli proteins involved in the amino acid efflux. FEBS Lett. 452:228-232. [DOI] [PubMed] [Google Scholar]