Abstract
Background and study aims The duodenum and colorectum are target organs for familial colorectal adenomatous polyposis, however, the association of duodenal epithelial tumors (DETs) and colorectal tumors is still controversial. The aim of our study was to elucidate the association between DET and colorectal tumor.
Patients and methods This was an exploratory cross-sectional study of patients with DETs treated by endoscopic resection at our hospital, between November 2018 and October 2022. Individuals who underwent colonoscopy as part of the health screening comprised the reference control group for comparison. In both groups, lesions suspected of being tumors were resected. The main outcome was the adenoma detection rate (ADR). Other outcomes were the detection rate for advanced neoplasia (AN) and risk factors for colorectal adenoma and AN, evaluated using univariate and multivariable analyses.
Results Analyses were based on data from 163 individuals in the DET group and 177 in the control group. ADR was higher in the DET (63.2%) than in the control (23.6%) group ( P < 0.001). AN and invasive cancer rates were also significantly higher in the DET than in the control group (AN: 20.9% vs 3.4%, respectively, P < 0.001; invasive cancer: 3.1% vs 0%, respectively, P < 0.001). On logistic regression analysis, DET was found to be associated with a 5-fold increase in the detection rate of adenoma and 6-fold increase in AN detection.
Conclusions The study revealed significant association between DET and high ADR and a higher frequency of AN and invasive cancer. Screening colonoscopy is suggested for patients with DETs.
Keywords: Endoscopy Lower GI Tract, Polyps / adenomas / ..., Colorectal cancer, Endoscopy Small Bowel, Neoplasia
Introduction
With recent improvements in endoscopy instruments and increased technical endoscopy skill among endoscopists, endoscopic treatment has become more prevalent for treatment of duodenal epithelial tumors (DET) 1 2 . Duodenal adenomas are common in patients with familial adenomatous polyposis 3 , associated with colorectal tumors. Indeed, adenoma detection rates (ADRs) have been reported to be significantly higher in patients with DETs compared with the general population 4 5 6 7 . However, because these studies used a retrospective case-control design, effects of bias and inappropriate selection of controls cannot be denied. Moreover, a recent multicenter retrospective study indicated that there was no significant difference in the incidence of colorectal tumors in patients with synchronous and metachronous duodenal lesions compared with those with a single DET 8 . Therefore, it remains controversial whether DETs are, in fact, associated with a higher risk for colorectal tumors. Accordingly, our aim was to conduct a cross-sectional study to evaluate the ADR for patients with DET compared with a general population control group consisting of individuals who underwent a regular health checkup to elucidate the association between DET and colorectal tumors.
Patients and methods
Study design and statement of ethics
This was an exploratory, cross-sectional, observational study conducted at our hospital. The study was conducted in accordance with the 2008 revision of the Declaration of Helsinki and the study protocol was approved by our Institutional Review Board (20190233). The study was registered in the University Hospital Medical Information Network (UMIN 000038749). Patients provided consent for use of their data for research and publication.
Study sample
The study sample for the DET group consisted of consecutive patients who underwent endoscopic treatment for their DETs which were not ampullary tumors at our hospital between November 2018 and October 2022. The reference control group for comparison consisted of individuals who were scheduled to undergo colonoscopy as part of their health screening but not as part of any treatment paid through health insurance during the same period and had no previous diagnosis of DET. The exclusion criteria for both groups were as follows: colonoscopy performed for any reason within the 3 years prior; previous colorectal resection excluding appendectomy; contraindication to discontinuing antithrombotic medications according to the guidelines of the Japan Gastroenterological Endoscopy Society 9 10 ; diagnosis of familial adenomatous polyposis or hereditary non-polyposis colorectal cancer; and history of inflammatory bowel disease. For patients with metachronous lesions in the DET group, findings of larger lesions are described.
Outcomes
The main outcome of this study was the ADR in both the DET and control groups. Other outcomes were the number and maximum diameter of adenomas per patient, as well as the percentage of advanced neoplasia (AN) and invasive cancer detected in both groups. Risk factors between individuals with and without colorectal adenomas and AN were evaluated in both groups.
Colonoscopy procedure
Before colonoscopy, individuals completed bowel preparation using an oral polyethylene glycol lavage solution. Colonoscopy was performed under conscious sedation, using benzodiazepines and/or pethidine. Scopolamine butyl bromide or glucagon was used as an antispasmodic agent. Colonoscopies were performed by 16 endoscopists with experience in performing > 300 colonoscopies. A high-definition endoscope with a water-jet function (PCF-Q290ZI, EVIS LUCERA ELITE, EVIS X1 endoscopic system; Olympus Medical Systems, Tokyo, Japan; EC-L600ZP7, ELUXEO 7000 endoscopic system; Fujifilm, Tokyo, Japan) was used in all cases. We measured time to withdrawal using white light. All lesions suspected of being tumors were resected during the examination, excluding those with endoscopic findings suggestive of hyperplastic polyps < 10 mm in size, located in the left colon segment. A pathological examination was performed on all resected lesions. Of note, a specific endoscopic resection modality, such as cold forceps polypectomy, cold snare polypectomy, endoscopic mucosal resection (EMR), underwater EMR, or endoscopic submucosal dissection, was not prescribed. For lesions considered to have submucosal invasion for which endoscopic resection was not indicated, surgical resection was subsequently performed, with pathological examination to confirm diagnosis.
Pathological diagnosis
Histopathological diagnosis of colorectal tumors was performed by a single pathologist (K.Y.), with gastroenterology specialization, using the World Health Organization classification 11 . AN was defined as an adenoma > 10 mm in size, high-grade adenoma, villous adenoma, or carcinoma. Histopathological diagnoses of DETs were made by three pathologists (A. M., R. K., and K. Y.) with gastroenterology specialization. Histological grades of DET were classified according to the Vienna classification 12 .
Statistical analysis
We consulted a statistician (Y. S.) about the analysis.
Based on previous reports 4 5 13 14 , we assumed an ADR of 0.55 for patients with DET and 0.4 for the control group. To identify a between-group difference with a power of 80% and type I error of 0.05, assuming a dropout rate of 5%, 180 individuals were included in each group.
We analyzed all data by full analysis set. For baseline variables, we constructed summary statistics, with frequencies and proportions for categorical data, and means and standard deviations (SDs) or median and interquartile range (IQR) for continuous variables. We compared patient characteristics using the Fisher’s exact test for categorical outcomes and t tests or the Wilcoxon rank sum test for continuous variables, as appropriate. Moreover, propensity-matched cohorts of the DET group and control group were derived and compared using a 1:1 ratio with greedy matching on the propensity score, with a caliper of 0.2 SDs of the propensity score logit with no replacement. We examined standardized differences and variance ratios to determine whether the matched cohort had balanced patient characteristics.
To analyze risk factors for adenoma or AN, we divided individuals into two groups, with or without adenoma and with or without AN, and thus, we performed logistic regression analysis. We chose factors that might be related to the adenoma or AN which were significantly more for presence of adenoma or AN in the univariate analysis and that had P < 0.06.
Statistical analyses were performed using JMP software ver. 16.2.0 and SAS ver.9.4 (SAS Institute, Inc., Cary, North Carolina, United States). All P values were two sided and P < 0.05 was deemed significant.
Results
Selection of the study sample
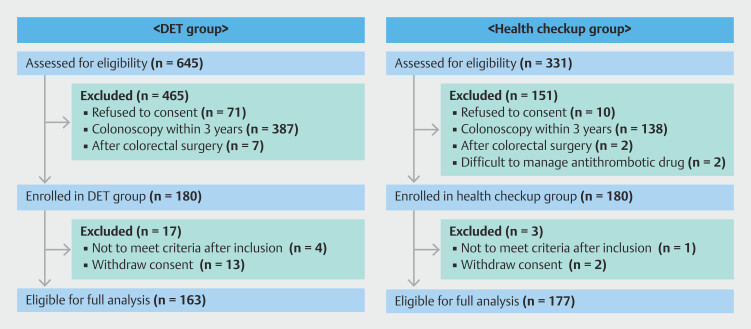
Selection of individuals for the DET and control groups is shown in Fig. 1 . Of the 645 patients with DET who underwent duodenal endoscopic resection at our facility during the study period, 465 were excluded based on our a priori selection area. Finally, of the 180 who met our selection criteria, 163 patients underwent their scheduled colonoscopy, and their data were used in the analysis. Similarly, for the control group, of the 331 individuals who underwent planned colonoscopic screening, 151 were excluded based on our selection criteria, leaving 180 of whom 177 underwent their scheduled colonoscopy and their data included in the analysis.
Fig. 1.
Relevant characteristics of the study sample before matching.
Characteristics of the study sample
Demographic, clinical, and lesion characteristics for the DET and comparative control groups are reported in Table 1 . Demographic (age, sex) and clinical characteristics (body mass index [BMI], comorbidities, and family history of colorectal cancer) did not differ between the two groups, with the exception of age (mean, 64 years, DET group, and 57 years, control group, P < 0.001). The most common location for DETs was the descending part and distal papilla of the duodenum, with a median (interquartile range [IQR]) lesion size 15 mm [range, 10–25]).
Table 1 Relevant characteristics of the study sample before matching.
| DET group (n = 163) | Control group (n = 177) | Odds ratio | 95% CI | P value | ||
| BMI, body mass index; CI, confidence interval DET, duodenal epithelial tumor; IQR, interquartile range; VC, Vienna classification. | ||||||
| Age (yr), median [IQR] | 64 [55–71] | 57 [49–67] | < 0.001 | |||
| Sex [male, n (%)] | 104 (63.8) | 115 (65.0) | 0.95 | 0.68–1.48 | 0.822 | |
| BMI median [IQR] | 23.2 [20.9–25.8] | 23.3 [20.9–25.8] | 0.991 | |||
| Comorbidity | Hypertension, n (%) | 48 (29.5) | 41 (23.0) | 1.38 | 0.85–2.25 | 0.178 |
| Hyperlipidemia, n (%) | 24 (14.7) | 40 (22.5) | 0.59 | 0.34–1.04 | 0.067 | |
| Diabetes, n (%) | 16 (9.8) | 12 (6.7) | 1.50 | 0.69–3.27 | 0.302 | |
| Ischemic heart disease, n (%) |
4 (2.5) | 1 (0.6) | 4.43 | 0.49–40.03 | 0.197 | |
| Cerebral infarction, n (%) | 4 (2.5) | 1 (0.6) | 4.43 | 0.49–40.03 | 0.197 | |
| Family history of colorectal cancer | n (%) | 20 (13.3) | 24 (13.6) | 0.98 | 0.52–1.86 | 0.952 |
| History of colonoscopy | Present, n (%) | 46 (28.2) | 75 (42.4) | 0.53 | 0.34–0.84 | 0.007 |
| History of polyp | Present, n (%) | 23 (14.1) | 18 (10.2) | 1.45 | 0.85–2.80 | 0.318 |
| Location of DET | Bulbs, n (%) | 27 (16.6) | ||||
| Descending part, proximal papilla, n (%) | 31 (19.0) | |||||
| Descending part, distal papilla, n (%) | 96 (58.9) | |||||
| Transverse, n (%) | 9 (5.5) | |||||
| Lesion size of DET | (mm), median [IQR] | 15 [10–25] | ||||
| Histopathology of DET | VC 3 (Low-grade adenoma), | 99 (60.7) | ||||
| n (%) | 47 (28.8) | |||||
| VC 4.1 (high-grade adenoma), n (%) | 12 (7.4) | |||||
| VC 4.2 (noninvasive carcinoma), n (%) | 2 (1.2) | |||||
| VC 5.1 (intramucosal carcinoma), n (%) | 3 (1.8) | |||||
Between-group differences in any tumors identified on colonoscopy
The ADR, the main outcome of the study, was 63.2% in the DET group and 23.6% in the control group (odds ratio [OR], 5.69; 95% confidence interval [CI], 3.55–9.13; P < 0.001; Table 2 ). The number of adenomas per patient was higher in the DET group (median 1; range 0–9) than the control group (median 0; range 0–7) ( P < 0.001). As well, the maximum diameter of adenomas per patient was larger in the DET group (median 4 mm; range 0–26 mm) than the control group (median 0; range 0–10 mm) ( P < 0.001). AN and invasive cancer rates were significantly higher in the DET than control group: AN, 20.9 % vs 3.4%, respectively (OR, 7.51; 95% CI, 3.06–18.42; P < 0.001); and invasive cancer, 3.1% vs 0%, respectively ( P =0.024).
Table 2 Between-group differences in detection of any tumors during colonoscopy.
| DET group | Control group | Odds ratio | 95% CI | P value | ||
| DET, duodenal epithelial tumor. | ||||||
| Adenoma | n, (%) | 103 (63.2) | 41 (23.0) | 5.69 | 3.55–9.13 | < 0.001 |
| Number of adenomas per patient | median [range] | 1 [0–9] | 0 [0–7] | < 0.001 | ||
| Maximum size of adenoma | (mm), median [range] | 4 [0–26] | 0 [0–10] | < 0.001 | ||
| Advanced neoplasia | n, (%) | 34 (20.9) | 6 (3.4) | 7.51 | 3.06–18.42 | < 0.001 |
| Invasive cancer | n, (%) | 5 (3.1) | 0 (0.0) | 12.32 | 0.68–224.56 | .024 |
Characteristics and main outcome after propensity score matching
The results of the propensity score matching test are shown in Table 3 . In each of the two groups, 124 individuals were matched. The analysis showed that ADR was higher in the DET group than in the control group (61.3% vs 23.4; OR, 5.19; 95%CI, 2.99–9.00; P < 0.001). Moreover, the detection rates for AN and invasive cancer were significantly higher in the DET group than in the control group: AN, 20.2 % vs 3.2%, respectively (OR, 7.58; 95%CI, 2.55–22.50; P < 0.001); and invasive cancer, 4.0% vs 0%, respectively ( P =0.006).
Table 3 Relevant characteristics and detection rate of study sample after matching.
|
DET group
(n = 124) |
Control group
(n = 124) |
Odds ratio | 95% CI | P value | ||
| BMI, body mass index; CI, confidence interval; DET, duodenal epithelial tumor; IQR, interquartile range. | ||||||
| Age (yr), median [IQR] | 60 [51–70] | 59 [51–69] | 0.567 | |||
| Sex [male, n (%)] | 77 (62.1) | 77 (62.1) | 1 | 0.60–1.67 | 1.000 | |
| BMI median [IQR] | 23.1 [21.0–25.8] | 22.9 [20.9–25.4] | 0.630 | |||
| Comorbidity | Hypertension, n (%) | 30 (24.2) | 31 (25.0) | 0.96 | 0.54–1.71 | 1.000 |
| Hyperlipidemia, n (%) | 20 (16.1) | 21 (16.9) | 0.94 | 0.48–1.84 | 1.000 | |
| Diabetes, n (%) | 10 (8.1) | 9 (7.3) | 1.12 | 0.44–2.86 | 1.000 | |
| Ischemic heart disease, n (%) |
1 (0.8) | 1 (0.8) | 1 | 0.06–16.17 | 1.000 | |
| Cerebral infarction, n (%) |
2 (1.6) | 1 (0.8) | 2.01 | 0.18–22.52 | 1.000 | |
| Family history of colorectal cancer | n (%) | 17 (13.7) | 20 (16.1) | 0.83 | 0.41–1.66 | 0.722 |
| Adenoma | n, (%) | 76 (61.3) | 29 (23.4) | 5.19 | 2.99–9.00 | < 0.001 |
| Number of adenomas per patient | median [range] | 1 [0–8] | 0 [0–7] | < 0.001 | ||
| Maximum size of adenoma | (mm), median [range] | 4 [0–26] | 0 [0–10] | < 0.001 | ||
| Advanced neoplasia | n, (%) | 25 (20.2) | 4 (3.2) | 7.58 | 2.55–22.50 | < 0.001 |
| Invasive cancer | n, (%) | 5 (4.0) | 0 (0.0) | 11.46 | 0.63–209.52 | 0.006 |
Univariate and multivariable analysis for adenoma and AN detection
We performed logistic regression analysis to confirm whether there was an association between adenoma/AN detection and DETs even after adjustment for confounding factors.
On univariate analysis, older age, male sex, and presence of DET were associated with adenoma detection. On multivariable analysis, older age, male sex, and presence of DET were retained as independent factors for a higher adenoma detection rate and DET was associated with a 5-fold higher rate of adenoma detection (OR, 5.43; 95%CI, 3.29–8.98; P < 0.001) even after adjusting for age, sex, BMI, and family history of colorectal cancer ( Table 4 ).
Table 4 Univariate and multivariable analyses of risk factors for adenoma.
| Factors | Univariate analysis | Multivariable analysis | |||||
| Odds ratio | 95% CI | P value | Adjusted odds ratio | 95% CI | P value | ||
| BMI, body mass index; CI, confidence interval. | |||||||
| Age | (each 10-year interval) | 1.63 | 1.28–1.91 | < 0.001 | 1.34 | 1.08–1.63 | 0.009 |
| Sex | Male Female |
1.73 1 |
1.09–2.75 | 0.018 | 1.88 1 |
1.08–3.26 | 0.025 |
| BMI | (each 5 kg/m2 interval) | 1.11 | 0.85–1.46 | 0.448 | 1.01 | 0.73–1.40 | 0.970 |
| Family history of colorectal cancer |
Present Absent |
1.11 1 |
0.58–2.11 | 0.750 | 1.07 1 |
0.52–2.24 | 0.846 |
| Duodenal epithelial tumor | Present Absent |
5.56 1 |
3.47–8.90 | < 0.001 | 5.43 1 |
3.29–8.98 | < 0.001 |
For AN detection rate, older age and presence of DET were increased on univariate analysis. On multivariable analysis, older age and presence of DET were independent factors for higher AN detection rate and 6-fold higher for AN detection (OR, 6.54; 95%CI, 2.62–16.27; P < 0.001) ( Table 5 ).
Table 5 Univariate and multivariable analyses of risk factors for advanced neoplasia.
| Factors | Univariate analysis | Multivariable analysis | |||||
| Odds ratio | 95% CI | P value | Adjusted odds ratio | 95% CI | P value | ||
| BMI, body mass index; CI, confidence interval. | |||||||
| Age | (each 10-year interval) | 1.82 | 1.31–2.57 | < 0.001 | 1.58 | 1.12–2.26 | 0.007 |
| Sex | Male Female |
2.05 1 |
1.08–3.26 | 0.057 | 2.02 1 |
0.89–4.57 | 0.078 |
| BMI | (each 5 kg/m2 interval) | 1.10 | 0.73–1.66 | 0.649 | |||
| Family history of colorectal cancer | Present Absent |
1.04 1 |
0.38–2.84 | 0.936 | |||
| Duodenal epithelial tumor | Present Absent |
7.56 1 |
3.08–18.54 | < 0.001 | 6.54 1 |
2.62–16.27 | < 0.001 |
Discussion
To the best of our knowledge, this is the first cross-sectional study to analyze the association between DET and colorectal tumors. We identified a significantly higher ADR in the DET group than in the control group, with the number of colorectal adenomas per patient being higher and the maximum diameter of colorectal adenomas being larger for the DET than for the control group. DET was associated with a 5-fold higher rate of adenoma detection and 6-fold more AN detection, even after adjusting for age, sex, and BMI.
The association between DET and colorectal tumors has not previously been specifically determined due to methodological issues, including use of retrospective study designs, which are susceptible to undetected bias effects 4 5 6 7 , and the various factors known to influence ADR, including individual background factors, such as age sex, BMI, history of colonoscopy, interval between colonoscopies 15 , and quality of colonoscopy 16 , such as use of image-enhanced endoscopy. To control for bias to the extent possible, we used an exploratory cross-sectional study design, with strict eligibility criteria defined a priori, including exclusion of individuals with a history of colonoscopy within 3 years prior to the study period. Therefore, the DET and control groups were comparable with regard to background characteristics. Moreover, the same endoscopy instruments and procedures, including pretreatment medications and polyp excision to confirm the pathological results, were used in both groups. Pathological diagnoses of DETs and colorectal tumors were performed by expert pathologists using evidence-based classifications. In particular, colorectal tumors were diagnosed by a single specialist. Therefore, we are confident about the stability of the diagnostic process, and our study provided an objective analysis of risk of colorectal tumors associated with DETs.
The positive association between DETs and colorectal tumors might indicate shared risk factors for these two conditions, such as smoking, overweight, and red meat consumption, which are known risk factors for colorectal tumors 17 18 . A systematic review regarding the risk factors for duodenal tumors 19 did not yield specific findings. In our study, we note that BMI in the DET and control groups did not differ; however, lifestyle habits were not assessed and, therefore, further investigation of risk factors for DETs is warranted.
With regard to application of findings to practice, the higher frequency of colorectal tumors in patients with DETs underscores the importance of colonoscopy examination for all patients with DET, regardless of age, sex, BMI, or family history of colorectal cancer. In fact, in our study, asymptomatic invasive cancer was found in the DET group but not in the control group. This practice would improve early detection of colorectal tumors in this clinical population and, ultimately, improve their prognosis.
The limitations of our study need to be acknowledged. First, the control group consisted of individuals who elected to undergo colonoscopy as part of their health screening. Therefore, it is possible that these individuals were more health-conscious than the general population. As such, rates of detection might not be representative of the general population. It is important to note, however, that ADR was no higher in the control group than in the DET group. Therefore, the difference between DET and the general population would not be less than that observed in this study. Second, the study included only individuals who had not undergone colonoscopy in 3 years prior to the study period. We based this decision on the Japanese guidelines, which recommend colonoscopy screening every 2 to 3 years after polypectomy 20 . We note that the American Cancer Society recommends endoscopy every 10 years 21 . Therefore, there are significant differences in surveillance intervals between Japan and the United States and, thus, it is unknown if our selection of a 3-year period is appropriate. Third, we controlled for age, sex, BMI, comorbidities of diabetes, and family history of colorectal cancer in our propensity score-matched analyses and multivariable analyses. However, there may be other potential confounding factors, such as aspirin use 22 , amount of meat intake 23 , and smoking status 24 , which were not considered. Moreover, potential adjustment bias also remained even with propensity score matching. Fourth, this study was not longitudinal and had no follow-up and the association between the two groups in this study represent a specific time point. Lastly, the dropout rate was higher than expected. The main reason for this was that the study period was during the COVID-19 epidemic and, therefore, many individuals selected to not follow through with scheduled colonoscopy for fear of infection. We excluded dropouts in our analysis; however, because colonoscopy was not performed on the excluded individuals, we were unable to perform an intention-to-treat analysis or sensitivity analysis.
Conclusions
In conclusion, our cross-sectional study identified a significantly higher ADR in patients with DETs. In addition, DETs were associated with a higher frequency of adenomas, AN, and invasive cancer. On the basis of these results, colonoscopy is suggested for patients with DETs.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Yahagi N, Kato M, Ochiai Y et al. Outcomes of endoscopic resection for superficial duodenal epithelial neoplasia. Gastrointest Endosc. 2018;88:676–682. doi: 10.1016/j.gie.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Yamasaki Y, Uedo N, Takeuchi Y et al. Current status of endoscopic resection for superficial nonampullary duodenal epithelial tumors. Digestion. 2018;97:45–51. doi: 10.1159/000484112. [DOI] [PubMed] [Google Scholar]
- 3.Serrano PE, Grant RC, Berk TC et al. Progression and management of duodenal neoplasia in familial adenomatous polyposis: A cohort study. Ann Surg. 2015;261:1138–1144. doi: 10.1097/SLA.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 4.Murray MA, Zimmerman MJ, Ee HC. Sporadic duodenal adenoma is associated with colorectal neoplasia. Gut. 2004;53:261–265. doi: 10.1136/gut.2003.025320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramsoekh D, van Leerdam ME, Dekker E et al. Sporadic duodenal adenoma and the association with colorectal neoplasia: a case-control study. Am J Gastroenterol. 2008;103:1505–1509. doi: 10.1111/j.1572-0241.2007.01775.x. [DOI] [PubMed] [Google Scholar]
- 6.Dariusz A, Jochen R. Increased prevalance of colorectal adenoma in patients with sporadic duodenal adenoma. Eur J Gastroenterol Hepatol. 2009;21:816–818. doi: 10.1097/MEG.0b013e328306c7cc. [DOI] [PubMed] [Google Scholar]
- 7.Pequin P, Manfredi S, Quentin V et al. Patients with sporadic duodenal adenoma are a high-risk group for advanced colorectal neoplasia: results of a case-control study. Aliment Pharmacol Ther. 2007;26:277–282. doi: 10.1111/j.1365-2036.2007.03359.x. [DOI] [PubMed] [Google Scholar]
- 8.Yamasaki Y, Kato M, Takeuchi Y et al. Characteristics of synchronous and metachronous duodenal tumors and association with colorectal cancer: a supplementary analysis. J Gastroenterol. 2023;58:459–469. doi: 10.1007/s00535-023-01964-1. [DOI] [PubMed] [Google Scholar]
- 9.Fujimoto K, Fujishiro M, Kato M et al. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment. Dig Endosc. 2014;26:1–14. doi: 10.1111/den.12183. [DOI] [PubMed] [Google Scholar]
- 10.Kato M, Uedo N, Hokimoto S et al. Guidelines for Gastroenterological Endoscopy in Patients Undergoing Antithrombotic Treatment: 2017 Appendix on Anticoagulants Including Direct Oral Anticoagulants. Dig Endosc. 2018;30:433–440. doi: 10.1111/den.13184. [DOI] [PubMed] [Google Scholar]
- 11.Nagtegaal ID, Odze RD, Klimstra D et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–188. doi: 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130–131. doi: 10.1136/gut.51.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbass R, Rigaux J, Al-Kawas FH. Nonampullary duodenal polyps: characteristics and endoscopic management. Gastrointest Endosc. 2010;71:754–759. doi: 10.1016/j.gie.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 14.Genta RM, Hurrell JM, Sonnenberg A. Duodenal adenomas coincide with colorectal neoplasia. Dig Dis Sci. 2014;59:2249–2254. doi: 10.1007/s10620-014-3131-5. [DOI] [PubMed] [Google Scholar]
- 15.Mangas-Sanjuan C, Seoane A, Alvarez-Gonzalez MA et al. Factors associated with lesion detection in colonoscopy among different indications. United European Gastroenterol J. 2022;10:1008–1019. doi: 10.1002/ueg2.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jrebi NY, Hefty M, Jalouta T et al. High-definition colonoscopy increases adenoma detection rate. Surg Endosc. 2017;31:78–84. doi: 10.1007/s00464-016-4986-7. [DOI] [PubMed] [Google Scholar]
- 17.Wei EK, Colditz GA, Giovannucci EL et al. Cumulative risk of colon cancer up to age 70 years by risk factor status using data from the Nurses' Health Study. Am J Epidemiol. 2009;170:863–872. doi: 10.1093/aje/kwp210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roos E, Seppä K, Pietiläinen O et al. Pairwise association of key lifestyle factors and risk of colorectal cancer: a prospective pooled multicohort study. Cancer Rep (Hoboken) 2022;5:e1612. doi: 10.1002/cnr2.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yabuuchi Y, Yoshida M, Kakushima N et al. Risk factors for non-ampullary duodenal adenocarcinoma: a systematic review. Dig Dis. 2022;40:147–155. doi: 10.1159/000516561. [DOI] [PubMed] [Google Scholar]
- 20.Saito Y, Oka S, Kawamura T et al. Colonoscopy screening and surveillance guidelines. J Jpn Soc Gastrointest Endosc. 2020;62:1519–1560. [Google Scholar]
- 21.Wolf AMD, Fontham ETH, Church TR et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68:250–281. doi: 10.3322/caac.21457. [DOI] [PubMed] [Google Scholar]
- 22.Guo CG, Ma W, Drew DA et al. Aspirin use and risk of colorectal cancer among older adults. JAMA Oncol. 2021;7:428–435. doi: 10.1001/jamaoncol.2020.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diallo A, Deschasaux M, Latino-Martel P et al. Red and processed meat intake and cancer risk: Results from the prospective NutriNet-Santé cohort study. Int J Cancer. 2018;142:230–237. doi: 10.1002/ijc.31046. [DOI] [PubMed] [Google Scholar]
- 24.Giovannucci E, Rimm EB, Stampfer MJ et al. A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in U.S. men. J Natl Cancer Inst. 1994;86:183–191. doi: 10.1093/jnci/86.3.183. [DOI] [PubMed] [Google Scholar]