Abstract
Nuclear protein of the testis carcinoma (NUTC) is a rare and aggressive malignancy. We herein report a case of NUTC in the lung characterized by a bronchial lesion and elevated alpha-fetoprotein levels. A 35-year-old Japanese man presented to our institution with suspected advanced lung cancer based on a histological examination. Subsequently, next-generation sequencing (NGS) yielded a positive BRD4-NUTM1 fusion. In addition, positive NUT immunostaining of the lung biopsy specimen confirmed NUTC in the lungs. Systemic chemotherapy and radiotherapy showed a temporary response, with decreased serum alpha-fetoprotein levels. We highlight this case of a prompt diagnosis by NGS of NUTC in a young individual with a rapidly progressing tumor.
Keywords: NUT carcinoma, BRD4-NUTM1, lung cancer, alpha-fetoprotein (AFP), immune checkpoint inhibitor
Introduction
Nuclear protein of the testis (NUT) carcinoma is a rare form of cancer characterized by chromosomal rearrangements involving the NUTM1 gene, also known as the NUT gene, located on chromosome 15q14. In approximately 70-80% of cases, NUTM1 undergoes translocation with the BRD4 gene (19p13.12), resulting in the formation of a BRD4-NUTM1 fusion oncogene. The first reported cases of mediastinal carcinoma associated with t(15;19) translocation, suggestive of NUT carcinoma, date back to 1991 (1,2). NUT carcinoma is an undifferentiated malignancy that exhibits aggressive progression and is particularly prevalent in young individuals. Typically, it arises in the head and neck region and mediastinal midline and is collectively referred to as NUT midline carcinoma (NMC).
Currently, there is no established treatment for NUT carcinoma, and the prognosis is exceedingly poor, with a median overall survival of 6.7 months (3). However, advancements in clinical practice have enabled the comprehensive analysis of multiple genes simultaneously, facilitating the early identification of rare driver genes and the prompt diagnosis of rare diseases. Confirmation of the BRD4-NUTM1 fusion gene and NUT immunostaining play a crucial role in the definitive diagnosis of NUT carcinoma. In addition, reports have suggested the potential utility of serum alpha-fetoprotein (AFP) as a diagnostic marker for NUT carcinoma and an indicator of disease progression (4). However, despite the resistance of NUT carcinoma to systemic chemotherapy, reports on the use of immune checkpoint inhibitors as a potential treatment approach are limited.
We herein report a case of NUT carcinoma with high AFP levels that was rapidly diagnosed and treated using genomic testing.
Case Report
A 35-year-old previously healthy Japanese man presented to a local physician with intermittent left chest pain persisting for 2 weeks. Computed tomography (CT) revealed a tumor in the left lower lobe, raising the suspicion of lung cancer. Consequently, the patient was referred to our hospital for the further evaluation of the suspected lung cancer. His Eastern Cooperative Oncology Group Performance Status score was 1, and he reported no fever, cough, or bloody sputum. Although he had no medical history and was not taking any regular medications, he had a history of smoking approximately 20 cigarettes per day for 15 years until the onset of symptoms. Blood tests revealed mild leukocytosis and thrombocytosis, indicating mild inflammation. In addition, his serum neuron-specific enolase (NSE) level was elevated, along with a markedly increased serum AFP level (Table).
Table.
Laboratory Data Findings on Admission.
| <Hematology> | <Tumor marker> | ||||||||
| WBC | 10,010 | /µL | CEA | 2.3 | ng/mL | ||||
| Neu | 69.0 | % | CYFRA | 6.4 | ng/mL | ||||
| Mon | 6.1 | % | ProGRP | 38.4 | pg/mL | ||||
| Lym | 21.3 | % | NSE | 35.4 | ng/mL | ||||
| Eos | 3.0 | % | PSA | 0.635 | ng/mL | ||||
| Bas | 0.6 | % | AFP | 3,335 | ng/mL | ||||
| RBC | 4.26 | ×106/µL | |||||||
| Hgb | 13.8 | g/dL | |||||||
| MCV | 99 | fL | |||||||
| Plt | 485 | ×103/µL | |||||||
| <Biochemistry> | |||||||||
| TP | 7.1 | g/dL | |||||||
| Alb | 3.5 | g/dL | |||||||
| T-Bil | 0.37 | mg/dL | |||||||
| AST | 14 | IU/L | |||||||
| ALT | 8 | IU/L | |||||||
| LDH | 415 | IU/L | |||||||
| ALP | 45 | IU/L | |||||||
| γ-GTP | 21 | IU/L | |||||||
| Cre | 0.77 | mg/dL | |||||||
| BUN | 14.6 | mg/dL | |||||||
| eGFR | 93.1 | mL/min/1.73 m2 | |||||||
| UA | 4.8 | mg/dL | |||||||
| Na | 141 | mEq/L | |||||||
| K | 4.3 | mEq/L | |||||||
| Cl | 108 | mEq/L | |||||||
| Ca | 9.1 | mg/dL | |||||||
| CRP | 0.64 | mg/dL | |||||||
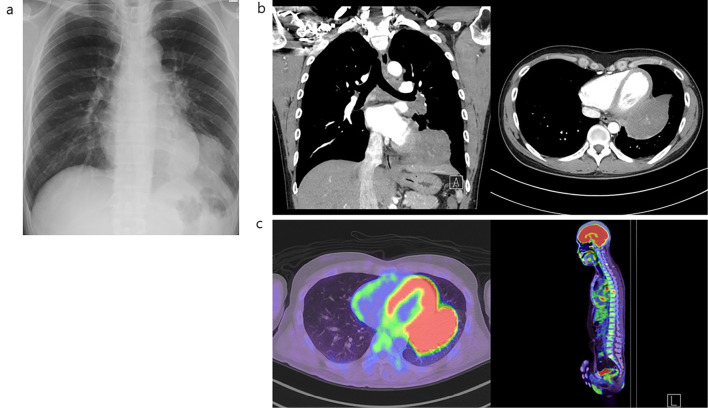
Chest radiography demonstrated decreased permeability in the left lower lung field, whereas computed tomography (CT) revealed a large pulmonary mass of approximately 10 cm in the left lower lobe, partially abutting the pericardium (Fig. 1a, b). A further evaluation with fluorodeoxyglucose-positron emission tomography/CT indicated the presence of a mass in the left lower lobe and involvement of the left hilar region, mediastinal lymph nodes, and multiple bone metastases (Fig. 1c). A bronchoscopic biopsy performed to establish a histological diagnosis revealed mucosal irregularities in a segment of the left lower lobe bronchus. Tumor lesions were biopsied using guide-sheath endobronchial ultrasound tomography (Fig. 2).
Figure 1.
Clinical images before treatment initiation. (a) Chest X-ray, (b) contrast-enhanced CT, and (c) 18F-FDG-PET/CT. CT: computed tomography, 18F-FDG-PET: fluorodeoxyglucose-positron emission tomography
Figure 2.

Bronchoscopy findings at the time of the diagnosis. The left inferior lobe branch showed some mucosal irregularities, and biopsy results of the same area show neoplastic changes, which were determined to be disseminated lesions.
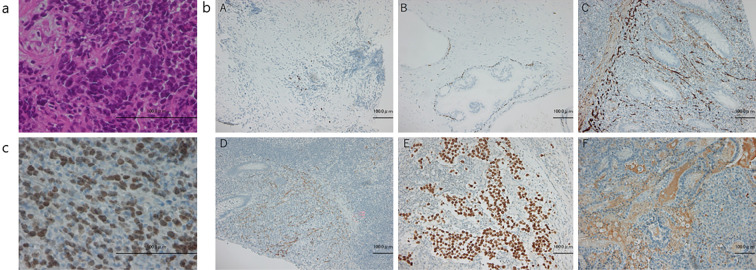
Initially we diagnosed him with non-small-cell lung cancer (NSCLC) by Hematoxylin and Eosin (H&E) staining of a transbronchial tumor biopsy specimen. As he was a relatively young patient, we expected to find driver gene mutations. Our facility participated in Lung Cancer Genomic Screening Project for Individualized Medicine in Asia (LC-SCRUM-Asia). Therefore, we immediately enrolled him in LC-SCRUM-Asia. LC-SCRUM-Asia was launched in 2013 as a large-scale, prospective, multicenter lung cancer genomic screening program to provide and develop optimal treatments for lung cancer patients with targeted gene mutations (5). An immunohistological examination performed a few days later was negative for TTF-1 and p40 and positive for AFP, CD56, synaptophysin, and Sall-4 (Fig. 3). Thus, the possibility of small-cell lung cancer was considered. Because of the rapid progression of the disease, chemotherapy was initiated immediately for advanced small-cell lung cancer. Approximately two weeks after LC-SCRUM-Asia registration, the BRD4-NUTM1 fusion gene was detected, and additional immunostaining for NUT was performed, confirming the diagnosis of NUT carcinoma.
Figure 3.
Histological images of the transbronchial biopsy specimens. (a) Hematoxylin and Eosin staining: testicular nuclear protein (NUT) carcinoma is a monomorphic, poorly differentiated form with varying degrees of squamous differentiation. It also has a histology similar to that of other undifferentiated neoplasms, such as germ cell tumors, Ewing sarcomas, and lymphomas. Bronchoscopic histology of our patient revealed a tumor with small-cell proliferation. (b) (A) TTF-1 stain; (B) p40 stain; (C) CD56 stain; (D) Synaptophysin stain; (E) SALL-4 stain; and (F) AFP stain. The results were negative for TTF-1 and p40 but positive for AFP, CD56, Synaptophysin, and SALL-4, and the histology was deemed unlikely to indicate a germ cell tumor; therefore, small-cell lung cancer was initially suspected, although the features were atypical. (c) Nuclear immunoreactivity to NUT was positive. AFP: alpha-fetoprotein
Since no established treatment standard exists for NUT carcinoma, we opted to follow a treatment approach similar to that for lung cancer with multiple distant metastases based on the histological findings. Systemic chemotherapy for advanced small-cell lung cancer, including cisplatin, etoposide, and durvalumab, was initiated. On day 7 of treatment, chest radiography revealed significant improvement in the previously observed area of decreased permeability of the left lower lung field, indicating a notable antitumor effect. Prior to initiating the initial therapy, the patient's serum AFP level had risen to 5,594 ng/mL, but as the tumor decreased in size, it declined to 2,401 ng/mL.
However, the serum AFP level increased again to 3,149 ng/mL before the start of the fourth course of systemic chemotherapy, with the tumor in the left lower lobe showing a growing trend. Three weeks after the fourth course of systemic chemotherapy, the serum AFP level had increased to 4,135 ng/mL, accompanied by further growth of the left lower lobe tumor and the appearance of new left pleural lesions (Fig. 4).
Figure 4.
Clinical course and serum AFP levels after treatment initiation. AFP: alpha-fetoprotein
Considering the efficacy of radiotherapy based on previous reports, we opted for a treatment regimen that could be combined with radiotherapy. The patient was started on systemic chemotherapy with cisplatin and docetaxel, and radiation therapy was initiated in the left lower lobe and hilar-mediastinal lymph nodes. The treatment yielded positive results, as the size of the left lower lobe tumor decreased, and the serum AFP level decreased to 2,038 ng/mL. However, the left pleural lesion showed enlargement, prompting us to include radiotherapy for the left pleural lesion as well.
Although this treatment approach allowed us to maintain an anti-tumor effect and temporarily control the disease, the effectiveness of our treatment was short-lived, and the tumor progressed at a rapid pace. Subsequently, the patient developed additional lesions, pleural lesions, and enlarged mediastinal lymph nodes, along with massive pleural and pericardial effusion. Additional radiotherapy was performed for the new lesions, and drainage was performed for the pleural and pericardial effusion, as required. Despite the patient's diligent adherence to the suggested treatments and our best supportive efforts, his condition gradually deteriorated, and he was unable to tolerate systemic chemotherapy and radiotherapy.
Following consultation with the patient, we decided to focus solely on symptomatic relief during the continued treatment. Despite this approach, the patient ultimately succumbed approximately six months after commencing the initial treatment.
Discussion
We encountered a rare case of NUT carcinoma with bronchial mucosal invasion promptly diagnosed using NGS. NUT carcinomas are difficult to diagnose and are rarely diagnosed by NGS alone. There are only a few reports of NUT carcinoma with bronchial lesions (6).
In approximately 70-80% of NUT carcinoma cases, the BRD4-NUTM1 fusion oncogene is formed by the fusion of NUTM1 (chromosome 15q14) and BRD4 (chromosome 19p13.12). In addition, approximately 15-30% of NUT carcinoma cases exhibit a mutant translocation involving exon 9 of BRD3 on chromosome 9q34.2, resulting in the formation of the BRD3-NUTM1 fusion oncogene. Other rare NUTM1 fusion genes, which are present in approximately 6% of cases, include NSD, ZNF532, ZNF592, and other unidentified genes (7). In the present case, the diagnosis was based on identification of the BRD4-NUTM1 fusion oncogene, which is considered the most common fusion gene in NUT carcinoma cases. Furthermore, our case involved a primary pulmonary NUT carcinoma. The thorax was the most common site for primary lesions (51%), followed by the head and neck region (41%) and bone and soft tissue (6%). Regardless of the specific fusion gene, primary thoracic NUT carcinoma has been associated with a poor prognosis. Furthermore, the presence of the BRD4-NUTM1 fusion gene is associated with a worse prognosis than other fusion gene groups (8). Given that our patient had primary lung involvement of the BRD4-NUTM1 fusion gene, his prognosis was considered poor.
NUT carcinoma often exhibits nonspecific histology, making it challenging to histologically diagnose or distinguish it from a poorly differentiated carcinoma. The most common histological subtype is the poorly differentiated type, with the majority lacking squamous differentiation (9,10). Since it is difficult to immediately suspect NUT carcinoma based on imaging and histological findings, we suggest that NUT carcinoma be considered when atypical poorly differentiated cancer is encountered. However, NUT immunostaining and genomic testing, which are crucial for the diagnosis of NUT carcinoma, may not be readily available at all hospitals. In our case, there was a significant increase in AFP levels before treatment, which correlated with the response to treatment in terms of tumor volume. Although reports on AFP trends during the course of NUT carcinoma treatment are limited, some studies have shown a correlation between AFP levels and treatment outcomes (4). Therefore, we propose that NUT carcinoma be considered as a differential diagnosis when a poorly differentiated tumor with elevated AFP levels is encountered.
In our case, although we initially suspected lung cancer in a young patient upon referral to our hospital, the serum AFP level was markedly elevated. Histopathological findings ruled out the possibility of a germ cell tumor, and a definitive diagnosis of NUT carcinoma was established through identification of the BRD4-NUTM1 fusion gene by NGS and positive NUT immunostaining. However, it should be noted that not all NUT carcinoma cases exhibit elevated AFP levels. Previously, our case would have been classified as a tumor with markedly elevated AFP levels, leading to the diagnosis of a germ cell tumor. However, genomic testing enabled the swift identification of NUT carcinoma before the initial treatment was commenced. In a literature review of 68 cases of NUT carcinoma, 7 were diagnosed using RNA-NGS. There was also a case of NUT carcinoma with false-negative NGS results owing to an insufficient RNA quality (6). Therefore, a diagnosis using both immunohistochemistry (or fluorescence in situ hybridization) and NGS, as in our case, is preferred.
The literature on bronchoscopy findings in cases of primary pulmonary NUT carcinomas is limited. One report described a case of NUT carcinoma arising from the bronchus that exhibited a stalked tumor with partial necrosis or a polypoid tumor; however, the characteristic features remain unknown (11). In our case, apart from the primary mass, we observed irregular mucosal findings in the bronchus, suggestive of endobronchial metastasis, which we considered valuable.
NUT carcinoma is a highly invasive and rapidly progressive tumor, and there is currently no consensus on surgical treatment. Intensive local therapies, such as gross total colectomy and radiation therapy, have been associated with a prolonged survival (3). Radiation therapy is expected to improve the overall survival of patients with primary tumors in the head, neck, and lungs, but its effectiveness is limited for primary mediastinal tumors (12). The systemic chemotherapy regimens for NUT carcinoma include cisplatin, carboplatin, cyclophosphamide, etoposide, doxorubicin, actinomycin D, vinorelbine, vinblastine, paclitaxel, docetaxel, 5-fluorouracil, S-1, bleomycin, vincristine, ifosfamide, gemcitabine, and bromodomain and extra-terminal (BET) inhibitors. However, resistance to treatment is commonly observed, despite the use of various drugs. Some reports have suggested the use of sarcoma-like therapy, with promising outcomes (12,13). The most common therapeutic approach for primary lung NUT carcinoma is taxane plus platinum followed by etoposide plus platinum and pemetrexed plus platinum. Pembrolizumab, nivolumab, and atezolizumab have shown moderate effectiveness, but their clinical efficacy has yet to be fully determined (14). BET inhibitors have demonstrated efficacy against various solid tumors; however, drug resistance remains a concern. BET inhibitors are believed to exert antitumor effects by rapidly dissociating BRD4-NUT from chromatin (15,16). Further clinical studies are needed to establish the use of BET inhibitors as a therapeutic option for NUT carcinoma.
Conclusion
We promptly diagnosed NUT carcinoma with a bronchial lesion and significantly elevated serum AFP levels through genomic testing before initiating systemic therapy. The patient was treated with a combination of systemic chemotherapy, including immune checkpoint inhibitors, and radiation therapy, which demonstrated temporary efficacy. However, complete control of the aggressive disease progression associated with NUT carcinoma is challenging. The use of immune checkpoint inhibitors in NUT carcinoma cases has been scarcely reported due to its rarity and rapid progression; therefore, the usefulness of immune checkpoints is unproven and requires further investigation. In addition, we anticipate future studies on the potential use of BET inhibitors in the management of NUT carcinomas.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We would like to thank the patient for providing consent to publish clinical information and data.
References
- 1.Kees UR, Mulcahy MT, Willoughby ML. Intrathoracic carcinoma in an 11-year-old girl showing a translocation t(15;19). Am J Pediatr Hematol Oncol 13: 459-464, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Kubonishi I, Takehara N, Iwata J, et al. Novel t(15;19)(q15;p13) chromosome abnormality in a thymic carcinoma. Cancer Res 51: 3327-3328, 1991. [PubMed] [Google Scholar]
- 3.Bauer DE, Mitchell CM, Strait KM, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res 18: 5773-5779, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Ambrosio L, Palesandro E, Moretti M, et al. Alpha-fetoprotein elevation in NUT midline carcinoma: a case report. BMC Cancer 17: 266, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohtsu A, Goto K, Yoshino T. Improvement of patient care using cancer genomic profiling: SCRUM-/CIRCULATE-Japan experience. Proc Jpn Acad Ser B 99: 241-253, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Wang J, Luo X, et al. Diagnosis of NUT carcinoma despite false-negative next-generation sequencing results: a case report and literature review. Onco Targets Ther 14: 4621-4633, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreno V, Saluja K, Pina-Oviedo S. NUT carcinoma: clinicopathologic features, molecular genetics and epigenetics. Front Oncol 12: 860830, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chau NG, Ma C, Danga K, et al. An anatomical site and genetic-based prognostic model for patients with nuclear protein in testis (NUT) midline carcinoma: analysis of 124 patients. JNCI Cancer Spectr 4: pkz094, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Han K, Dong X, et al. Case report and literature review: primary pulmonary NUT-midline carcinoma. Front Oncol 11: 700781, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.French CA. Demystified molecular pathology of NUT midline carcinomas. J Clin Pathol 63: 492-496, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe S, Hirano S, Mine S, et al. A case of endobronchial NUT midline carcinoma with intraluminal growth. Anticancer Res 35: 1607-1612, 2015. [PubMed] [Google Scholar]
- 12.Giridhar P, Mallick S, Kashyap L, Rath GK. Patterns of care and impact of prognostic factors in the outcome of NUT midline carcinoma: a systematic review and individual patient data analysis of 119 cases. Eur Arch Otorhinolaryngol 275: 815-821, 2018. [DOI] [PubMed] [Google Scholar]
- 13.Storck S, Kennedy AL, Marcus KJ, et al. Pediatric NUT-midline carcinoma: therapeutic success employing a sarcoma based multimodal approach. Pediatr Hematol Oncol 34: 231-237, 2017. [DOI] [PubMed] [Google Scholar]
- 14.Yuan J, Xu Z, Guo Y. Diagnosis, treatment and prognosis of primary pulmonary NUT carcinoma: a literature review. Curr Oncol 29: 6807-6815, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y, Vakoc CR. Targeting cancer cells with BET bromodomain inhibitors. Cold Spring Harb Perspect Med 7: a026674, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.French CA, Cheng ML, Hanna GJ, et al. Report of the first international symposium on NUT carcinoma. Clin Cancer Res 28: 2493-2505, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]