Abstract
Processes by which fecal bacteria enter overland flow and their transportation state to surface waters are poorly understood, making the effectiveness of measures designed to intercept this pathway, such as vegetated buffer strips, difficult to predict. Freshly made and aged (up to 30 days) cowpats were exposed to simulated rainfall, and samples of the cowpat material and runoff were collected. Escherichia coli in the runoff samples were separated into attached (to particles) and unattached fractions, and the unattached fraction was analyzed to determine if the cells were clumped. Within cowpats, E. coli grew for 6 to 14 days, rather than following a typical logarithmic die-off curve. E. coli numbers in the runoff correlated with numbers inside the cowpat. Most of the E. coli organisms eroded from the cowpats were transported as single cells, and only a small percentage (about 8%) attached to particles. The erosion of E. coli from cowpats and the state in which the cells were transported did not vary with time within a single rainfall event or over time as the cowpats aged and dried out. These findings indicate that cowpats can remain a significant source of E. coli in overland flow for more than 30 days. As well, most of the E. coli organisms eroded from cowpats will occur as readily transportable single cells.
Vegetated buffer strips (VBS) have been promoted as a way to reduce runoff pollution from agricultural land (19), but their effectiveness in removing bacteria has varied from study to study (9, 16). This variation is believed to be due to the different soils, slopes, and flow rates used across experiments (1) and to differences in the degrees of bacterial attachment to particles (31). To develop effective strategies for controlling runoff and to monitor the performance of such systems, an improved understanding of how bacteria enter overland flow and are transported within it is required (15, 35). For example, one study (20) reported that in a VBS, only soil particles of >63 μm in diameter settle out and that grass filters out only large clumps of cells (>500 μm in diameter), implying that individual bacterial cells will be transported through a VBS. Studies of the attached and unattached fractions of bacteria in urban storm water support this possibility. These studies have found that although the bacterial cells attached to particles had enhanced settling rates, these attached cells make up only a small percentage of the total population, and therefore, settling had only a minimal effect on reducing bacterial numbers in storm water (7, 11, 32).
Environmental agricultural research in the Northern Hemisphere has focused on the impacts of spreading manures or slurries of fecal material from animal housing facilities onto land at high loading rates (30). In New Zealand and other Southern Hemisphere countries, farm animals are generally grazed on pasture all year round, and the fecal material is deposited directly on the land from the animals. The erosion of bacteria from individual droppings, such as cowpats, is therefore a controlling step in the subsequent contamination of waterways during rainfall events. Published studies of the impact of erosion of fecal microorganisms from individual cowpats have not investigated the effect of bacterial attachment to particles or of cell clumping (12, 24, 34).
The present study developed a method to separate Escherichia coli organisms in runoff samples into attached (to dense particles) and unattached fractions and determined the degree of clumping in the unattached fraction. This new method was then used to investigate the state of E. coli cells eroded in runoff directly from fresh and aged cowpats.
MATERIALS AND METHODS
Development of a method to separate attached and unattached cells.
Two local silt loam soils were used in this study: Pukemutu soil (New Zealand classification, argillic mottled fragic pallic soil) and Waikiwi soil (New Zealand classification, typic firm brown soil) (22). These two soils are representative of the two predominant soil types used for agriculture in the Southland and Otago regions of New Zealand. Where required, the soils were sterilized by autoclaving approximately 500-g lots at 121°C for 15 min. Soil slurries were prepared by diluting the soil (1 g [wet weight]) with sterile water (100 ml) and blending the slurry at low speed for 1 min (model no. KB290; Kambrook, Oakleigh, Victoria, Australia). The E. coli isolates used in this study have been previously described (28). Isolates were stored in cryovials (MicroBank; Pro-Lab Diagnostics, Richmond Hill, Ontario, Canada), resuscitated on Trypticase soy agar (Difco), and grown in Trypticase soy broth (Difco) for 16 to 18 h at 37°C before use. Cell numbers were measured by the drop plate technique (6) on Trypticase soy agar (Difco). Nycodenz solution was made by mixing 8 g of Nycodenz powder (also called Histodenz [Sigma catalog no. D2158]) with 10 ml of water and heating the mixture to dissolve the powder. The Nycodenz solution was stored at 4°C for up to a month before use.
The buoyant-density separation procedure, adapted from reference 5, involved placing 10 ml of soil and/or bacterial suspension into a 15-ml centrifuge tube with a V-shaped tip (Falcon; Becton Dickinson, Franklin Lakes, NJ) and injecting 1 ml of the Nycodenz solution below the suspension. Suspensions were centrifuged for 20 min at 3,000 × g and 5°C in a swing-out rotor centrifuge, after which the supernatant and Nycodenz layer were drawn off with a pipette and the pellet was resuspended into sterile water.
To determine the percentage of the mineral fraction of the soil that migrated into the pellet during centrifugation, 10 ml of a soil slurry was separated on Nycodenz and both the supernatant (without any contamination from the Nycodenz layer) and the initial slurry were analyzed for total suspended solids (TSS) and volatile suspended solids (VSS) (3).
To determine if unattached bacteria migrate through the Nycodenz layer, five 10-ml aliquots of overnight E. coli broths (about 1010 cells) were centrifuged with Nycodenz and cells in the supernatant and pellet were enumerated by the drop plate technique.
To determine if attached bacteria migrate to the pellet with soil particles and to determine the precision of the Nycodenz separation technique, 0.1 ml of an overnight broth of E. coli was added to 0.5 g of sterile soil in a 15-ml centrifuge tube and left for 20 to 30 min. Ten milliliters of sterile water was added to the tubes, and then the attached and unattached fractions were separated on Nycodenz and enumerated by the drop plate technique. This was repeated to create 62 duplicate results by using the full range of combinations of the five E. coli isolates previously described in reference 28 and the Waikiwi and Pukemutu soils.
To determine if Nycodenz inhibited the recovery of E. coli in selective media, seven different samples (two cowpats, one tap water sample contaminated with fecal material, and two samples each of Pukemutu and Waikiwi soils contaminated with fecal material) were enumerated in the presence and absence of 10% Nycodenz using both the Colilert technique and a miniaturized most probable number (MPN) technique (28). The counts with and without the addition of Nycodenz were compared by regressing their differences against their means (2).
Determination of the proportions of unattached cells that are clumped.
The degree of clumping of bacterial cells in the unattached fraction was determined by enumerating before and after disrupting the clumps by vortexing a 10-ml subsample in the presence of 0.1 g of microbeads (Sigma glass beads, 150 to 212 μm in diameter) for 90 seconds (8).
Rainfall simulator design.
The rainfall simulator used one TeeJet 1/4HH-SS30WSQ nozzle (Spraying Systems Co., Wheaton, IL) sited approximately 250 cm above the soil surface to gain terminal velocity (25). Simulated rainfall had a drop size, velocity, and impact energy approximating those of natural rainfall (33). The target rainfall rate in all experiments was 25 mm h−1, equivalent to an 8-year event in the South Otago region.
Cowpat runoff experiments.
Artificial cowpats were made from either dairy cow fecal material only (experiments 1, 2, and 3) or a 50:50 fecal-material-soil mix (experiment 2). Fecal material was collected from the concrete base of a dairy shed holding pen. The feces (or feces-soil mixture) were then mixed until it appeared homogeneous before 1 kg (wet weight) was placed into a metal tray (250 mm long and 200 mm wide with sides that were 50 mm high) to form the artificial cowpat. The tray had an approximately 10% slope, which allowed runoff to be collected from one of its corners.
Three experiments were conducted. For all three, simulated rainfall was applied on days 0, 2, 6, 14, and 30, and E. coli numbers, in all samples, were determined by the Colilert method (28). To separate attached and unattached cells, 20 ml of sample and 2 ml of Nycodenz were placed in 30-ml tubes with V-shaped tips and centrifuged at 3,000 × g for 20 min. Additional trays of replicate artificial cowpats were destructively sampled each day to determine the total solids content (3) and E. coli numbers.
In experiment 1, runoff from duplicate trays of artificial cowpats, stored outside exposed to the weather, was collected as a series of three composite samples after 0 to 10 min, 10 to 20 min, and 20 to 30 min of simulated rain. Each runoff sample was analyzed for attached and unattached E. coli cells using the Nycodenz separation. The degree of cell clumping was determined in this experiment only.
In experiment 2, cowpat samples consisted of either fecal material alone or 0.5 kg (wet weight) of fecal material mixed with either 0.5 kg (wet weight) of Pukemutu or Waikiwi soils. In New Zealand farming systems, animals are often fed on brassica crops, resulting in exposed soil that is heavily trampled by hoofs. The 50:50 mixtures of feces and soil were used to simulate feces being trampled into the exposed soil. The soils were sieved (filter pore size, <5 mm) before being mixed with the fecal material. Trays were stored outside exposed to the weather. Duplicate trays of the cowpats and the cowpats mixed with soil were used. During the simulated rain, a single composite of the first 20 min of runoff was collected. Runoff samples were analyzed for attached and unattached E. coli cells by Nycodenz separation.
In experiment 3, artificial cowpats were stored in a glasshouse, protected from the weather. Runoff from the cowpats was collected as a single composite sample over the first 30 min of simulated rainfall. Triplicate samples on each day were analyzed for attached and unattached E. coli cells by Nycodenz separation.
RESULTS
Development of a method to separate attached and unattached cells.
Centrifugation caused all of the soil particles from a soil slurry to form a pellet below the Nycodenz cushion. The Nycodenz solution had a TSS component itself, which meant that the pellet of soil particles could not be analyzed directly. However, analysis of the supernatant fraction showed that centrifugation removed 98% of the total suspended solids (TSS) and 100% of the mineral fraction (TSS-VSS) from the initial slurry (data not shown).
E. coli cells from a broth culture (mean ± standard error, 1.50 × 1010 ± 0.05 × 1010 cells) centrifuged using a Nycodenz cushion formed a visible layer above the Nycodenz layer, with no pellet observed below the Nycodenz layer. The Nycodenz layer and the supernatant above it contained 1.52 × 1010 ± 0.07 × 1010 cells. The number of cells recovered from the centrifuge tube (presumably lining the walls of the tube) after extraction of the supernatant and Nycodenz layer was 4.4 × 108 ± 0.5 × 108 cells, which, while being equal to 2.9% of the total number recovered, is less than the standard error of the count from the supernatant fraction. This result indicates that 3% attachment would be the lower detection limit for this separation method.
Initial studies indicated that the percentage of E. coli cells that attached to the soil particles varied between different isolates, from a low of 17% (laboratory isolate) to a high of 98% (recent isolate from cow feces) attached (data not shown). The percentages of attached cells from 62 duplicate analyses were logit transformed {ln [P/(1 − P])}, and the duplicate standard deviation was calculated as 0.34 on this scale. This standard deviation corresponds to a 95% confidence interval for an individual sample, ranging from 34 to 66% for a sample with a mean of 50% attached cells to 5 to 18% for a sample with a mean of 10% attached cells.
The addition of Nycodenz did not significantly (P > 0.05) affect the recovery of E. coli by the Colilert and miniaturized MPN techniques. The mean and standard deviation of the log difference between the samples, analyzed with and without the addition of 10% Nycodenz, was −0.04 ± 0.14 and 0.34 ± 0.34 for the Colilert and miniaturized MPN medium techniques, respectively.
Cowpat runoff experiments.
The percentage of attached E. coli cells in the runoff from aged (2 to 30 days old) artificial cowpats ranged from 2 to 26%, with an overall average of about 8% (Table 1). In experiment 2, percentages of attachment were not significantly different (P > 0.05) for cowpats mixed with soil and cowpats containing only fecal material. The attachment data for fresh cowpats are not presented, as organic material in the runoff from fresh cowpats formed a dense layer (about 8 mm thick) on top of the Nycodenz layer that was difficult to extract with a pipette. In samples from aged cowpats, this organic layer was <2 mm thick and was easily extracted. Subsequent development of the Nycodenz separation technique showed that samples with high organic matter content could be successfully separated if the samples were diluted (1:10) prior to the Nycodenz centrifugation step (data not shown).
TABLE 1.
Range of the percentages of E. coli organisms attached to particles in runoff for each day and overall means and standard deviations for each experimenta
| Day | % of E. coli organisms attached to particles
|
||||
|---|---|---|---|---|---|
| Expt 1 (feces) | Expt 2 (feces) | Expt 2 (feces- Pukemutu soil) | Expt 2 (feces- Waikiwi soil) | Expt 3 (feces) | |
| 2 | 5-11 | 10-12 | 6-7 | 7-11 | 9-11 |
| 6 | 2-7 | 6-9 | 10-13 | 5-7 | 2-9 |
| 14 | 8-26 | 8-13 | 9-14 | 8-9 | 2-2 |
| 30 | 3-9 | 5-19 | 8-9 | 13-16 | 2-6 |
| Mean | 7.9 | 10.0 | 9.4 | 9.6 | 5.1 |
| SD | 5.6 | 4.4 | 3.7 | 2.9 | 3.5 |
The ranges are based on six analyses in experiment 1, duplicates in experiment 2, and triplicates in experiment 3. Day 0 data are omitted; see the text for an explanation.
The E. coli cells in the runoff from the cowpats were not being transported in clumps. Vortexing the unattached fraction of the runoff samples in the presence of microbeads did not significantly (P > 0.05) increase the number of E. coli organisms in the runoff samples in experiment 1. Vortexing actually decreased the number recovered in some samples, and the overall average increased by only 6% after vortexing. Microscopic examination of some samples confirmed that there were few large clumps in the samples either before or after vortexing.
In experiment 1, the average total number of E. coli cells in runoff from artificial cowpats did not vary with time within a single rainfall event (data not shown). Analysis of variance of the concentrations of E. coli organisms in the runoff indicated that there was a significant difference between days (P < 0.05) but not between the three time intervals of collection (P > 0.05).
The fresh fecal material used in the cowpats contained about 11% total solids, and the total solid content in all of the cowpats increased over time to approximately 50% in experiments 1 and 2. The cowpats in experiment 3, however, increase to a high of 91% total solid content on day 30, as they had been held in a glasshouse protected from natural rainfall. The cowpats made by mixing soil with fecal material in experiment 2 had a higher initial total solid content of approximately 40% due to the addition of the soil material, and by day 30, this value had increased to approximately 70%.
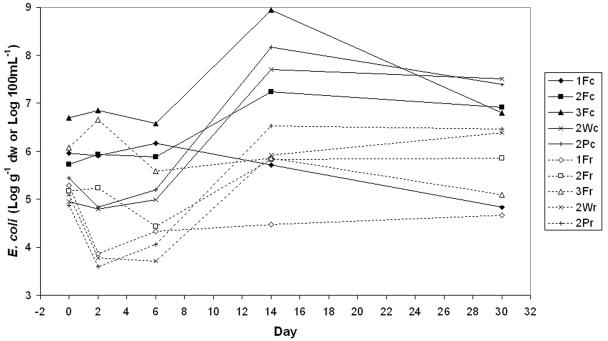
Figure 1 summarizes the microbial data for cowpats and runoff over time for the three runoff experiments. The E. coli concentrations in the fresh cowpats on day 0 varied from 105 to 107 g−1 dry weight. On subsequent days, microbial numbers did not follow typical “log-linear” die-off curves. Instead, E. coli concentrations in the cowpats increased to a maximum on day 6 or 14, and in most series the concentration on day 30 was higher than the initial concentration (Fig. 1). In experiment 2, the E. coli concentrations (g−1 dry weight) in the fecal material mixed with soil (Waikiwi soil and cowpat feces and Pukemutu soil and cowpat feces) were initially lower than those for the all-feces cowpats due to a dilution effect from the soil, but by day 14, E. coli numbers had increased to be greater than the number in the all-feces cowpats. The E. coli concentration in the fecal material used in experiment 3 was considerably higher than for the other experiments and subsequently remained higher until day 30 (Fig. 1).
FIG. 1.
E. coli concentrations in cowpats (g−1 dry weight [dw]) and the mean total E. coli concentration (attached plus unattached cells) in the runoff (100 ml−1) from cowpats measured with a series of aged cowpats. In the key, the number is the experiment number, the first letter denotes the sample source material (F is for feces, W is for the mixture of feces and Waikiwi soil, and P is for the mixture of feces and Pukemutu soil), and the second letter denotes the sample type (c is for cowpat and r is for runoff).
The average daily temperature, rainfall, and solar radiation data for experiments 1 and 2 are shown in Table 2 (note that experiment 3 samples were stored in a glasshouse protected from natural rainfall). The changes in E. coli concentration in the cowpats did not appear to be related to any weather variable or to any weather change that occurred in the 24 h prior to sampling.
TABLE 2.
Averaged weather data for the time period between simulated rainfall events on the cowpats exposed to the elements in experiments 1 and 2
| Expt | Time period (days) | Mean air temp (°C) | Mean daily rainfall (mm) | Mean daily solar radiation (MJ m−2) |
|---|---|---|---|---|
| 1 | 0-2 | 12.5 | 0 | 15.0 |
| 2-6 | 6.7 | 4.2 | 11.4 | |
| 6-14 | 6.6 | 3.2 | 12.7 | |
| 14-30 | 11.0 | 2.0 | 16.1 | |
| 2 | 0-2 | 6.8 | 8.2 | 8.4 |
| 2-6 | 5.5 | 2.3 | 11.8 | |
| 6-14 | 8.1 | 2.4 | 15.3 | |
| 14-30 | 10.9 | 1.7 | 16.6 |
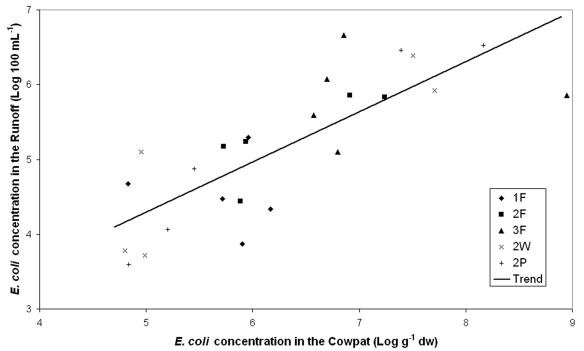
The MPN E. coli numbers in runoff direct from the cowpats varied from 103 to 106 100 ml−1 (Fig. 1). The changes in concentration in the runoff over time tended to follow the changes in concentration in the cowpat material (Fig. 1). This pattern can be observed in a scatter plot of the mean concentrations in the runoff against the mean concentrations in the cowpat for the different sample types on different days (Fig. 2). Relationships between the mean number of E. coli cells in the cowpats and the mean number of E. coli cells in the runoff were examined by regression models where different slopes and intercepts for each experiment and/or sample type were compared. However, the different slopes and intercepts were not significantly different (P > 0.05). The strongest relationship incorporating all the data was as follows: runoff (log 100 ml−1) = [0.672 × cowpat (log g−1 dry weight)] + 0.934. The relationship was statistically significant (P < 0.001).
FIG. 2.
Relationship between the E. coli concentration measured in cowpats and the mean E. coli concentration measured in the runoff. Also shown is the overall trend line, which was statistically significant (P < 0.001). dw, dry weight. See the legend of Fig. 1 for an explanation of the key.
DISCUSSION
At present our understanding of the movement of bacteria in overland flow is limited due to uncertainty over how the bacteria are transported and the relative importance of each potential mechanism (18). Bacterial cells can be transported as individual cells, cells attached to soil particles, or cells in clumps (35). In this study we developed an effect method to separate E. coli cells into fractions of either unattached cells, cells attached to soil, or cells in clumps and investigated the assumption that the cells would be transported at different rates (20).
The percentages of E. coli cells attached to particles were varied but tended to be low, with an overall mean of only 8% attached. The variation is probably a reflection of the small number of dense particles in feces and the wide confidence interval of the Nycodenz separation technique. Despite the inherent variation in the Nycodenz technique, the accumulated data strongly suggest that the level of attachment is low (<25%). These findings are similar to those published from studies of the attachment of cells to particles in storm water (7, 11, 32).
It was found that the majority of the E. coli cells in the unattached fraction from runoff from the cowpats were individual cells, not clumps. This result surprised us, as microscopic examination of samples from environments as diverse as the large intestine (26), leaf surfaces (27), soil (21), and freshwater and marine systems (14, 23, 29) indicate that bacteria readily form large clumps. The lack of clumping observed in samples eroded from cowpats may be due to the energy impact of the raindrops being high enough to break up the clumps prior to transport.
Bacteria in the surface crusts of cowpats are exposed to drying and UV radiation (10), so it is logical to expect greater bacterial die-off initially on the surface of the cowpat than on the inside. Since rainfall will predominantly affect the surface of the cowpat, it was anticipated that the concentration of E. coli cells in the runoff would markedly decrease over time and that it would be independent of the concentration of E. coli cells in the bulk of the cowpat. Our results did not support this hypothesis or the assumption that the bacterial die-off curves will follow a log-linear curve, as reported for other studies of the survival of fecal bacteria in the environment (4, 10, 24, 34). This study suggests that using log-liner models to predict bacterial die-off may not always be appropriate. In practical terms, this means that old cowpats from the previous grazing round (30 days earlier) may contribute a number of E. coli cells to runoff from these pastures equivalent to that contributed by fresh cowpats.
This work suggests that E. coli cells in cowpats may not only survive for longer than currently anticipated but also may, during rainfall events, become deposited onto soil systems as highly mobile unattached cells. These findings may explain the occurrence of high “background” concentrations of fecal bacteria in runoff from agricultural land that has not been recently impacted with feces (13, 15, 17).
Acknowledgments
This work was funded in part by the New Zealand Government through the Foundation for Research, Science and Technology (grant number C10X0320). Richard Muirhead received a Ph.D. scholarship from the University of Otago.
Thanks go to Roger Littlejohn of AgResearch for the statistical analysis.
REFERENCES
- 1.Abu-Zreig, M., R. P. Rudra, and H. R. Whiteley. 2001. Validation of a vegetated filter strip model (VFSMOD). Hydrol. Process. 15:729-742. [Google Scholar]
- 2.Altman, D. G., and J. M. Bland. 1983. Measurement in medicine: the analysis of method comparison studies. Statistician 32:307-317. [Google Scholar]
- 3.Anonymous. 1992. Physical and aggregate properties (part 2000), p. 2-54-2-60. In A. E. Greenburg, L. S. Clesceri, and A. D. Eaton (ed.), Standard methods for the examination of water and wastewater, 18th ed. American Public Health Association, Washington, D.C.
- 4.Avery, S. M., A. Moore, and M. L. Hutchison. 2004. Fate of Escherichia coli originating from livestock faeces deposited directly onto pasture. Lett. Appl. Microbiol. 38:355-359. [DOI] [PubMed] [Google Scholar]
- 5.Bakken, L. R., and V. Lindahl. 1995. Recovery of bacterial cells from soil, p. 9-27. In J. T. Trevors and J. D. van Elsas (ed.), Nucleic acids in the environment: methods and applications. Springer-Verlag, Berlin, Germany.
- 6.Barboas, H. R., M. F. A. Rodrigues, C. C. Campos, M. E. Chaves, I. Nunes, Y. Juliano, and N. F. Novo. 1995. Counting of viable cluster-forming and non cluster-forming bacteria: a comparison between the drop and spread plate methods. J. Microbiol. Methods 22:39-50. [Google Scholar]
- 7.Borst, M., and A. Selvakumar. 2003. Particle-associated microorganisms in stormwater runoff. Water Res. 37:215-223. [DOI] [PubMed] [Google Scholar]
- 8.Bremer, P. J., I. Monk, and C. M. Osborne. 2001. Survival of Listeria monocytogenes attached to stainless steel surfaces in the presence or absence of Flavobacterium spp. J. Food Prot. 64:1369-1376. [DOI] [PubMed] [Google Scholar]
- 9.Crane, S. R., J. A. Moore, M. E. Grismer, and J. R. Miner. 1983. Bacterial pollution from agricultural sources: a review. Trans. Am. Soc. Agric. Eng. 26:858-866. [Google Scholar]
- 10.Crane, S. R., and J. A. Moore. 1986. Modeling enteric bacterial die-off: a review. Water Air Soil Pollut. 27:411-439. [Google Scholar]
- 11.Davies, C. M., and H. J. Bavor. 2000. The fate of stormwater-associated bacteria in constructed wetland and water pollution control pond systems. J. Appl. Microbiol. 89:349-360. [DOI] [PubMed] [Google Scholar]
- 12.Davies, C. M., C. M. Ferguson, C. Kaucner, M. Krogh, N. Altavilla, D. A. Deere, and N. J. Ashbolt. 2004. Dispersion and transport of Cryptosporidium oocysts from fecal pats under simulated rainfall events. Appl. Environ. Microbiol. 70:1151-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doran, J. W., and D. M. Lin. 1979. Bacteriological quality of runoff water from pastureland. Appl. Environ. Microbiol. 37:985-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Droppo, I. G., and E. D. Ongley. 1994. Flocculation of suspended sediment in rivers of southeastern Canada. Water Res. 28:1799-1809. [Google Scholar]
- 15.Edwards, D. R., B. T. Larson, and T. T. Lin. 2000. Runoff nutrient and fecal coliform content from cattle manure application to fescue plots. J. Am. Water Resource Assoc. 36:711-721. [Google Scholar]
- 16.Entry, J. A., R. K. Hubbard, J. E. Thies, and J. J. Fuhrman. 2000. The influence of vegetation in riparian filterstrips on coliform bacteria: 1. Movement and survival in water. J. Environ. Qual. 29:1206-1214. [Google Scholar]
- 17.Fajardo, J. J., J. W. Bauder, and S. D. Cash. 2001. Managing nitrate and bacteria in runoff from livestock confinement areas with vegetative filter strips. J. Soil Water Conserv. 56:185-191. [Google Scholar]
- 18.Ferguson, C., A. M. de Roda Husman, N. Altavilla, D. Deere, and N. Ashbolt. 2003. Fate and transport of surface water pathogens in watersheds. Crit. Rev. Environ. Sci. Technol. 33:299-361. [Google Scholar]
- 19.Ferguson, C., N. Altavilla, N. J. Ashbolt, and D. A. Deere. 2003. Prioritizing watershed pathogen research. J. Am. Water Works Assoc. 95:92-102. [Google Scholar]
- 20.Fiener, P., and K. Auerswald. 2003. Effectiveness of grassed waterways in reducing runoff and sediment delivery from agricultural watersheds. J. Environ. Qual. 32:927-936. [DOI] [PubMed] [Google Scholar]
- 21.Guy, E. M., B. K. G. Theng, and G. D. Walker. 1980. Interactions of Escherichia coli and Streptococcus bovis with soil clay surfaces as revealed by scanning electron microscopy. Z. Pflanzenernaehr. Bodenkd. 143:194-199. [Google Scholar]
- 22.Hewitt, A. E. 1998. New Zealand soil classification. Landcare Research Science Series no. 1, 2nd ed. Manaaki Whenua Press, Lincoln, New Zealand.
- 23.Kiørboe, T., H.-P. Grossart, H. Ploug, and K. Tang. 2002. Mechanisms and rates of bacterial colonization of sinking aggregates. Appl. Environ. Microbiol. 68:3996-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kress, M., and G. F. Gifford. 1984. Fecal coliform release from cattle fecal deposits. Water Resource Bull. 20:61-66. [Google Scholar]
- 25.McDowell, R. W., and A. N. Sharpley. 2002. The effect of antecedent moisture conditions on sediment and phosphorus loss during overland flow: Mahantango Creek catchment, Pennsylvania, USA. Hydrol. Process. 16:3037-3050. [Google Scholar]
- 26.McFarlane, S., J. H. Cumming, and J. T. McFarlane. 1999. Bacterial colonisation of surfaces in the large intestine, p. 71-87. In G. R. Gibson and M. B. Roberfroid (ed.), Colonic microbiota, nutrition and health. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 27.Monier, J.-M., and S. E. Lindow. 2004. Frequency, size, and localization of bacterial aggregates on bean leaf surfaces. Appl. Environ. Microbiol. 70:346-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muirhead, R. W., R. P. Littlejohn, and P. J. Bremer. 2004. Evaluation of the effectiveness of a commercially available defined substrate medium and enumeration system for measuring Escherichia coli numbers in faeces and soil samples. Lett. Appl. Microbiol 39:383-387. [DOI] [PubMed] [Google Scholar]
- 29.Paerl, H. W. 1975. Microbial attachment to particles in marine and freshwater systems. Microb. Ecol. 2:73-83. [DOI] [PubMed] [Google Scholar]
- 30.Quinton, J. N., S. F. Tyrrel, and M. C. Ramos. 2003. The effect of incorporating slurries on the transport of faecal coliforms in overland flow. Soil Use Manag. 9:185-186. [Google Scholar]
- 31.Reddy, K. R., R. Khaleel, and M. R. Overcash. 1981. Behavior and transport of microbial pathogens and indicator organisms in soils treated with organic wastes. J. Environ. Qual. 10:255-265. [Google Scholar]
- 32.Schillinger, J. E., and J. J. Gannon. 1985. Bacterial adsorption and suspended particles in urban storm water. J. Water Pollut. Control Fed. 57:384-389. [Google Scholar]
- 33.Shelton, C. H., R. D. von Bernuth, and S. P. Rajbhandari. 1985. A continuous-application rainfall simulator. Trans. Am. Soc. Agric. Eng. 28:1115-1119. [Google Scholar]
- 34.Thelin, R., and G. J. Gifford. 1983. Fecal coliform release patterns from fecal material of cattle. J. Environ. Qual. 12:57-63. [Google Scholar]
- 35.Tyrrel, S. F., and J. N. Quinton. 2003. Overland flow transport of pathogens from agricultural land receiving faecal wastes. J. Appl. Microbiol. 94:87s-93s. [DOI] [PubMed] [Google Scholar]