Abstract
The influence of bacterial growth stage and the evolution of surface macromolecules on cell adhesion have been examined by using a mutant of Escherichia coli K-12. To better understand the adhesion kinetics of bacteria in the mid-exponential and stationary growth phases under flow conditions, deposition experiments were conducted in a well-controlled radial stagnation point flow (RSPF) system. Complementary cell characterization techniques were conducted in combination with the RSPF experiments to evaluate the hydrophobicity, electrophoretic mobility, size, and titratable surface charge of the cells in the two growth phases considered. It was observed that cells in stationary phase were notably more adhesive than those in mid-exponential phase. This behavior is attributed to the high degree of local charge heterogeneity on the outer membranes of stationary-phase cells, which results in decreased electrostatic repulsion between the cells and a quartz surface. The mid-exponential-phase cells, on the other hand, have a more uniform charge distribution on the outer membrane, resulting in greater electrostatic repulsion and, subsequently, less adhesion. Our results suggest that the macromolecules responsible for this phenomenon are outer membrane-bound proteins and lipopolysaccharide-associated functional groups.
Pathogenic microorganisms in groundwater are estimated to cause 750,000 to 5 million illnesses per year in the United States (38). The fate and transport of these microbes are dependent on their propensity to adhere to mineral surfaces. By studying this phenomenon, we gain insight not only into the mechanisms influencing pathogen transport but also into processes such as the initiation of infection (36, 56), biofilm formation (18, 45), and the colonization of plant roots (3).
Escherichia coli, a gram-negative bacterium, is considered an ideal indicator of fecal contamination (27) and was therefore the organism employed in our study. The outer membrane of a gram-negative bacterium—a lipid bilayer—primarily contains lipopolysaccharides (LPS) and proteins. The LPS molecule extends into the surrounding medium and is anchored to the outer membrane by a lipid moiety known as lipid A (39). Adjacent to lipid A is the 2-keto-3-deoxyoctonic acid; this molecule links lipid A to the core polysaccharide region of the LPS (10, 39), which consists of heptose, glucose, galactose, and N-acetylglucosamine molecules (29, 39). The outermost part of the LPS assembly is a lengthy sugar chain called the O-polysaccharide or O-antigen, for which the exact size and composition are strain specific (34). The types of proteins that exist on the outer membrane include murein lipoproteins, porins (i.e., OmpC, OmpF, OmpA, and PhoE), diffusion proteins, enzymes, and structural molecules (44). Portions of these proteinaceous molecules are exposed to the external environment. In addition, many gram-negative cells exude extracellular polymeric substances (EPS), which typically consist of proteins, polysaccharides, and nucleic acids (47). Previous studies have addressed the role of these macromolecules on cell adhesion. Specifically, the adhesive nature of bacteria has been attributed to such features as LPS (1, 19, 34, 67), outer membrane proteins (41, 51), fimbriae (50, 63), flagella (13, 55), and EPS (20, 65).
One major limitation to the evaluation of previous adhesion studies is the wide variation in experimental protocols and approaches. Much of the previous work addressing adhesion has utilized batch systems, where adhesion to a submerged collector substrate occurs in either a static or gently shaken reactor (1, 37). Other studies have quantified bacterial adhesion in flowing systems, including packed columns (1, 7, 58), parallel plate flow chambers (6, 23), and radial stagnation point flow systems (58, 67, 68). The type of solution in which cells are suspended has also varied. Work has been conducted in such solutions as buffers (1, 7, 33, 37), simple salts (58, 67), and native environmental samples (12, 46). Adhesion has also been related to the measured interaction forces by the use of atomic force microscopy (1, 7, 54, 66). The range of media utilized for adhesion studies includes such collector media as natural sediment cores (12, 15, 21), glass (33, 51, 53), modified glass (23, 37), quartz (1, 67), and stainless steel (5, 46).
The growth phase of cells has also been a source of variation in the literature. Some studies have described the adhesive nature of cells at mid-exponential phase (6, 33, 48, 67) and stationary growth phase (6, 11), whereas others have not attempted to characterize the exact time of growth phase, preferring to define the time of growth as “overnight” (46, 50) or “late exponential” phase (1). Bacteria are inherently dynamic organisms, and their protein coverage (30, 43) and LPS molecule conformation evolve as a function of growth phase (30, 31). The extent to which these modifications alter the adhesive nature of the cell has rarely been addressed (6, 40).
The objective of this paper is to evaluate the role of growth phase in cellular adhesion to solid surfaces in an aquatic environment. A radial stagnation point flow (RSPF) system with well-defined hydrodynamics was used to determine the kinetics of bacterial adhesion to a quartz surface over a wide range of ionic strengths. Through the utilization of cell characterization techniques in combination with RSPF adhesion studies, the evolving nature of bacterial cell surface molecules and their role in adhesion are assessed.
MATERIALS AND METHODS
Bacterial cell growth and preparation.
Escherichia coli K-12 D21, the strain used for this study, was obtained from the E. coli Genetic Stock Center at Yale University. This particular K-12 mutant has been reported to produce little or no EPS (57). For visualization of the cells in adhesion studies, a plasmid coding for an enhanced green fluorescent protein and gentamicin resistance (64) was introduced into native D21 cells by electroporation (60). The resulting transformed D21 cell line is referred to as D21g. Cells were grown in Luria-Bertani broth (Fisher Scientific, Fair Lawn, N.J.) supplemented with 0.03 mg/liter gentamicin (Sigma, St. Louis, Mo.) at 37°C until they reached the desired growth stage (3 and 18 h, corresponding to the mid-exponential and stationary phases, respectively), at which time they were harvested for use.
Cells were pelleted by centrifugation (Sorvall RC26 Plus centrifuge) for 15 min at 3,823 × g via an SS34 rotor (Kendro Laboratory Products, Newtown, Conn.). The growth medium was decanted and the pellet was resuspended in a KCl electrolyte solution (10−2 M). The cells were pelleted and rinsed with fresh electrolyte solution in this manner two additional times to remove all traces of the growth medium.
Viability tests for the D21g cells were performed using a Live/Dead BacLight kit (L-7012; Molecular Probes, Eugene, Oreg.) under the solution conditions used for the adhesion experiments and cell characterization techniques. Specifically, viability was evaluated in salt solutions ranging from 10−2 to 10−0.5 M KCl and in solutions containing 2% molecular-biology-grade disodium EDTA (American Bioanalytical, Natick, Mass.). The viabilities of the 3- and 18-h cell cultures averaged 79% and 80%, respectively; cell suspensions exposed to EDTA averaged 76% viability.
Bacterial cell characterization.
The electrophoretic mobility of the bacterial cells was determined by diluting the rinsed cell pellet in a KCl electrolyte solution to a final concentration of 105 to 106 cells/ml. Electrolyte solutions were prepared with deionized water (Barnstead Thermolyne Corporation, Dubuque, Iowa) and reagent-grade KCl (Fisher Scientific), with no pH adjustment (pH 5.6 to 5.8). Electrophoretic mobility measurements were conducted at 25°C using a ZetaPALS analyzer (Brookhaven Instruments Corporation, Holtsville, N.Y.) and were repeated a minimum of three times at each ionic strength with freshly rinsed cells. Electrophoretic mobilities were converted to zeta potentials by use of the tabulated numerical calculations of Ottewill and Shaw, which account for retardation and relaxation effects (49).
An inverted fluorescence microscope (Axiovert 200m; Zeiss, Thornwood, N.Y.) operating in phase-contrast mode was used to take images of D21g cells harvested after 3 and 18 h of growth following resuspension in an electrolyte solution (ca. 107 cells/ml in 10−2 M KCl). The images were imported into an image processing program (ImageJ; National Institutes of Health) and analyzed using the built-in particle analysis routines. From the measured cell lengths and widths, the average equivalent spherical radii of the D21g cells were determined to be 0.87 and 0.93 μm for mid-exponential-phase and stationary-phase cells, respectively.
EDTA extractions were conducted to collect cell-bound carbohydrate or protein molecules for analysis. The original cell pellet was resuspended in a 1:1 (vol/vol) mixture of 10−2 M KCl and 2% EDTA. The EDTA-cell suspension was incubated at 4°C for 30 min, followed by centrifugation at 10,426 × g for 50 min at 4°C (5804R; Eppendorf, Hamburg, Germany) (69). After centrifugation, the supernatant was collected for subsequent analysis. Total protein content was determined for both filtered (0.45-μm Millex-HA membrane; Millipore Corp., Bedford, Mass.) and unfiltered samples of the supernatant solutions according to the method of Bradford (4). Human serum albumin (HSA) was used as the reference standard. Total carbohydrate content analysis of the filtered and unfiltered supernatants utilized the phenol-sulfuric acid (PSA) method (16), with xanthan gum as the standard.
The hydrophobicities of the mid-exponential- and late-stationary-phase (3 and 18 h, respectively) cells were measured using the microbial adhesion to hydrocarbons (MATH) test (52) with n-dodecane (laboratory grade; Fisher Scientific). Samples were prepared by transferring 4 ml of a cell solution (optical density at 546 nm of 0.2 to 0.25 in 10−2 M KCl) to test tubes containing 1 ml of dodecane. The test tubes were vortexed (Touch Mixer model 231; Fisher Scientific) for 2 min, followed by a 15-min rest period to allow for phase separation. The optical density of the cells in the aqueous phase was measured at 546 nm (Hewlett-Packard model 8453) to determine the extent of bacterial cell partitioning between the dodecane and the electrolyte. Hydrophobicities are reported as the percentages of total cells partitioned into the hydrocarbon (52).
Potentiometric titrations of mid-exponential- and stationary-phase cells were conducted to determine the relative acidities of the bacterial surfaces using a microtitrator (794 Basic Titrino; Metroohm, Switzerland). Prior to titration, the solution pH was lowered to 4 by the addition of HCl and the solution was purged with N2 gas to remove any dissolved carbon dioxide present. Titrations were performed on suspensions of bacteria (between 108 and 1010 cells/ml) in 10−2 M KCl. The acidity was determined from the amount of NaOH consumed during titration between pHs 4 and 10 (61).
Bacterial adhesion experimental setup.
Bacterial deposition experiments were conducted in a radial stagnation point flow (RSPF) system (67). This system comprised a specially blown glass flow chamber installed on the stage of an inverted fluorescence microscope (Axiovert 200m; Zeiss, Thornwood, N.Y.). The bacterial suspension entered the radially symmetric flow cell through a capillary tube (2-mm inner diameter). Flow impinged upon a quartz microscope cover glass 2 mm below the capillary opening and traveled radially along the cover slide, exiting the RSPF chamber through a separate capillary tube. An LD Achroplan 40× objective (1.8-mm working distance) located beneath the flow cell was focused on the inner surface of the quartz coverslip. Fluorescent cells were imaged using a fluorescent filter set with an excitation wavelength of 480 nm and an emission wavelength of 510 nm (Chroma Technology Corp., Brattleboro, Vt.).
Preparation of solid substrate for adhesion experiments.
Round quartz coverslips with a 25-mm diameter and a 0.1-mm thickness (Electron Microscopy Sciences, Ft. Washington, Pa.) were cleaned by soaking them initially in a 2% Extran MA02 solution (EM Science, Gibbstown, N.J.), followed by a thorough rinsing with ethanol (Pharmco Products, Inc., Brookfield, Conn.) and deionized water. The coverslips were sonicated for 10 to 15 min while submerged in a 2% RBS 35 detergent solution (Pierce, Rockford, Ill.), followed by a second rinse with ethanol and deionized (DI) water. The coverslips were soaked overnight in NOCHROMIX solution (Godax Laboratories, Inc., Takoma Park, Md.). After removal from the NOCHROMIX solution and a rinse with DI water, the coverslips were mounted in the glass flow cell used in the radial stagnation point flow system. For adhesion experiments under favorable (nonrepulsive) electrostatic conditions, the slides were chemically modified with aminosilane. One side of each quartz coverslip was exposed to a 0.2% (vol/vol) mixture of (aminoethylaminomethyl)-phenethyltrimethoxysilane (Gelest, Inc., Tullytown, Pa.) in ethanol for 3 to 5 min at room temperature and then cured for 90 min at 130°C. The slide was then rinsed with DI water and installed in the radial stagnation point flow cell.
Determination of bacterial adhesion rate.
The deposition of bacterial cells was recorded with a digital camera (AxioCam MR monochrome; Zeiss) acquiring images every 20 seconds over the course of a 20-min injection period and was analyzed with the supplied software (AxioVision 3.1; Zeiss). The number of deposited bacterial cells was determined for each image by comparing the changes between successive images. Bacterial cell deposition experiments were conducted with cells harvested after 3 and 18 h of growth and were done with a range of ionic strengths (10−2, 10−1.5, 10−1, and 10−0.5 M KCl) at an unadjusted pH (5.6 to 5.8) and ambient temperature (22 to 25°C). Inlet concentrations for experiments were 107 to 108 cells/ml, as determined by directly visualizing cells in a counting chamber (Buerker-Tuerk chamber; Marienfield Laboratory Glassware, Lauda-Königshofen, Germany). A flow rate of 5 ml/min, corresponding to an average capillary flow velocity of 0.0265 m/s, was employed.
The bacterial adhesion kinetics in the RSPF system is quantified by calculating the bacterial transfer rate coefficient, kRSPF, as follows: kRSPF = J/C0, where C0 is the bacterial bulk concentration and J is the deposition flux of bacteria to the coverslip. For determination of the deposition flux, J, the observed deposition rate of bacteria onto the coverslip (i.e., the initial slope of the number of deposited bacteria versus time) was normalized to the microscope viewing area (210 μm by 165 μm). The reported kRSPF values represent the averages of at least three different runs, with each run utilizing a fresh cell suspension.
RESULTS AND DISCUSSION
Adhesion of E. coli D21g in mid-exponential and stationary growth phases.
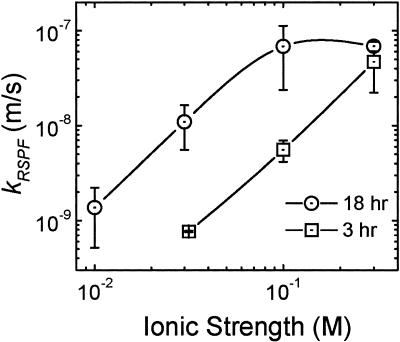
Figure 1 indicates that stationary-phase cells are substantially more adhesive than mid-exponential-phase cells. The rate of bacterial deposition onto the quartz surface was higher for stationary-phase cells across the range of solution ionic strengths examined. Deposition rates for cells grown for 3 and 18 h (referred to as 3- and 18-h cells, respectively) were governed by the ionic strength, with higher ionic strengths resulting in greater deposition. As can be seen in Fig. 1, the kRSPF for 18-h cells reached a plateau between 10−1 and 10−0.5 M. This indicates entrance into the mass-transfer-limited regimen, for which a further increase in ionic strength will not affect the deposition rate. The deposition of 3-h cells at strengths below 10−1.5 M was statistically insignificant, even when the bulk cell concentration was increased to 108 cells/ml.
FIG. 1.
Bacterial deposition kinetics in the radial stagnation point flow system, presented as cell transfer rate coefficients (kRSPF) as a function of ionic strength. Data are shown for E. coli D21g cells harvested in the mid-exponential and stationary phases (3 and 18 h, respectively). The experimental conditions were as follows: flow velocity = 0.0265 m/s and pH = 5.6 to 5.8. Error bars indicate 1 standard deviation.
Bacterial adhesion mechanisms.
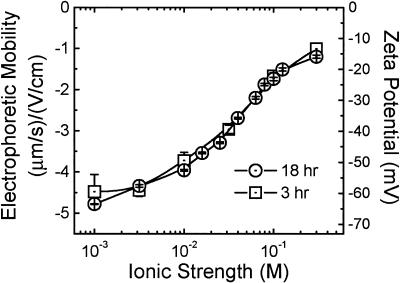
The observed sensitivity of E. coli adhesion to ionic strength suggests that electrostatic forces dominate interactions between bacterial cells and quartz. As indicated by the measured electrophoretic mobilities and corresponding zeta potentials (Fig. 2), the bacterial cells were negatively charged under all of the conditions of our adhesion experiments. Because the quartz surface also exhibits a net negative charge under the solution conditions analyzed (58), repulsive electrostatic interactions should dominate cell deposition. Repulsive electrostatic interactions are sensitive to the solution’s ionic strength such that with an increase in the ionic strength of the background solution, the repulsive force is lessened. This is the case for E. coli-quartz interactions—an increase in ionic strength results in greater adhesion or a higher bacterial transfer rate (kRSPF). It must be noted that although the measured electrophoretic mobility values for 3- and 8-h cells were virtually identical, the much lower observed kRSPF values for 3-h cells imply a greater repulsive force between quartz and the cells across the range of ionic strength conditions. This phenomenon will be addressed further in the discussion of bacterial characterization.
FIG. 2.
Electrophoretic mobilities and zeta potentials of E. coli D21g cells harvested at 3 and 18 h (corresponding to mid-exponential- and stationary-phase cells) as a function of ionic strength (KCl). Experiments were conducted at ambient pH (5.6 to 5.8) and temperature (25°C). Error bars indicate 1 standard deviation.
To gain further insight into the forces governing D21g cell deposition, the classic Derjaguin-Landau-Verwey-Overbeek theory for colloidal stability (14) has been applied to calculate the total interaction energy as a function of the separation distance between the bacterium and the quartz surface (58). Essentially, the total interaction energy, namely, the sum of attractive van der Waals (24) and repulsive electrostatic (28) interactions, is determined by assuming that the bacterium-quartz slide approximates a sphere-plate interaction. The resulting interaction energy profile exhibits one of the following trends (58, 67). First, when repulsive electrostatic forces are suppressed, no energy barrier to deposition exists, implying irreversible attachment of the bacterium to the quartz surface in a deep primary minimum. This scenario existed for our D21g-quartz under ionic strength conditions of 10−1 M and higher (58, 67). Second, for solution ionic strengths of ≤10−1.5 M, there exists an insurmountable electrostatic energy barrier to deposition. It has been shown that in the presence of such a substantial repulsive energy barrier to bacterial deposition, it is unlikely that bacterial cells will deposit in the primary energy minimum at the quartz surface (58). However, under the latter solution conditions, a shallow energy well—the “secondary minimum”—exists at greater separation distances from the quartz surface. This energy well is sufficiently deep to allow entrainment of the D21g bacterium near the quartz (26, 58).
Bacteria entrained in a secondary energy minimum are at greater separation distances from the quartz collector than those in primary minima (26, 58). In the case of the RSPF system, bacterial cells held in the secondary minimum experience a hydrodynamic force due to the radial component of flow parallel to the quartz surface (2). Consequently, such cells are swept away from the stagnation point region and the microscope's field of view. On the other hand, cells bound in a primary minimum remain firmly attached to the quartz surface, within the field of view. Only cells that are irreversibly deposited in a primary energy minimum, and not those entrained in the secondary minimum, are enumerated with the RSPF system. Accordingly, Fig. 1 can be considered a comparison of levels of primary-minimum deposition as a function of the bacterial growth phase and the solution ionic strength.
The electrophoretic mobilities (or zeta potentials) are virtually identical for the mid-exponential- and stationary-phase cells (Fig. 2). Thus, the deposition trends in Fig. 1 cannot be explained by electrostatic interactions determined from average zeta potentials alone. As discussed in detail later, the disparity is attributed to exposed functional groups on the cell surface which contribute to local charge inhomogeneity. The presence of surface charge heterogeneity would decrease electrostatic repulsion and increase the rate of irreversible attachment in the primary minimum (17, 62) compared to Derjaguin-Landau-Verwey-Overbeek theory predictions. At higher ionic strengths, when no barrier to deposition exists, deposition occurs regardless of charge heterogeneity on the bacterial surface.
The predominant functional groups exposed on the outer membrane of E. coli include amino and carboxyl groups on exposed proteins as well as phosphate and carboxyl groups on both LPS- and EPS-associated carbohydrates (22, 31, 42). The pKa values of the various exposed groups indicate that, at the pH of our experiments (pH 5.6 to 5.8), the dominant ionic species exposed on the outer membranes of the bacteria arise from negatively charged phosphate groups associated with the LPS (42, 59) and from carboxyl groups associated with both proteins and polysaccharides (59). Additionally, phosphate species may exist on the nucleic acids associated with any EPS (47). Because the experiments with 3- and 18-h cells were conducted with the same solution chemistry, it was anticipated that both would have phosphate and carboxyl groups present. Therefore, the higher rate of adhesion observed for the 18-h cells was not attributed to the type of ionic species; rather, we propose that it was due to the greater amount of heterogeneity in the distribution of functional groups exposed on cellular structures. To establish the extent to which these functional groups contribute to inhomogeneity on the cell surface and, consequently, to deposition trends, we utilized traditional tools for cell characterization.
Bacterial surface characterization.
A possible contributing factor to the deposition behavior of E. coli is the difference in cell transfer rate related to the sizes of individual cells. In order to determine whether cell size changes notably with the growth stage, the equivalent spherical radii were calculated from the lengths and widths of cells (Table 1). The equivalent radii for the 3- and 18-h cells were quite similar, at 0.87 and 0.93 μm, respectively. To ensure that this minor difference in cell size is not the factor determining the extent of the cell transfer rate, experiments were carried out under favorable (nonrepulsive) conditions with positively charged, aminosilane-modified quartz slides. The subsequent interaction between oppositely charged surfaces allowed for the determination of the kRSPF under mass-transport-limited conditions. This favorable deposition scenario produced virtually identical bacterial transfer rates (reported in Table 1), confirming that the difference between 3- and 18-h cell deposition is not size dependent.
TABLE 1.
Characterization of E. coli D21g cells as a function of growth stage
| Growth stage | Growth time (h) | Ionic strength (M) | % Live cellsa | Cell radius (μm)b | MATH result (%)c | kRSPF,fav (m/s) | Acidity (meq/108 cell)d | Surfae charge (μC/cm2)e |
|---|---|---|---|---|---|---|---|---|
| Mid-exponential | 3 | 10−3 | 80 | |||||
| 3 | 10−2 | 80 | 0.87 | 16 ± 1 | 2.5 × 10−7 | 7.1 × 10−5 | 796.8 | |
| 3 | 10−1 | 77 | ||||||
| Stationary | 18 | 10−3 | 82 | |||||
| 18 | 10−2 | 86 | 0.93 | 34 ± 6 | 2.6 × 10−7 | 8.4 × 10−6 | 87.9 | |
| 18 | 10−1 | 73 |
Percentage of the cell population determined to be viable based on Live/Dead BacLight kit results.
Value for equivalent spherical radius calculated from the experimentally measured lengths and widths of individual cells. The average lengths and widths of the cells were 3.7 and 1.2 μm, respectively, for 3-h cells and 3.6 and 1.3 μm, respectively, for 18-h cells.
The microbial adhesion to hydrocarbons (MATH) test indicates the relative hydrophobicity of the cell as the percentage of cells partitioned into dodecane versus an electrolyte (10−2 M KCl).
Acidity was determined from the amount of NaOH consumed during a titration between pH 4 and 10 for cells suspended in 10−2 M KCl.
The surface charge indicates the density of charged functional groups across the cell surface. Values were determined from experimentally measured acidities, accounting for the exposed surface area of the cells (calculated for a spherical cell) and using the Faraday constant of 96,485 C/mol.
Previously, cellular deposition and the strengthening of attachment have been attributed to the presence of EPS (20, 46). EPS has also been credited with contributing to the overall heterogeneous nature of bacterial surfaces (20, 47, 65). The amount of EPS was analyzed by using standard characterization techniques. Quantification of the carbohydrate fraction of EPS was achieved by the phenol-sulfuric acid (PSA) method, using xanthan gum as a standard (reported in standard units of xanthan gum equivalence [XGE]). The quantities of EPS collected and measured at the exponential and stationary phases revealed values that were at or near the detection limit (1 μg XGE/ml). The average normalized cell values were 0.6 μg XGE/ml and 5 μg XGE/ml for 3- and 18-h cells, respectively (±11 μg XGE/ml error for the PSA method). The Bradford assay was employed to quantify the protein content of the EPS, using human serum albumin (HSA) as a standard. For unfiltered samples, normalized values were 0.1 μg HSA/ml for 3-h cells and 1 μg HSA/ml for 18-h cells (±1 μg HSA/ml error for the Bradford assay). These values were also at or near the detection limit. These extremely low measurements were not surprising since the E. coli K-12 strain used in this study has been reported to produce little or no EPS (57). Reports of other E. coli K-12 strains have been published, confirming their low production of EPS (12, 33). Because the values for EPS were so insignificant and since it is doubtful that over the time course of our experimentation the bacteria would form EPS, we do not attribute the adhesion behavior of 3- and 18-h cells to this phenomenon.
Further characterization of the 3- and 18-h cells was performed by measuring the hydrophobicity of E. coli D21g, a bulk property which captures the evolving presence of the chemical species on the surfaces of the cells. Hydrophobicity was determined to change with growth phase (Table 1), as measured by the MATH test. It was determined that 16% of the 3-h cells partitioned into dodecane but that 34% of the 18-h cells partitioned into the hydrocarbon. The outer membrane proteins of E. coli are mostly hydrophilic (acidic) and decrease in number with culture age (30, 42), yielding a corresponding increase in hydrophobicity. Our MATH test data confirmed this trend. Additional evidence of the change in bacterial hydrophobicity due to the presence of protein can be found in the literature (6, 8, 25, 32).
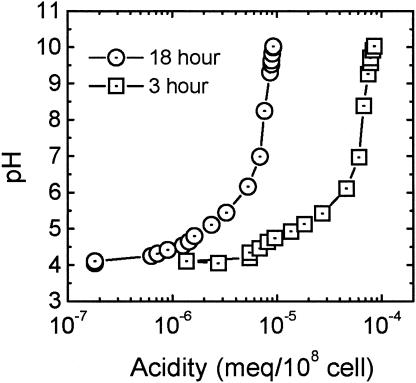
One final characterization tool, potentiometric titration, was used to evaluate the distribution of charge across the cell surface. The results of the titrations are presented in Fig. 3 as acidity, or titrated charge (in milliequivalents per cell), as a function of pH. Experiments confirmed that mid-exponential-phase cells are substantially more acidic than stationary-phase cells. As indicated in Table 1, the corresponding titrated surface charges are 796.84 μC/cm2 and 87.93 μC/cm2 for 3- and 18-h cells, respectively. The much higher value for 3-h cells indicates that the number of dissociable functional groups on the bacterial surface is greater than that for 18-h cells, implying a greater charge density on the outer membrane as well. We attribute these results to the presence of outer membrane proteins, which are more abundant on the surfaces of mid-exponential-phase cells (30). Notably, the shapes of the titration curves for the 3- and 18-h cells are comparable, suggesting that the types of functional groups do not change with the growth phase. These phenomena are explained further below.
FIG. 3.
Potentiometric titration of mid-exponential- and stationary-phase E. coli D21g cells. Acidities are presented in milliequivalents per 108 bacterial cells, as determined from the amount of NaOH consumed during titration. Titrations were performed in 10−2 M KCl and at a temperature of 25°C.
Relationship among adhesion behavior, growth phase, and surface heterogeneity of E. coli.
Irrespective of the growth phase, the dominant ionic species exposed on the outer surfaces of E. coli cells have previously been shown to be a combination of protein and LPS-associated functional groups. Our characterization techniques support this assertion. Both the titration and MATH test results indicate that mid-exponential-phase cells have greater amounts of polar molecules at the cell surface. We propose that the extra ionic species on mid-exponential-phase cells compared to stationary-phase cells originate from the presence of a greater number of proteins. Consequently, to a collector quartz surface, 3-h cells will appear more uniformly charged than 18-h cells (9), despite the uneven distribution of LPS (35). In contrast, 18-h cells—with fewer exposed proteins—will have a more heterogeneous distribution of charge, with most charged groups originating from the core of the LPS molecules. This is further compounded by the E. coli cells exposing a greater number of phosphate groups on the LPS at later growth stages (30, 31), even though the number of LPS molecules remains constant.
In conclusion, experiments were conducted to quantitatively evaluate the role of growth phase on cellular adhesion. The RSPF system allowed for the quantification of deposition kinetics and has given greater insight into the mechanisms involved in bacterial adhesion. The RSPF system, coupled with complementary cell characterization techniques, has led us to hypothesize that the evolution of the cell with the growth phase, as manifested by subtle alterations in cell surface charge heterogeneity, dramatically alters the adhesive nature of E. coli.
Acknowledgments
We acknowledge funding from the National Science Foundation (Collaborative Research Activities in Environmental Molecular Sciences;grant CHE-0089156) and the National Water Research Institute.
The use of the enhanced green fluorescent protein-expressing plasmid was provided by H. P. Spaink (Leiden University, Leiden, The Netherlands).
REFERENCES
- 1.Abu-Lail, N. I., and T. A. Camesano. 2003. Role of lipopolysaccharides in the adhesion, retention, and transport of Escherichia coli JM109. Environ. Sci. Technol. 37:2173-2183. [DOI] [PubMed] [Google Scholar]
- 2.Adamczyk, Z., B. Siwek, P. Warszynski, and E. Musial. 2001. Kinetics of particle deposition in the radial impinging-jet cell. J. Colloid Interface Sci. 242:14-24. [DOI] [PubMed] [Google Scholar]
- 3.Bloemberg, G. V., G. A. Otoole, B. J. J. Lugtenberg, and R. Kolter. 1997. Green fluorescent protein as a marker for Pseudomonas spp. Appl. Environ. Microbiol. 63:4543-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Briandet, R., T. Meylheuc, C. Maher, and M. N. Bellon-Fontaine. 1999. Listeria monocytogenes Scott A: cell surface charge, hydrophobicity, and electron donor and acceptor characteristics under different environmental growth conditions. Appl. Environ. Microbiol. 65:5328-5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruinsma, G. M., M. Rustema-Abbing, H. C. van der Mei, and H. J. Busscher. 2001. Effects of cell surface damage on surface properties and adhesion of Pseudomonas aeruginosa. J. Microbiol. Methods 45:95-101. [DOI] [PubMed] [Google Scholar]
- 7.Burks, G. A., S. B. Velegol, E. Paramonova, B. E. Lindenmuth, J. D. Feick, and B. E. Logan. 2003. Macroscopic and nanoscale measurements of the adhesion of bacteria with varying outer layer surface composition. Langmuir 19:2366-2371. [Google Scholar]
- 8.Chavant, P., B. Martinie, T. Meylheuc, M. N. Bellon-Fontaine, and M. Hebraud. 2002. Listeria monocytogenes LO28: surface physicochemical properties and ability to form biofilms at different temperatures and growth phases. Appl. Environ. Microbiol. 68:728-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, J. Y., J. F. Klemic, and M. Elimelech. 2002. Micropatterning microscopic charge heterogeneity on flat surfaces for studying the interaction between colloidal particles and heterogeneously charged surfaces. Nano Lett. 2:393-396. [Google Scholar]
- 10.Coughlin, R. T., S. Tonsager, and E. J. McGroarty. 1983. Quantitation of metal-cations bound to membranes and extracted lipopolysaccharide of Escherichia coli. Biochemistry 22:2002-2007. [DOI] [PubMed] [Google Scholar]
- 11.Daughney, C. J., D. A. Fowle, and D. E. Fortin. 2001. The effect of growth phase on proton and metal adsorption by Bacillus subtilis. Geochim. Cosmochim. Acta 65:1025-1035. [Google Scholar]
- 12.DeFlaun, M. F., S. R. Oppenheimer, S. Streger, C. W. Condee, and M. Fletcher. 1999. Alterations in adhesion, transport, and membrane characteristics in an adhesion-deficient pseudomonad. Appl. Environ. Microbiol. 65:759-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deflaun, M. F., A. S. Tanzer, A. L. McAteer, B. Marshall, and S. B. Levy. 1990. Development of an adhesion assay and characterization of an adhesion-deficient mutant of Pseudomonas fluorescens. Appl. Environ. Microbiol. 56:112-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derjaguin, B. V., and L. Landau. 1941. Theory of the stability of strongly charged lyophobic sols and the adhesion of strongly charged particles in solutions of electrolytes. Acta Physicochim. U.S.S.R. 14:733-763. [Google Scholar]
- 15.Dong, H., R. Rothmel, T. C. Onstott, M. E. Fuller, M. F. DeFlaun, S. H. Streger, R. Dunlap, and M. Fletcher. 2002. Simultaneous transport of two bacterial strains in intact cores from Oyster, Virginia: biological effects and numerical modeling. Appl. Environ. Microbiol. 68:2120-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28:350-356. [Google Scholar]
- 17.Elimelech, M., M. Nagai, C. H. Ko, and J. N. Ryan. 2000. Relative insignificance of mineral grain zeta potential to colloid transport in geochemically heterogeneous porous media. Environ. Sci. Technol. 34:2143-2148. [Google Scholar]
- 18.Espinosa-Urgel, M., A. Salido, and J. L. Ramos. 2000. Genetic analysis of functions involved in adhesion of Pseudomonas putida to seeds. J. Bacteriol. 182:2363-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flemming, C. A., R. J. Palmer, A. A. Arrage, H. C. Van der Mei, and D. C. White. 1999. Cell surface physicochemistry alters biofilm development of Pseudomonas aeruginosa lipopolysaccharide mutants. Biofouling 13:213-231. [Google Scholar]
- 20.Frank, B. P., and G. Belfort. 2003. Polysaccharides and sticky membrane surfaces: critical ionic effects. J. Membr. Sci. 212:205-212. [Google Scholar]
- 21.Fuller, M. E., H. L. Dong, B. J. Mailloux, T. C. Onstott, and M. F. DeFlaun. 2000. Examining bacterial transport in intact cores from Oyster, Virginia: effect of sedimentary facies type on bacterial breakthrough and retention. Water Resour. Res. 36:2417-2431. [Google Scholar]
- 22.Gmeiner, J., and S. Schlecht. 1980. Molecular composition of the outer-membrane of Escherichia coli and the importance of protein-lipopolysaccharide interactions. Arch. Microbiol. 127:81-86. [DOI] [PubMed] [Google Scholar]
- 23.Gottenbos, B., H. C. van der Mei, and H. J. Busscher. 2000. Initial adhesion and surface growth of Staphylococcus epidermidis and Pseudomonas aeruginosa on biomedical polymers. J. Biomed. Mater. Res. 50:208-214. [DOI] [PubMed] [Google Scholar]
- 24.Gregory, J. 1981. Approximate expressions for retarded van der Waals interaction. J. Colloid Interface Sci. 83:138-145. [Google Scholar]
- 25.Haake, D. A., C. Martinich, T. A. Summers, E. S. Shang, J. D. Pruetz, A. M. McCoy, M. K. Mazel, and C. A. Bolin. 1998. Characterization of leptospiral outer membrane lipoprotein LipL36: downregulation associated with late-log-phase growth and mammalian infection. Infect. Immun. 66:1579-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hahn, M. W., and C. R. O'Melia. 2004. Deposition and reentrainment of Brownian particles in porous media under unfavorable chemical conditions: some concepts and applications. Environ. Sci. Technol. 38:210-220. [DOI] [PubMed] [Google Scholar]
- 27.Havelaar, A., U. J. Blumenthal, M. Strauss, D. Kay, and J. Bartram. 2001. Guidelines: the current position, p. 17-42. .In L. Fewtrell and J. Bartram (ed.), World Health Organization water quality: guidelines, standards and health. IWA Publishing, London, United Kingdom.
- 28.Hogg, R., T. W. Healy, and D. W. Fuersten. 1966. Mutual coagulation of colloidal dispersions. Trans. Faraday Soc. 62:1638-1651. [Google Scholar]
- 29.Holst, O., A. J. Ulmer, H. Brade, H. D. Flad, and E. T. Rietschel. 1996. Biochemistry and cell biology of bacterial endotoxins. FEMS Immunol. Med. Microbiol. 16:83-104. [DOI] [PubMed] [Google Scholar]
- 30.Huisman, G. W., D. A. Siegele, M. M. Zambrano, and R. Kolter. 1996. Morphological and physiological changes during stationary phase, p. 1672-1682. .In F. C. Neidhardt (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 31.Ivanov, A. Y., and V. M. Fomchenkov. 1989. Dependence of surfactant damage to Escherichia coli cells on culture-growth phase. Microbiology 58:785-791.2699649 [Google Scholar]
- 32.Jana, T. K., A. K. Srivastava, K. Csery, and D. K. Arora. 2000. Influence of growth and environmental conditions on cell surface hydrophobicity of Pseudomonas fluorescens in non-specific adhesion. Can. J. Microbiol. 46:28-37. [PubMed] [Google Scholar]
- 33.Jones, J. F., J. D. Feick, D. Imoudu, N. Chukwumah, M. Vigeant, and D. Velegol. 2003. Oriented adhesion of Escherichia coli to polystyrene particles. Appl. Environ. Microbiol. 69:6515-6519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kannenberg, E. L., and R. W. Carlson. 2001. Lipid A and O-chain modifications cause Rhizobium lipopolysaccharides to become hydrophobic during bacteroid development. Mol. Microbiol. 39:379-391. [DOI] [PubMed] [Google Scholar]
- 35.Kotra, L. P., D. Golemi, N. A. Amro, G. Y. Liu, and S. Mobashery. 1999. Dynamics of the lipopolysaccharide assembly on the surface of Escherichia coli. J. Am. Chem. Soc. 121:8707-8711. [Google Scholar]
- 36.Lamba, N. M. K., J. N. Baumgartner, and S. L. Cooper. 2000. The influence of thrombus components in mediating bacterial adhesion to biomaterials. J. Biomater. Sci.-Polym. 11:1227-1237. [DOI] [PubMed] [Google Scholar]
- 37.Li, B. K., and B. E. Logan. 2004. Bacterial adhesion to glass and metal-oxide surfaces. Colloids Surf. B 36:81-90. [DOI] [PubMed] [Google Scholar]
- 38.Macler, B. A., and J. C. Merkle. 2000. Current knowledge on groundwater microbial pathogens and their control. Hydrogeol. J. 8:29-40. [Google Scholar]
- 39.Madigan, M. T., J. M. Martinko, and J. Parker. 1997. Brock biology of microorganisms, 8th ed. Prentice Hall, Upper Saddle River, N.J.
- 40.Manas, P., and B. M. Mackey. 2004. Morphological and physiological changes induced by high hydrostatic pressure in exponential- and stationary-phase cells of Escherichia coli: relationship with cell death. Appl. Environ. Microbiol. 70:1545-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navarre, W. W., and O. Schneewind. 1999. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 63:174-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikaido, H. 1996. Outer membrane, p. 29-47. .In F. C. Neidhardt (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 44.Nikaido, H., and M. Vaara. 1985. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 49:1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okte, E., N. Sultan, B. Dogan, and S. Asikainen. 1999. Bacterial adhesion of Actinobacillus actinomycetemcomitans serotypes to titanium implants: SEM evaluation. A preliminary report. J. Periodontol. 70:1376-1382. [DOI] [PubMed] [Google Scholar]
- 46.Olofsson, A. C., M. Hermansson, and H. Elwing. 2003. N-Acetyl-l-cysteine affects growth, extracellular polysaccharide production, and bacterial biofilm formation on solid surfaces. Appl. Environ. Microbiol. 69:4814-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Omoike, A., and J. Chorover. 2004. Spectroscopic study of extracellular polymeric substances from Bacillus subtilis: aqueous chemistry and adsorption effects. Biomacromolecules 5:1219-1230. [DOI] [PubMed] [Google Scholar]
- 48.Ong, Y. L., A. Razatos, G. Georgiou, and M. M. Sharma. 1999. Adhesion forces between E. coli bacteria and biomaterial surfaces. Langmuir 15:2719-2725. [Google Scholar]
- 49.Ottewill, R. H., and J. N. Shaw. 1972. Electrophoretic studies on polystyrene lattices. J. Electroanal. Chem. 37:133. [Google Scholar]
- 50.Otto, J., J. Norbeck, T. Larsson, K. A. Karlsson, and M. Hermansson. 2001. Adhesion of type 1-fimbriated Escherichia coli to abiotic surfaces leads to altered composition of outer membrane proteins. J. Bacteriol. 183:2445-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otto, K., H. Elwing, and M. Hermansson. 1999. The role of type 1 fimbriae in adhesion of Escherichia coli to hydrophilic and hydrophobic surfaces. Colloids Surf. B 15:99-111. [Google Scholar]
- 52.Pembrey, R. S., K. C. Marshall, and R. P. Schneider. 1999. Cell surface analysis techniques: what do cell preparation protocols do to cell surface properties? Appl. Environ. Microbiol. 65:2877-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poortinga, A. T., R. Bos, and H. J. Busscher. 2001. Lack of effect of an externally applied electric field on bacterial adhesion to glass. Colloids Surf. B 20:189-194. [DOI] [PubMed] [Google Scholar]
- 54.Poortinga, A. T., R. Bos, W. Norde, and H. J. Busscher. 2002. Electric double layer interactions in bacterial adhesion to surfaces. Surf. Sci. Rep. 47:3-32. [Google Scholar]
- 55.Prigent-Combaret, C., G. Prensier, T. T. Le Thi, O. Vidal, P. Lejeune, and C. Dorel. 2000. Developmental pathway for biofilm formation in curli-producing Escherichia coli strains: role of flagella, curli and colanic acid. Environ. Microbiol. 2:450-464. [DOI] [PubMed] [Google Scholar]
- 56.Razatos, A., Y. L. Ong, M. M. Sharma, and G. Georgiou. 1998. Evaluating the interaction of bacteria with biomaterials using atomic force microscopy. J. Biomater. Sci.-Polym. 9:1361-1373. [DOI] [PubMed] [Google Scholar]
- 57.Razatos, A., Y. L. Ong, M. M. Sharma, and G. Georgiou. 1998. Molecular determinants of bacterial adhesion monitored by atomic force microscopy. Proc. Natl. Acad. Sci. USA 95:11059-11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Redman, J. A., S. L. Walker, and M. Elimelech. 2004. Bacterial adhesion and transport in porous media: role of the secondary energy minimum. Environ. Sci. Technol. 38:1777-1785. [DOI] [PubMed] [Google Scholar]
- 59.Rijnaarts, H. H. M., W. Norde, J. Lyklema, and A. J. B. Zehnder. 1995. The isoelectric point of bacteria as an indicator for the presence of cell-surface polymers that inhibit adhesion. Colloids Surf. B 4:191-197. [Google Scholar]
- 60.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., vol. 3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 61.Shim, Y., H. J. Lee, S. Lee, S. H. Moon, and J. Cho. 2002. Effects of natural organic matter and ionic species on membrane surface charge. Environ. Sci. Technol. 36:3864-3871. [DOI] [PubMed] [Google Scholar]
- 62.Song, L. F., P. R. Johnson, and M. Elimelech. 1994. Kinetics of colloid deposition onto heterogeneously charged surfaces in porous media. Environ. Sci. Technol. 28:1164-1171. [DOI] [PubMed] [Google Scholar]
- 63.Soto, G. E., and S. J. Hultgren. 1999. Bacterial adhesins: common themes and variations in architecture and assembly. J. Bacteriol. 181:1059-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stuurman, N., C. P. Bras, H. R. M. Schlaman, A. H. M. Wijfjes, G. Bloemberg, and H. P. Spaink. 2000. Use of green fluorescent protein color variants expressed on stable broad-host-range vectors to visualize rhizobia interacting with plants. Mol. Plant-Microbe Interact. 13:1163-1169. [DOI] [PubMed] [Google Scholar]
- 65.Tsuneda, S., H. Aikawa, H. Hayashi, A. Yuasa, and A. Hirata. 2003. Extracellular polymeric substances responsible for bacterial adhesion onto solid surface. FEMS Microbiol. Lett. 223:287-292. [DOI] [PubMed] [Google Scholar]
- 66.Velegol, S. B., and B. E. Logan. 2002. Contributions of bacterial surface polymers, electrostatics, and cell elasticity to the shape of AFM force curves. Langmuir 18:5256-5262. [Google Scholar]
- 67.Walker, S. L., J. Redman, and M. Elimelech. 2004. Role of cell surface lipopolysaccharides (LPS) in Escherichia coli K12 adhesion and transport. Langmuir 20:7736-7746. [DOI] [PubMed] [Google Scholar]
- 68.Yang, J. L., R. Bos, G. F. Belder, J. Engel, and H. J. Busscher. 1999. Deposition of oral bacteria and polystyrene particles to quartz and dental enamel in a parallel plate and stagnation point flow chamber. J. Colloid Interface Sci. 220:410-418. [DOI] [PubMed] [Google Scholar]
- 69.Zhang, X. Q., P. L. Bishop, and B. K. Kinkle. 1999. Comparison of extraction methods for quantifying extracellular polymers in biofilms. Water Sci. Technol. 39:211-218. [Google Scholar]