Abstract
Background and study aims: This study aimed to conduct a clinical audit of adverse events (AEs) arising from gastrointestinal endoscopy, utilizing the AGREE classification for AEs and establishing its correlation with the ASGE classification. This study further integrated the economic repercussions of AEs into the AGREE classification through the AIG-AGREE modification.
Patients and methods: A prospective observational study was conducted at the Asian Institute of Gastroenterology, Hyderabad, India, from July 1, 2021, to December 31, 2021. The study included all patients who underwent diagnostic or therapeutic endoscopic procedures. AEs were categorized using the American Society of Gastrointestinal Endoscopy (ASGE) and AGREE classifications. A quality indicator questionnaire containing 15 questions was graded based on the latest ASGE and European Society of Gastrointestinal Endoscopy guidelines. The grading scale ranged from 1 to 3 (poor), 4 to 6 (average), 7 to 9 (excellent), to 10 (outstanding). In addition, the AIG-AGREE modification divided the economic impact into five scales (α, β, γ, δ, and ε) based on multiples of the baseline amount. (ClinicalTrials.gov Identifier: NCT05228353)
Results: Over the 6-month study period, a total of 42,471 endoscopic procedures were performed, identifying 220 AEs. Analysis revealed a significant positive correlation (Pearson correlation coefficient = 0.79; P < 0.001) between the grades of AEs in the AGREE and ASGE classifications. The median score for all quality indicators was 8, indicating excellent services based on feedback from 13,042 surveyed patients. Notably, patients with more severe AEs (AGREE III-V) exhibited higher economic impact categories (β, γ, δ, ε) compared with those with less severe AEs (AGREE I-II).
Conclusions: The AIG-AGREE modification stands as a pioneering effort that highlights the importance of considering economic factors in the evaluation of AEs in gastrointestinal endoscopy.
Keywords: Quality and logistical aspects, Quality management, Performance and complications
Introduction
Endoscopy plays a pivotal role in management of gastrointestinal diseases. It is an invasive procedure, associated with a relatively low risk for diagnostic endoscopy and a higher risk for therapeutic procedures 1 2 3 4 . Multiple society guidelines emphasize the importance of reporting and monitoring adverse events (AEs) in gastrointestinal endoscopy 3 5 . Incidence of AEs varies due to a lack of uniform definition consensus 4 6 . The quality of endoscopy is integral to the audit of the endoscopy unit and is often measured through quality indicators (QIs).
Gastrointestinal endoscopy has made significant progress over the last two decades owing to the minimally invasive nature of therapeutic endoscopy compared with surgery. While the Clavien-Dindo classification is widely accepted for reporting adverse surgical events, it is crucial to note that surgery is typically an inpatient procedure, whereas most endoscopic procedures are performed on an outpatient basis 7 8 . In this study, we utilized a novel AGREE classification, addressing the shortcomings of the previously used classification 9 . It is a validated scale and enables performance comparison between different endoscopy services, countries, and potentially between various disciples. However, the AGREE classification does not consider the financial burden on the patient or the system. Therefore, we proposed a modified AGREE classification—a unique approach incorporating a suffix denoting the financial burden on the patient and healthcare system. Our aim was a clinical audit of gastrointestinal endoscopy AEs, with a distinctive focus on QI and integrating the economic impact on patients within the AGREE classification.
Methods
Patient selection
This prospective observational study was conducted at the Asian Institute of Gastroenterology, Hyderabad, India, from July 1, 2021, to December 31, 2021. It included consecutive patients undergoing any gastrointestinal endoscopic procedure over 6 months who developed AEs. Patients who did not provide consent were excluded. The study adhered to the ethical principles for human subjects outlined in the Declaration of Helsinki and received approval from the institutional ethical committee (ECR/346/Inst/AP/2013/RR-19) on May 21, 2021, with informed consent obtained from all participants. The study is registered at clinicaltrials.gov (NCT05228353).
Aims and objectives
The primary objective of this study was to conduct a clinical audit of AEs arising in the gastrointestinal endoscopy unit of a tertiary care center. Secondary objectives included comparing AE grades between the AGREE and American Society of Gastrointestinal Endoscopy (ASGE) classifications and examining QIs of gastrointestinal endoscopy. In addition, the economic impact of AEs on patients and healthcare was integrated within the AGREE classification using the AIG-AGREE modification.
Study procedure
The audit encompassed all patients who underwent any gastrointestinal endoscopic procedure within the study period and subsequently developed AEs. These procedures included diagnostic and therapeutic upper gastrointestinal endoscopy, diagnostic and therapeutic colonoscopy, endoscopic retrograde cholangiopancreatography (ERCP), endoscopic ultrasound (EUS), peroral endoscopic myotomy (POEM), various forms of enteroscopy (antegrade, retrograde, or spiral), endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), submucosal tunnel endoscopic resection (STER), anti-reflux mucosal ablation (ARMA), GERD-X, endoscopic sleeve gastroplasty (ESG), and intragastric balloon insertion.
The gastrointestinal endoscopy unit at our facility, one of the largest globally, is equipped with state-of-the-art technology. It includes 10 endoscopy rooms, four colonoscopy rooms, and five advanced therapeutic endoscopy rooms, with dedicated spaces for ERCP, EUS, and third-space procedures. The unit is supported by three pre- and post-anesthetic care units for gastroscopy, colonoscopy, and advanced procedures, staffed by specialized anesthesiologists and a dedicated nursing team. A mobile endoscopy unit facilitates bedside procedures in intensive care.
In terms of sedation and recovery, patient American Society of Anesthesiologists physical status is evaluated pre-procedure. Sedation, either propofol or non-propofol, is administered based on anesthesiologist discretion, with most procedures performed under conscious sedation, except for advanced third-space procedures like POEM, STER, EMR/ESD, and ESG, which require general anesthesia. An anesthetist was present during all cases, irrespective of whether propofol or non-propofol sedation was used. Antibiotic prophylaxis is administered when necessary. Post-procedure, patients are monitored in recovery units, assessed for endoscopic AEs, and provided with detailed post-procedure instructions. Special consideration is given to patients undergoing ERCP and other high-risk procedures, who may be scheduled for 24-hour post-procedural observation or transferred to intensive care if AEs arise. Prophylactic pancreatic duct stenting is considered for high-risk ERCP patients.
An AE in this study was defined as any negative outcome that either precluded a planned endoscopic procedure or deviated from the standard post-procedural course. These events were documented in a comprehensive manner for all procedures, including those interrupted during the preparatory phase. However, events occurring during the procedure that did not alter the standard post-procedural outcome were not classified as adverse. Notably, any events within 30 days post-procedure were considered adverse, regardless of their direct relation to the procedure, with a focus on minimizing subjective interpretation. Intra-procedure and immediate post-procedure complications, including serious sedation-related events, were detailed in the procedure report. Each patient was evaluated by the primary investigator 24 hours post-procedure for potential symptoms and complications. Follow-up also involved a standardized telephone interview and electronic medical record review 30 days or later post-procedure. Patients readmitted due to procedure complications received systematic follow-up by the gastroenterology service, in coordination with other medical, radiological, and surgical teams. Delayed complications were meticulously recorded in the patient's electronic record. An exhaustive AE form, encompassing both immediate and delayed events, was completed for each incident. Sedation-related events were also overseen by a multidisciplinary committee. Complications were categorized according to the ASGE classification and AGREE criteria 9 10 .
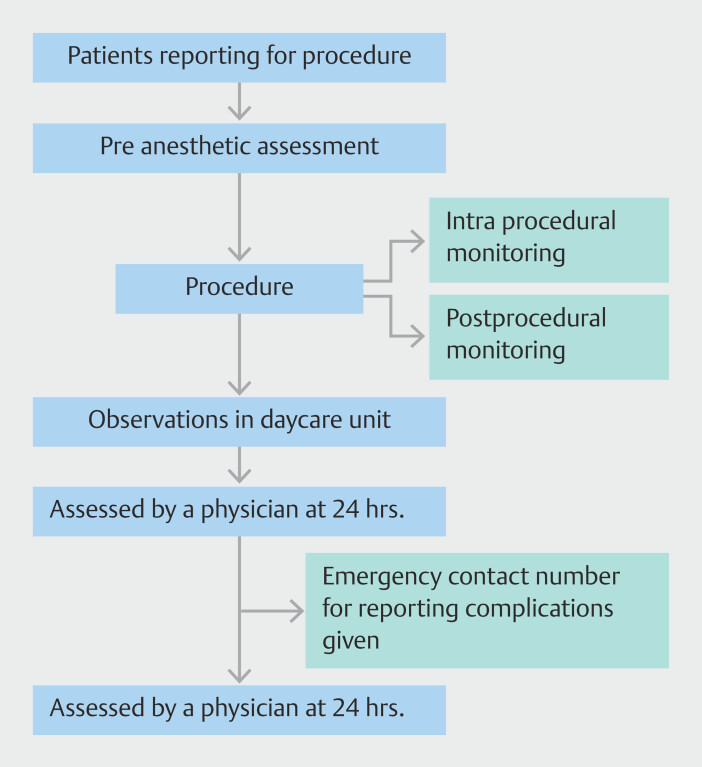
For each AE, data collected included procedure details, indications, use of antiplatelets/anticoagulants, comorbidities, operator characteristics, procedure timing and duration, sedation type, complication detection time, hospital/ICU stay duration, mortality, 30-day follow-up, readmission rates, ASA physical status classification, and relevant procedure images and videos. AEs were recorded as per ASGE guidelines, encompassing a range of issues from perforation to mortality. The study's methodology is illustrated in Fig. 1 .
Fig. 1.
Flowchart showing study outline.
Quality indicators
We designed a questionnaire based on the latest ASGE 11 and ESGE 12 guidelines, aiming to enhance patient outcomes. QIs of gastrointestinal endoscopy were taken from those proposed by the ASGE endoscopy unit QIs task force 11 . The domains included patient experiences, employee experience, efficiency and operations, procedure-related aspects, safety, and infection control. The employee experience domain was excluded as most employees were not willing to participate in the study. A formal list of 15 QIs from four domains was shortlisted by a task force consisting of gastroenterologists, nursing heads, and the hospital management team. They were given scores from 1 to 10, and the quality of services was graded as follows: 1 to 3 (poor), 4 to 6 (average), 7 to 9 (excellent), and 10 (outstanding). The data were entered on a tablet-based questionnaire in four different languages: English, Hindi, Telugu, and Bengali, covering the base population. The scores were collected by a designated person not involved in the study.
AIG-AGREE modification
The AIG-AGREE modification extends the existing AGREE classification by recognizing the necessity of incorporating economic considerations alongside clinical outcomes. This advancement is achieved by introducing a suffix that indicates the financial burden on the patient, contributing significantly to a more thorough assessment of AEs. The incorporation of five scales—α, β, γ, δ, and ε—based on multiples of the baseline amount establishes a structured framework for evaluating the diverse degrees of economic impact associated with various AEs. The baseline amount, set at three thousand rupees (33€ or $36) for shared room accommodation with intravenous fluids and required drugs, serves as the reference point.
α (single multiple-x): This category signifies cases where the economic impact of endoscopy complications corresponds to a single multiple of the baseline amount.
β (2–5x): The economic impact falls within the range of two to five times the baseline amount.
γ (6–20x): Economic impacts within the range of six to 20 times the baseline amount are categorized under this scale.
δ (21–50x): Economic impacts ranging from 21 to 50 times the baseline amount are considered in this category.
ε (> 50x): The highest economic impact category denotes cases where the economic impact surpasses 50 times the baseline amount.
This modification not only enhances the precision of economic assessments related to endoscopy complications but also facilitates a more nuanced understanding of the varying financial burdens experienced by patients in different scenarios.
Statistical analysis
Baseline characteristics were summarized using descriptive statistics. Categorical variables were presented as frequencies and percentages and compared using either the Fisher exact test or Chi-square test, as appropriate. Ordinal data were expressed as the median with interquartile range (IQR). To assess the correlation between two variables, the Pearson correlation coefficient was employed. Agreement between variables was evaluated using Cohen's kappa coefficient. For paired data comparisons, the Wilcoxon signed-rank test was utilized. This non-parametric test was chosen due to its suitability for analyzing paired data that did not conform to normal distribution assumptions. Statistical significance was set at a 5% level, with P < 0.05 considered statistically significant.
Results
During the study period, 42,471 endoscopic procedures were conducted, yielding 220 AEs, 41 of which were severe, necessitating repeat procedures, intensive care unit care, or resulting in mortality. Table 1 summarizes the AGREE III-V AEs that occurred during the study period. Specifically, complication rates were as follows: 0.03% for upper gastrointestinal (UGI) endoscopies, 0.6% for therapeutic endoscopies, and 4.4% for UGI polypectomies. Post-endoscopic variceal ligation (EVL) bleeding occurred in 1% of EVL cases. In the 13,043 colonoscopies performed, serious complications occurred in 0.06% of cases. Anesthesia-related AEs included hepatocellular jaundice after a colonic polypectomy and sudden cardiac arrest following a colonoscopy.
Table 1 Summary of the AGREE III-V complications and AIG-AGREE modification that occurred during the study period.
| Procedure type | No | Complication n (%) | Severe complications | Management strategy | AGREE grade | AIG-AGREE modification (α,β,γ,δ,ε) |
| *A patient with lower gastrointestinal bleeding, classified as ASA IV with left ventricular dysfunction, experienced sudden cardiac arrest post-colonoscopy. Despite resuscitation and 1 day in the intensive care unit, the patient succumbed to a second cardiac arrest. ARMA, anti-reflux mucosal ablation; EUS, endoscopic ultrasound; EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; ICU, intensive care unit; SEMS, self-expanding metal stent; TIPs, transjugular intrahepatic portosystemic shunt; WOPN, walled-off pancreatic necrosis. | ||||||
| Endoscopy | 23,947 | 6 (0.03%) | Post polypectomy bleed – 2 | Endoscopic Mx | 2 IIIa | 2 IIIa(β) |
| Post EVL bleeding – 4 | 1 – Endoscopic Mx 1 – TIPS 2 – Dannis Ella stent & TIPS |
IIIa | IIIa(δ) IIIa(ε) 2 IIIa(ε) |
|||
| Colonoscopy | 13,043 | 8 (0.06%) | Post polypectomy bleed – 3 | 3 – Endoscopic Mx | IIIa | 3 IIIa(β) |
| Perforation – 3 | 2 – Endoscopic Mx 1 – Surgery |
IIIa – 2 IIIb – 1 |
IIIa(γ) IIIa(δ) IIIb(ε) |
|||
| Acute hepatitis – 1 | Supportive care (ICU) | IVa | IVa(δ) | |||
| Death – 1* | V | V(γ) | ||||
| Enteroscopy | Single balloon – 113 | 6 (5.3%) | Nil | – | – | – |
| Motor spiral – 65 | 5 (7.7%) | – | ||||
| EUS | 1916 | 2 | Perforation – 1 | Endoscopic Mx | IIIa | IIIa(δ) |
| Internal migration of SEMS during WOPN drainage – 1 | Endoscopic Mx | IIIa | IIIa(γ) | |||
| ERCP | 2716 | 183 (164 AGREE I or II & 19 III–V) | Post-sphincterotomy bleeding (n=4) | Endoscopic Mx – 4 | IIIa – 4 | 3 IIIa(β), IIIa(γ) |
| Post-ERCP pancreatitis (n=7) | 1 – Radiological intervention 1 – surgery 5 – Supportive care (ICU) |
IIIa IIIb 4 IVa & 1 IVb |
IIIa(δ) IIIa(ε) 3 IVa(γ), 1 IVa(δ) 1 IVb(δ) |
|||
| Cholangitis (n=8) | 3 – Endoscopic Mx 3 – Supportive care (ICU) 2 – Death |
3 IIIa 2 IVa & 1 IVb 2 V |
1 IIIa(γ), 2 IIIa(δ) 2 IVa(γ) & 1 IVb(δ) V(δ), V(ε) |
|||
| Third Space Endoscopy | 120 POEM | 6 | Mucosal injury – 2 Delayed bleeding – 1 |
2 – Endoscopic Mx 1 – Supportive care (ICU) |
2 IIIa 1 IVa |
IIIa(β), IIIa(γ) IVa(γ) |
| 28 EMR | – | – | – | – | – | |
| 42 ESD | 1 | Perforation – 1 | 1 – Endoscopic Mx | IIIa | IIIa(β) | |
| Endoscopic necrosectomy | 78 | 2 | Bleeding – 1 | 1 – Endoscopic Mx | IIIa | IIIa(γ) |
| Anti-reflux procedures | ARMA – 47 | 1 | – | – | – | |
| GERDx – 37 | 1 | Hydropneumothorax – 1 | 1 – Radiological intervention | IIIa | IIIa(δ) | |
Among other procedures, single balloon enteroscopy and novel motorized spiral enteroscopy (NMSE) reported no serious complications, with minor issues occurring in 5.3% and 7.7% of cases, respectively. Endoscopic retrograde cholangiopancreatography (ERCP) had a 6.7% complication rate, predominantly mild, with 0.7% being serious. Mild grade (AGREE grade I or II) complications included abdominal pain, diarrhea, fever, and mild acute pancreatitis, managed conservatively with supportive medications. Two patients with metal stents for cholangiocarcinoma experienced stent obstruction due to disease progression and could not be salvaged despite interventions.
EUS examinations showed a 0.05% complication rate. Direct endoscopic necrosectomy had a low complication incidence, with bleeding observed in two patients. Third-space endoscopic procedures recorded a 5% complication rate in peroral endoscopic myotomy (POEM), with no complications in EMR and STER. Anti-reflux procedures had one complication each in ARMA and GERD-X treatments.
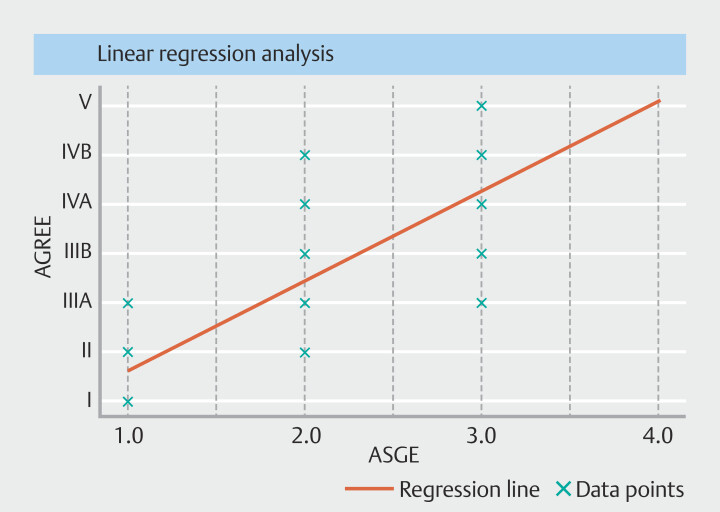
Of the 220 total AEs, the Pearson correlation coefficient ( P = 0.79; P < 0.01) suggested a positive correlation between AE grade in the AGREE classification as compared with ASGE classification ( Fig. 2 ).
Fig. 2.
Scatter plot showing a significant positive correlation (Pearson correlation coefficient = 0.79; P < 0.001) between the grades of adverse events in the AGREE and ASGE classifications.
Quality indicators
The median score for all 15 indicators was 8, reflecting excellent service based on a survey of 13,042 patients. Waiting time for procedures maintained a median score of around 7 for 2 consecutive months. Following an internal audit, measures were implemented to alleviate patient wait times. These measures included commencing procedures 1 hour earlier in the morning and enhancing the availability of endoscopes and endoscopists, resulting in an improved patient experience. In the specific month under review, the median score (interquartile range) surged to 9 (8–10), representing a significant enhancement compared with the previous month's score of 7 (7–9) ( P < 0.005). Other indicators with lower scores included accessibility to facilities such as parking and access to a quiet area for discussions with doctors. Steps were taken to enhance accessibility to facilities, and provisions were made for a dedicated quiet area for consultations with doctors. Key QIs include the availability of language translation services (score: 8 8 9 ), clarity of information regarding endoscopic procedure indications (score: 8 [8–8]), effectiveness of pre-procedure reviews in communicating key procedure elements (score: 8 [8–8]), and quality of discharge instructions provided (score: 8 [8–8]). A summary of median scores with interquartile ranges for all QIs is presented in Table 2 .
Table 2 Quality indicators for endoscopy.
| No. | Questions | Jul 2021 (n = 1667) |
Aug 2021 (n = 1625) |
Sep 2021 (n = 2164) |
Oct 2021 (n = 2422) |
Nov 2021 (n = 2184) |
Dec 2021 (n = 2980) |
Median (IQR) |
| IQR, interquartile range. | ||||||||
| 1 | Rate the Availability of language translation services. | 8 (8–9) |
8 (8–9) |
8 (8–8) |
8 (8–9) |
8 (8–8) |
9 (8–10) |
8
(8–9) |
| 2 | Rate the information regarding the indication of the endoscopic procedure. | 8 (8–8) |
8 (8–8) |
8 (8–8) |
8 (8–9) |
8 (8–8) |
9 (8–9) |
8
(8–8) |
| 3 | Rate the Information of the appointment . | 8 (7–8) |
8 (7–8) |
8 (8–8) |
8 (8–9) |
8 (8–8) |
8 (8–9) |
8
(8–8) |
| 4 | Rate the explanation for the cost of the procedure and transparency | 8 (8–8) |
8 (8–8) |
8 (8–8) |
8 (8–9) |
8 (8–8) |
8 (8–9) |
8
(8–8) |
| 5 | Rate clarifying all the doubts before the procedure | 8 (7–8) |
8 (7–8) |
8 (8–8) |
8 (7–9) |
8 (8–8) |
8 (8–9) |
8
(7–8) |
| 6 | Rate explanation about the consent for the procedure | 8 (8–8) |
8 (7–8) |
8 (8–8) |
8 (8–9) |
8 (8–8) |
9 (8–9) |
8
(8–8) |
| 7 | Rate the pre-procedure review communicating about key elements of the procedure | 8 (8–8) |
8 (7–8) |
8 (8–8) |
8 (7–9) |
8 (8–8) |
8 (8–9) |
8
(8–8) |
| 8 | Rate the opportunity to speak with the provider who performed the procedure before the discharge | 8 (8–8) |
8 (8–8) |
8 (8–8) |
8 (8–9) |
8 (8–8) |
8 (8–9) |
8
(8–8) |
| 9 | Rate the process of receiving the final reports | 8 (8–8) |
8 (7–8) |
8 (8–8) |
8 8–9 |
8 (8–8) |
8 (8–9) |
8
(8–8) |
| 10 | Rate the discharge instructions provided | 8 (8–8) |
8 (8–8) |
8 (8–8) |
8 8–10 |
8 (8–8) |
9 (8–9) |
8
(8–8) |
| 11 | Rate the basic monitoring of patient comfort and pain levels before, during, and after the procedure | 8 (8–8) |
8 (7–8) |
8 (8–8) |
8 (8–10) |
8 (8–8) |
8 (8–9) |
8
(8–8) |
| 12 | Rate the waiting time for the procedure upon arrival at endoscopy unit | 8 (7–8) |
8 (7–8) |
8 (7–9) |
7 (7–10) |
7 (7–9) |
9 (8–10) |
8
(7–8) |
| 13 | Rate the accessibility to facilities (i.e., parking, finding your way | 8 (7–9) |
8 (7–8) |
8 (7–8) |
8 8–10 |
7 (7–8) |
8 (8–9) |
8
(7–8) |
| 14 | Rate access to a quiet area for discussion with the doctor | 8 (7–9) |
8 (7–8) |
7 (7–8) |
8 (7–9) |
8 (7–9) |
8 (8–9) |
8
(7–8) |
| 15 | Rate the recovery space (clean, functional, quiet, ensure patient privacy, post-procedure monitoring for patients | 8 (8–8) |
8 (7–8) |
8 (8–8) |
8 (8–9) |
8 (8–8) |
9 (8–9) |
8
(8–8) |
AIG-AGREE modification
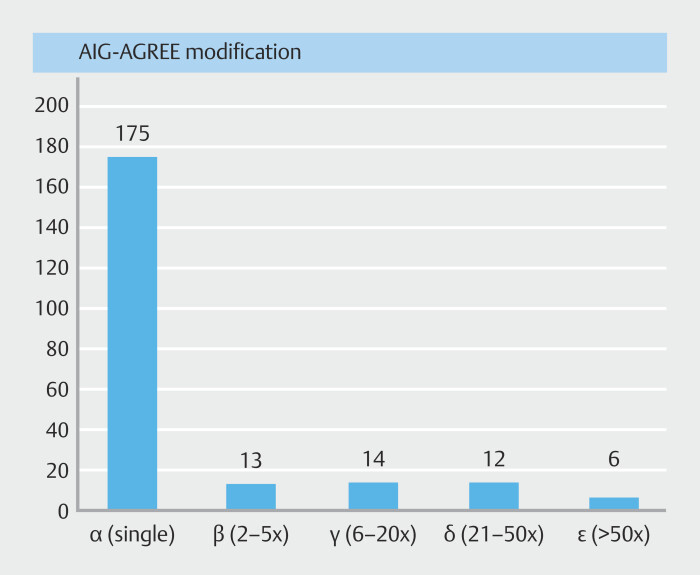
This denotes the economic aspect of endoscopy complications, categorizing them into five scales (α, β, γ, δ, and ε) based on multiples of the baseline amount. Fig. 3 shows a bar diagram denoting the distribution of patients across different economic impact categories.
Fig. 3.
Bar diagram showing the distribution of patients across different economic impact categories.
Patients with more severe AEs (AGREE III-V) typically fall into higher economic impact categories (β, γ, δ, ε) compared with those with less severe AEs (AGREE I-II), who are predominantly in category α. While less severe AEs mainly fall into the α category (97.7%), it is noteworthy that 2.2% of AGREE I-II complications can be associated with a higher economic impact category. Similarly, AGREE V, the highest severity category, does not necessarily imply the highest economic impact. AGREE V cases included one each in the γ, δ, and ε categories.
The relationship between AGREE classification and AIG-AGREE modification is elucidated in Table 3 . This type of analysis is valuable for understanding the economic implications of endoscopy complications across different severity levels.
Table 3 Relationship between AGREE classification and AIG-AGREE modification.
| AGREE classification | AIG-AGREE modification | ||||
| α | β | γ | δ | ε | |
| I | 88 | 1 | – | – | – |
| II | 87 | 2 | 1 | 0 | 0 |
| IIIa | 0 | 10 | 6 | 6 | 3 |
| IIIb | – | – | – | – | 2 |
| IVa | – | – | 6 | 3 | 0 |
| IVb | – | – | – | 2 | 0 |
| V | – | – | 1 | 1 | 1 |
Discussion
This prospective study, conducted over 6 months, aimed to assess the AE rates for endoscopic procedures and to evaluate the applicability of the latest AGREE classification in comparison with the traditional ASGE classification. Our study underscores the feasibility of maintaining high-quality care despite the substantial volume of procedures. We observed an overall AE rate of 0.5% (220 cases) across all grades as per the AGREE classification, with Grade III/IV complications, including mortality (3 cases, 0.007%), accounting for a rate of approximately 0.1% (41 cases). Notably, diagnostic procedures such as gastroscopy, colonoscopy (3 cases), and diagnostic EUS (1 case) demonstrated a markedly lower AE rate (4 cases of 37,489, 0.01%) compared with therapeutic endoscopic interventions (216 cases of 4,982, 4.3%).
In our findings, therapeutic upper gastrointestinal endoscopy, therapeutic colonoscopy, and diagnostic EUS proved to be relatively safe, with AE rates of 0.6%, 1%, and 0.05%, respectively. However, as anticipated, incidence of complications was higher for certain procedures. Specifically, ERCP presented a comprehensive AE rate of 6.7%, with post-ERCP pancreatitis (PEP) occurring in 0.7% of the patients. In the case of peroral endoscopic myotomy (POEM), it was observed to be 5%. These results highlight the varying risk profiles across different endoscopic procedures and underscore the importance of tailored risk management strategies in clinical practice.
In the context of endoscopic retrograde cholangiopancreatography (ERCP), risk of PEP historically varies widely, with reported rates ranging from less than 1% to as high as 40% in different studies 13 14 15 16 . In our current investigation, we observed a notably lower rate of PEP, a finding that can be attributed to several key factors. Primarily, 68% of all ERCPs performed in our study were for pancreatic indications, predominantly chronic pancreatitis. This specific patient demographic is largely due to the availability of the latest extracorporeal shock wave lithotripsy device (Delta III, Dornier Medtech, Wessling, Germany) at our tertiary care center, which has become a referral hub for chronic pancreatitis cases. Furthermore, implementation of proactive strategies, such as careful screening of high-risk patients, universal administration of prophylactic rectal indomethacin, and a low threshold for placement of prophylactic pancreatic stents, particularly in biliary cases, has significantly contributed to reduction in risk of PEP.
Regarding peroral endoscopic myotomy (POEM), the surgical risk observed in our study aligns with previously reported data, where risk rates fluctuate between 1% and 26%. 17 18 19 20 21 22 These AEs encompass a spectrum of complications including bleeding, mucosal injury, pneumonia, pneumoperitoneum, and gastric/esophagal perforation. Notably, mucosal injury emerged as the most common complication in our study.
In addition, post-EVL ulcer bleeding presents as a formidable complication, historically associated with a high mortality rate of up to 22.3%. Incidence of post-EVL ulcer bleeds typically ranges between 3.6% and 15% 23 24 . In contrast, our study documented a 1% incidence of post-EVL ulcer bleeds with a mortality rate of 25%. The relatively lower frequency of post-EVL bleeds in our study can be largely credited to the majority of EVL banding procedures being conducted by endoscopists with more than 2 years of experience and use of sucralfate solution post-banding to prevent post EVL ulcer-related bleeding.
Introduction of NMSE in the field of gastroenterology was initially met with optimism, substantiated by its efficacy in several studies 25 26 . However, its recent market withdrawal due to safety concerns marks a significant development in device-assisted enteroscopy, the sole modality for examining the small bowel. 27 Our study observed a 7.7% incidence of minor AEs associated with NMSE, notably lower than the rates reported in a comprehensive meta-analysis, which documented major AEs like perforation, pancreatitis, and hemorrhage in 1% of cases, and minor AEs in 16% 28 .
Traditionally, the ASGE classification has been a standard for categorizing AEs in endoscopic procedures, analogous to the Clavien-Dindo classification in surgery. 10 However, these classifications have limitations when applied to endoscopy-related complications. Our study endeavors to validate the recently introduced AGREE classification in a tertiary care setting. Analyzing 220 AEs, we observed a considerable correlation between the AGREE and ASGE classifications. Furthermore, we propose incorporating cost as a critical factor, considering the substantial financial impact on patients. The concept of quality in endoscopy was first emphasized by the ASGE/American College of Gastroenterology task force in 2006, laying the foundation for a remarkable transformation in this field 29 30 . Subsequent recommendations for QIs in gastrointestinal endoscopy by the ASGE endoscopy unit QIs task force in 2017 further advanced this transformation 11 . However, adherence to these QIs among endoscopists has been suboptimal in various studies 31 . Our study, a pioneering effort from a major endoscopy unit, examines these QIs, setting a benchmark for other units to enhance care quality, particularly in high-volume settings. Although our study did not encompass employee experience, it introduces the AIG-AGREE modification, incorporating the economic burden on patients. This inclusion sheds light on the relationship between complication severity and financial strain, underscoring the need to consider both clinical and economic outcomes in patient care.
The AIG-AGREE classification complements the AGREE classification by adding a suffix to indicate the economic burden. The economic impact of an AE can be merged with the AGREE classification and denoted in brackets (e.g., AIG-AGREE 3a [gamma]).
While our study provides valuable insights into the correlation between the AGREE and ASGE classifications, there are several limitations worth considering. First, the baseline amount used in the AIG-AGREE modification may not be universally applicable across all healthcare systems. However, multiples of the baseline amount, as employed in the AIG-AGREE modification, can be adapted to suit diverse healthcare systems. Second, the AIG-AGREE modification can have both positive and negative effects, because economic considerations might influence clinical decisions and patient care. Healthcare decisions should prioritize clinical severity over economic reasons to prevent inadvertently exacerbating healthcare inequalities. Balancing economic factors with clinical severity is essential for equitable patient care. Third, because this is a new scale, it should undergo rigorous validation to establish its reliability and utility. Loss of earnings for patients was not calculated and requires further studies for a comprehensive assessment. We also did not look into quality-of-life aspects in patients who developed endoscopy-related AEs. In addition, because ours is a tertiary referral center, the study population may be heterogeneous and not generalizable to other centers. Finally, AEs did not include near misses, which are valuable learning opportunities for colleagues and trainees. An attempt should be made to record near misses in future studies.
Conclusions
In summary, our findings highlight a significant correlation between AGREE and ASGE classifications. Consistent achievement of high scores on QIs demonstrates our institution's dedication to upholding superior care standards. Introduction of the AIG-AGREE modification, which considers economic impact, is an innovative approach in assessment of gastrointestinal endoscopy AEs. Future research should focus on the broader applicability and relevance of the AIG-AGREE modification across varied healthcare contexts and populations.
Acknowledgement
We acknowledge the departments of Surgical Gastroenterology, Radiology and Pathology for their support during the study.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Supplementary Material
References
- 1.Bisschops R, Areia M, Coron E et al. Performance measures for upper gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) quality improvement initiative. Endoscopy. 2016;48:843–864. doi: 10.1055/s-0042-113128. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Menachem T, Decker GA, Early DS et al. Adverse events of upper GI endoscopy. Gastrointest Endosc. 2012;76:707–718. doi: 10.1016/j.gie.2012.03.252. [DOI] [PubMed] [Google Scholar]
- 3.Kaminski MF, Thomas-Gibson S, Bugajski M et al. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) quality improvement initiative. Endoscopy. 2017;49:378–397. doi: 10.1055/s-0043-103411. [DOI] [PubMed] [Google Scholar]
- 4.Reumkens A, Rondagh EJ, Bakker MC et al. Post-colonoscopy complications: a systematic review, time trends, and meta-analysis of population-based studies. Am J Gastroenterol. 2016;111:1092–1101. doi: 10.1038/ajg.2016.234. [DOI] [PubMed] [Google Scholar]
- 5.Fisher DA, Maple JT, Ben-Menachem T et al. Complications of colonoscopy. Gastrointest Endosc. 2011;74:745–752. doi: 10.1016/j.gie.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 6.Kim SY, Kim H-S, Park HJ. Adverse events related to colonoscopy: Global trends and future challenges. World J Gastroenterol. 2019;25:190. doi: 10.3748/wjg.v25.i2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clavien PA, Barkun J, De Oliveira ML et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 8.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nass KJ, Zwager LW, van der Vlugt M et al. Novel classification for adverse events in GI endoscopy: the AGREE classification. Gastrointest Endosc. 2022;95:1078–1085 e1078. doi: 10.1016/j.gie.2021.11.038. [DOI] [PubMed] [Google Scholar]
- 10.Cotton PB, Eisen GM, Aabakken L et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–454. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 11.ASGE Endoscopy Unit Quality Indicator Taskforce . Day LW, Cohen J et al. Quality indicators for gastrointestinal endoscopy units. VideoGIE. 2017;2:119–140. doi: 10.1016/j.vgie.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valori R, Cortas G, De Lange T et al. Performance measures for endoscopy services: a European Society of Gastrointestinal Endoscopy (ESGE) quality improvement initiative. Endoscopy. 2018;50:1186–1204. doi: 10.1055/a-0755-7515. [DOI] [PubMed] [Google Scholar]
- 13.Cotton PB, Elmunzer BJ. Adverse events: definitions, avoidance, and management. ERCP: The Fundamentals. 2020;2020:357–384. [Google Scholar]
- 14.Guda NM, Freeman ML. Overview of ERCP complications: prevention and management. ERCP and EUS: A case-based approach. ERCP EUS. 2015;2015:37–56. [Google Scholar]
- 15.Wang P, Li Z-S, Liu F et al. Risk factors for ERCP-related complications: a prospective multicenter study. Am J Gastroenterol. 2009;104:31–40. doi: 10.1038/ajg.2008.5. [DOI] [PubMed] [Google Scholar]
- 16.Elmunzer BJ. Reducing the risk of post-endoscopic retrograde cholangiopancreatography pancreatitis. Digest Endosc. 2017;29:749–757. doi: 10.1111/den.12908. [DOI] [PubMed] [Google Scholar]
- 17.Nabi Z, Ramchandani M, Chavan R et al. Peroral endoscopic myotomy in treatment-naïve achalasia patients versus prior treatment failure cases. Endoscopy. 2018;50:358–370. doi: 10.1055/s-0043-121632. [DOI] [PubMed] [Google Scholar]
- 18.Inoue H, Sato H, Ikeda H et al. Per-oral endoscopic myotomy: a series of 500 patients. J Am Coll Surg. 2015;221:256–264. doi: 10.1016/j.jamcollsurg.2015.03.057. [DOI] [PubMed] [Google Scholar]
- 19.Haito-Chavez Y, Inoue H, Beard KW et al. Comprehensive analysis of adverse events associated with per oral endoscopic myotomy in 1826 patients: an international multicenter study. Am J Gastroenterol. 2017;112:1267–1276. doi: 10.1038/ajg.2017.139. [DOI] [PubMed] [Google Scholar]
- 20.Von Renteln D, Fuchs KH, Fockens P et al. Peroral endoscopic myotomy for the treatment of achalasia: an international prospective multicenter study. Gastroenterology. 2013;145:309–3.11E305. doi: 10.1053/j.gastro.2013.04.057. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X-C, Li Q-L, Xu M-D et al. Major perioperative adverse events of peroral endoscopic myotomy: a systematic 5-year analysis. Endoscopy. 2016;48:967–978. doi: 10.1055/s-0042-110397. [DOI] [PubMed] [Google Scholar]
- 22.Werner YB, von Renteln D, Noder T et al. Early adverse events of per-oral endoscopic myotomy. Gastrointest Endosc. 2017;85:708–INF. doi: 10.1016/j.gie.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 23.de Brito Nunes M, Knecht M, Wiest R et al. Predictors and management of post-banding ulcer bleeding in cirrhosis: A systematic review and meta-analysis. Liver Int. 2023;43:1644–1653. doi: 10.1111/liv.15621. [DOI] [PubMed] [Google Scholar]
- 24.Bambha K, Kim WR, Pedersen R et al. Predictors of early re-bleeding and mortality after acute variceal haemorrhage in patients with cirrhosis. Gut. 2008;57:814–820. doi: 10.1136/gut.2007.137489. [DOI] [PubMed] [Google Scholar]
- 25.Ramchandani M, Rughwani H, Inavolu P et al. Diagnostic yield and therapeutic impact of novel motorized spiral enteroscopy in small-bowel disorders: a single-center, real-world experience from a tertiary care hospital (with video) Gastrointest Endosc. 2021;93:616–626. doi: 10.1016/j.gie.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Rughwani H, Singh AP, Ramchandani M et al. A randomized, controlled trial, comparing the total enteroscopy rate and diagnostic efficacy of novel motorized spiral enteroscopy and single balloon enteroscopy in patients with small-bowel disorders-THE MOTOR TRIAL ( NCT 05548140) Am J Gastroenterol. 2022;10:14309. doi: 10.14309/ajg.0000000000002409. [DOI] [PubMed] [Google Scholar]
- 27.Pal P, Rebala P, Nabi Z et al. Small-bowel transection after peroral motorized spiral enteroscopy. iGIE. 2023;2:271–272. [Google Scholar]
- 28.Papaefthymiou A, Ramai D, Maida M et al. Performance and safety of motorized spiral enteroscopy: a systematic review and meta-analysis. Gastrointest Endosc. 2023;97:849–INF. doi: 10.1016/j.gie.2023.01.048. [DOI] [PubMed] [Google Scholar]
- 29.Bjorkman DJ, Popp JW., Jr Measuring the quality of endoscopy. Gastrointest Endosc. 2006;101:864–865. doi: 10.1016/j.gie.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 30.Faigel DO, Pike IM, Baron TH et al. Quality indicators for gastrointestinal endoscopic procedures: an introduction. Gastrointest Endosc. 2006;101:866–872. doi: 10.1016/j.gie.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 31.Zagari RM, Frazzoni L, Fuccio L et al. Adherence to European Society of Gastrointestinal Endoscopy quality performance measures for upper and lower gastrointestinal endoscopy: a nationwide survey from the Italian Society of Digestive Endoscopy. Front Med. 2022;9:868449. doi: 10.3389/fmed.2022.868449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.